Does Tobacco Smoking Affect Vaccine-Induced Immune Response? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
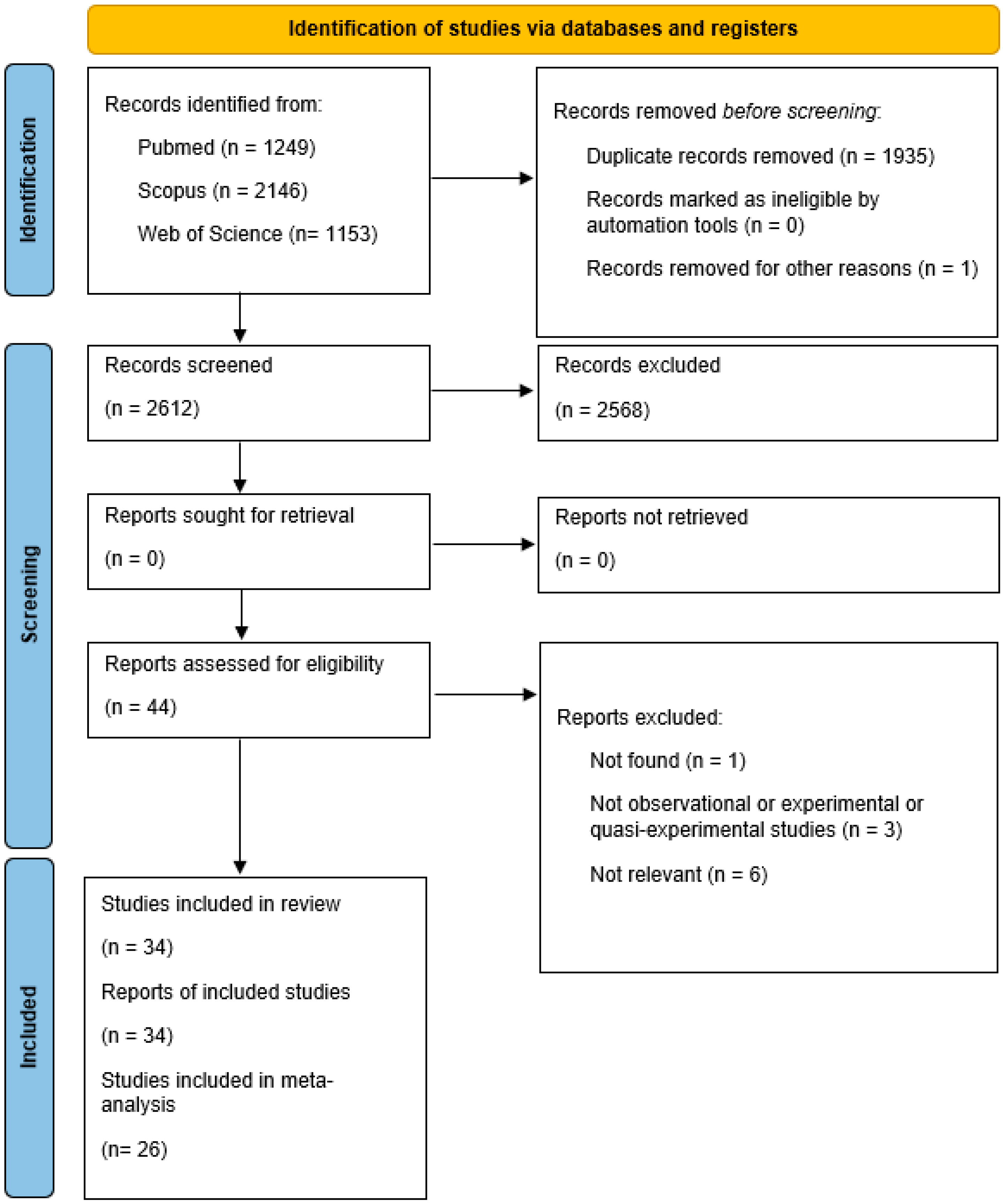
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
- Population: all people (individuals of all gender, age, ethnicity and health conditions) vaccinated against any vaccine-preventable disease.
- Intervention: active and/or passive tobacco smoking.
- Control: age-, gender- and condition-matched non-smoking control group (if present).
- Outcomes: effects of active and/or passive tobacco smoking on vaccine-induced immune response.
- Study: observational studies, semi-experimental and experimental studies on humans. All studies that did not satisfy the inclusion criteria were excluded.
2.3. Risk of Bias Assessment
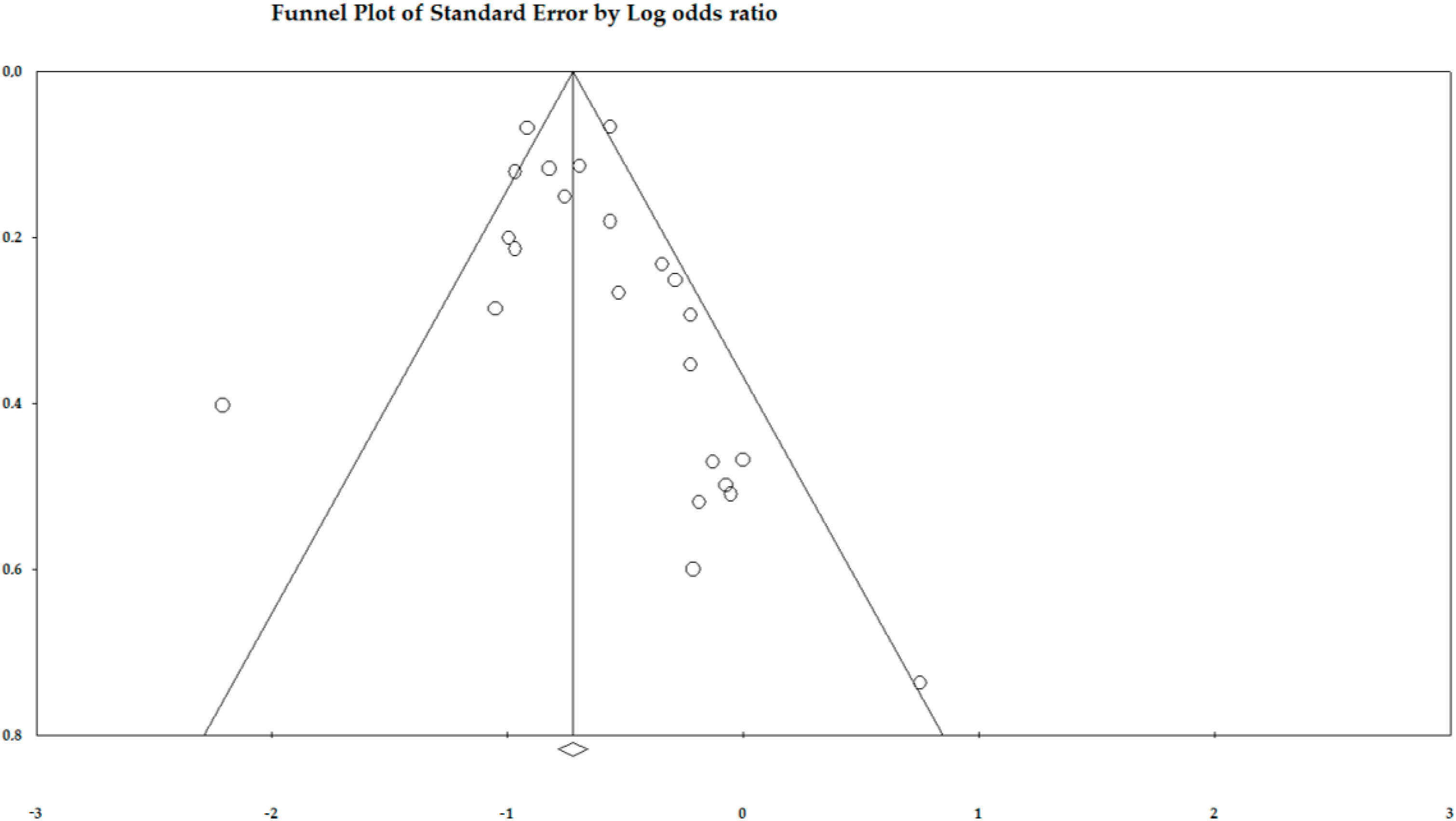
2.4. Data Synthesis
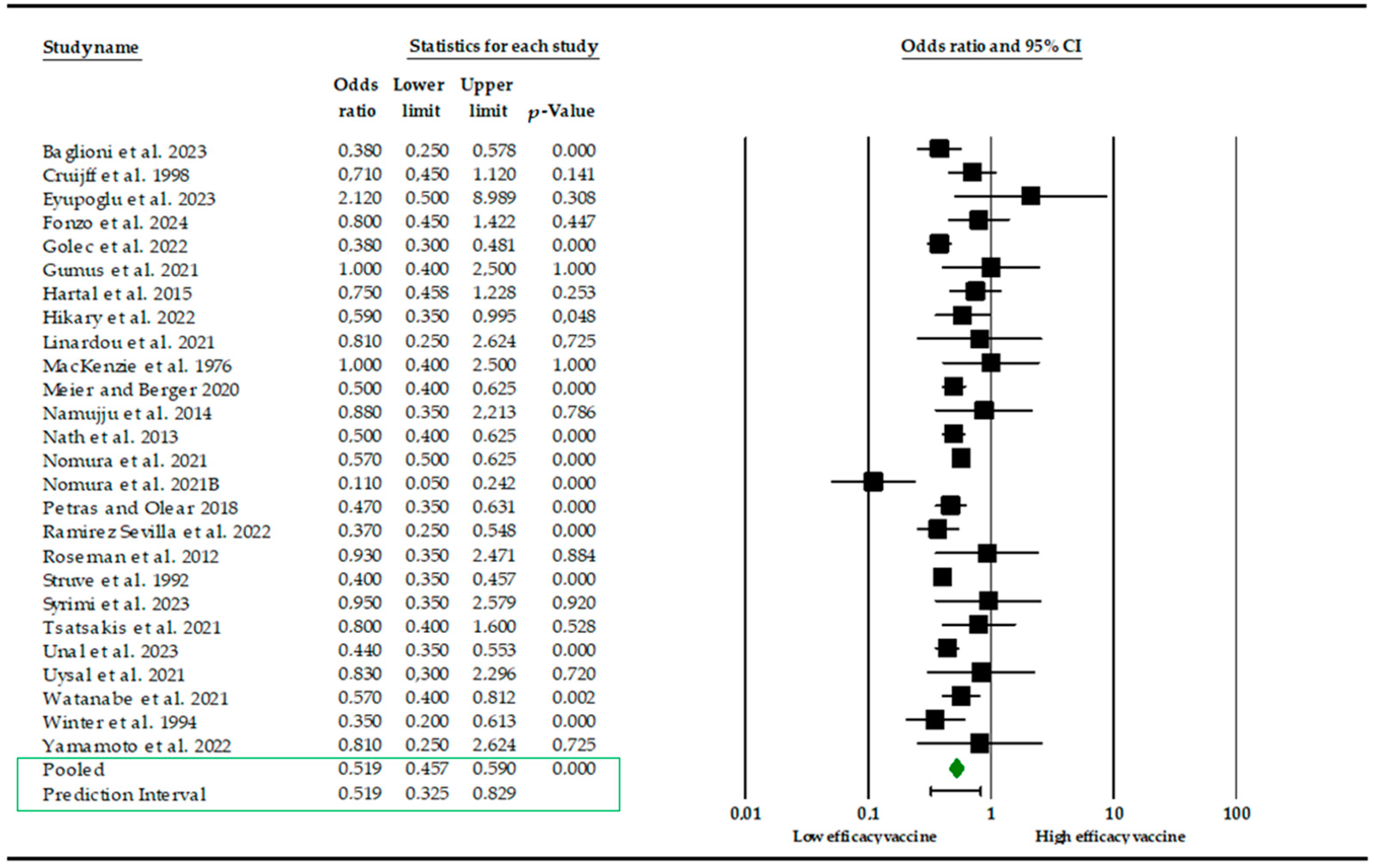
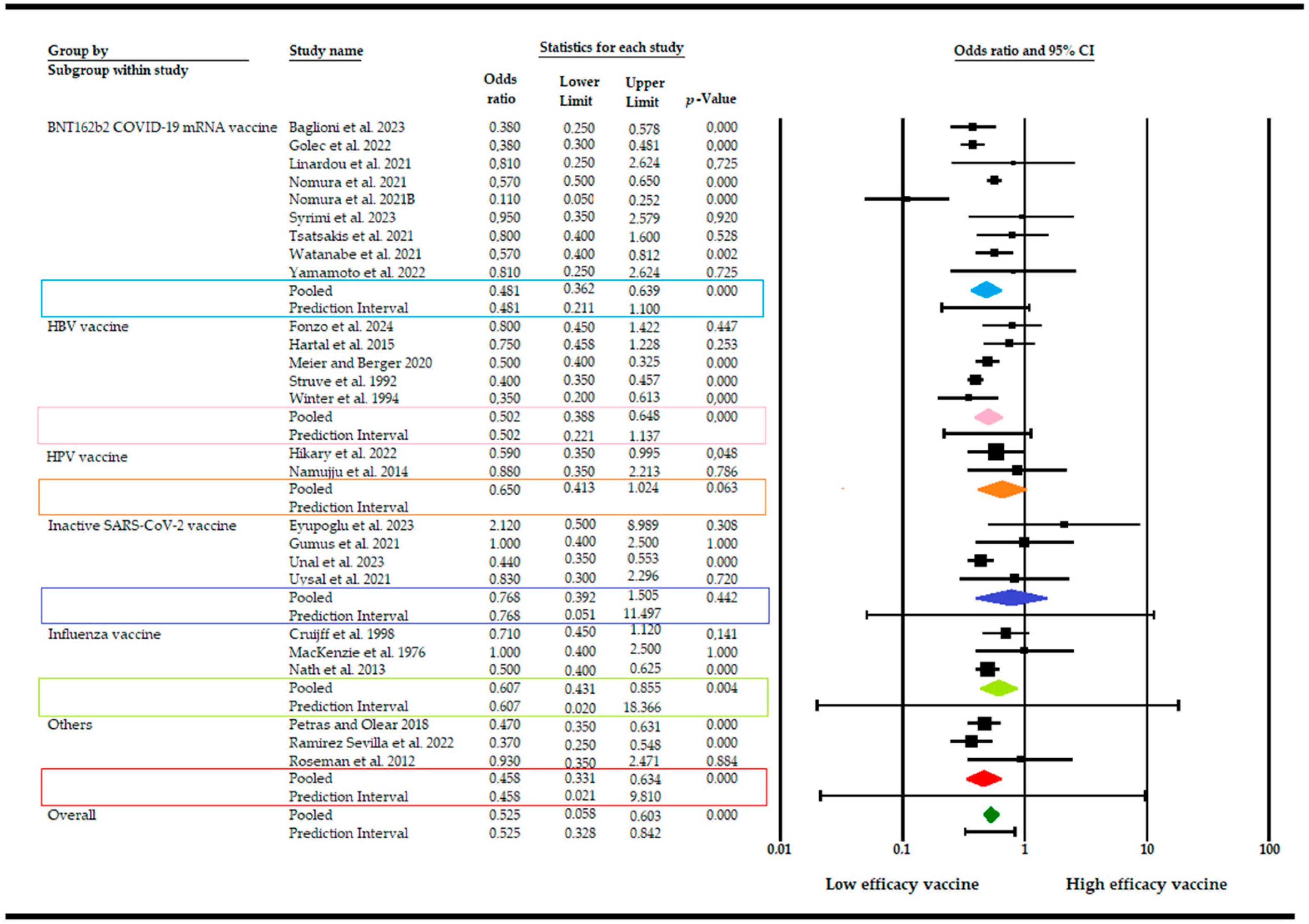
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- World Health Organization Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 6 September 2024).
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, temporal, and demographic patterns in the prevalence of smoking tobacco use and attributable burden of disease in 204 countries and territories, 1990–2019: A systematic review of the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef] [PubMed]
- WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products. Available online: https://www.who.int/publications/i/item/9789240032095 (accessed on 6 September 2024).
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation Findings from the Global Burden of Disease Study 2021. Available online: https://www.healthdata.org/research-analysis/gbd (accessed on 6 September 2024).
- Taucher, E.; Mykoliuk, I.; Lindenmann, J.; Smolle-Juettner, F.M. Implications of the Immune Landscape in COPD and Lung Cancer: Smoking Versus Other Causes. Front. Immunol. 2022, 13, 846605. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The effect of smoking on the humoral system Response to COVID-19 vaccines: A systematic review of epidemiological studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef]
- Kastratovic, N.; Zdravkovic, N.; Cekerevac, I.; Sekerus, V.; Harrell, C.R.; Mladenovic, V.; Djukic, A.; Volarevic, A.; Brankovic, M.; Gmizic, T.; et al. Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases. Diseases 2024, 12, 144. [Google Scholar] [CrossRef]
- Qi, X.; Fu, J.; Liu, J.; Wu, X.; Zheng, X.; Wang, S. Association between secondhand smoke exposure and rheumatoid arthritis in US never-smoking adults: A cross-sectional study from NHANES. Sci. Rep. 2024, 14, 11061. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, P.; Liu, H.; Wang, Z.; Hu, P.; Ye, P.; Xia, J.; Chen, S. Assessing Causality Between Second-Hand Smoking and Potentially Associated Diseases in Multiple Systems: A Two-Sample Mendelian Randomization Study. Nicotine Tob. Res. 2024, 26, 678–684. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Bender, R.; Friede, T.; Koch, A.; Kuss, O.; Schlattmann, P.; Schwarzer, G.; Skipka, G. Methods for Evidence Synthesis in the Case of Very Few Studies. Res. Synth. Methods 2018, 9, 382–392. [Google Scholar] [CrossRef]
- MacKenzie, J.S.; MacKenzie, I.H.; Holt, P.G. The effect of cigarette smoking on susceptibility to epidemic influenza and on serological responses to live attenuated and killed subunit influenza vaccines. J. Hyg. 1976, 77, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Struve, J.; Aronsson, B.; Frenning, B.; Granath, F.; von Sydow, M.; Weiland, O. Intramuscular versus intradermal administration of a recombinant hepatitis B vaccine: A comparison of response rates and analysis of factors influencing the antibody response. Scand. J. Infect. Dis. 1992, 24, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.P.; Follett, E.A.C.; McIntyre, J.; Stewart, J.; Symington, I.S. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine 1994, 12, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Cruijff, M.; Thijs, C.; Govaert, T.; Aretz, K.; Dinant, G.J.; Knottnerus, A. The effect of smoking on influenza, influenza vaccination efficacy and on the antibody response to influenza vaccination. Vaccine 1999, 17, 426–432. [Google Scholar] [CrossRef]
- Baynam, G.; Khoo, S.K.; Rowe, J.; Zhang, G.; Laing, I.; Hayden, C.; Kusel, M.; DeKlerk, N.; Sly, P.; Goldblatt, J.; et al. Parental smoking impairs vaccine responses in children with atopic genotypes. J. Allergy Clin. Immunol. 2007, 119, 366–374. [Google Scholar] [CrossRef]
- Roseman, C.; Truedsson, L.; Kapetanovic, M.C. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Res. Ther. 2012, 14, R170. [Google Scholar] [CrossRef]
- Nath, K.D.; Burel, J.G.; Shankar, V.; Pritchard, A.L.; Towers, M.; Looke, D.; Davies, J.M.; Upham, J.W. Clinical factors associated with the humoral immune response to influenza vaccination in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Namujju, P.B.; Pajunen, E.; Simen-Kapeu, A.; Hedman, L.; Merikukka, M.; Surcel, H.M.; Kirnbauer, R.; Apter, D.; Paavonen, J.; Hedman, K.; et al. Impact of smoking on the quantity and quality of antibodies induced by human papillomavirus type 16 and 18 AS04-adjuvanted virus-like-particle vaccine—A pilot study. BMC Res. Notes 2014, 7, 445. [Google Scholar] [CrossRef]
- Hartal, M.; Yavnai, N.; Galor, I.; Avramovich, E.; Sela, T.; Kayouf, R.; Tzurel-Ferber, A.; Greenberg, L.J.; Halperin, T.; Levine, H. Seroprevalence of anti-HBs antibodies at young adulthood, before and after a booster vaccine dose, among medical personnel vaccinated in infancy. Vaccine 2015, 33, 4878–4885. [Google Scholar] [CrossRef]
- Petráš, M.; Oleár, V. Predictors of the immune response to booster immunisation against tetanus in Czech healthy adults. Epidemiol. Infect. 2018, 146, 2079–2085. [Google Scholar] [CrossRef]
- Meier, M.A.; Berger, C.T. A simple clinical score to identify likely hepatitis B vaccination non-responders—Data from a retrospective single center study. BMC Infect. Dis. 2020, 20, 891. [Google Scholar] [CrossRef] [PubMed]
- Gümüş, H.H.; Ödemiş, İ.; Alışka, H.E.; Karslı, A.; Kara, S.; Özkale, M.; Gül, E. Side effects and antibody response of an inactive severe acute respiratory syndrome coronavirus 2 vaccine among health care workers. Rev. Assoc. Med. Bras. 2021, 67, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Linardou, H.; Spanakis, N.; Koliou, G.A.; Christopoulou, A.; Karageorgopoulou, S.; Alevra, N.; Vagionas, A.; Tsoukalas, N.; Sgourou, S.; Fountzilas, E.; et al. Responses to SARS-CoV-2 Vaccination in Patients with Cancer (ReCOVer Study): A Prospective Cohort Study of the Hellenic Cooperative Oncology Group. Cancers 2021, 13, 4621. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines 2021, 9, 1042. [Google Scholar] [CrossRef]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; Sugiyama, K. Attenuation of Antibody Titers from 3 to 6 Months after the Second Dose of the BNT162b2 Vaccine Depends on Sex, with Age and Smoking Risk Factors for Lower Antibody Titers at 6 Months. Vaccines 2021, 9, 1500. [Google Scholar] [CrossRef]
- Pitzalis, M.; Idda, M.L.; Lodde, V.; Loizedda, A.; Lobina, M.; Zoledziewska, M.; Virdis, F.; Delogu, G.; Pirinu, F.; Marini, M.G.; et al. Effect of Different Disease-Modifying Therapies on Humoral Response to BNT162b2 Vaccine in Sardinian Multiple Sclerosis Patients. Front. Immunol. 2021, 12, 781843. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Vakonaki, E.; Tzatzarakis, M.; Flamourakis, M.; Nikolouzakis, T.K.; Poulas, K.; Papazoglou, G.; Hatzidaki, E.; Papanikolaou, N.C.; Drakoulis, N.; et al. Immune response (IgG) following full inoculation with BNT162b2 COVID-19 mRNA among healthcare professionals. Int. J. Mol. Med. 2021, 48, 200. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.C.; Mantovani, L.G.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Golec, M.; Fronczek, M.; Zembala-John, J.; Chrapiec, M.; Konka, A.; Wystyrk, K.; Botor, H.; Brzoza, Z.; Kasperczyk, S.; Bułdak, R.J. Early and Longitudinal Humoral Response to the SARS-CoV-2 mRNA BNT162b2 Vaccine in Healthcare Workers: Significance of BMI, Adipose Tissue and Muscle Mass on Long-Lasting Post-Vaccinal Immunity. Viruses 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- Hikari, T.; Honda, A.; Hashiguchi, M.; Okuma, R.; Kurihara, M.; Fukuda, A.; Okuma, E.; Nakao, Y.; Yokoyama, M. The difference in the effectiveness of human papillomavirus vaccine based on smoking status. J. Obstet. Gynaecol. Res. 2022, 48, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Tanaka, M.; Kozai, H.; Hotta, K.; Aoyama, Y.; Shigeno, Y.; Aoike, M.; Kawamura, H.; Tsurudome, M.; Ito, M. Antibody response of smokers to the COVID-19 vaccination: Evaluation based on cigarette dependence. Drug Discov. Ther. 2022, 16, 78–84. [Google Scholar] [CrossRef]
- Ramírez Sevilla, C.; Gómez Lanza, E.; Llopis Manzanera, J.; Cetina Herrando, A.; Puyol Pallàs, J.M. A Focus on Long-Term Follow-Up of Immunoprophylaxis to Recurrent Urinary Tract Infections: 10 Years of Experience with MV140 Vaccine in a Cohort of 1003 Patients Support High Efficacy and Safety. Arch. Esp. Urol. 2022, 75, 753–757. [Google Scholar] [CrossRef]
- Trontzas, I.P.; Vathiotis, I.; Economidou, C.; Petridou, I.; Gomatou, G.; Grammoustianou, M.; Tsamis, I.; Syrigos, N.; Anagnostakis, M.; Fyta, E.; et al. Assessment of Seroconversion after SARS-CoV-2 Vaccination in Patients with Lung Cancer. Vaccines 2022, 10, 618. [Google Scholar] [CrossRef]
- Toda, M.; Yoshifuji, A.; Kikuchi, K.; Koinuma, M.; Komatsu, M.; Fujii, K.; Kato, A.; Kikuchi, T.; Nakazawa, A.; Ryuzaki, M. Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin. Exp. Nephrol. 2022, 26, 571–580, Erratum in Clin. Exp. Nephrol. 2022, 26, 581–582. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tanaka, A.; Ohmagari, N.; Yamaguchi, K.; Ishitsuka, K.; Morisaki, N.; Kojima, M.; Nishikimi, A.; Tokuda, H.; Inoue, M.; et al. Use of heated tobacco products, moderate alcohol drinking, and anti-SARS-CoV-2 IgG antibody titers after BNT162b2 vaccination among Japanese healthcare workers. Prev. Med. 2022, 161, 107123. [Google Scholar] [CrossRef]
- Asmar, I.; Almahmoud, O.; Yaseen, K.; Jamal, J.; Omar, A.; Naseef, H.; Hasan, S. Assessment of immunoglobin G (spike and nucleocapsid protein) response to COVID-19 vaccination in Palestine. Clin. Epidemiol. Glob. Health 2023, 22, 101330. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, I.; Galli, A.; Gatti, C.; Tufoni, S.; Rocchi, R.; Lattanzi, F.; Toia, F.; Santarelli, A.; Marcelli, S. Evaluation of antibody response anti SARS-Cov-2: A retrospective observational study (Marche-Italy). Ig. Sanita Pubbl. 2023, 80, 13–26. [Google Scholar]
- Eyupoglu, G.; Guven, R.; Karabulut, N.; Cakir, A.; Sener, K.; Yavuz, B.G.; Tekyol, D.; Avci, A. Humoral responses to the CoronoVac vaccine in healthcare workers. Rev. Soc. Bras. Med. Trop. 2023, 56, e0209. [Google Scholar] [CrossRef]
- Prather, A.A.; Dutcher, E.G.; Robinson, J.; Lin, J.; Blackburn, E.; Hecht, F.M.; Mason, A.E.; Fromer, E.; Merino, B.; Frazier, R.; et al. Predictors of long-term neutralizing antibody titers following COVID-19 vaccination by three vaccine types: The BOOST study. Sci. Rep. 2023, 13, 6505. [Google Scholar] [CrossRef] [PubMed]
- Syrimi, N.; Sourri, F.; Giannakopoulou, M.C.; Karamanis, D.; Pantousas, A.; Georgota, P.; Rokka, E.; Vladeni, Z.; Tsiantoula, E.; Soukara, E.; et al. Humoral and Cellular Response and Associated Variables Nine Months following BNT162b2 Vaccination in Healthcare Workers. J. Clin. Med. 2023, 12, 3172. [Google Scholar] [CrossRef] [PubMed]
- Ünal, G.; Sezgin, S.D.; Sancar, M. Evaluation of SARS-CoV-2 Antibody Levels in Pharmacists and Pharmacy Staff Following CoronaVac Vaccination. Turk. J. Pharm. Sci. 2024, 26, 347–351, Erratum in Turk. J. Pharm. Sci. 2024, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Fonzo, M.; Palmisano, A.; Trevisan, A.; Bertoncello, C. The Impact of Smoking on Long-Term Protection Following Hepatitis B Vaccination: A 24-Year Cohort Study. Viruses 2024, 16, 1137. [Google Scholar] [CrossRef]
- Saulyte, J.; Regueira, C.; Montes-Martínez, A.; Khudyakov, P.; Takkouche, B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001611. [Google Scholar] [CrossRef]
- Pedersen, K.M.; Çolak, Y.; Ellervik, C.; Hasselbalch, H.C.; Bojesen, S.E.; Nordestgaard, B.G. Smoking and Increased White and Red Blood Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 965–977. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef]
- Sopori, M.L. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- Hernandez, C.P.; Morrow, K.; Velasco, C.; Wyczechowska, D.D.; Naura, A.S.; Rodriguez, P.C. Effects of cigarette smoke extract on primary activated T cells. Cell Immunol. 2013, 282, 38–43. [Google Scholar] [CrossRef]
- Piaggeschi, G.; Rolla, S.; Rossi, N.; Brusa, D.; Naccarati, A.; Couvreur, S.; Spector, T.D.; Roederer, M.; Mangino, M.; Cordero, F.; et al. Immune Trait Shifts in Association with Tobacco Smoking: A Study in Healthy Women. Front. Immunol. 2021, 12, 637974. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, L.; Yang, H.; Wu, X.; Luo, X.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; Chen, Y.; et al. Dysregulation of immunity by cigarette smoking promotes inflammation and cancer: A review. Environ. Pollut. 2023, 339, 122730. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yang, D.; Cao, D.; Gong, Z.; He, F.; Hou, Y.; Lin, S. Effect of smoking status on immunotherapy for lung cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1422160. [Google Scholar] [CrossRef]
- Peer, N.; Nguyen, K.A.; Peprah, E.; Xu, H.; Matsha, T.E.; Chegou, N.N.; Kengne, A.P. Exploring the associations of tobacco smoking and serum cotinine levels with selected inflammatory markers in adults with HIV in South Africa. Sci. Rep. 2024, 14, 25772. [Google Scholar] [CrossRef]
- Thomas, S.; Rouilly, V.; Patin, E.; Alanio, C.; Dubois, A.; Delval, C.; Marquier, L.G.; Fauchoux, N.; Sayegrih, S.; Vray, M.; et al. Milieu Intérieur Consortium. The Milieu Intérieur study—An integrative approach for study of human immunological variance. Clin. Immunol. 2015, 157, 277–293. [Google Scholar] [CrossRef]
- Lugade, A.A.; Bogner, P.N.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P.; Thanavala, Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J. Immunol. 2014, 192, 5226–5235. [Google Scholar] [CrossRef]
- Mertens, T.C.J.; van der Does, A.M.; Kistemaker, L.E.; Ninaber, D.K.; Taube, C.; Hiemstra, P.S. Cigarette smoke differentially affects IL-13-induced gene expression in human airway epithelial cells. Physiol. Rep. 2017, 5, e13347. [Google Scholar] [CrossRef]
- Saint-André, V.; Charbit, B.; Biton, A.; Rouilly, V.; Possémé, C.; Bertrand, A.; Rotival, M.; Bergstedt, J.; Patin, E.; Albert, M.L.; et al. Smoking changes adaptive immunity with persistent effects. Nature 2024, 626, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Piqueras, L.; Sanz, M.J. An updated overview of e-cigarette impact on human health. Respir. Res. 2021, 22, 151. [Google Scholar] [CrossRef]
- McGrath, J.J.C.; Rebuli, M.E.; Cass, S.P.; Loeb, M.; Jaspers, I. Smoking and e-cigarette use: Key variables in testing IgA-oriented intranasal vaccines. Lancet Respir. Med. 2022, 10, 822–824. [Google Scholar] [CrossRef]
- Sullivan, L.; Crotty Alexander, L.E. A Problem for Generations: Impact of E-Cigarette Type on Immune Homeostasis. Am. J. Respir. Crit. Care Med. 2022, 206, 1195–1197. [Google Scholar] [CrossRef]
- Suzuki, F.; Maeyama, J.I.; Kubota, A.; Nishimune, A.; Horiguchi, S.; Takii, T.; Urasaki, Y.; Shimada, I.; Iho, S. Effect of cigarette smoke on mucosal vaccine response with activation of plasmacytoid dendritic cells: The outcomes of in vivo and in vitro experiments. Vaccine 2023, 41, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Şen, S.; Arslan, G.; Tütüncü, M.; Demir, S.; Dinç, Ö.; Gündüz, T.; Uzunköprü, C.; Gümüş, H.; Tütüncü, M.; Akçin, R.; et al. The Effect of Smoking on Inactivated and mRNA Vaccine Responses Applied to Prevent COVİD-19 in Multiple Sclerosis. Noro Psikiyatr. Ars. 2023, 60, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, D.; Losa, L.; Campagna, D.; Magliuolo, R.; Vitale, A.; Cacciapuoti, D.; Zampella, A.; Alleanza, L.; Schiavone, B.; Spicuzza, L.; et al. Smoking habits predict adverse effects after mRNA COVID-19 vaccine: Empirical evidence from a pilot study. Public Health 2023, 219, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Sangani, R.G.; Deepak, V.; Anwar, J.; Patel, Z.; Ghio, A.J. Cigarette Smoking, and Blood Monocyte Count Correlate with Chronic Lung Injuries and Mortality. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 431–446. [Google Scholar] [CrossRef]
| Author, Year, Country | Study Design | Sample Size, Type of Vaccine, Study Population, Type of Smoking, Immune Response Indicators | Main Results | Quality/Risk of Bias |
|---|---|---|---|---|
| MacKenzie et al. [14] 1976 UK | RCT | 799 Influenza vaccine Group A: 259 (smokers 42.9%), two doses of a live influenza A virus vaccine administered intra-nasally; Group B: 264, two doses of a saline control; Group C: 276 (smokers 32.2%), two doses of a killed subunit influenza vaccine administered by deep subcutaneous injection Active tobacco cigarette smoking Serum antibody levels | After receiving the live vaccine, smokers seroconverted significantly higher with respect to non-smokers (p < 0.05). Subunit vaccines determined a similar trend. After vaccination, the persistence of the immunity was assessed for 50 weeks. The persistence of haemagglutination-inhibiting (HI) antibody was not significantly different between smoker and non-smoker subjects who received the live vaccine, while a significant depression in the persistence of HI antibody by 50 weeks was observed in smokers who received subunit vaccines. The persistence in volunteers with residual immunity before vaccination was not influenced by smoking. | Medium risk |
| Struve et al. [15] 1992 Sweden | Non-randomized CT | 595 Hepatitis B vaccine: 257 intramuscular route, 338 intradermal route Female (74.8%); median age 34–42 years; smokers (39%) Active tobacco cigarette smoking Serum antibody levels | Smokers and non-smokers presented a percentage of protective anti-HBs levels (≥10 IU/I) respectively equal to 39% and 61%. Female sex, intramuscular vaccination, young age, and being a non-smoker were linked to a higher geometric mean anti-HBs titre and a higher response rate with respect to males, intradermal vaccination, old age and being a smoker. The vaccination’s failure rate was equal to an OR 1.6 (95% CI 0.9–2.9, NS) comparing smokers vs. non-smokers. | Medium risk |
| Winter et al. [16] 1994 UK | RCT | 115 Hepatitis B vaccine Group A: 56 (rapid schedule, vaccination at 0, 1, 2 and 12 months); Group B: 59 (standard schedule, vaccination at 0, 1 and 6 months) Female (69.6%); smokers (32.2%); Active tobacco cigarette smoking Serum antibody levels | Regardless of vaccination schedule, smokers (standard vaccination: 28.6%, rapid vaccination: 5.6%) failed to seroconvert and achieve higher antibody levels with respect to non-smokers (standard vaccination: 5.6%, rapid vaccination: 2.8%) (p = 0.0003). | Medium risk |
| Cruijff et al. [17] 1999 Netherlands | Double-blind RCT | 1531 Influenza vaccine Group A: 927 Purified split-virion vaccine, A/Singapore/6/86 (H1N1), A/Beijing/353/89 (H3N2), B/Panama/45/90 and B/Beijing/1/87; Group B: 911 intramuscular placebo physiological saline solution Female (52.3%); age range 60–91 years; cigarette smokers (321), pipe and/or cigar smokers (58), non-smokers (1152); 490 patients with heart conditions, lung conditions, or diabetes mellitus Cigarette, pipe and cigar active smoking Serum antibody levels | The efficacy of vaccination was found to be statistically significantly different between smokers and non-smokers (p < 0.0001; corrected for age, sex and risk group). No notable discrepancies were observed in pre-titres between smokers and non-smokers, with adjustments made for age, sex, and risk group. The antibody titre exhibited a statistically significant increase following vaccination in smokers for the A/Singapore/6/86 and B/Beijing/11/87 strains, but not for the A/Beijing/353/89 and B/Panama/45/90 strains. The decline in titre at the conclusion of the post-vaccination period was marginally more pronounced in smokers for all strains. The end-titre was observed to be marginally elevated in smoker with respect to non-smoker individuals both for B/Panama/45/90 and B/Beijing/11/87. In addition, the end-titre adjusted for age, sex and risk group was significantly higher for A/Singapore/6/86 (p = 0.04). | Low risk |
| Baynam et al. [18] Australia 2007 | Cross-sectional study | 200 with parental atopic history Pertussis, polio, haemophilus influenzae type b, measles–mumps-rubella vaccines Female: no parental smoking (39%), parental smoking (55%); mean age 2 years and 15 days; parental smoking: 158 (79%) not exposed, 42 (21%) exposed Passive tobacco cigarette smoking Serum antibody levels | The unexposed subjects with IL-4 2589 CT/TT genotypes exhibited heightened antibody responses to diphtheria (p = 0.005) and tetanus (p = 0.04) compared with those with the CC genotype. Individuals with IL-4Ra I5OV II/IV genotypes exhibited heightened antibody responses to diphtheria compared with subjects with the VV genotype, though this did not reach statistical significance (NS). Subjects who had been exposed and who carried the IL-4 2589 CT/TT genotypes exhibited diminished antibody responses to tetanus in comparison with subjects with the CC genotype (p = 0.02). Individuals with IL-4Ra Q551R QR/RR genotypes exhibited diminished antibody responses relative to subjects with the QQ genotype (diphtheria, p = 0.03; tetanus, p = 0.02). Increased vaccine response was associated with single nucleotide polymorphisms in the IL-4 and IL-4Ra genes linked to atopy in children of non-smokers and lowered responses were observed in children of smokers. | Good |
| Roseman et al. [19] 2012 Sweden | Cohort study | 505 Pneumococcal conjugate vaccine Female (63%); smokers (17.4%); ex-smokers (40%); 253 rheumatoid arthritis; 252 spondylarthropathy Active tobacco cigarette smoking Serum geometric mean concentration of antibody responses; serum antibody levels | Serotype specific IgG against pneumococcal polysaccharide serotypes 23F and 6B resulted as follows: 9.9 g/L in smokers (p = 0.028), 10.6 g/L in non-smokers (NS), 10.4 g/L in ex-smokers (NS). Geometric mean concentration (GMC) of antibody responses were significantly higher in non-smokers for both serotypes. GMC immune response 23F: smokers 2.6 (p = 0.028), non-smokers 3.7 (NS), ex-smokers 3.3 (NS); GMC immune response 6B: smokers 1.8 (p = 0.0042), non-smokers 2.4 (NS), ex-smokers 2.2 (NS). Current smokers presented lower IgG serum levels than non-smokers independently of ongoing treatment or diagnosis (p = 0.028). Lower IgG level was statistically significantly associated with higher numbers of cigarettes smoked daily and number of packs smoked annually (p = 0.003 and p = 0.006, respectively). | Good |
| Nath et al. [20] 2014 Australia | Cohort study | 34 (20 COPD, 14 healthy) Influenza vaccine Female: COPD (35%), non-COPD (42.9%); mean age: COPD 66 years, non-COPD 54 years; smokers: COPD 45%, non-COPD 0% Active tobacco cigarette smoking Serum antibody levels | Vaccine response (expressed as Ab titres 28 days post-vaccination) was not associated with the habit of smoking. | Fair |
| Namujju et al. [21] 2014 Finland | Cohort study | 216 Human papilloma virus vaccine Female (100%); age range 16–17 years; smokers: 46.6% interventions, 56.6% controls; 103 HPV vaccine (interventions), 113 HAV vaccine (controls) Active tobacco cigarette smoking Serum antibody levels; serum antibody avidity | After month 7 post vaccination women who smoked (cotinine level > 20 ng/mL) presented levels of anti-HPV16/18 antibodies similar to those received for non-smoking women. Low-avidity HPV16/18 IgG antibodies were observed in 16% of the vaccinated women, and active smoking determined a three-fold increased risk (95% CI 1.0–9.3) of low-avidity antibodies. Mean absorbance of anti-HPV16 antibodies was equal to 1.97 (±0.78) among non-smokers and 1.88 (±0.73) among smokers, but differences were not significant. Mean absorbance of anti-HPV18 antibodies was 1.44 (±0.85) among non-smokers and 1.36 (±0.76) among smokers, but also in this case differences were not significant. | Good |
| Hartal et al. [22] 2015 Israel | Cohort study | 617 Hepatitis B vaccine Female (0%); age range 18–20 years; 156 smokers (25.7%): 132 from 1–19/day and 24 heavy smokers >20/day Active tobacco cigarette smoking Serum antibody levels | Heavy smokers had a lower rate of seropositivity at encounter 2 one month after the first booster dose (66.7% vs. 88.4%, RR 0.75, p = 0.016). Heavy smokers were 5 times less likely to present detectable antibodies after a single booster dose (OR 0.196, 95% CI 0.060–0.641, p = 0.007). | Good |
| Petras and Olear [23] 2018 Czech Republic/Slovakia Work supported by Biodrug s.r.o. Slovakia | Single blind RCT | 200 Tetanus vaccines Female (50%); age range 24–65 years; smokers (20.5%) Active tobacco cigarette smoking Serum geometric mean concentration of antibody responses; serum antibody levels; pre-to post-booster level ratio | Smokers had a significantly reduced seroconversion rate both considering crude (0.47; 95% Cl 0.23–0.96) and adjusted ORs (aORs) (0.11; 95% Cl 0.02–0.69). Only 56% of smokers (95% CI 40–72%) had a fourfold increase in antibodies with respect to 73% of non-smokers (95% CI 65–80%) (p = 0.019). Any significant difference in post-booster geometric mean concentrations (GMCs) of tetanus antibody (7.3 in smokers with respect to 8.0 in non-smokers, NS) and pre-to post-booster level ratio, including the 95%CI (6.7 in smokers with respect to 7.8 in non-smokers, NS). | Low risk |
| Meier and Berger [24] 2020 Switzerland | Cohort study | 247 (40 non responders NR, 73 low responders LR, 134 responders R) Hepatitis B vaccine Smokers: NR (57.1%), LR (52.5%) R (30.8%) Active tobacco cigarette smoking Serum antibody levels | Smoking presented a strong association with predicted non-response (p = 0.011). Individuals was grouped as follows: non-smoker (0 points), 1–9 cigarettes a day (1 point), and ≥10 cigarettes a day (2 points), resulting in a potential score between 0 and 6. Vaccine responders presented a median score equal to 1 (IQR 0–2), low-responders equal to 2 (IQR 1–3), and non-responders equal to 2.5 (IQR 1–4). Only about 5% of the responders, but 35% of the low−/non-responders, presented a score equal to or higher than 4, while about 85% of the responders had a score equal to or less than 2. In an ROC analysis, a high score predicted non-response with a specificity equal to 85% and a sensitivity equal to 47%. | Good |
| Gumus et al. [25] 2021 Turkey | Cross-sectional study | 94 Inactive SARS-CoV-2 vaccine Female (45.7%); mean age 41.0 ± 7.74 years; smokers (36.2%) Active tobacco cigarette smoking Serum antibody levels | Seropositivities predominant in non-smokers compared with smokers after each dose of vaccine (75–64.1% and 25.0–35.9%, p = 0.555 and p = 0.999, respectively). | Good |
| Linardou et al. [26] 2021 Greece | Cohort study | 288 (189 cancer patients and 99 healthy controls) BNT162b2 COVID-19 mRNA, mRNA-1273 and AZD1222 vaccines Female (56.9%); age from 18 to >85; 159 smokers (55.2%): 88 current smokers and 71 previous smokers Active tobacco cigarette smoking Serum antibody levels | Significant association between IgG titres and smoking status (p = 0.017). Post-hoc analysis revealed that those who had never smoked presented significantly higher antibody titres with respect to current smokers (p = 0.006). | Good |
| Nomura et al. [27] September 2021 Japan | Cohort study | 378 BNT162b2 COVID-19 mRNA vaccine Female (67.5%); median age 44 years; smokers (40.7%) Active tobacco cigarette smoking Serum median antibody titres; serum antibody levels | The age-adjusted median antibody titres (interquartile range) were found to be equal to −174 and 90, respectively, in those who had always smoked and those who had never smoked (p < 0.0001). In both genders, the age-adjusted median antibody titres were significantly lower in individuals who had always smoked than in those who had never smoked. The age-adjusted median antibody titres in males were −246 and 49, and in females were −140 and 95, respectively. The antibody titres were found to be significantly lower in current smokers than in ex-smokers (p = 0.019). The age-adjusted median antibody titres were found to be significantly lower in individuals who had always smoked than in those who had never smoked, with a p-value of 0.0007 for males and 0.0023 for females. The age-adjusted median antibody titres between current smokers and never smokers were found to be significantly different (p < 0.0001), as were the titres between ex-smokers and those who had never smoked (p = 0.0019). | Good |
| Nomura et al. [28] December 2021 Japan | Cohort study | 365 BNT162b2 COVID-19 mRNA vaccine Female (68.5%); mean age 43.9 years; 135 smokers (37%): 90 current smokers and 45 ex-smokers Active tobacco cigarette smoking Serum median antibody titres; serum antibody levels | The age-adjusted median (interquartile range) of antibody titres at six months was found to be −97 (−277 to 184) in those who had always smoked and 56 (−182 to 342) in those who had never (p < 0.0001). Antibody titres in current smokers were −205, while ex-smokers exhibited a value of −72. Antibody titres in ex-smokers were found to be significantly lower than in those who had never smoked (p = 0.0203), and current smokers exhibited lower titres than those who had never smoked (p < 0.0001). The median percentage change in antibody titres from three to six months was 28.4% for those who had always smoked and 30.3% for those who had never smoked (p = 0.3051). The median percentage change in antibody titres from three to six months was 31.7% for current smokers and 27.4% for ex-smokers (p = 0.3853). The median percentage change in antibody titres from three to six months was 31.7% for ex-smokers and 27.4% for those who had never smoked (p = 0.2914). The median percentage change in antibody titres from three to six months was 31.7% for current smokers and 27.4% for those who had never smoked (p = 0.8809). Significant differences were observed between males and females with regard to age-adjusted median antibody titres, according to smoking status. However, no significant differences were identified in the median percentage change in antibody titres by smoking status, when stratified by sex. Both the groups of those who had always smoked and those who had never smoked had significant sex differences in the median percentage change in Ab titres. Age-adjusted median Ab titres and percentage changes between sex and smoking were found as follows: individuals who had always smoked—Ab, median (IQR), U/mL male/female −120/−68 (p = 0.5709), percentage change, median (IQR) −25.9%/−30.5% (p = 0.0400); those who had never smoked—115/46 (p = 0.4700), −24.0%/−31.7% (p = 0.0050); p-value for Ab titre, male p = 0.0040, female p = 0.0120; p-value for percentage change male p = 0.7613, female p = 0.7018. | Good |
| Pitzalis et al. [29] 2021 Italy | Cohort study | 975 (912 multiple sclerosis patients and 63 healthy controls) BNT162b2 COVID-19 mRNA vaccine Female (73.1%); mean age 48.8 years in multiple sclerosis patients and 52.1 years in controls; smokers (28.6%) Active tobacco cigarette smoking Serum antibody levels | Effects of cigarette smoking on humoral response to SARS-CoV-2 vaccine in a subset of 535 multiple sclerosis patients negative for Anti-N antibody production for whom smoking status was 28.6% for active smokers. Among multiple sclerosis patients, reduced Anti-S antibody production in smokers (median = 719 U/mL) with respect to non-smokers (median = 1054 U/mL) in response to BNT162b2 vaccine (p < 0.001). | Good |
| Tsatsakis et al. [30] 2021 Greece | Cross-sectional study | 517 BNT162b2 COVID-19 mRNA vaccine Female (66.3%); mean age 47.7 years; smokers (34.4%) Active tobacco cigarette smoking Serum antibody levels | Significantly higher Ab titres in non-smokers with respect to smokers (4.48 ± 2.79 vs. 3.80 ± 2.64, respectively, p = 0.003). | Good |
| Ferrara et al. [31] 2022 Italy | Cohort study | 162 BNT162b2 COVID-19 mRNA vaccine Female (58.0%); mean age 42.4 years; smokers (30.2%) Active tobacco cigarette smoking Serum median antibody titres; serum antibody levels | A median antibody titre of 211.80 AU/mL (interquartile range [IQR] 149.80–465.50) was observed in 30.2% of current smokers and 69.8% of non-smokers (p = 0.002) 60 days after the completion of the vaccination cycle. A median antibody titre of 487.50 AU/mL (IQR 308.45–791.65) was observed in 48.7% of current smokers and 51.3% of non-smokers (p = 0.002) 60 days after the completion of the vaccination cycle. The notable disparity in vaccine-induced IgG titres between current smokers and non-smokers persisted even after adjusting for age, sex, and previous SARS-CoV-2 infection. | Good |
| Uysal et al. [32] 2022 Turkey | Cohort study | 314 Inactive SARS-CoV-2 vaccine Female (57.6%); median age: female 39 years, male 41 years; smokers (32.6%) Active tobacco cigarette smoking Serum antibody levels | Significantly lower Ab titres were recovered in smokers [1–125 U/mL (40.0%), 126–250 U/mL (24.7%), >250 U/mL (27.5%)] with respect to non-smokers [1–125 U/mL (60.0%), 126–250 U/mL (75.3%), >250 U/mL (72.5%)] (p = 0.032). | Good |
| Watanabe et al. [33] 2022 Italy | Cohort study | 86 BNT162b2 COVID-19 mRNA vaccine Female (60.5%); median age 43 years; smokers (31.7%); Caucasian ethnicity; hypertension (15.3%) type 2 diabetes (2.4%), dyslipidaemia (7.1%) Active tobacco cigarette smoking Serum antibody levels | Significantly lower antibody levels were found in smokers with respect to non-smokers [1099 (1350) vs. 1921 (1375), respectively, (p = 0.007)]. | Good |
| Golec et al. [34] 2022 Poland | Cohort study | 243 BNT162b2 COVID-19 mRNA vaccine Female (76.95%); mean age 47.42 years; smokers (18.52%) Active tobacco cigarette smoking Serum antibody levels | Following vaccination, smokers exhibit diminished long-lasting immunity relative to non-smokers (OR 0.38, 95% CI 0.17–0.85, p = 0.02). The temporal changes in IgG titre are contingent upon the status of smoking. | Good |
| Hikary et al. [35] 2022 Spain | Cross-sectional study | 2467 Human papilloma virus vaccine Female (100%); age range 20–24 years; smokers (18.7%) Active tobacco cigarette smoking Incidence of HPV-related lesions | HPV bivalent or quadrivalent vaccination is effective in protecting against CIN but insufficient in smokers. In non-smokers, HPV vaccination significantly reduced the incidence of HSIL+ from 0.42% to 0.1% (OR 0.21, 95% CI, 0.05–0.95), but not in smokers (OR 0.59, 95% CI, 0.22–1.56). In vaccinated women, the incidence of CIN2+ was equal to 0.20% in non-smokers and 0.87% in smokers (OR 0.22, 95% CI, 0.05–0.89, p = 0.02). In the vaccinated cohort, the incidence of CIN1+ was found to be equal to 4.8% in smokers and 1.9% in non-smokers, while the incidence of CIN2+ was 0.87% and 0.20%, respectively. The odds ratio (OR) for non-smokers with respect to smokers in the development of CIN1+ was 0.38 (95% CI, 0.22–0.65, p = 0.0003), while the OR for CIN2+ was 0.22 (95% CI, 0.05–0.89, p = 0.02). | Good |
| Mori et al. [36] 2022 Japan | Cross-sectional study | 55 BNT162b2 COVID-19 mRNA vaccine Female (0%); age range 20–69 years; smokers (100%): cigarette 25.5%, heat-not-burn tobacco 52.7%, combination 21.8% Active tobacco cigarette smoking, heat-not-burn tobacco Serum antibody levels | Fagerstrom test for nicotine dependence showed a significant negative relationship with IgG levels (ρ = −0.426, p = 0.001) and a weak negative relationship between serum cotinine level and IgG concentrations (ρ = −0.156, p = 0.256). | Good |
| Ramirez Sevilla et al. [37] 2022 Spain | Cohort study | 1003 MV140 vaccine Female (82.7%); mean age 78 years; smokers (24.6%) Active tobacco cigarette smoking Incidence of urinary tract infections | Smoking did not degrade the response of MV140 in preventing recurrent urinary tract infections. Efficacy was respectively equal to 0–1 urinary tract infections (UTIs) in 80.2% (3 months), 65.5% (6 months), 53.9% (12 months) for smokers and 0–1 UTIs in 85.8% (3 months), 66.8% (6 months), 20% (12 months) for non-smokers (NS). | Good |
| Trontzas et al. [38] 2022 Greece | Cohort study | 246 BNT162b2 COVID-19 mRNA, mRNA-1273 and AZD1222 vaccines Group A: 125 patients with lung cancer and ongoing anticancer therapy, female (25.7%), median age 68 years, smokers (16.0%), ex-smokers (80.0%), those who had never smoked (4.0%); Group B: 35 non-lung cancer patients, female (54.3%), median age 59 years, smokers (14.3%), ex-smokers (68.6%), those who had never smoked (17.1%); Group C: 86 healthy controls; female (72.1%), median age 50 years, smokers (29.1%), ex-smokers (22.1%), those who had never smoked (48.8%) Active tobacco cigarette smoking Serum antibody levels | Significant reduction of Ab titres for lung cancer patients who were smokers with respect to those patients with lung cancer who were former smokers or who had never smoked at T2 (p = 0.04), T3 (p = 0.04) and T4 (p < 0.0001); no significant reduction of Ab titres was recovered for healthy patients smokers (NS). | Poor |
| Toda et al. [39] 2022 Japan | Cohort study | 139 (104 haemodialysis patients and 35 controls) BNT162b2 COVID-19 mRNA vaccine Mean age: haemodialysis patients 70.4 years old, controls 40.3 years old; smokers (9.35%) Active tobacco cigarette smoking Serum antibody levels | Smokers presented significantly lower Ab titres compared with non-smokers (p = 0.007) | Fair |
| Yamamoto et al. [40] 2022 Japan | Cross-sectional study | 3433 BNT162b2 COVID-19 mRNA vaccine Female (72.0%); median age 41 years; those who had never smoked (82.0%); ex-smokers (11.0%); smokers (6.0%); exclusive HTPs user (2.0%); dual user of HTPs and cigarettes (1.0%); exclusive cigarette smoker (3.0%); hypertension (8.0%); diabetes (2.0%); cancer (1.0%) Active tobacco cigarette smoking, including heated tobacco products (HTPs) Serum antibody levels; serum geometric mean titres | The antibody geometric mean titre (GMT) was observed to be significantly lower in smokers compared with those who had never smoked (101 vs. 96, respectively; ratio of means, 0.85 [95% CI: 0.78–0.93]). Cigarette smokers exhibited significantly lower GMT than those who had never smoked (adjusted GMT: 118 versus 96; ratio of means: 0.81 [95% CI: 0.71–0.92], p < 0.01). Furthermore, exclusive HTP users and dual users exhibited a comparable reduction in adjusted GMT (103 and 107, respectively) relative to those who had never smoked (ratio of means 0.87 [95% CI: 0.75–1.02] and 0.90 [95% CI: 0.75–1.07], respectively, NS). | Fair |
| Asmar et al. [41] 2023 Palestine | Cross-sectional study | 172 COVID-19 mRNA and inactive vaccines Female (45.3%); mean age 39.7 years; smokers (39.5%) Active tobacco cigarette smoking Serum antibody levels | Lower levels of vaccine-induced Ab in smokers with respect to non-smokers with a mean of AU/mL (SD) IgG-S equal to 11,219 (12094) and 13,415 (12972), respectively (NS) | Good |
| Baglioni et al. [42] 2023 Italy | Cohort study | 1115 BNT162b2 COVID-19 mRNA vaccine Female (69.1%); mean age 48.1 years; smokers (23.8%) Active tobacco cigarette smoking Serum antibody levels | Mean Ab response was lower in smokers compared with non-smokers, both at 120 days (787 binding antibody units/mL vs. 949 BAU/mL; p < 0.001) and at 180 days from the second dose (493 BAU/mL vs. 657 BAU/mL; p < 0.001) | Good |
| Eyupoglu et al. [43] 2023 Turkey | Cohort study | 224 (113 vaccinated) Inactive SARS-CoV-2 vaccine Female (56.3%); median age: female 25.0 years, male 27.0 years; smokers (34.8%); 10 with a history of nCoV (−) inactive vaccine (one dose); 103 with a history of nCoV (−) inactive (two doses) Active tobacco cigarette smoking Serum antibody levels | IgG response rate among participants with a history of nCoV (−) inactive vaccine (one dose) (n = 10) was equal to 0.6 (0.3–4.3) in non-smokers and 0.4 (0.1–18.0) in smokers (NS). IgG response rate in participants with history of nCoV (−) inactive vaccine (two doses) (n = 103) was equal to 49.0 (11.5–160.5) in non-smokers and 23.1 (7.4–98.5) in smokers (NS). | Good |
| Prather et al. [44] 2023 USA | Cohort study | 498 BNT162b2 COVID-19 mRNA, mRNA-1273, Ad26.COV2.S vaccines Female (64.3%); mean age 55; smokers (2%) Active tobacco cigarette smoking Serum antibody levels | Non-smokers presented 2.4-fold higher neutralizing Ab than smokers (mean difference = −0.37, CI −0.64 to −0.10; p = 0.007). | Fair |
| Syrimi et al. [45] 2023 Greece | Cohort study | 204 (204 at 4-month timepoint and 189 at 9-month timepoint) BNT162b2 COVID-19 mRNA vaccine Female (51.3%); median age 43 years; smokers (31.8%); Caucasian ethnicity; hypertensive on medication (11), dyslipidaemia (17), autoimmunity (7), immunosuppression (4) Active tobacco cigarette smoking Serum antibody levels | Ab levels increased in non-smokers at the first month [mean 159 U/mL (100–195), p = 0.009], at 4 months [mean 24 U/mL (13–49), p < 0.001], and at 9 months after the second dose [mean 4.78 U/mL (2.84–8.22), p < 0.001] with respect to smokers. | Good |
| Unal et al. [46] 2024 Turkey | Cross-sectional study | 329 Inactive SARS-CoV-2 vaccine Female (71.4%); mean age 49.7 ± 13.7 years; smokers (30.3%) Active tobacco cigarette smoking Serum antibody levels | Ab levels (AU/mL) of individuals with a positive Ab response after vaccination were analyzed by two laboratories, Lab A (183 results) and Lab B (39 results). The resuls were as follows: Lab A: non-smokers (583.38 ± 531.32) and smokers (446.44 ± 392.31) (NS); Lab B: non-smokers (61.07 ± 58.92) and smokers (15.24 ± 16.86) (NS). | Good |
| Fonzo et al. [47] 2024 Italy | Cohort study | 2133 Hepatitis B vaccine Non-smokers (85.8%, female 64.2%), smokers (14.2%, female 53.3%); mean age 20.28 ± 0.92 years Active tobacco cigarette smoking Serum antibody levels | Overall, there was a non-significant difference in the percentage of subjects with Ab levels below 10 IU/L between non-smokers and smokers (50.6% vs. 56.6%). Smokers were more likely to have non-protective Ab levels than non-smokers (AOR: 1.291; 95% CI: 1.006–1.657, p = 0.0045). | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valeriani, F.; Protano, C.; Pozzoli, A.; Vitale, K.; Liguori, F.; Liguori, G.; Gallè, F. Does Tobacco Smoking Affect Vaccine-Induced Immune Response? A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 1260. https://doi.org/10.3390/vaccines12111260
Valeriani F, Protano C, Pozzoli A, Vitale K, Liguori F, Liguori G, Gallè F. Does Tobacco Smoking Affect Vaccine-Induced Immune Response? A Systematic Review and Meta-Analysis. Vaccines. 2024; 12(11):1260. https://doi.org/10.3390/vaccines12111260
Chicago/Turabian StyleValeriani, Federica, Carmela Protano, Angela Pozzoli, Katia Vitale, Fabrizio Liguori, Giorgio Liguori, and Francesca Gallè. 2024. "Does Tobacco Smoking Affect Vaccine-Induced Immune Response? A Systematic Review and Meta-Analysis" Vaccines 12, no. 11: 1260. https://doi.org/10.3390/vaccines12111260
APA StyleValeriani, F., Protano, C., Pozzoli, A., Vitale, K., Liguori, F., Liguori, G., & Gallè, F. (2024). Does Tobacco Smoking Affect Vaccine-Induced Immune Response? A Systematic Review and Meta-Analysis. Vaccines, 12(11), 1260. https://doi.org/10.3390/vaccines12111260










