Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies
Abstract
1. Introduction
2. HPV Infection: General Aspects and Virology of HPV
3. Global and Local Burden of HPV Infection: Epidemiology, Prevention, and Socioeconomic Impact
4. Diagnostic Approaches and HPV Prevention: Microbiological Testing and Vaccination Strategies
5. Genetic Aspects of HPV Infection: Viral Variants and Individual Predisposition
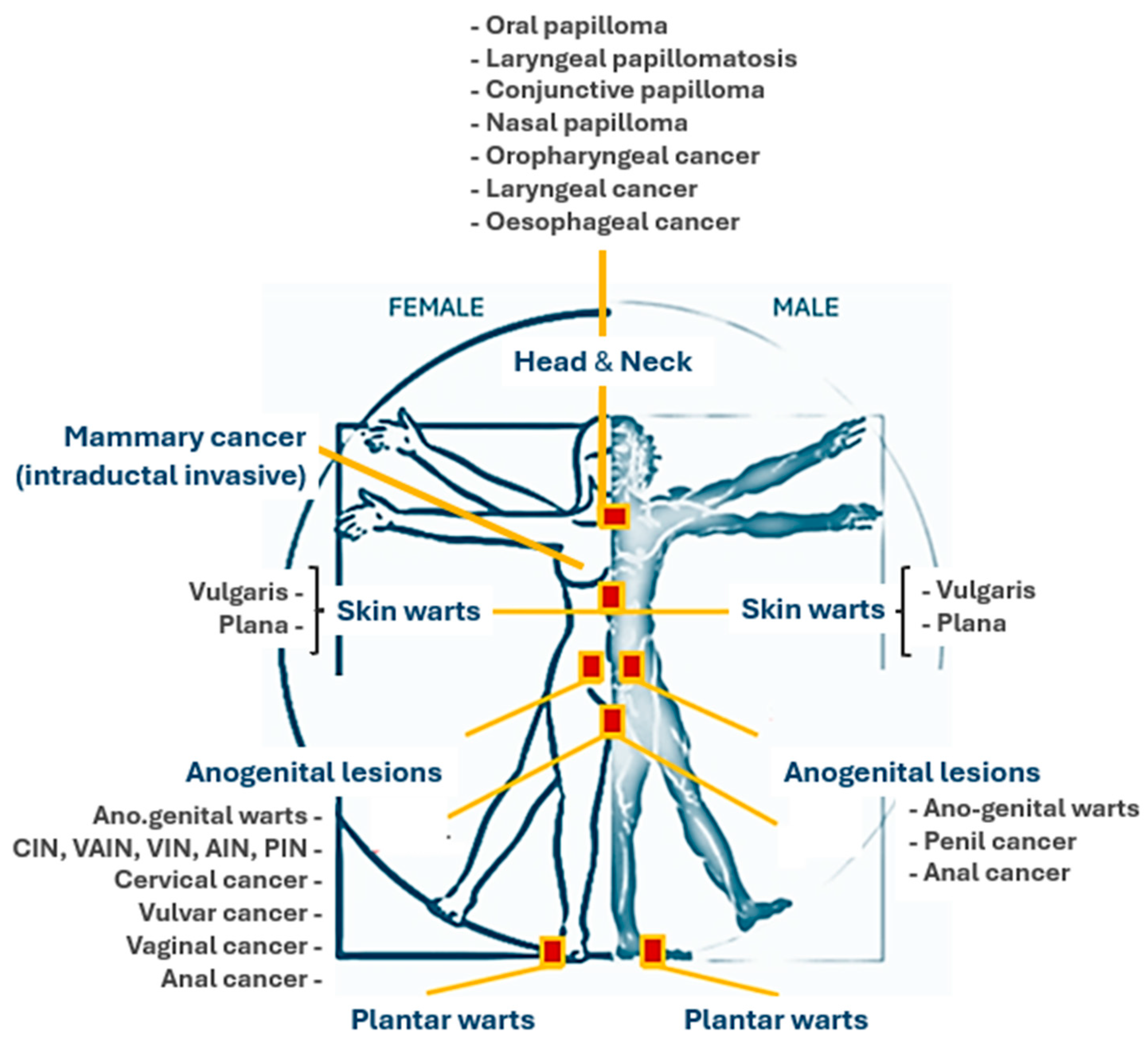
6. Clinical Manifestations of HPV Infection
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vaccines to Treat Human Papillomavirus Could Be a Significant Innovation in the Fight Against Cervical Cancer. Available online: https://www.who.int/news/item/03-07-2024-vaccines-to-treat-human-papillomavirus-could-be-a-significant-innovation-in-the-fight-against-cervical-cancer (accessed on 18 October 2024).
- Swanson, A.A.; Pantanowitz, L. The evolution of cervical cancer screening. J. Am. Soc. Cytopathol. 2024, 13, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Barroeta, J.E. The Future Role of Cytology in Cervical Cancer Screening in the Era of HPV Vaccination. Acta Cytol. 2023, 67, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Heal. 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Human Papillomavirus and Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 14 October 2024).
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Malagón, T.; Franco, E.L.; Tejada, R.; Vaccarella, S. Epidemiology of HPV-associated cancers past, present and future: Towards prevention and elimination. Nat. Rev. Clin. Oncol. 2024, 21, 522–538. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Tsu, V.; Deeks, S.L.; Cubie, H.; Wang, S.A.; Vicari, A.S.; Brotherton, J.M. Human papillomavirus vaccine introduction–the first five years. Vaccine 2012, 30, F139–F148. [Google Scholar] [CrossRef]
- Reuschenbach, M.; Doorbar, J.; del Pino, M.; Joura, E.A.; Walker, C.; Drury, R.; Rauscher, A.; Saah, A.J. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine 2023, 41, 6194–6205. [Google Scholar] [CrossRef]
- Khalil, A.I.; Zhang, L.; Muwonge, R.; Sauvaget, C.; Basu, P. Efficacy and safety of therapeutic HPV vaccines to treat CIN 2/CIN 3 lesions: A systematic review and meta-analysis of phase II/III clinical trials. BMJ Open 2023, 13, e069616. [Google Scholar] [CrossRef]
- WHO Preferred Product Characteristics for Therapeutic HPV Vaccines. Available online: https://www.who.int/publications/i/item/9789240092174 (accessed on 19 October 2024).
- Ebrahimi, N.; Yousefi, Z.; Khosravi, G.; Malayeri, F.E.; Golabi, M.; Askarzadeh, M.; Shams, M.H.; Ghezelbash, B.; Eskandari, N. Human papillomavirus vaccination in low-and middle-income countries: Progression, barriers, and future prospective. Front. Immunol. 2023, 14, 1150238. [Google Scholar] [CrossRef]
- Pavia, G.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Marascio, N.; Quirino, A.; Matera, G.; et al. Integrating Digital Health Solutions with Immunization Strategies: Improving Immunization Coverage and Monitoring in the Post-COVID-19 Era. Vaccines 2024, 12, 847. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.-U.; Chan, P.K.S.; DeSalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV virus taxonomy profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Kostrzewska-Poczekaj, M.; Wierzbicka, M.; Brenner, J.C.; Giefing, M. HPV-driven oncogenesis—Much more than the E6 and E7 oncoproteins. J. Appl. Genet. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Day, P.M.; Trus, B.L. The papillomavirus major capsid protein L1. Virology 2013, 445, 169–174. [Google Scholar] [CrossRef]
- Wang, J.W.; Roden, R.B. L2, the minor capsid protein of papillomavirus. Virology 2013, 445, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Bzhalava, D.; Eklund, C.; Dillner, J. International standardization and classification of human papillomavirus types. Virology 2015, 476, 341–344. [Google Scholar] [CrossRef]
- de Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- A Population-Based Prospective Study of Carcinogenic Human Papillomavirus Variant Lineages, Viral Persistence, and Cervical Neoplasia—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20354192/ (accessed on 19 October 2024).
- Ibeanu, O.A. Molecular pathogenesis of cervical cancer. Cancer Biol. Ther. 2011, 11, 295–306. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Mirabello, L.; Yeager, M.; Cullen, M.; Boland, J.F.; Chen, Z.; Wentzensen, N.; Zhang, X.; Yu, K.; Yang, Q.; Mitchell, J.; et al. HPV16 sublineage associations with histology-specific cancer risk using HPV whole-genome sequences in 3200 women. JNCI J. Natl. Cancer Inst. 2016, 108, djw100. [Google Scholar] [CrossRef]
- Chauhan, S.C.; Jaggi, M.; Bell, M.C.; Verma, M.; Kumar, D. Epidemiology of human papilloma virus (HPV) in cervical mucosa. Methods Mol Biol. 2009, 471, 439–456. [Google Scholar]
- Galati, L.; Brancaccio, R.N.; Robitaille, A.; Cuenin, C.; Luzi, F.; Fiorucci, G.; Chiantore, M.V.; Marascio, N.; Matera, G.; Liberto, M.C.; et al. Detection of human papillomaviruses in paired healthy skin and actinic keratosis by next generation sequencing. Papillomavirus Res. 2020, 9, 100196. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Muñoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 22 October 2024).
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; Ali, H.; Boily, M.C.; Baldo, V.; Brassard, P.; Brotherton, J.M.; Callander, D.; et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, National Vaccine Prevention Plan. Available online: https://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4828&area=vaccinazioni&menu=vuoto (accessed on 21 October 2024).
- Carozzi, F.M.; Tornesello, M.L.; Burroni, E.; Loquercio, G.; Carillo, G.; Angeloni, C.; Scalisi, A.; Macis, R.; Chini, F.; Buonaguro, F.M.; et al. Prevalence of human papillomavirus types in high-grade cervical intraepithelial neoplasia and cancer in Italy. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef]
- Llave, C.L.; Uy, M.E.V.; Lam, H.Y.; Aldaba, J.G.; Yacapin, C.C.; Miranda, M.B.; Valverde, H.A.; Silva, W.T.; Nawaz, S.; Slavkovsky, R.C.; et al. The cost-effectiveness of human papillomavirus vaccination in the Philippines. Vaccine 2022, 40, 3802–3811. [Google Scholar] [CrossRef]
- Evans, M.R.; James, C.D.; Loughran, O.; Nulton, T.J.; Wang, X.; Bristol, M.L.; Windle, B.; Morgan, I.M. An oral keratinocyte life cycle model identifies novel host genome regulation by human papillomavirus 16 relevant to HPV positive head and neck cancer. Oncotarget 2017, 8, 81892. [Google Scholar] [CrossRef]
- Ursu, R.G.; Onofriescu, M.; Nemtescu, D.; Nemtescu, R.; Iancu, L.S. Detection of HPV 16 and HPV 18 viral loads by real time PCR in women with cervical dysplasia. J. Exp. Mol. Biol. 2011, 12. [Google Scholar]
- Cancer Genome Atlas Research Network. Albert Einstein College of Medicine; Analytical Biological Services; Barretos Cancer Hospital; Baylor College of Medicine; Beckman Research Institute of City of Hope. Buck Inst. Res. Aging 2017, 16, 378–384. [Google Scholar]
- Coquillard, G.; Palao, B.; Patterson, B.K. Quantification of intracellular HPV E6/E7 mRNA expression increases the specificity and positive predictive value of cervical cancer screening compared to HPV DNA. Gynecol. Oncol. 2011, 120, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Derbie, A.; Mekonnen, D.; Woldeamanuel, Y.; Van Ostade, X.; Abebe, T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): A systematic review. Infect. Agents Cancer 2020, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- von Knebel Doeberitz, M.; Reuschenbach, M.; Schmidt, D.; Bergeron, C. Biomarkers for cervical cancer screening: The role of p16INK4a to highlight transforming HPV infections. Expert Rev. Proteom. 2012, 9, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zou, M.; Xu, N.; Liu, Y.; Wang, Y. Portable, and ultrasensitive HR-HPV tests based on nucleic acid biosensors. Front. Cell. Infect. Microbiol. 2024, 14, 1357090. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.S.; Chan, T.L.; Au, C.H.; Leung, C.P.; To, M.Y.; Ng, M.K.; Leung, S.M.; Chan, M.K.M.; Ma, E.S.K.; Tang, B.S.F. An economical Nanopore sequencing assay for human papillomavirus (HPV) genotyping. Diagn. Pathol. 2020, 15, 45. [Google Scholar] [CrossRef]
- Liu, S.S.; Chan, K.K.L.; Wei, T.N.; Tse, K.Y.; Ngu, S.F.; Chu, M.M.Y.; Lau, L.S.K.; Cheung, A.N.Y.; Ngan, H.Y.S. Clinical performance of the Roche Cobas 4800 HPV test for primary cervical cancer screening in a Chinese population. PLoS ONE 2022, 17, e0272721. [Google Scholar] [CrossRef]
- Alameda, F.; Garrote, L.; Mojal, S.; Sousa, C.; Muset, M.; Lloveras, B.; Bellosillo, B.; Saldanha, C.; Carreras, R.; Serrano, S. Cervista HPV HR test for cervical cancer screening: A comparative study in the Catalonian population. Archives of Pathology and Laboratory Medicine. Arch. Pathol. Lab. Med. 2015, 139, 241–244. [Google Scholar] [CrossRef]
- Guo, M.; Khanna, A.; Feng, J.; Patel, S.; Zhang, W.; Gong, Y.; Huo, L.; Staerkel, G. Analytical performance of cervista HPV 16/18 in S ure P ath pap specimens. Diagn. Cytopathol. 2015, 43, 301–306. [Google Scholar] [CrossRef]
- Arbyn, M.; Simon, M.; Peeters, E.; Xu, L.; Meijer, C.J.; Berkhof, J.; Cuschieri, K.; Bonde, J.; Vanlencak, A.O.; Zhao, F.-H.; et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin. Microbiol. Infect. 2021, 27, 1083–1095. [Google Scholar] [CrossRef]
- Skolnik, J.M.; Morrow, M.P. Vaccines for HPV-associated diseases. Mol. Asp. Med. 2023, 94, 101224. [Google Scholar] [CrossRef]
- Wang, W.; Kothari, S.; Skufca, J.; Giuliano, A.R.; Sundström, K.; Nygård, M.; Koro, C.; Baay, M.; Verstraeten, T.; Luxembourg, A.; et al. Real-world impact and effectiveness of the quadrivalent HPV vaccine: An updated systematic literature review. Expert Rev. Vaccines 2022, 21, 1799–1817. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, A.; Popovsky, D.; Hmood, L.; Kim, A.S.; Akman, A.E.; Yuan, H. The nonavalent vaccine: A review of high-risk HPVs and a plea to the CDC. Am. J. Stem Cells 2019, 8, 52. [Google Scholar] [PubMed]
- Meites, E.; Wilkin, T.J.; Markowitz, L.E. Review of human papillomavirus (HPV) burden and HPV vaccination for gay, bisexual, and other men who have sex with men and transgender women in the United States. Hum. Vaccines Immunother. 2022, 18, 2016007. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cowell, L.G.; Tomkies, A.; Day, A.T. Therapeutic vaccination for HPV-mediated cancers. Curr. Otorhinolaryngol. Rep. 2023, 11, 44–61. [Google Scholar] [CrossRef]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E.; et al. Im-munotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012, 4, 155ra138. [Google Scholar] [CrossRef]
- Alouini, S.; Pichon, C. Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review. Vaccines 2024, 12, 428. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Espinoza, H.; Ha, K.T.; Pham, T.T.; Espinoza, J.L. Genetic predisposition to persistent human papillomavirus-infection and vi-rus-induced cancers. Microorganisms 2021, 9, 2092. [Google Scholar] [CrossRef]
- Alhamlan, F.S.; Alfageeh, M.B.; Al Mushait, M.A.; Al-Badawi, I.A.; Al-Ahdal, M.N. Human papillomavirus-associated cancers. Microb. Pathog. Infect. Immun. 2021, 1–4. [Google Scholar]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef]
- Bordignon, V.; Di Domenico, E.G.; Trento, E.; D’agosto, G.; Cavallo, I.; Pontone, M.; Pimpinelli, F.; Mariani, L.; Ensoli, F. How human papillomavirus replication and immune evasion strategies take advantage of the host DNA damage repair machinery. Viruses 2017, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R.; Venuti, A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71. [Google Scholar] [CrossRef]
- Nicolás-Párraga, S.; Gandini, C.; Pimenoff, V.N.; Alemany, L.; de Sanjosé, S.; Bosch, F.X.; Bravo, I.G.; the RIS HPV TT and HPV VVAP study groups. HPV16 variants distribution in invasive cancers of the cervix, vulva, vagina, penis, and anus. Cancer Med. 2016, 5, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Rosa, B.A.; Lameiras, S.; Cuninghame, S.; Bernard, J.; Floriano, W.B.; Lambert, P.F.; Nicolas, A.; Zehbe, I. Functional variants of human papillomavirus type 16 demonstrate host genome integration and transcriptional alterations corresponding to their unique cancer epidemiology. BMC Genom. 2016, 17, 851. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Shin, H.J.; Park, B.; Park, S.Y.; Yoo, C.W.; Yoon, K.A.; Kong, S.Y.; Kim, Y.J.; Kim, S.S.; Kim, J.Y. Integration pattern of human papillo-mavirus is a strong prognostic factor for disease-free survival after radiation therapy in cervical cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Crux, N.B.; Elahi, S. Human leukocyte antigen (HLA) and immune regulation: How do classical and non-classical HLA alleles modulate immune response to human immunodeficiency virus and hepatitis C virus infections? Front. Immunol. 2017, 8, 832. [Google Scholar] [CrossRef]
- Adebamowo, S.N.; Adeyemo, A.; Adebayo, A.; Achara, P.; Alabi, B.; Bakare, R.A.; Famooto, A.O.; Obende, K.; Offiong, R.; Olaniyan, O.; et al. Genome, HLA and polygenic risk score analyses for prevalent and persistent cervical human papillomavirus (HPV) infections. Eur. J. Hum. Genet. 2024, 32, 708–716. [Google Scholar] [CrossRef]
- Ramachandran, D.; Schürmann, P.; Mao, Q.; Wang, Y.; Bretschneider, L.; Speith, L.; Hülse, F.; Enßen, J.; Bousset, K.; Jentschke, M.; et al. Association of genomic variants at the human leukocyte antigen locus with cervical cancer risk, HPV status and gene expression levels. Int. J. Cancer 2020, 147, 2458–2468. [Google Scholar] [CrossRef]
- Fernandez-Avila, L.; Castro-Amaya, A.M.; Molina-Pineda, A.; Hernández-Gutiérrez, R.; Jave-Suarez, L.F.; Aguilar-Lemarroy, A. The value of CXCL1, CXCL2, CXCL3, and CXCL8 as potential prognosis markers in cervical cancer: Evidence of E6/E7 from HPV16 and 18 in chemokines regulation. Biomedicines 2023, 11, 2655. [Google Scholar] [CrossRef]
- El-Tahan, R.R.; Ghoneim, A.M.; El-Mashad, N. TNF-α gene polymorphisms and expression. Springerplus 2016, 5, 1508. [Google Scholar] [CrossRef]
- Gusho, E.; Laimins, L. Human papillomaviruses target the DNA damage repair and innate immune response pathways to allow for persistent infection. Viruses 2021, 13, 1390. [Google Scholar] [CrossRef]
- Porter, V.L.; Marra, M.A. The drivers, mechanisms, and consequences of genome instability in HPV-driven cancers. Cancers 2022, 14, 4623. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, M.D.; Fradet-Turcotte, A. Virus DNA replication and the host DNA damage response. Annu. Rev. Virol. 2018, 5, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, D.; Banerjee, A.; Pathak, S.; Jain, S.K.; Singh, N. Decreased expression of DNA repair genes (XRCC1, ERCC1, ERCC2, and ERCC4) in squamous intraepithelial lesion and invasive squamous cell carcinoma of the cervix. Mol. Cell. Biochem. 2013, 377, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Mostaid, S.; Mumu, S.B.; Haque, A.; Sharmin, S.; Jamiruddin, M.R.; Rahman, G.M.S.; Reza, H.M. Elevated serum expression of p53 and association of TP53 codon 72 polymorphisms with risk of cervical cancer in Bangladeshi women. PLoS ONE 2021, 16, e0261984. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Travier, N.; Waterboer, T.; De Sanjosé, S.; Bosch, F.X.; Pawlita, M.; Pala, V.; Weiderpass, E.; Margall, N.; Dillner, J.; et al. The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: Results from the EPIC cohort. PLoS ONE 2016, 11, e0147029. [Google Scholar]
- Kumar, M.M.; Davuluri, S.; Poojar, S.; Mukherjee, G.; Bajpai, A.K.; Bafna, U.D.; Devi, U.K.; Kallur, P.P.R.; Kshitish, A.K.; Jayshree, R.S. Role of estrogen receptor alpha in human cervical cancer-associated fibroblasts: A transcriptomic study. Tumor Biol. 2016, 37, 4409–4420. [Google Scholar] [CrossRef]
- Moarcăs, M.; Georgescu, I.; Brătilă, E.; Badea, M.; Cîrstoiu, E. Clinical significance of HPV-DNA testing for precancerous cervical lesions. J. Med. Life 2014, 7, 37–39. [Google Scholar]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–7. [Google Scholar] [CrossRef]
- Plotzker, R.E.; Vaidya, A.; Pokharel, U.; Stier, E.A. Sexually transmitted human papillomavirus: Update in epidemiology, prevention, and management. Infect. Dis. Clin. 2023, 37, 289–310. [Google Scholar]
- Wolf, J.; Kist, L.F.; Pereira, S.B.; Quessada, M.A.; Petek, H.; Pille, A.; Maccari, J.G.; Mutlaq, M.P.; Nasi, L.A. Human papillomavirus infection: Epidemiology, biology, host interactions, cancer development, prevention, and therapeutics. Rev. Med. Virol. 2024, 34, e2537. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.K. Human Papillomavirus (HPV) Clinical Presentation. Medscape eMedicine. 2024. Available online: https://emedicine.medscape.com/article/219110-overview?form=fpf (accessed on 29 October 2024).
- Luria, L.; Cardoza-Favarato, G. Human Papillomavirus; [Updated 2021 Jan 24]. Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shetty, S.; Alvarado, P.C.; Pettie, D.; Collier, J.H. Next-Generation Vaccine Development with Nanomaterials: Recent Advances, Possibilities, and Challenges. Annu. Rev. Biomed. Eng. 2024, 26, 273–306. [Google Scholar] [CrossRef] [PubMed]
| Type of Assays | Tests | Targets | Sensibility (%) | Specificity (%) | References |
|---|---|---|---|---|---|
| DNA-based assay | Digene Hybrid Capture 2 high-risk HPV DNA test Cervista HPV HR and Genfind DNA extraction kit Cobas HPV test | 13 high-risk HPV types using multigene probes L1, E6, E7 genes and 14 high-risk HPV types L1 gene, 14 high-risk HPV types, and HPV16 and Hpv18 | 92.86 (CIN2) 89.47 (CIN3) 98.4 82.14 (CIN2) 78.95 (CIN3) | 43.67 (CIN2) 42.86 (CIN3) 85.2 66.46 (CIN2) 65.42 (CIN3) | [42] [43] [42] |
| HPV genotyping assays | Cervista HPV16/18 | Detects and differentiates HPV16 and HPV18 | 77% | [44] | |
| E6/E7 mRNA-based assay | Aptima HPV assay | E6/E7 viral mRNA and 14 high-risk HPV types | 95.5% | 94.5% | [45] |
| HPV Type and Disease Association [75] | Characteristics of HPV Related Diseases [76,77,78] | Clinical Presentation [76,77,78] |
|---|---|---|
| Anogenital Warts —————- A total of 90% are caused by HPV types 6 or 11. Other types are 30, 42, 43, 45, 51, 54, 55, and 70. | Localization: Primarily affecting moist areas such as the perianal region, vaginal introitus, vagina, labia, and vulva. However, they can also arise on dry skin surfaces, including the penile shaft. Morphology: The spectrum ranges from smooth papular warts to keratotic warts, the latter resembling common cutaneous warts due to their thickened, irregular texture. Flat condylomata (squamous intraepithelial neoplasia) typically manifest as white, plaque-like lesions, most commonly on the cervix but also potentially involving the vulva, anus, and male genitalia. Giant condyloma:
| Typically, they occur several months after HPV inoculation. They pursue a slow, indolent course, often spreading through autoinoculation between adjacent skin surfaces. Condylomata acuminata are frequently asymptomatic but can cause pruritus. Bleeding may arise from lesion confluence and irritation by clothing. |
| Cervical HPV Infection and Disease —————- HPV types Low-risk 6, 11, (31, 33, 35, 42, 43, 44, 45, 51, 52, 74) High-risk 16, 18, (6, 11, 31, 34, 33, 35, 39, 42, 44, 45, 51, 52, 56, 58, 66) | Localization: cervical region of uterus. Morphology: low-grade squamous intraepithelial lesion or high-grade squamous intraepithelial lesion (Papanicolaou smear screening). Acetowhite changes and abnormal vascular patterns are indicative of HPV-associated dysplasia (3–5% acetic acid and colposcopy). HSIL can progress to invasive cervical cancer. | Most cervical HPV infections are latent or subclinical and therefore asymptomatic. Symptoms at cancer stage may include intermenstrual or postcoital bleeding, dyspareunia, and pelvic fullness. |
| Anal Cancer —————- HPV types 16, 18, (31, 45, 33, 35, 39, 51, 52, 56, 58, 66, 68, 70) | Localization: anal region. Morphology: HPV-associated inflammation can lead to anal intraepithelial neoplasia (AIN). AIN is graded I-III based on the degree of abnormality in squamous cell differentiation and maturation, mitotic activity, nuclear membrane changes, and the depth of these abnormalities within the epithelium. AIN can progress to invasive squamous cell carcinoma (SCC) in approximately 10–11% of cases. A history of anorectal warts is more common in homosexual men (50%) with anal SCC compared to women and heterosexual men (20%) | Asymptomatic or a range of symptoms including anal bleeding, anal or pelvic pain, weight loss, the sensation of anal or rectal mass, anal irritation, tissue prolapse, flatus or fecal incontinence, and constipation. SCC is often misdiagnosed as hemorrhoids. |
| Non-anogenital Mucosal Disease —————- HPV types 6, 11 | Involve various non-anogenital mucosal surfaces, including the nares, mouth, larynx, and conjunctiva. Oral warts are indicative of HPV infection of the oral mucosa. Focal epithelial hyperplasia, a disseminated HPV infection of the oral mucosa, is primarily associated with HPV types 13 and 32 and may exhibit a familial predisposition. | Oral warts are relatively common but often subtle and easily missed. |
| Non-genital Cutaneous HPV —————- Verruca vulgaris 1, 2, 4, 7 Palmoplantar warts 1, 2, 4, 63 Verruca plana 2, 3, 10 | Verruca Vulgaris: These warts typically occur on keratinized skin, presumably at the site of viral inoculation. Autoinoculation can lead to the development of adjacent warts. They present as circumscribed, rough, hyperkeratotic papulonodular lesions or plaques with irregular, scaly surfaces, most commonly on the hands, fingers, feet, and knees. | While generally asymptomatic, they can be painful with applied pressure and are typically benign and self-limiting. |
| Palmoplantar Warts: Affecting the acral surfaces of the hands and feet, these warts are characteristically thick, posing therapeutic challenges. Thrombosed capillaries can appear as small, black “seeds” within the wart. | Deep plantar warts often present as solitary lesions that may become blackened and painful before spontaneously regressing. | |
| Verruca Plana: These frequently manifest as clusters of small plaques (less than 5 mm in diameter) on the face and hands. They typically regress spontaneously within several years. | Often preceded by pruritus or erythema, they can cause significant pigmentary changes. | |
| Malignant transformation of skin lesions: Typically begins in the fourth and fifth decades of life. Premalignant lesions often first appear on sun-exposed areas such as the forehead. | These lesions range in clinical severity from benign papillomas and seborrheic keratoses to premalignant actinic keratoses and squamous cell carcinoma. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branda, F.; Pavia, G.; Ciccozzi, A.; Quirino, A.; Marascio, N.; Gigliotti, S.; Matera, G.; Romano, C.; Locci, C.; Azzena, I.; et al. Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies. Vaccines 2024, 12, 1293. https://doi.org/10.3390/vaccines12111293
Branda F, Pavia G, Ciccozzi A, Quirino A, Marascio N, Gigliotti S, Matera G, Romano C, Locci C, Azzena I, et al. Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies. Vaccines. 2024; 12(11):1293. https://doi.org/10.3390/vaccines12111293
Chicago/Turabian StyleBranda, Francesco, Grazia Pavia, Alessandra Ciccozzi, Angela Quirino, Nadia Marascio, Simona Gigliotti, Giovanni Matera, Chiara Romano, Chiara Locci, Ilenia Azzena, and et al. 2024. "Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies" Vaccines 12, no. 11: 1293. https://doi.org/10.3390/vaccines12111293
APA StyleBranda, F., Pavia, G., Ciccozzi, A., Quirino, A., Marascio, N., Gigliotti, S., Matera, G., Romano, C., Locci, C., Azzena, I., Pascale, N., Sanna, D., Casu, M., Ceccarelli, G., Ciccozzi, M., & Scarpa, F. (2024). Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies. Vaccines, 12(11), 1293. https://doi.org/10.3390/vaccines12111293
















