Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies
Abstract
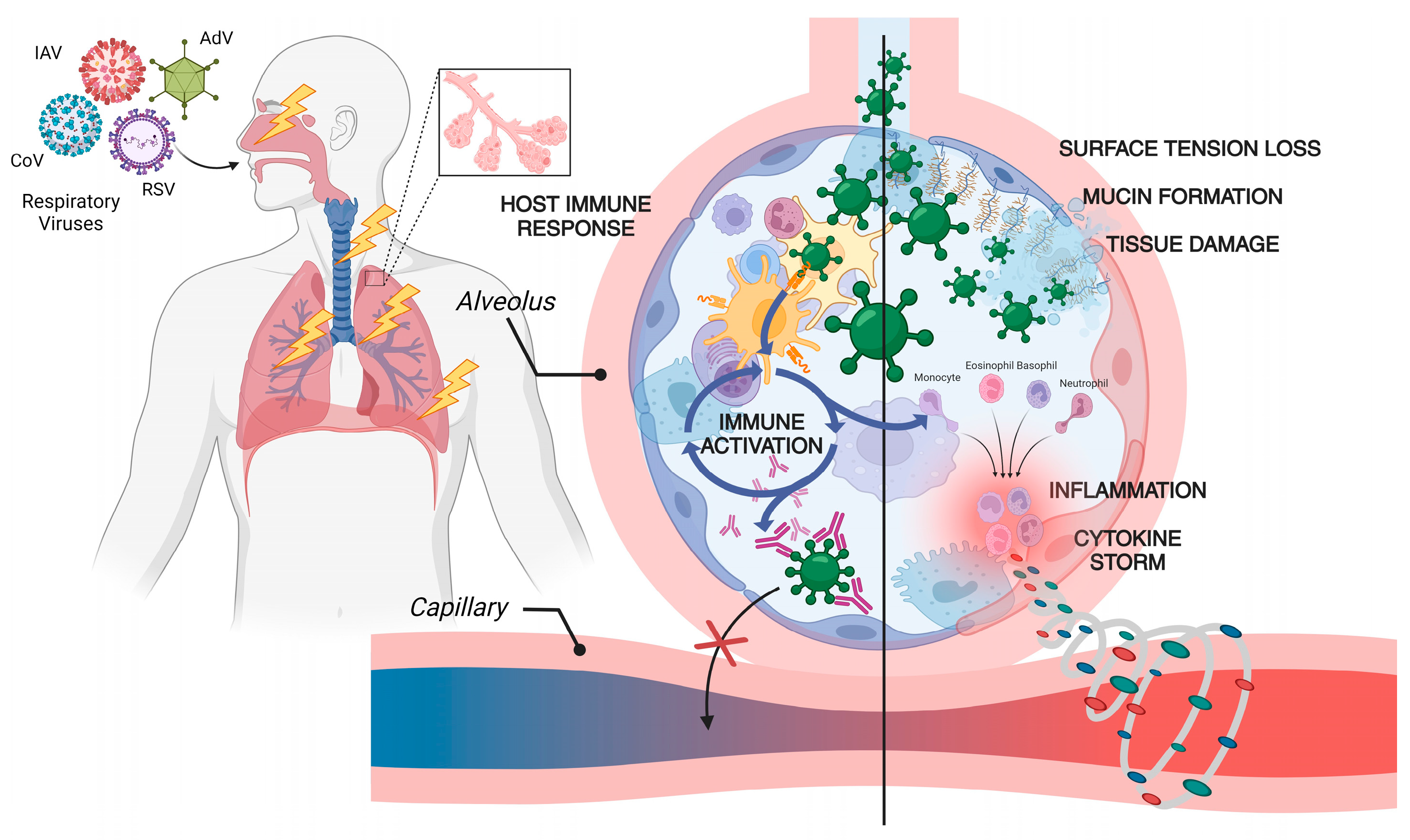
:1. Respiratory Viruses: An Overview
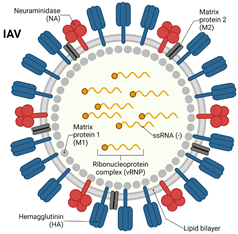
2. Virology of Respiratory Viruses: Influenza and Coronavirus
3. Immune Response Against Respiratory Viruses: Influenza and Coronavirus
4. Epidemiology and Transmission Dynamics: Influenza and Coronavirus
5. Antiviral Therapies for Respiratory Viruses: Influenza and Coronavirus
5.1. Antiviral Drug Options for Influenza Virus
| Antiviral Agents | Virus Target | Mechanism of Action | Route of Administration | Category | Target | Indication | Approval Status | References |
|---|---|---|---|---|---|---|---|---|
| Favipiravir (Avigan®, Toyama Chemical, Tokyo, Japan) | Influenza A/B, SARS-CoV-2 | RNA-dependent RNA polymerase inhibitor | Oral | Nucleoside analog | RdRp inhibitor | Investigational for COVID-19 and influenza | CT approved | [104] |
| Oseltamivir (Tamiflu®, Roche, Basel, Switzerland) | Influenza A/B | Neuraminidase inhibitor (prevents viral release) | Oral | Neuraminidase inhibitor | Neuraminidase | Treatment and prophylaxis of influenza A and B | FDA approved | [97] |
| Zanamivir (Relenza®, GSK, Middlesex, UK) | Influenza A/B | Neuraminidase inhibitor | Inhalation | Neuraminidase inhibitor | Virus budding inhibitor | Treatment of influenza A and B | CT approved | [105] |
| Peramivir (Rapivab®, BioCryst Pharmaceuticals, Durham, NC, USA) | Influenza A/B | Neuraminidase inhibitor | Intravenous (IV) | Neuraminidase inhibitor | Virus budding inhibitor | Acute, uncomplicated influenza | FDA approved | [106] |
| Baloxavir marboxil (Xofluza, Shionogi, Osaka, Japan) | Influenza A/B | Enzyme inhibitor, targeting the influenza virus’ cap-dependent endonuclease activity | Oral | Cap-dependent endonuclease inhibitor | RNA polymerase inhibitor | For individuals who are twelve years of age or older that have presented symptoms of this infection for no more than 48 h. | CT approved | [107] |
5.2. Antiviral Drug Options for Coronavirus (SARS-CoV-2)
5.2.1. Viral RNA-Dependent RNA Polymerase (RdRp) Targeting Drugs
- (a)
- Remdesivir
- (b)
- Molnupiravir
5.2.2. Other Antiviral Drugs
- (a)
- Favipiravir
- (b)
- Ribavirin
- (c)
- Lopinavir/Ritonavir
- (d)
- Nirmatrelvir/Ritonavir (Paxlovid)
5.2.3. Convalescent Plasma
5.2.4. Monoclonal Antibodies
| Antiviral Agents | Virus Target | Mechanism of Action | Route of Administration | Category | Target | Indication | Approval Status | References |
|---|---|---|---|---|---|---|---|---|
| Remdesivir (Veklury®) | SARS-CoV-2 | Inhibits RNA-dependent RNA polymerase (RdRp) | Intravenous (IV) | Adenosine analog | RdRp inhibitor | Hospitalized and non-hospitalized adults and pediatrics at high risk of progression to severe disease | FDA approved | [146] |
| Molnupiravir (Lagevrio®) | SARS-CoV-2 | Induces viral RNA mutagenesis | Oral | Nucleoside analog | RdRp inhibitor | Adults with mild-to-moderate COVID-19 are at high risk of progression to severe disease | EUA and approved in many countries | [147] |
| Nirmatrelvir + Ritonavir (Paxlovid®) | SARS-CoV-2 | Protease inhibitor (inhibits viral replication) | Oral | Protease inhibitor | Mpro | Mild-to-moderate COVID-19 patients at risk of progression to severe disease | Approved in the United States, the United Kingdom, and EU; EUA in many countries | [148] |
| Bebtelovimab | SARS-CoV-2 (all variants) | Monoclonal antibody (targets spike protein) | Intravenous (IV) | mAb | S-protein | Treatment of COVID-19 in non-hospitalized patients | EUA by US FDA | [143] |
| Sotrovimab (Xevudy) | SARS-CoV-2 | Monoclonal antibody (targets highly conserved sequences) | Intravenous (IV) | mAb | S-protein | Mild-to-moderate COVID-19 patients at risk of progression to severe disease | EUA or approved in many countries | [149] |
| Casirivimab and imdevimab (REGEN-COV) | SARS-CoV-2 | Monoclonal antibody (targets spike protein) | Intravenous (IV) and subcutaneous (SC) | mAb | S-protein | Mild or moderate COVID-19, conditional approval for the prophylaxis and treatment of acute COVID-19 in the United Kingdom | EUA in many countries | [141] |
| Ensitrelvir (Xocova) | SARS-CoV-2 | 3C-like protease inhibitor | Oral | Small molecule | Mpro | May be effective in treating smell and taste loss from the COVID-19 infection | Approved in Japan | [150] |
| Simnotrelvir + ritonavir (Xiannuoxin) | SARS-CoV-2 | Protease inhibitor | Oral | Small molecule | Mpro | Mild-to-moderate COVID-19 | Approved in China | [151] |
| VV116 | SARS-CoV-2 | Nucleoside analogue antiviral drug | Oral | Small molecule | RdRp inhibitor | Non-hospitalized adults with mild-to-moderate disease | Approved in China | [152] |
6. Clinical Studies and Approval of Antiviral Treatments for RVs
7. Antiviral Vaccines for Respiratory Virus: Influenza and Coronavirus
7.1. Vaccine Types
7.1.1. Whole Pathogen Vaccines
7.1.2. Subunit Vaccines
7.1.3. Nucleic Acid Vaccines
7.2. Viral Vaccines Combating Influenza Virus
7.2.1. Inactivated Influenza Vaccine
7.2.2. Live Attenuated Influenza Vaccines
7.2.3. Recombinant HA Vaccine
7.3. Viral Vaccines Combating Coronavirus
7.3.1. Inactivated Whole Virus Vaccines
7.3.2. Protein Subunit Vaccine
7.3.3. mRNA Vaccines
7.3.4. Viral Vector-Based Vaccines
8. Lesson(s) Learnt from These Outbreaks
9. Combating Future RV Outbreak
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- van Doorn, H.R.; Yu, H. 33—Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: London, UK, 2020; pp. 284–288. [Google Scholar]
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.M.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.J.; Jia, N.; Richardson, S.; Huang, C. Viral respiratory infections in a rapidly changing climate: The need to prepare for the next pandemic. EBioMedicine 2023, 93, 104593. [Google Scholar] [CrossRef]
- Kessler, S.; Harder, T.C.; Schwemmle, M.; Ciminski, K. Influenza A Viruses and Zoonotic Events—Are We Creating Our Own Reservoirs? Viruses 2021, 13, 2250. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef]
- Escudero-Pérez, B.; Lalande, A.; Mathieu, C.; Lawrence, P. Host–Pathogen Interactions Influencing Zoonotic Spillover Potential and Transmission in Humans. Viruses 2023, 15, 599. [Google Scholar] [CrossRef]
- Jelinek, H.F.; Mousa, M.; Alefishat, E.; Osman, W.; Spence, I.; Bu, D.; Feng, S.F.; Byrd, J.; Magni, P.A.; Sahibzada, S.; et al. Evolution, Ecology, and Zoonotic Transmission of Betacoronaviruses: A Review. Front. Vet. Sci. 2021, 8, 644414. [Google Scholar] [CrossRef]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Sooksawasdi Na Ayudhya, S.; Kuiken, T. Reverse Zoonosis of COVID-19: Lessons From the 2009 Influenza Pandemic. Vet. Pathol. 2021, 58, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Dharmarajan, G.; Khan, I. Evolution of pathogen tolerance and emerging infections: A missing experimental paradigm. Elife 2021, 10, e68874. [Google Scholar] [CrossRef]
- Furuse, Y.; Oshitani, H. Viruses That Can and Cannot Coexist With Humans and the Future of SARS-CoV-2. Front. Microbiol. 2020, 11, 583252. [Google Scholar] [CrossRef]
- Mihaescu, G.; Chifiriuc, M.C.; Iliescu, C.; Vrancianu, C.O.; Ditu, L.-M.; Marutescu, L.G.; Grigore, R.; Berteșteanu, Ș.; Constantin, M.; Gradisteanu Pircalabioru, G. SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management. Microorganisms 2020, 8, 1468. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef]
- Bhadoria, P.; Gupta, G.; Agarwal, A. Viral Pandemics in the Past Two Decades: An Overview. J. Fam. Med. Prim. Care 2021, 10, 2745–2750. [Google Scholar] [CrossRef]
- Makarov, V.; Riabova, O.; Ekins, S.; Pluzhnikov, N.; Chepur, S. The past, present and future of RNA respiratory viruses: Influenza and coronaviruses. Pathog. Dis. 2020, 78, ftaa046. [Google Scholar] [CrossRef]
- Šimičić, P.; Židovec-Lepej, S. A Glimpse on the Evolution of RNA Viruses: Implications and Lessons from SARS-CoV-2. Viruses 2023, 15, 1. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Z.; Zhao, S.; Gao, R. A Comparison of Etiology, Pathogenesis, Vaccinal and Antiviral Drug Development between Influenza and COVID-19. Int. J. Mol. Sci. 2023, 24, 6369. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Nair, A.; Mohagaonkar, S.; Yadav, P.; Gambhir, K.; Tyagi, N.; Sharma, R.K.; Butola, B.S.; Sharma, N. Threat, challenges, and preparedness for future pandemics: A descriptive review of phylogenetic analysis based predictions. Infect. Genet. Evol. 2022, 98, 105217. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Branda, F.; Cella, E.; Scarpa, F.; Bazzani, L.; Ciccozzi, A.; Slavov, S.N.; Benvenuto, D.; Sanna, D.; Casu, M.; et al. Epidemic history and evolution of an emerging threat of international concern, the severe acute respiratory syndrome coronavirus 2. J. Med. Virol. 2023, 95, e29012. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.N.; Kackos, C.M.; Webby, R.J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021, 53, 737–749. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- McCaughey, C. Influenza: A virus of our times. Ulster Med. J. 2010, 79, 46–51. [Google Scholar]
- Alsobaie, S. Understanding the Molecular Biology of SARS-CoV-2 and the COVID-19 Pandemic: A Review. Infect. Drug Resist. 2021, 14, 2259–2268. [Google Scholar] [CrossRef]
- Kaye, A.D.; Cornett, E.M.; Brondeel, K.C.; Lerner, Z.I.; Knight, H.E.; Erwin, A.; Charipova, K.; Gress, K.L.; Urits, I.; Urman, R.D.; et al. Biology of COVID-19 and related viruses: Epidemiology, signs, symptoms, diagnosis, and treatment. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 269–292. [Google Scholar] [CrossRef]
- Moghadami, M. A Narrative Review of Influenza: A Seasonal and Pandemic Disease. Iran. J. Med. Sci. 2017, 42, 2–13. [Google Scholar]
- Taubenberger, J.K.; Morens, D.M. Influenza: The once and future pandemic. Public Health Rep. 2010, 125 (Suppl. 3), 16–26. [Google Scholar] [CrossRef]
- Caini, S.; Kroneman, M.; Wiegers, T.; El Guerche-Séblain, C.; Paget, J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: A systematic literature review. Influenza Other Respir. Viruses 2018, 12, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Mettelman, R.C.; Thomas, P.G. Human Susceptibility to Influenza Infection and Severe Disease. Cold Spring Harb. Perspect. Med. 2021, 11, a038711. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.K.W.; Poon, L.L.M. Biology of Influenza A Virus. Ann. N. Y. Acad. Sci. 2007, 1102, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Singhal, T. The Emergence of Omicron: Challenging Times Are Here Again! Indian J. Pediatr. 2022, 89, 490–496. [Google Scholar] [CrossRef]
- Allan, M.; Lièvre, M.; Laurenson-Schafer, H.; de Barros, S.; Jinnai, Y.; Andrews, S.; Stricker, T.; Formigo, J.P.; Schultz, C.; Perrocheau, A.; et al. The World Health Organization COVID-19 surveillance database. Int. J. Equity Health 2022, 21, 167. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.; Somogyvári, F.; Virok, D.P.; Noseda, M.; McLean, G.R. Decoding COVID-19 with the SARS-CoV-2 Genome. Curr. Genet. Med. Rep. 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318. [Google Scholar] [CrossRef]
- Brant, A.C.; Tian, W.; Majerciak, V.; Yang, W.; Zheng, Z.-M. SARS-CoV-2: From its discovery to genome structure, transcription, and replication. Cell Biosci. 2021, 11, 136. [Google Scholar] [CrossRef]
- Jiang, X.; Li, D.; Maghsoudloo, M.; Zhang, X.; Ma, W.; Fu, J. Targeting furin, a cellular proprotein convertase, for COVID-19 prevention and therapeutics. Drug Discov. Today 2024, 29, 104026. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Y.; Rui, X.; Yang, Y.; Ling, C.; Liu, S.; Liu, S.; Wang, Y. Animal models for COVID-19: Advances, gaps and perspectives. Signal Transduct. Target. Ther. 2022, 7, 220. [Google Scholar] [CrossRef]
- Borkotoky, S.; Dey, D.; Hazarika, Z. Interactions of angiotensin-converting enzyme-2 (ACE2) and SARS-CoV-2 spike receptor-binding domain (RBD): A structural perspective. Mol. Biol. Rep. 2023, 50, 2713–2721. [Google Scholar] [CrossRef]
- Nguyen, H.; Nguyen, H.L.; Lan, P.D.; Thai, N.Q.; Sikora, M.; Li, M.S. Interaction of SARS-CoV-2 with host cells and antibodies: Experiment and simulation. Chem. Soc. Rev. 2023, 52, 6497–6553. [Google Scholar] [CrossRef]
- Bejoy, J.; Williams, C.I.; Cole, H.J.; Manzoor, S.; Davoodi, P.; Battaile, J.I.; Kaushik, A.; Nikolaienko, S.I.; Brelidze, T.I.; Gychka, S.G.; et al. Effects of spike proteins on angiotensin converting enzyme 2 (ACE2). Arch. Biochem. Biophys. 2023, 748, 109769. [Google Scholar] [CrossRef] [PubMed]
- Barthe, M.; Hertereau, L.; Lamghari, N.; Osman-Ponchet, H.; Braud, V.M. Receptors and Cofactors That Contribute to SARS-CoV-2 Entry: Can Skin Be an Alternative Route of Entry? Int. J. Mol. Sci. 2023, 24, 6253. [Google Scholar] [CrossRef] [PubMed]
- Costa Dos Santos, J.; Ximenes Rabelo, M.; Mattana Sebben, L.; de Souza Carneiro, M.V.; Bosco Lopes Botelho, J.; Cardoso Neto, J.; Nogueira Barbosa, A.; Monteiro de Carvalho, D.; Pontes, G.S. Persistence of SARS-CoV-2 Antigens in the Nasal Mucosa of Eight Patients with Inflammatory Rhinopathy for over 80 Days following Mild COVID-19 Diagnosis. Viruses 2023, 15, 899. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.P.Y.; Cheung, M.C.; Perera, R.; Ng, K.C.; Bui, C.H.T.; Ho, J.C.W.; Ng, M.M.T.; Kuok, D.I.T.; Shih, K.C.; Tsao, S.W.; et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: An analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 2020, 8, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Cilloniz, C.; Luna, C.M.; Hurtado, J.C.; Marcos, M.; Torres, A. Respiratory viruses: Their importance and lessons learned from COVID-19. Eur. Respir. Rev. 2022, 31, 220051. [Google Scholar] [CrossRef]
- Memoli, M.J.; Athota, R.; Reed, S.; Czajkowski, L.; Bristol, T.; Proudfoot, K.; Hagey, R.; Voell, J.; Fiorentino, C.; Ademposi, A.; et al. The Natural History of Influenza Infection in the Severely Immunocompromised vs Nonimmunocompromised Hosts. Clin. Infect. Dis. 2013, 58, 214–224. [Google Scholar] [CrossRef]
- Walsh, K.A.; Spillane, S.; Comber, L.; Cardwell, K.; Harrington, P.; Connell, J.; Teljeur, C.; Broderick, N.; de Gascun, C.F.; Smith, S.M.; et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J. Infect. 2020, 81, 847–856. [Google Scholar] [CrossRef]
- Noh, H.-E.; Rha, M.-S. Mucosal Immunity against SARS-CoV-2 in the Respiratory Tract. Pathogens 2024, 13, 113. [Google Scholar] [CrossRef]
- Ahmed-Hassan, H.; Sisson, B.; Shukla, R.K.; Wijewantha, Y.; Funderburg, N.T.; Li, Z.; Hayes, D., Jr.; Demberg, T.; Liyanage, N.P.M. Innate Immune Responses to Highly Pathogenic Coronaviruses and Other Significant Respiratory Viral Infections. Front. Immunol. 2020, 11, 1979. [Google Scholar] [CrossRef]
- Davidson, S.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, K.; Gusarova, G.A.; Islam, M.N.; Subramanian, M.; Cohen, T.S.; Prince, A.S.; Bhattacharya, J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014, 506, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.J.; Angel, N.; Kato, M.; López, J.A.; MacDonald, K.; Vuckovic, S.; Hart, D.N.J. The role of dendritic cells in the innate immune system. Microbes Infect. 2000, 2, 257–272. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.-L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 860915. [Google Scholar] [CrossRef]
- Yunis, J.; Short, K.R.; Yu, D. Severe respiratory viral infections: T-cell functions diverging from immunity to inflammation. Trends Microbiol. 2023, 31, 644–656. [Google Scholar] [CrossRef]
- Braciale, T.J.; Sun, J.; Kim, T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 2012, 12, 295–305. [Google Scholar] [CrossRef]
- Wang, L.; Nicols, A.; Turtle, L.; Richter, A.; Duncan, C.J.; Dunachie, S.J.; Klenerman, P.; Payne, R.P. T cell immune memory after covid-19 and vaccination. BMJ Med. 2023, 2, e000468. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Tonacci, A.; Isola, S.; Allegra, A.; Gangemi, S. The Potential Role of Cytokine Storm Pathway in the Clinical Course of Viral Respiratory Pandemic. Biomedicines 2021, 9, 1688. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 589095. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.F.; Brienen, N.; Kretzschmar, M.E. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics 2010, 2, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, Z. Transmission and infection risk of COVID-19 when people coughing in an elevator. Build. Environ. 2023, 238, 110343. [Google Scholar] [CrossRef]
- Agrawal, A.; Bhardwaj, R. Probability of COVID-19 infection by cough of a normal person and a super-spreader. Phys. Fluids (1994) 2021, 33, 031704. [Google Scholar] [CrossRef]
- Thangavel, R.R.; Bouvier, N.M. Animal models for influenza virus pathogenesis, transmission, and immunology. J. Immunol. Methods 2014, 410, 60–79. [Google Scholar] [CrossRef]
- van Doorn, A.S.; Meijer, B.; Frampton, C.M.A.; Barclay, M.L.; de Boer, N.K.H. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment. Pharmacol. Ther. 2020, 52, 1276–1288. [Google Scholar] [CrossRef]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 269–283. [Google Scholar] [CrossRef]
- Al Khatib, H.A.; Coyle, P.V.; Al Maslamani, M.A.; Al Thani, A.A.; Pathan, S.A.; Yassine, H.M. Molecular and biological characterization of influenza A viruses isolated from human fecal samples. Infect. Genet. Evol. 2021, 93, 104972. [Google Scholar] [CrossRef]
- Kutti-Sridharan, G.; Vegunta, R.; Vegunta, R.; Mohan, B.P.; Rokkam, V.R.P. SARS-CoV2 in Different Body Fluids, Risks of Transmission, and Preventing COVID-19: A Comprehensive Evidence-Based Review. Int. J. Prev. Med. 2020, 11, 97. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Ren, M.; Dai, J. Influenza A (H1N1). Radiol. Infect. Dis. 2015, 1, 465–514. [Google Scholar] [CrossRef]
- Near, A.M.; Tse, J.; Young-Xu, Y.; Hong, D.K.; Reyes, C.M. Burden of influenza hospitalization among high-risk groups in the United States. BMC Health Serv. Res. 2022, 22, 1209. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Nalla, L.V.; Sharma, M.; Sharma, N.; Singh, A.A.; Malim, F.M.; Ghatage, M.; Mukarram, M.; Pawar, A.; Parihar, N.; et al. Association of COVID-19 with Comorbidities: An Update. ACS Pharmacol. Transl. Sci. 2023, 6, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Khadela, A.; Soni, S.; Megha, K.; Bhagat, S.; Chavda, V.P. A Review on the Impact of the SARS-CoV-2 Omicron Subvariant on Elderly Patients with Diverse Co-Morbidities. Biologics 2023, 3, 138–157. [Google Scholar] [CrossRef]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef]
- De Serres, G.; Rouleau, I.; Hamelin, M.E.; Quach, C.; Skowronski, D.; Flamand, L.; Boulianne, N.; Li, Y.; Carbonneau, J.; Bourgault, A.; et al. Contagious period for pandemic (H1N1) 2009. Emerg. Infect. Dis. 2010, 16, 783–788. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Radhi, A.S. Uncommon Presentation of COVID-19 With Long Incubation Period and Only Gastrointestinal Symptoms in a Fully Vaccinated Patient: Is There a Relation? Cureus 2021, 13, e16028. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The 1918 Influenza Pandemic and Its Legacy. Cold Spring Harb. Perspect. Med. 2020, 10, a038695. [Google Scholar] [CrossRef]
- Worldometer, D. COVID-19 Coronavirus Pandemic. World Health Organization. 2020. Available online: https://www.worldometers.info/coronavirus/ (accessed on 24 October 2024).
- Fritz, M.; Gries, T.; Redlin, M. The effectiveness of vaccination, testing, and lockdown strategies against COVID-19. Int. J. Health Econ. Manag. 2023, 23, 585–607. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.A.; Castro, R.d.C.; Masters, H.L.; Carrico, R. Influenza Management During the COVID-19 Pandemic: A Review of Recent Innovations in Antiviral Therapy and Relevance to Primary Care Practice. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 974–991. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Tiwari, R.; Sharun, K.; Dhama, K.; Harapan, H. Antivirals for COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 9, 90–98. [Google Scholar] [CrossRef]
- Jones, J.C.; Yen, H.-L.; Adams, P.; Armstrong, K.; Govorkova, E.A. Influenza antivirals and their role in pandemic preparedness. Antivir. Res. 2023, 210, 105499. [Google Scholar] [CrossRef]
- von Itzstein, M.; Wu, W.Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Van Phan, T.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef]
- Świerczyńska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. [Google Scholar] [CrossRef]
- Singh, P.P.; Sodhi, K.K.; Bali, A.K.; Shree, P. Influenza A virus and its antiviral drug treatment options. Med. Microecol. 2023, 16, 100083. [Google Scholar] [CrossRef]
- Davies, B.E. Pharmacokinetics of oseltamivir: An oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 2010, 65 (Suppl. 2), ii5–ii10. [Google Scholar] [CrossRef]
- Yu, Y.; Garg, S.; Yu, P.A.; Kim, H.J.; Patel, A.; Merlin, T.; Redd, S.; Uyeki, T.M. Peramivir use for treatment of hospitalized patients with influenza A(H1N1)pdm09 under emergency use authorization, October 2009–June 2010. Clin. Infect. Dis. 2012, 55, 8–15. [Google Scholar] [CrossRef]
- Sorbello, A.; Jones, S.C.; Carter, W.; Struble, K.; Boucher, R.; Truffa, M.; Birnkrant, D.; Gada, N.; Camilli, S.; Chan, I.; et al. Emergency use authorization for intravenous peramivir: Evaluation of safety in the treatment of hospitalized patients infected with 2009 H1N1 influenza A virus. Clin. Infect. Dis. 2012, 55, 1–7. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef] [PubMed]
- Colman, P.M. Zanamivir: An influenza virus neuraminidase inhibitor. Expert. Rev. Anti Infect. Ther. 2005, 3, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Peramivir: A Review in Uncomplicated Influenza. Drugs 2018, 78, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, R.; Shaw, M.L. Baloxavir marboxil: The new influenza drug on the market. Curr. Opin. Virol. 2019, 35, 14–18. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Vicenti, I.; Zazzi, M.; Saladini, F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin. Ther. Pat. 2021, 31, 325–337. [Google Scholar] [CrossRef]
- Bekheit, M.S.; Panda, S.S.; Girgis, A.S. Potential RNA-dependent RNA polymerase (RdRp) inhibitors as prospective drug candidates for SARS-CoV-2. Eur. J. Med. Chem. 2023, 252, 115292. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Tian, F.; Feng, Q.; Chen, Z. Efficacy and Safety of Molnupiravir Treatment for COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Antimicrob. Agents 2023, 62, 106870. [Google Scholar] [CrossRef]
- Pourkarim, F.; Pourtaghi-Anvarian, S.; Rezaee, H. Molnupiravir: A new candidate for COVID-19 treatment. Pharmacol. Res. Perspect. 2022, 10, e00909. [Google Scholar] [CrossRef]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H., 3rd; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhong, W. Mechanism of action of favipiravir against SARS-CoV-2: Mutagenesis or chain termination? Innovation 2021, 2, 100165. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Sreekanth Reddy, O.; Lai, W.F. Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options. Chembiochem 2021, 22, 939–948. [Google Scholar] [CrossRef]
- Xu, Y.; Li, M.; Zhou, L.; Liu, D.; He, W.; Liang, W.; Sun, Q.; Sun, H.; Li, Y.; Liu, X. Ribavirin Treatment for Critically Ill COVID-19 Patients: An Observational Study. Infect. Drug Resist. 2021, 14, 5287–5291. [Google Scholar] [CrossRef]
- Hung, I.F.; Lung, K.C.; Tso, E.Y.; Liu, R.; Chung, T.W.; Chu, M.Y.; Ng, Y.Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Banerjee, R.; Perera, L.; Tillekeratne, L.M.V. Potential SARS-CoV-2 main protease inhibitors. Drug Discov. Today 2021, 26, 804–816. [Google Scholar] [CrossRef]
- Chandwani, A.; Shuter, J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Ther. Clin. Risk Manag. 2008, 4, 1023–1033. [Google Scholar] [CrossRef]
- Loos, N.H.C.; Beijnen, J.H.; Schinkel, A.H. The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism? Int. J. Mol. Sci. 2022, 23, 9866. [Google Scholar] [CrossRef]
- Lamb, Y.N. Nirmatrelvir Plus Ritonavir: First Approval. Drugs 2022, 82, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Feys, J.R. SARS-CoV-2 Mpro Inhibitors: Achieved Diversity, Developing Resistance and Future Strategies. Future Pharmacol. 2023, 3, 80–107. [Google Scholar] [CrossRef]
- Vries, M.d.; Mohamed, A.S.; Prescott, R.A.; Valero-Jimenez, A.M.; Desvignes, L.; O’Connor, R.; Steppan, C.; Devlin, J.C.; Ivanova, E.; Herrera, A.; et al. A Comparative Analysis of SARS-CoV-2 Antivirals Characterizes 3CL<sup>pro</sup> Inhibitor PF-00835231 as a Potential New Treatment for COVID-19. J. Virol. 2021, 95, 10. [Google Scholar] [CrossRef]
- Ripoll, J.G.; van Helmond, N.; Senefeld, J.W.; Wiggins, C.C.; Klassen, S.A.; Baker, S.E.; Larson, K.F.; Murphy, B.M.; Andersen, K.J.; Ford, S.K.; et al. Convalescent Plasma for Infectious Diseases: Historical Framework and Use in COVID-19. Clin. Microbiol. Newsl. 2021, 43, 23–32. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Pau, A.K.; Aberg, J.; Baker, J.; Belperio, P.S.; Coopersmith, C.; Crew, P.; Grund, B.; Gulick, R.M.; Harrison, C.; Kim, A.; et al. Convalescent Plasma for the Treatment of COVID-19: Perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann. Intern. Med. 2021, 174, 93–95. [Google Scholar] [CrossRef]
- Sanz, C.; Nomdedeu, M.; Pereira, A.; Sauleda, S.; Alonso, R.; Bes, M.; Brillembourg, H.; García-Vidal, C.; Millan, A.; Martínez-Llonch, N.; et al. Efficacy of early transfusion of convalescent plasma with high-titer SARS-CoV-2 neutralizing antibodies in hospitalized patients with COVID-19. Transfusion 2022, 62, 974–981. [Google Scholar] [CrossRef]
- Libster, R.; Marc, G.P.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M.; Pirofski, L.A.; Burnouf, T.; Paneth, N.; Joyner, M.J.; Casadevall, A. COVID-19 Convalescent Plasma and Clinical Trials: Understanding Conflicting Outcomes. Clin. Microbiol. Rev. 2022, 35, e0020021. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, Z.; Ma, H.; Shao, S.; Kang, H.; Tong, Z. The efficiency of convalescent plasma in COVID-19 patients: A systematic review and meta-analysis of randomized controlled clinical trials. Front. Immunol. 2022, 13, 964398. [Google Scholar] [CrossRef]
- Focosi, D.; McConnell, S.; Casadevall, A.; Cappello, E.; Valdiserra, G.; Tuccori, M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Infect. Dis. 2022, 22, e311–e326. [Google Scholar] [CrossRef] [PubMed]
- Wynia, M.K.; Beaty, L.E.; Bennett, T.D.; Carlson, N.E.; Davis, C.B.; Kwan, B.M.; Mayer, D.A.; Ong, T.C.; Russell, S.; Steele, J.D.; et al. Real-World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID-19 Outpatients. Chest 2023, 163, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- An EUA for Bamlanivimab-A Monoclonal Antibody for COVID-19. JAMA 2021, 325, 880–881. [CrossRef] [PubMed]
- An EUA for casirivimab and imdevimab for COVID-19. Med. Lett. Drugs Ther. 2020, 62, 201–202.
- Razonable, R.R.; Pawlowski, C.; O’Horo, J.C.; Arndt, L.L.; Arndt, R.; Bierle, D.M.; Borgen, M.D.; Hanson, S.N.; Hedin, M.C.; Lenehan, P.; et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 2021, 40, 101102. [Google Scholar] [CrossRef]
- Deeks, E.D. Casirivimab/Imdevimab: First Approval. Drugs 2021, 81, 2047–2055. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef]
- Hayek, S.; Ben-Shlomo, Y.; Dagan, N.; Reis, B.Y.; Barda, N.; Kepten, E.; Roitman, A.; Shapira, S.; Yaron, S.; Balicer, R.D.; et al. Effectiveness of REGEN-COV antibody combination in preventing severe COVID-19 outcomes. Nat. Commun. 2022, 13, 4480. [Google Scholar] [CrossRef]
- Molina, K.C.; Kennerley, V.; Beaty, L.E.; Bennett, T.D.; Carlson, N.E.; Mayer, D.A.; Peers, J.L.; Russell, S.; Wynia, M.K.; Aggarwal, N.R.; et al. Real-world evaluation of bebtelovimab effectiveness during the period of COVID-19 Omicron variants, including BA.4/BA.5. Int. J. Infect. Dis. 2023, 132, 34–39. [Google Scholar] [CrossRef]
- Liew, M.N.Y.; Kua, K.P.; Lee, S.W.H.; Wong, K.K. SARS-CoV-2 neutralizing antibody bebtelovimab—A systematic scoping review and meta-analysis. Front. Immunol. 2023, 14, 1100263. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, F.; Mirandola, M.; Savoldi, A.; De Nardo, P.; Morra, M.; Tebon, M.; Armellini, M.; De Luca, G.; Calandrino, L.; Sasset, L.; et al. Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern. eLife 2022, 11, e79639. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Remdesivir: A Review in COVID-19. Drugs 2023, 83, 1215–1237. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Molnupiravir: First Approval. Drugs 2022, 82, 455–460. [Google Scholar] [CrossRef]
- Harris, E. FDA Grants Full Approval to Paxlovid, COVID-19 Antiviral Treatment. JAMA 2023, 329, 2118. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Sotrovimab: First Approval. Drugs 2022, 82, 477–484. [Google Scholar] [CrossRef]
- Syed, Y.Y. Ensitrelvir Fumaric Acid: First Approval. Drugs 2024, 84, 721–728. [Google Scholar] [CrossRef]
- Zhu, K.W. Deuremidevir and Simnotrelvir-Ritonavir for the Treatment of COVID-19. ACS Pharmacol. Transl. Sci. 2023, 6, 1306–1309. [Google Scholar] [CrossRef]
- Fan, X.; Dai, X.; Ling, Y.; Wu, L.; Tang, L.; Peng, C.; Huang, C.; Liu, H.; Lu, H.; Shen, X.; et al. Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: A multicentre, double-blind, phase 3, randomised controlled study. Lancet Infect. Dis. 2024, 24, 129–139. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Wilson-Welder, J.H.; Torres, M.P.; Kipper, M.J.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Vaccine adjuvants: Current challenges and future approaches. J. Pharm. Sci. 2009, 98, 1278–1316. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Gillim-Ross, L.; Subbarao, K. Emerging respiratory viruses: Challenges and vaccine strategies. Clin. Microbiol. Rev. 2006, 19, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Ellis, D.; King, N.P. New Vaccine Design and Delivery Technologies. J. Infect. Dis. 2019, 219, S88–S96. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Florez, M.I.; Stergiopoulos, S.; Peña, Y.; Smith, Z.; Wilkinson, M.; Getz, K.A. Development Times and Approval Success Rates for Drugs to Treat Infectious Diseases. Clin. Pharmacol. Ther. 2020, 107, 324–332. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O., 3rd. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Calina, D.; Docea, A.O.; Petrakis, D.; Egorov, A.M.; Ishmukhametov, A.A.; Gabibov, A.G.; Shtilman, M.I.; Kostoff, R.; Carvalho, F.; Vinceti, M.; et al. Towards effective COVID-19 vaccines: Updates, perspectives and challenges (Review). Int. J. Mol. Med. 2020, 46, 3–16. [Google Scholar] [CrossRef]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Elveborg, S.; Monteil, V.M.; Mirazimi, A. Methods of Inactivation of Highly Pathogenic Viruses for Molecular, Serology or Vaccine Development Purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Vartak, A.; Sucheck, S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef]
- Foged, C. Subunit vaccines of the future: The need for safe, customized and optimized particulate delivery systems. Ther. Deliv. 2011, 2, 1057–1077. [Google Scholar] [CrossRef]
- Bolhassani, A.; Yazdi, S.R. DNA immunization as an efficient strategy for vaccination. Avicenna J. Med. Biotechnol. 2009, 1, 71–88. [Google Scholar]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Wahren, B.; Liu, M.A. Gene-based vaccines: Recent technical and clinical advances. Trends Mol. Med. 2006, 12, 216–222. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Kutikuppala, L.V.S.; Kourampi, I.; Kanagala, R.S.D.; Bhattacharjee, P.; Boppana, S.H. Prospects and Challenges in Developing mRNA Vaccines for Infectious Diseases and Oncogenic Viruses. Med. Sci. 2024, 12, 28. [Google Scholar] [CrossRef]
- Deering, R.P.; Kommareddy, S.; Ulmer, J.B.; Brito, L.A.; Geall, A.J. Nucleic acid vaccines: Prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 2014, 11, 885–899. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Potter, C.W. A history of influenza. J. Appl. Microbiol. 2001, 91, 572–579. [Google Scholar] [CrossRef]
- Smyk, J.M.; Szydłowska, N.; Szulc, W.; Majewska, A. Evolution of influenza viruses—Drug resistance, treatment options, and prospects. Int. J. Mol. Sci. 2022, 23, 12244. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Saunders-Hastings, P.R.; Krewski, D. Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission. Pathogens 2016, 5, 66. [Google Scholar] [CrossRef]
- Coletti, P.; Poletto, C.; Turbelin, C.; Blanchon, T.; Colizza, V. Shifting patterns of seasonal influenza epidemics. Sci. Rep. 2018, 8, 12786. [Google Scholar] [CrossRef]
- Ali, S.T.; Cowling, B.J. Influenza Virus: Tracking, Predicting, and Forecasting. Annu. Rev. Public Health 2021, 42, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.C. The pathogenesis of influenza in humans. Rev. Med. Virol. 2001, 11, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Jolly, J.; Lawrence, D.A. Cellular and Molecular Immunity to Influenza Viruses and Vaccines. Vaccines 2024, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Krammer, F.; Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015, 14, 167–182. [Google Scholar] [CrossRef]
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Zargar, M.; Ghaemi, A. Inactivation methods for whole influenza vaccine production. Rev. Med. Virol. 2019, 29, e2074. [Google Scholar] [CrossRef]
- Guan, L.; Ping, J.; Lopes, T.J.S.; Fan, S.; Presler, R.; Neumann, G.; Kawaoka, Y. Development of an Enhanced High-Yield Influenza Vaccine Backbone in Embryonated Chicken Eggs. Vaccines 2023, 11, 1364. [Google Scholar] [CrossRef]
- Xue, J.; Chambers, B.S.; Hensley, S.E.; López, C.B. Propagation and Characterization of Influenza Virus Stocks That Lack High Levels of Defective Viral Genomes and Hemagglutinin Mutations. Front. Microbiol. 2016, 7, 326. [Google Scholar] [CrossRef]
- Uddback, I.E.M.; Pedersen, L.M.I.; Pedersen, S.R.; Steffensen, M.A.; Holst, P.J.; Thomsen, A.R.; Christensen, J.P. Combined local and systemic immunization is essential for durable T-cell mediated heterosubtypic immunity against influenza A virus. Sci. Rep. 2016, 6, 20137. [Google Scholar] [CrossRef]
- Wong, S.S.; Webby, R.J. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef]
- Sun, K.; Ye, J.; Perez, D.R.; Metzger, D.W. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J. Immunol. 2011, 186, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.; Brokstad, K.A.; Islam, S.; Oftung, F.; Tøndel, C.; Aarstad, H.J.; Cox, R.J. Early induction of cross-reactive CD8+ T-cell responses in tonsils after live-attenuated influenza vaccination in children. J. Infect. Dis. 2020, 221, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Coelingh, K. Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol. 2002, 15, 295–323. [Google Scholar] [CrossRef] [PubMed]
- Maassab, H. Biologic and immunologic characteristics of cold-adapted influenza virus. J. Immunol. 1969, 102, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Hollister, J.R. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 2009, 37, 182–189. [Google Scholar] [CrossRef]
- Geisler, C.; Jarvis, D.L. Adventitious viruses in insect cell lines used for recombinant protein expression. Protein Expr. Purif. 2018, 144, 25–32. [Google Scholar] [CrossRef]
- Grohskopf, L.A. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 influenza season. MMWR Recomm. Rep. 2019, 68, 1–24. [Google Scholar] [CrossRef]
- Richards, K.; Moritzky, S.; Shannon, I.; Fitzgerald, T.; Yang, H.; Branche, A.; Topham, D.; Treanor, J.; Nayak, J.; Sant, A.J. Recombinant HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody responses in humans. npj Vaccines 2020, 5, 77. [Google Scholar] [CrossRef]
- Gutierrez, A.F.; Sahly, H.E. Recombinant hemagglutinin protein vaccine: A new option in immunization against influenza. Future Virol. 2015, 10, 1057–1067. [Google Scholar] [CrossRef]
- Sun, P.; Lu, X.; Xu, C.; Sun, W.; Pan, B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020, 92, 548–551. [Google Scholar] [CrossRef]
- Agor, J.K.; Özaltın, O.Y. Models for predicting the evolution of influenza to inform vaccine strain selection. Hum. Vaccin. Immunother. 2018, 14, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hanney, S.R.; Wooding, S.; Sussex, J.; Grant, J. From COVID-19 research to vaccine application: Why might it take 17 months not 17 years and what are the wider lessons? Health Res. Policy Syst. 2020, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Approved Vaccines. Available online: https://covid19.trackvaccines.org/vaccines/approved/#vaccine-list (accessed on 2 December 2022).
- Khoshnood, S.; Arshadi, M.; Akrami, S.; Koupaei, M.; Ghahramanpour, H.; Shariati, A.; Sadeghifard, N.; Heidary, M. An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J. Clin. Lab. Anal. 2022, 36, e24418. [Google Scholar] [CrossRef] [PubMed]
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated virus vaccines from chemistry to prophylaxis: Merits, risks and challenges. Expert Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef]
- WHO. WHO COVID-19 Dashboard. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 28 March 2024).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Nguyen, B.T.; Duong, H.N.V.; Lenh, P.T.; Tran, K.T.; Tran, H.M.; Nguyen, T.C.; Nguyen, D.P.; Ta, M.N.; Trieu, N.N.M.; et al. Side effects following first dose of COVID-19 vaccination in Ho Chi Minh City, Vietnam. Hum. Vaccin. Immunother. 2023, 19, 2176066. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Asokan, M.S.; Joan, R.F.; Babji, S.; Dayma, G.; Nadukkandy, P.; Subrahmanyam, V.; Pandey, A.; Malagi, G.; Arya, P.; Mahajan, V.; et al. Immunogenicity of SARS-CoV-2 vaccines BBV152 (COVAXIN®) and ChAdOx1 nCoV-19 (COVISHIELD™) in seronegative and seropositive individuals in India: A multicentre, nonrandomised observational study. Lancet Reg. Health-Southeast Asia 2024, 22, 100361. [Google Scholar] [CrossRef]
- Vikkurthi, R.; Ansari, A.; Pai, A.R.; Jha, S.N.; Sachan, S.; Pandit, S.; Nikam, B.; Kalia, A.; Jit, B.P.; Parray, H.A.; et al. Inactivated whole-virion vaccine BBV152/Covaxin elicits robust cellular immune memory to SARS-CoV-2 and variants of concern. Nat. Microbiol. 2022, 7, 974–985. [Google Scholar] [CrossRef]
- Zasada, A.A.; Darlińska, A.; Wiatrzyk, A.; Woźnica, K.; Formińska, K.; Czajka, U.; Główka, M.; Lis, K.; Górska, P. COVID-19 Vaccines over Three Years after the Outbreak of the COVID-19 Epidemic. Viruses 2023, 15, 1786. [Google Scholar] [CrossRef] [PubMed]
- Gordeychuk, I.V.; Kozlovskaya, L.I.; Siniugina, A.A.; Yagovkina, N.V.; Kuzubov, V.I.; Zakharov, K.A.; Volok, V.P.; Dodina, M.S.; Gmyl, L.V.; Korotina, N.A.; et al. Safety and Immunogenicity of Inactivated Whole Virion COVID-19 Vaccine CoviVac in Clinical Trials in 18–60 and 60+ Age Cohorts. Viruses 2023, 15, 1828. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ulitzky, L.; Silberstein, E.; Taylor, D.R.; Viscidi, R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013, 26, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Dabrera, G.; Ramsay, M.; Lopez Bernal, J. Effectiveness of the Sanofi/GSK (VidPrevtyn Beta) and Pfizer-BioNTech (Comirnaty Original/Omicron BA.4-5) bivalent vaccines against hospitalisation in England. eClinicalMedicine 2024, 71, 102587. [Google Scholar] [CrossRef]
- Dayan, G.H.; Rouphael, N.; Walsh, S.R.; Chen, A.; Grunenberg, N.; Allen, M.; Antony, J.; Asante, K.P.; Bhate, A.S.; Beresnev, T.; et al. Efficacy of a bivalent (D614 + B.1.351) SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant in adults: A phase 3, parallel, randomised, modified double-blind, placebo-controlled trial. Lancet Respir. Med. 2023, 11, 975–990. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Echaide, M.; Chocarro de Erauso, L.; Bocanegra, A.; Blanco, E.; Kochan, G.; Escors, D. mRNA vaccines against SARS-CoV-2: Advantages and caveats. Int. J. Mol. Sci. 2023, 24, 5944. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck Jr, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Shabu, A.; Nishtala, P.S. Safety outcomes associated with the moderna COVID-19 vaccine (mRNA-1273): A literature review. Expert Rev. Vaccines 2023, 22, 393–409. [Google Scholar] [CrossRef]
- Bull, J.J.; Nuismer, S.L.; Antia, R. Recombinant vector vaccine evolution. PLoS Comput. Biol. 2019, 15, e1006857. [Google Scholar] [CrossRef]
- Tang, P.C.H.; Ng, W.H.; King, N.J.; Mahalingam, S. Can live-attenuated SARS-CoV-2 vaccine contribute to stopping the pandemic? PLoS Pathog. 2022, 18, e1010821. [Google Scholar] [CrossRef]
- Fano, V.; Crielesi, A.; Coviello, E.; Fabiani, M.; Salvatore Miglietta, A.; Colaiocco, G.; Moretti, I.; Pasqua, C.; Vivaldi, F.; De Angelis, G.; et al. Effectiveness of the Comirnaty and the Vaxzevria vaccines in preventing SARS-CoV-2 infection among residents in Lazio region (Italy). Vaccine 2022, 40, 2540–2545. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Huiberts, A.J.; de Gier, B.; Hoeve, C.E.; de Melker, H.E.; Hahné, S.J.M.; den Hartog, G.; Grobbee, D.E.; van de Wijgert, J.H.H.M.; van den Hof, S.; Knol, M.J. Vaccine effectiveness of primary and booster COVID-19 vaccinations against SARS-CoV-2 infection in the Netherlands from July 12, 2021 to June 6, 2022: A prospective cohort study. Int. J. Infect. Dis. 2023, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- Lundstrom, K. COVID-19 Vaccines: Where Did We Stand at the End of 2023? Viruses 2024, 16, 203. [Google Scholar] [CrossRef]
- Elrashdy, F.; Redwan, E.M.; Uversky, V.N. Why COVID-19 Transmission Is More Efficient and Aggressive Than Viral Transmission in Previous Coronavirus Epidemics? Biomolecules 2020, 10, 1312. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the next viral threat with broad-spectrum antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Hagey, R.J.; Elazar, M.; Pham, E.A.; Tian, S.; Ben-Avi, L.; Bernardin-Souibgui, C.; Yee, M.F.; Moreira, F.R.; Rabinovitch, M.V.; Meganck, R.M.; et al. Programmable antivirals targeting critical conserved viral RNA secondary structures from influenza A virus and SARS-CoV-2. Nat. Med. 2022, 28, 1944–1955. [Google Scholar] [CrossRef]
- Tripathi, D.; Sodani, M.; Gupta, P.K.; Kulkarni, S. Host directed therapies: COVID-19 and beyond. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100058. [Google Scholar] [CrossRef]
- Ren, Z.; Shen, C.; Peng, J. Status and Developing Strategies for Neutralizing Monoclonal Antibody Therapy in the Omicron Era of COVID-19. Viruses 2023, 15, 1297. [Google Scholar] [CrossRef] [PubMed]
- Mengist, H.M.; Kombe Kombe, A.J.; Mekonnen, D.; Abebaw, A.; Getachew, M.; Jin, T. Mutations of SARS-CoV-2 spike protein: Implications on immune evasion and vaccine-induced immunity. Semin. Immunol. 2021, 55, 101533. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.Y.; Farouk, I.A.; Lal, S.K. Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses 2020, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Zabidi, N.Z.; Liew, H.L.; Farouk, I.A.; Puniyamurti, A.; Yip, A.J.W.; Wijesinghe, V.N.; Low, Z.Y.; Tang, J.W.; Chow, V.T.K.; Lal, S.K. Evolution of SARS-CoV-2 Variants: Implications on Immune Escape, Vaccination, Therapeutic and Diagnostic Strategies. Viruses 2023, 15, 944. [Google Scholar] [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate change increases cross-species viral transmission risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; de Sant’Anna Carvalho, A.M.; Hultquist, J.F.; Ozer, E.A. SARS-CoV-2 genomics and impact on clinical care for COVID-19. J. Antimicrob. Chemother. 2023, 78, ii25–ii36. [Google Scholar] [CrossRef]
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef]
- Mishra, N.T.P.; Das, S.S.; Yadav, S.; Khan, W.; Afzal, M.; Alarifi, A.; kenawy, E.-R.; Ansari, M.T.; Hasnain, M.S.; Nayak, A.K. Global impacts of pre- and post-COVID-19 pandemic: Focus on socio-economic consequences. Sens. Int. 2020, 1, 100042. [Google Scholar] [CrossRef]
- dos Santos, W.G. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed. Pharmacother. 2021, 136, 111272. [Google Scholar] [CrossRef]
- Williams, B.A.; Jones, C.H.; Welch, V.; True, J.M. Outlook of pandemic preparedness in a post-COVID-19 world. npj Vaccines 2023, 8, 178. [Google Scholar] [CrossRef]
- Sharan, M.; Vijay, D.; Yadav, J.P.; Bedi, J.S.; Dhaka, P. Surveillance and response strategies for zoonotic diseases: A comprehensive review. Sci. One Health 2023, 2, 100050. [Google Scholar] [CrossRef]

| Features | Influenza A Virus | SARS-CoV-2 |
|---|---|---|
 |  | |
| Year and pandemic name | 1918 (H1N1), 1957 (H2N2), 1968 (H3N2), 2009 (H1N1), and Flu pandemics | 2019 and COVID-19 |
| Virus family | Orthomyxoviridae | Coronaviridae (genus β-CoVs) |
| Structure | An enveloped, negative-sense, and single-stranded RNA virus; slightly ovoid or mostly round; diameter of 80–120 nm | An enveloped, positive-sense, and single-stranded RNA virus; spherical or round in shape; diameter of 60–140 nm |
| Genome size | 13.5 kb | 29.9 kb |
| Mode of transmission | Droplet, aerosol, direct contact, and fecal–oral route | Droplet, aerosol, direct contact, and fecal–oral route |
| Replication sites | Upper respiratory tract and, in severe cases, lower respiratory tract | Starts from the upper respiratory tract, infects the lower respiratory tract, and spreads to other organs (cardiovascular, intestinal, kidney, and nervous system) |
| Incubation period | 1–7 days | 2–14 days (a maximum of 24 days) |
| Host receptor and entry | Terminal glycosides of sialic acid | ACE2 and TMPRSS2 |
| Cellular tropism | Epithelial cells of Respiratory tract: Alveolar Epithelial cells and ciliated cells | Epithelial cells of the respiratory tract: alveolar epithelial cells, ciliated cells, basal cells of the olfactory epithelium, intestinal epithelial cells, renal parenchymal cells, and endothelial cells |
| Viral protein binding to host receptor | HA | Spike (S) protein |
| Replication | Nuclear | Cytoplasm |
| Symptom | Fever, dry cough, sore throat, fatigue, and nasal congestion | High fever, dry cough, fatigue, ARDS, and anosmia |
| Extrapulmonary complications | In rare cases, myocarditis and encephalitis | In most cases, anosmia, thrombosis, stroke, encephalitis, and diarrhea |
| Target for neutralizing antibodies | HA and NA | RBD of the spike protein |
| Hematological parameters | Lymphopenia and CRP ↑ | Type I interferon ↓, neutrophil counts ↑, and significant lymphopenia |
| Variants of concern (VOCs) | 1957 H2N2, 1968 H3N2, and 2009 H1N1 | Alpha, Beta, Gamma, Delta, and Omicron |
| Mortality rate | 0.05–0.1% (seasonal influenza) | ~1–3.4% (higher in early wave) |
| Vaccine availability | Annual seasonal vaccines (inactivated, live) | Multiple vaccines (mRNA, vector-based, inactivated) |
| Mutations/variants | Antigenic shift and drift | Frequent mutations with variants of concern (e.g., Delta and Omicron) |
| Treatment options | Antivirals (e.g., oseltamivir and zanamivir) | Antivirals (e.g., remdesivir, molnupiravir, and Paxlovid), mAbs |
| Complications | Pneumonia and secondary bacterial infections | Pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ damage |
| References | [26,27] | [28,29] |
| Drug | Virus Target | Clinical Trial Identifier | Phase | Classification | Function |
|---|---|---|---|---|---|
| Camostat mesylate | Influenza virus A/B/SARS-CoV-2 | NCT04470544 | II | TMPRSS2 serine protease inhibitor | Blocking the virus activating host cell protease TMPRSS2 |
| Baloxavir marboxil | Influenza virus A/B | NCT03684044 | III | Polymerase acidic (PA) endonuclease inhibitor | Inhibits viral replication |
| Pimodivir | Influenza virus A/B | NCT02262715 NCT02342249 | I II | PB2 inhibitor | Inhibits viral replication |
| Enisamium iodide | Influenza virus A/B | NCT04682444 NCT04682873 | II/III III | RNA polymerase inhibitor | Inhibits viral replication |
| DAS181 | Influenza virus A/B | NCT01173224 NCT01651494 NCT00527865 NCT01037205 | I | Entry inhibitor | Removes sialic acid from epithelial cells, preventing viral entry |
| Remdesivir | SARS-CoV-2 | NCT04345419 NCT04678739 | II/III | RdRp inhibitor | Inhibit viral RNA replication by binding with viral RNA |
| Favipiravir | SARS-CoV-2 | NCT04303299 NCT04351295 NCT04600999 NCT04346628 NCT04387760 NCT04542694 NCT03394209 | III II/III III II II III II | RdRp inhibitor | Inhibits viral replication and genetic transversion |
| Ribavirin | SARS-CoV-2 | NCT01097395 NCT01497366 NCT04276688 NCT04563208 | IV III II II | RdRp inhibitor | Inhibits viral RNA synthesis and immunomodulation |
| Ivermectin | SARS-CoV-2 | NCT04403555 NCT04381884 NCT04646109 NCT04591600 | II/III II III I/II | Viral protease inhibitor | Inhibits viral protein transport to nucleus |
| Ritonavir | SARS-CoV-2 | NCT04303299 | III | Viral protease inhibitor | Inhibits viral Plpro protease activity |
| Lopinavir | SARS-CoV-2 | NCT04276688 NCT04252885 | II IV | Viral protease inhibitor | Inhibits viral 3CLpro activity |
| Eculizumab | SARS-CoV-2 | NCT04346797 | II | Monoclonal antibody | Prevents the activation of inflammation by inhibiting the C5 complement protein |
| Bevacizumab | SARS-CoV-2 | NCT04275414 | II | Monoclonal antibody | Preventing acute lung injury in ARDS and suppression of pulmonary edema |
| Meplazumab | SARS-CoV-2 | NCT04275245 | II/III | Monoclonal antibody | Prevents viral entry and inflammation |
| Ramipril | SARS-CoV-2 | NCT04366050 | II | ACE inhibitor | Ras inhibitor to stop heart failure |
| Azithromycin | SARS-CoV-2 | NCT04332107 NCT04381962 | III III | RNA inhibitor | Regulates cytokine storm |
| Chloroquine | SARS-CoV-2 | NCT04420247 NCT04353336 | III II/III | Derivatives of quinine compounds | Inhibit virion formation and MAPK activation |
| Colchicine | SARS-CoV-2 | NCT04322682 NCT04472611 | III III | Anti-inflammatory drug | Inactivates pro-inflammatory cytokines and migration of leukocytes |
| Baricitinib | SARS-CoV-2 | NCT04421027 NCT04373044 | II/III II | JAK1/AAK1 inhibitor | Suppresses inflammatory factors (IL-6 and IL7) |
| Methylprednisolone | SARS-CoV-2 | NCT04244591 NCT04263402 NCT04273321 | II/III/IV | Corticosteroid | Lowers the viral lung damage |
| APN01-COVID-19 | SARS-CoV-2 | NCT04335136 | II | Recombinant human angiotensin-converting enzyme 2 (rhACE2) | Prevents viral entry and viral replication |
| Vaccine Name | Manufacturer | Vaccine Type | Approved Country |
|---|---|---|---|
| Influgen | Lupin Laboratories Ltd., Mumbai, India | Inactivated influenza vaccine | India |
| Fluzone Quadrivalent | Sanofi Pasteur, Inc., Swiftwater, PA, USA | Inactivated influenza vaccine | USA |
| FluQuadri | Sanofi-Aventis, Macquarie Park, Australia | Inactivated influenza vaccine | Australia |
| Vaxigrip Tetra | Sanofi-Aventis, Macquarie Park, Australia | Inactivated influenza vaccine | Australia |
| Fluarix Tetra | Glaxo-SmithKline Biologicals, Rixensart, Belgium | Inactivated influenza vaccine | Australia |
| Afluria Quadrivalent | Seqirus-Pty. Ltd., Parkville, Australia | Inactivated influenza vaccine | USA |
| Fluarix Quadrivalent | Glaxo-SmithKline Biologicals | Inactivated influenza vaccine | USA |
| Fluad Quadrivalent | Seqirus, Inc., Holly Springs, NC, USA | Inactivated influenza vaccine | USA |
| Agripal | Chiron Panacea Vaccines Pvt. Ltd., New Delhi, India | Inactivated influenza vaccine | India |
| Influvac Tetra | Mylan Health, Canonsburg, PA, USA | Inactivated influenza vaccine | Australia |
| FluLaval-Quadrivalent | ID-Biomedical, Laval, QC, Canada | Inactivated influenza vaccine | USA |
| FlublokQuadrivalent | Sanofi Pasteur, Inc. | Recombinant influenza vaccine | USA |
| Cadiflu-S Vaccine | CPL Biologicals Pvt Ltd., Ahmedabad, India | Inactivated influenza vaccine | India |
| FluMist-Quadrivalent | Med-Immune, LLC., Gaithersburg, MD, USA | Live attenuated influenza vaccine | USA |
| Flucelvax-Quadrivalent | Seqirus, Inc. | Inactivated influenza vaccine | USA |
| Nasovac S Vaccine | Serum Institute of India Ltd., Pune, India | Inactivated influenza vaccine | India |
| Types of Vaccine | Vaccine Name | Produced by | Approval Status | Trials | No. of Countries Allowed Trials | Approved Countries |
|---|---|---|---|---|---|---|
| Inactivated vaccine | Covaxin | Bharat Biotech, Hyderabad, India | Approved | 16 | 2 | 14 |
| Physically or chemically inactivated viral vaccines | KoviVac | Chumakov Center, Moscow, Russia | Approved | 5 | 1 | 3 |
| Turkovac | Health Institutes of Turkey, Ankara, Turkey | Approved | 8 | 1 | 1 | |
| FAKHRAVAC (MIVAC) | Organization of Defensive Innovation and Research, Tehran, Iran | Approved | 3 | 1 | 1 | |
| QazVac | Research Institute for Biological Safety Problems (RIBSP), Gvardeyskiy, Kazakhstan | Approved | 3 | 1 | 2 | |
| KCONVAC | Shenzhen Kangtai Biological Products Co., Shenzhen, China | Approved | 7 | 1 | 2 | |
| COVIran Barekat | Shifa Pharmed Industrial Co, Tehran, Iran | Approved | 6 | 1 | 1 | |
| Covilo | Sinopharm, Beijing, China | Approved | 39 | 18 | 93 | |
| Inactivated (Vero Cells) | Sinopharm, Wuhan, China | Approved | 9 | 7 | 2 | |
| CoronaVac | Sinovac, Beijing, China | Approved | 42 | 10 | 56 | |
| SKYCovione | SK Bioscience Co. Ltd., Seongnam, South Korea | Approved | 7 | 6 | 1 | |
| VLA2001 | Valneva, Saint-Herblain, France | Approved | 9 | 4 | 33 | |
| Protein subunit vaccines | Zifivax | Anhui Zhifei Longcom, Hefei, China | Approved | 21 | 5 | 4 |
| Use a protein fragment or viral spike proteins as the antigen to trigger an immune response | Noora vaccine | Bagheiat-allah University of Medical Sciences, Tehran, Iran | Approved | 3 | 1 | 1 |
| Corbevax | Biological E Limited, Hyderabad, India | Approved | 7 | 1 | 2 | |
| Soberana 02 | Instituto Finlay de Vacunas, Havana, Cuba | Approved | 7 | 2 | 4 | |
| Soberana Plus | Instituto Finlay de Vacunas | Approved | 5 | 1 | 2 | |
| V-01 | Livzon Mabpharm Inc., Zhuhai, China | Approved | 7 | 3 | 1 | |
| MVC-COV1901 | Medigen, Taipei, Taiwan | Approved | 15 | 4 | 4 | |
| Recombinant SARS-CoV-2 Vaccine (CHO Cell) | National Vaccine and Serum Institute, Beijing, China | Approved | 3 | 2 | 1 | |
| Nuvaxovid | Novavax, Gaithersburg, MD | Approved | 22 | 14 | 40 | |
| IndoVac | PT Bio Farma, Bandung, Indonesia | Approved | 4 | 1 | 1 | |
| Razi Cov Pars | Razi Vaccine and Serum Research Institute, Karaj, Iran | Approved | 5 | 1 | 1 | |
| VidPrevtyn Beta | Sanofi/GSK, Lyon, France/Brentford, UK | Approved | 3 | 2 | 30 | |
| COVOVAX | Serum Institute of India, Pune, India | Approved | 7 | 3 | 6 | |
| TAK-019 (Novavax formulation) | Takeda, Tokyo, Japan | Approved | 3 | 1 | 1 | |
| SpikoGen | Vaxine/CinnaGen Co., Adelaide, Australia | Approved | 8 | 2 | 1 | |
| Aurora-CoV | Vector State Research Center of Virology and Biotechnology, Koltsovo, Russia | Approved | 2 | 1 | 1 | |
| EpiVacCorona | Vector State Research Center of Virology and Biotechnology | Approved | 4 | 1 | 4 | |
| Non-replicating viral vector vaccines | iNCOVACC | Bharat Biotech | Approved | 4 | 1 | 1 |
| Bioengineered viral vectors that are unable to express and clone antigens originating from the targeted virus | Convidecia | CanSino, Tianjin, China | Approved | 14 | 6 | 10 |
| Convidecia Air | CanSino | Approved | 5 | 4 | 2 | |
| Abdala | Center for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba | Approved | 5 | 1 | 6 | |
| Gam-COVID-Vac | Gamaleya, Moscow, Russia | Approved | 2 | - | 1 | |
| Sputnik Light | Gamaleya | Approved | 7 | 3 | 26 | |
| Sputnik V | Gamaleya | Approved | 25 | 8 | 74 | |
| Jcovden | Janssen (Johnson & Johnson), New Brunswick, NJ | Approved | 26 | 25 | 113 | |
| Vaxzevria | Oxford/AstraZeneca, Oxford, UK | Approved | 73 | 34 | 149 | |
| Covishield | Serum Institute of India | Approved | 6 | 1 | 49 | |
| RNA vaccines | GEMCOVAC-19 | Gennova Biopharmaceuticals Limited, Pune, India | Approved | 2 | 1 | 1 |
| Vaccines based on mRNA that are combined with injectable nanoparticles to efficiently transfer the mRNA into target cells and induce adaptive immunity | Spikevax | Moderna, Cambridge, MA, USA | Approved | 70 | 24 | 88 |
| Spikevax Bivalent Original/Omicron BA.1 | Moderna | Approved | 5 | 4 | 38 | |
| Spikevax Bivalent Original/Omicron BA.4/BA.5 | Moderna | Approved | 2 | 1 | 33 | |
| Comirnaty | Pfizer/BioNTech, New York, NY, USA/Mainz, Germany | Approved | 100 | 31 | 149 | |
| Comirnaty Bivalent Original/Omicron BA.1 | Pfizer/BioNTech | Approved | 3 | 5 | 35 | |
| Comirnaty Bivalent Original/Omicron BA.4/BA.5 | Pfizer/BioNTech | Approved | 4 | 1 | 33 | |
| TAK-919 (Moderna formulation) | Takeda | Approved | 2 | 1 | 1 | |
| AWcorna | Walvax, Kunming, China | Approved | 4 | 3 | 1 | |
| DNA vaccines | ZyCoV-D | Zydus Cadila, Ahmedabad, India | Approved | 6 | 1 | 1 |
| DNA vaccines comprise of a plasmid that has been genetically modified to encode the antigen unique to the disease and a vector that transports the plasmid into the host cell. | ||||||
| VLP vaccines | Covifenz | Medicago, Quebec City, QC, Canada | Approved | 6 | 6 | 1 |
| Spontaneously assembled from several structural proteins of the virus to stimulate the immune system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukutty, P.; MacDonald, J.; Yoo, S.Y. Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies. Vaccines 2024, 12, 1220. https://doi.org/10.3390/vaccines12111220
Muthukutty P, MacDonald J, Yoo SY. Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies. Vaccines. 2024; 12(11):1220. https://doi.org/10.3390/vaccines12111220
Chicago/Turabian StyleMuthukutty, Palaniyandi, Jaime MacDonald, and So Young Yoo. 2024. "Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies" Vaccines 12, no. 11: 1220. https://doi.org/10.3390/vaccines12111220
APA StyleMuthukutty, P., MacDonald, J., & Yoo, S. Y. (2024). Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies. Vaccines, 12(11), 1220. https://doi.org/10.3390/vaccines12111220







