Approaches to Enhance the Potency of Vaccines in Chickens
Abstract
:1. Introduction
2. Current Poultry Vaccine Technology
2.1. Current Use of Inactivated Virus Vaccines in Poultry
2.2. Research and Current Use of Live Attenuated Vaccines in Poultry
2.3. Research into Using Subunit Vaccines in Poultry
2.4. Research into Virus-like Particles (VLPs) Vaccines in Poultry
2.5. Research into Using DNA Vaccines in Poultry
2.6. Research into Using mRNA Vaccines in Poultry
2.7. Research and Current Use of Recombinant Viral Vectored Vaccines in Poultry
2.8. Research into the Use of Nanotechnology in Poultry Vaccines
2.9. Current Poultry Vaccine Technology Conclusions
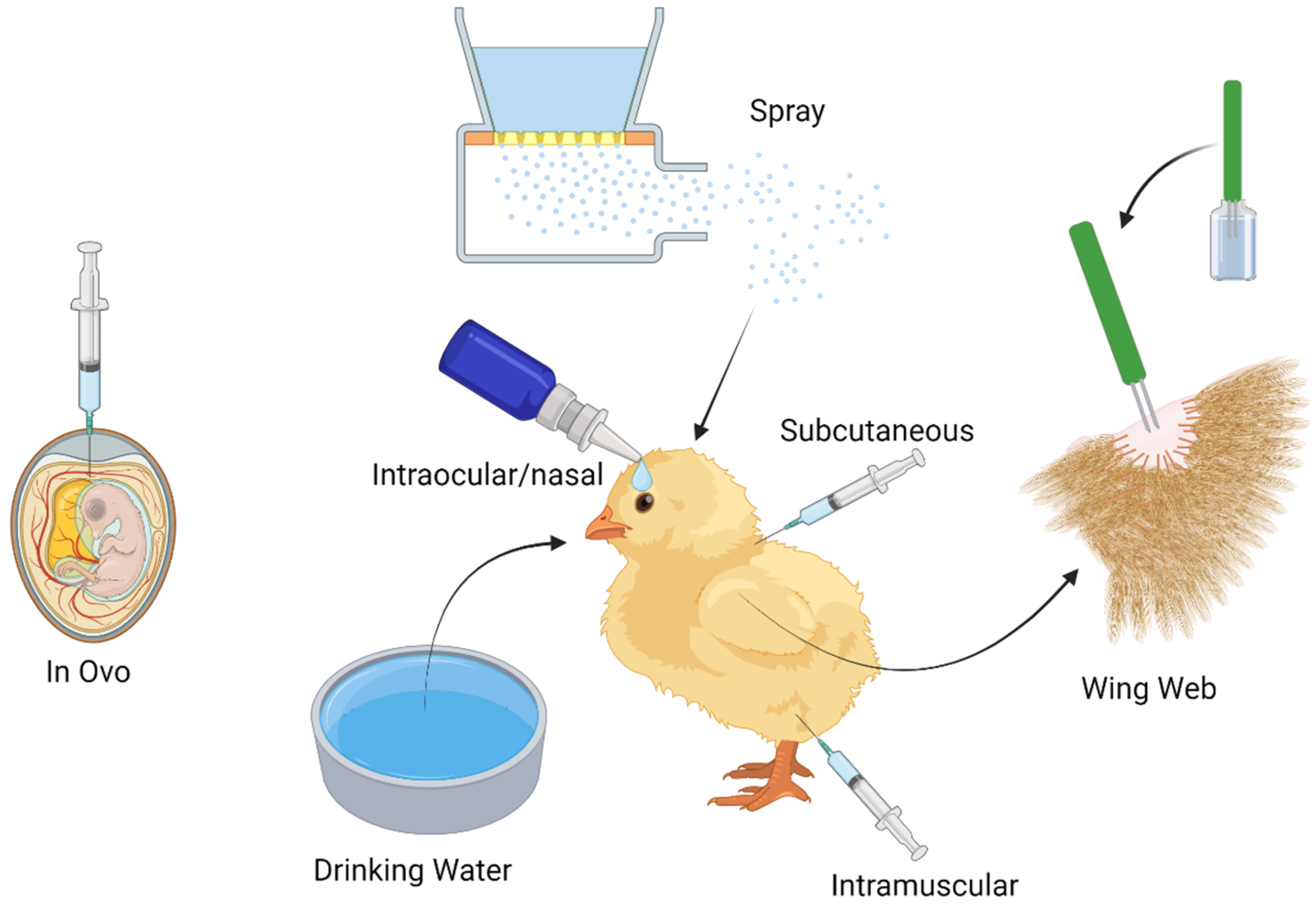
3. Delivery Mechanisms
3.1. The Current Use of in Ovo Vaccination in Poultry
3.2. The Current and Future Use of Spray Vaccine Administration in Poultry
3.3. Current Use of Drinking Water Vaccine Administration in Poultry
3.4. Current Use of Subcutaneous/Intramuscular Vaccine Administration in Poultry
3.5. Current Use of Intraocular/Nasal Drop Vaccine Administration in Poultry
3.6. Current Use of Wing Web Vaccine Administration in Poultry
3.7. Research into Intracloacal Administration of Poultry Vaccines
3.8. Delivery Mechanism Conclusions
4. The Use of Adjuvants in Poultry Vaccines
4.1. Current and Future Uses of Emulsions as Adjuvants (Delivery Agent and Immunostimulant)
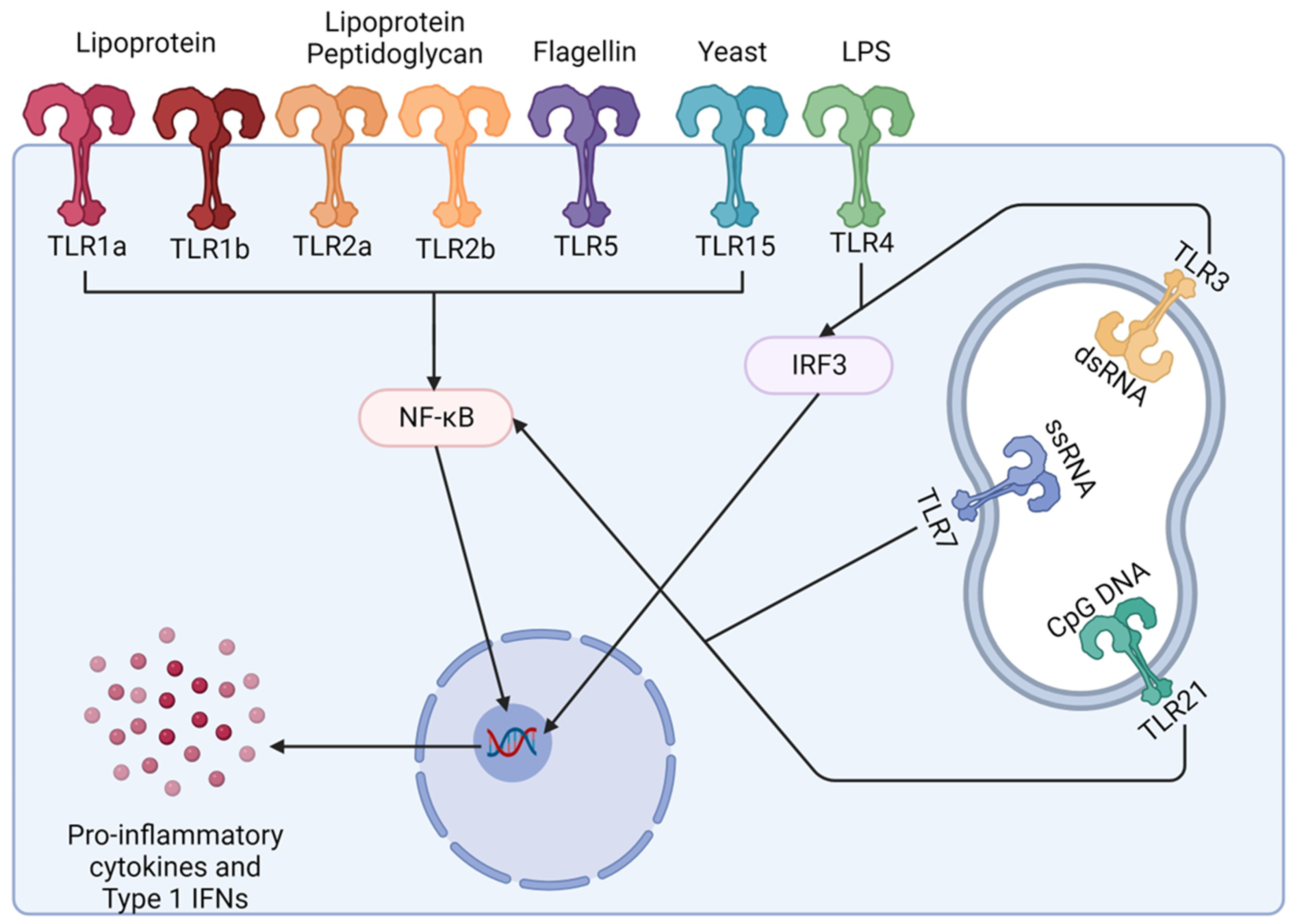
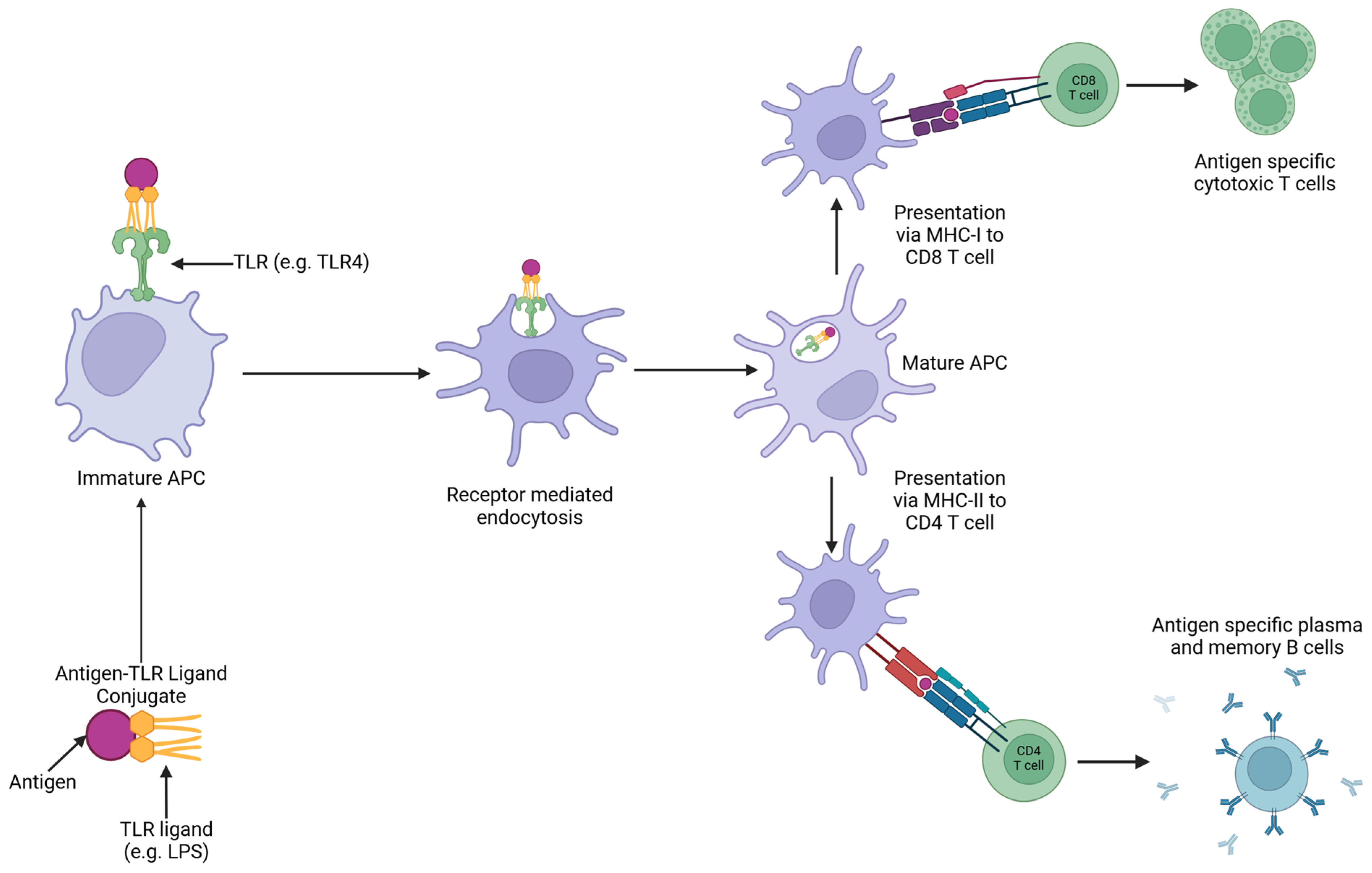
4.2. Research into Using Toll-like Receptors (TLRs) as Adjuvants (Immunostimulatory) in Poultry Vaccines
4.3. Research into Using Cytokines as Adjuvants (Immunostimulatory) in Poultry Vaccines
4.4. Use of Adjuvants in Poultry Conclusions
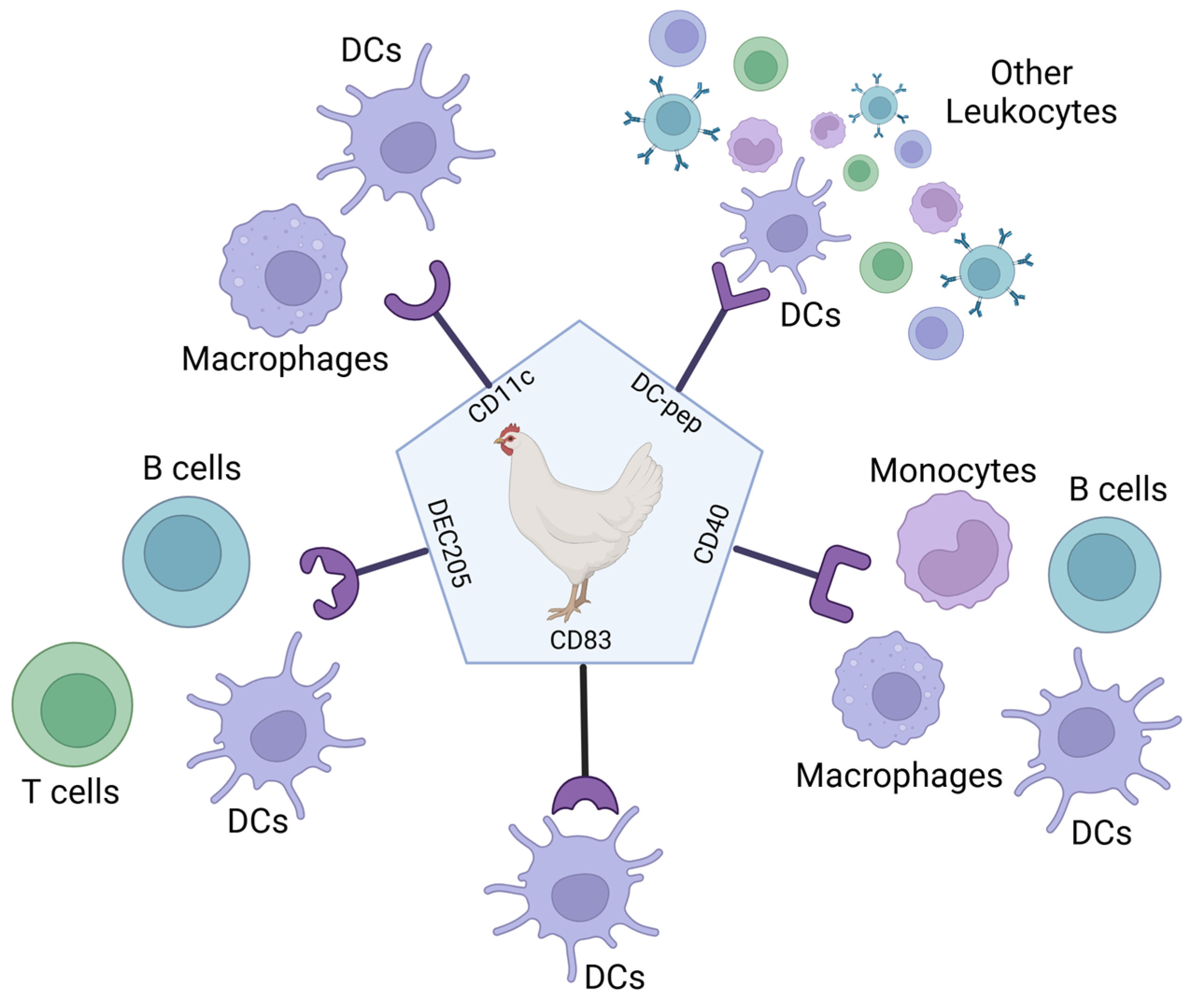
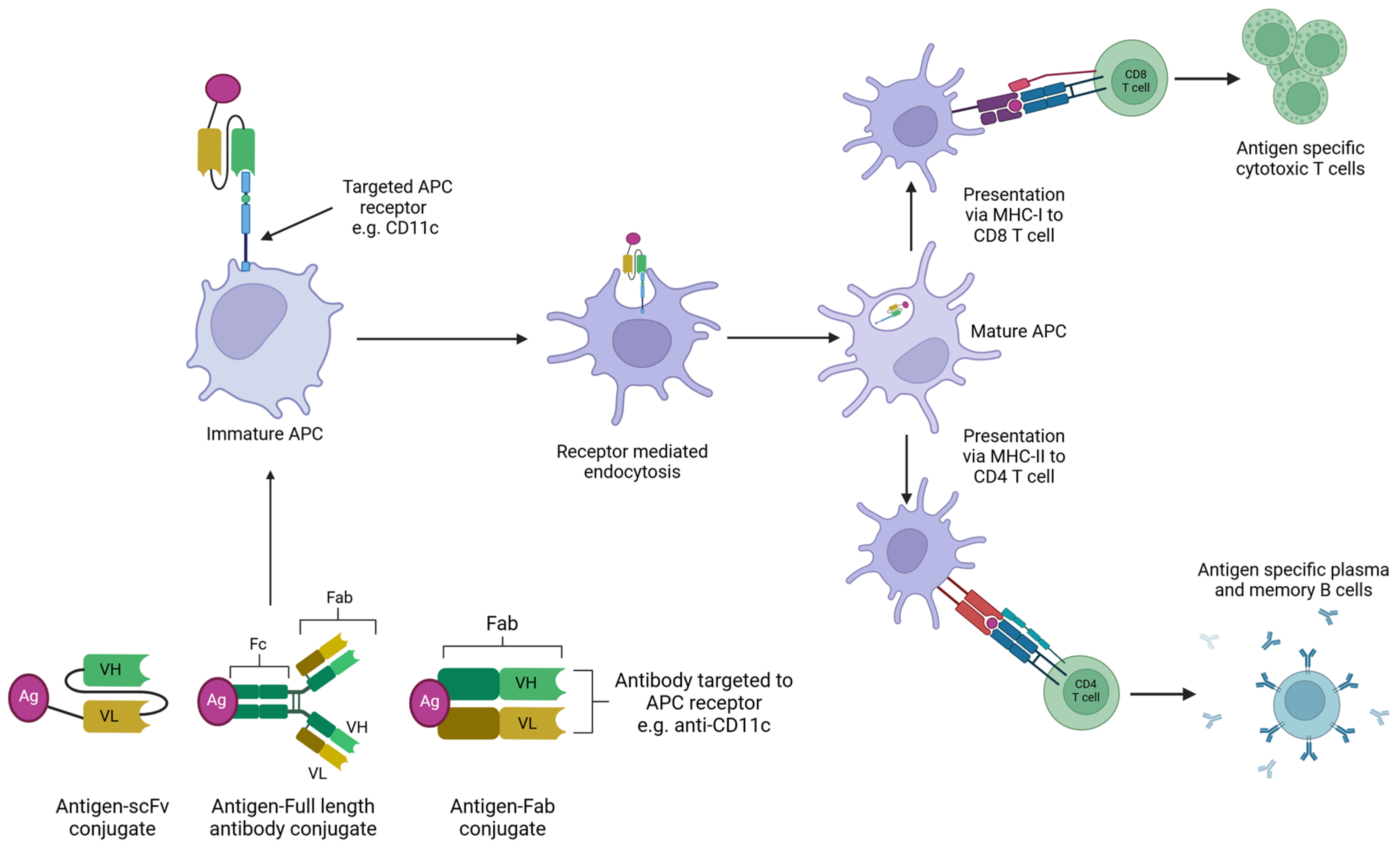
5. Targeting the Antigen to Antigen-Presenting Cells
5.1. Research into Using Ligand-Based Targeting of APCs
5.2. Research into Using Antibody-Based Targeting of APCs in Poultry Vaccines
6. Research into Using Probiotics in the Enhancement of Vaccines in Poultry
7. Enhancement of Poultry Vaccine Efficacy by the Incorporation of Multiple Antigens
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. MEAT MARKET REVIEW: Emerging Trends and Outlook 2022. Available online: https://openknowledge.fao.org/items/0b06ce2a-4221-47bf-aad5-8e0e8c8b8ba7 (accessed on 20 November 2024).
- OCED and Food Agricultural Organization of the United Nations. OECD-FAO Agricultural Outlook 2020–2029; OECD-FAO Agricultural Outlook; OECD: Paris, France, 2020; ISBN 9789264317673. [Google Scholar]
- Ravikumar, R.; Chan, J.; Prabakaran, M. Vaccines against Major Poultry Viral Diseases: Strategies to Improve the Breadth and Protective Efficacy. Viruses 2022, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Charkhkar, S.; Bashizade, M.; Sotoudehnejad, M.; Ghodrati, M.; Bulbuli, F.; Akbarein, H. The Evaluation and Importance of Newcastle Disease’s Economic Loss in Commercial Layer Poultry. J. Poult. Sci. Avian Dis. 2024, 2, 1–4. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- And Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Dejyong, T.; Chanachai, K.; Prarakamawongsa, T.; Kongkaew, W.; Thiptara, A.; Songserm, T.; Rukkwamsuk, T.; TagoPacheco, D.; Phimpraphai, W. Economic and Value Chain Analysis to Support an Investigation and Risk Mitigation Efforts on Marek’s Disease in Layers in the Southern Part of Thailand. Vet. World 2023, 16, 35–45. [Google Scholar] [CrossRef]
- Gashaw, M. A Review on Avian Influenza and Its Economic and Public Health Impact. Int. J. Vet. Sci. Technol. 2020, 4, 15–27. [Google Scholar]
- Shrestha, A.; Sadeyen, J.-R.; Iqbal, M. Enhancing Protective Efficacy of Poultry Vaccines through Targeted Delivery of Antigens to Antigen-Presenting Cells. Vaccines 2018, 6, 75. [Google Scholar] [CrossRef]
- Abdul-Cader, M.S.; Palomino-Tapia, V.; Amarasinghe, A.; Ahmed-Hassan, H.; De Silva Senapathi, U.; Abdul-Careem, M.F. Hatchery Vaccination Against Poultry Viral Diseases: Potential Mechanisms and Limitations. Viral Immunol. 2018, 31, 23–33. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Singer, R.S.; Johnson, T.J. Antimicrobial Therapy (Including Resistance). In Diseases of Poultry; John Wiley and Sons, Incorporated: Hoboken, NJ, USA, 2013; pp. 40–43. [Google Scholar]
- Swayne, D.E. Impact of Vaccines and Vaccination on Global Control of Avian Influenza. Avian Dis. 2012, 56, 818–828. [Google Scholar] [CrossRef]
- Romanutti, C.; Keller, L.; Zanetti, F.A. Current Status of Virus-Vectored Vaccines against Pathogens That Affect Poultry. Vaccine 2020, 38, 6990–7001. [Google Scholar] [CrossRef]
- Swayne, D.E.; Kapczynski, D.R. Vaccines and Vaccination for Avian Influenza in Poultry. In Avian Influenza; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 378–434. [Google Scholar]
- Veterinary Medicines Directorate. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase (accessed on 17 June 2024).
- Swayne, D.E. Avian Influenza Vaccines and Therapies for Poultry. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 351–363. [Google Scholar] [CrossRef]
- Jang, H.; Elaish, M.; Mahesh, K.C.; Abundo, M.C.; Ghorbani, A.; Ngunjiri, J.M.; Lee, C.W. Efficacy and Synergy of Live-Attenuated and Inactivated Influenza Vaccines in Young Chickens. PLoS ONE 2018, 13, e0195285. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Brokstad, K.; Cox, R. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Peek, H.W.; Landman, W.J.M. Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Lowen, A.C.; Pena, L.; Angel, M.; Solórzano, A.; Albrecht, R.; Perez, D.R.; García-Sastre, A.; Palese, P. Live Attenuated Influenza Viruses Containing NS1 Truncations as Vaccine Candidates against H5N1 Highly Pathogenic Avian Influenza. J. Virol. 2009, 83, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Grødeland, G.; Fossum, E.; Bogen, B. Polarizing T and B Cell Responses by APC-Targeted Subunit Vaccines. Front. Immunol. 2015, 6, 367. [Google Scholar] [CrossRef]
- Meunier, M.; Chemaly, M.; Dory, D. DNA Vaccination of Poultry: The Current Status in 2015. Vaccine 2016, 34, 202–211. [Google Scholar] [CrossRef]
- Lauer, K.B.; Borrow, R.; Blanchard, T.J. Multivalent and Multipathogen Viral Vector Vaccines. Clin. Vaccine Immunol. 2017, 24, e00298-16. [Google Scholar] [CrossRef]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S.; et al. Efficacy of a Bivalent L1 Virus-like Particle Vaccine in Prevention of Infection with Human Papillomavirus Types 16 and 18 in Young Women: A Randomised Controlled Trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef]
- Keating, G.M.; Noble, S. Recombinant Hepatitis B Vaccine (Engerix-B): A Review of Its Immunogenicity and Protective Efficacy against Hepatitis B. Drugs 2003, 63, 1021–1051. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like Particles: Fundamentals and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 8579. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qian, J.; Qin, L.; Li, J.; Xue, C.; Ding, J.; Wang, W.; Ding, W.; Yin, R.; Jin, N.; et al. Chimeric Newcastle Disease Virus-like Particles Containing DC-Binding Peptide-Fused Haemagglutinin Protect Chickens from Virulent Newcastle Disease Virus and H9N2 Avian Influenza Virus Challenge. Virol. Sin. 2020, 35, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Firouzamandi, M.; Helan, J.A.; Moeini, H.; Soleimanian, A.; Khatemeh, S.; Hosseini, S.D. Developing a Vaccine against Velogenic Sub-Genotype Seven of Newcastle Disease Virus Based on Virus-like Particles. AMB Express 2023, 13, 114. [Google Scholar] [CrossRef]
- Noh, J.Y.; Park, J.K.; Lee, D.H.; Yuk, S.S.; Kwon, J.H.; Lee, S.W.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Chimeric Bivalent Virus-like Particle Vaccine for H5N1 HPAI and ND Confers Protection Against a Lethal Challenge in Chickens and Allows a Strategy of Differentiating Infected from Vaccinated Animals (DIVA). PLoS ONE 2016, 11, e0162946. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Y.; Hu, Y.; Qiu, J.; Lei, W.; Ji, W.; Li, X.; Wu, Q.; Shi, X.; Li, Z. Protection of Chickens against Infectious Bronchitis Virus with a Multivalent DNA Vaccine and Boosting with an Inactivated Vaccine. J. Vet. Sci. 2013, 14, 53–60. [Google Scholar] [CrossRef]
- Faurez, F.; Dory, D.; Le Moigne, V.; Gravier, R.; Jestin, A. Biosafety of DNA Vaccines: New Generation of DNA Vectors and Current Knowledge on the Fate of Plasmids after Injection. Vaccine 2010, 28, 3888–3895. [Google Scholar] [CrossRef]
- Robinson, H.L.; Hunt, L.A.; Webster, R.G. Protection against a Lethal Influenza Virus Challenge by Immunization with a Haemagglutinin-Expressing Plasmid DNA. Vaccine 1993, 11, 957–960. [Google Scholar] [CrossRef]
- Bublot, M. Poultry Vaccine Technology Platforms. Avian Dis. 2023, 67, 380–401. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Poh, C.L. Recent Advances in Delivery of Veterinary DNA Vaccines against Avian Pathogens. Vet. Res. 2019, 50, 78. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of Virus-specific Cytotoxic T Lymphocytes in Vivo by Liposome-entrapped MRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine into MRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for Non-Viral Intracellular Delivery of Messenger RNA Therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Chen, N.; Xia, P.; Li, S.; Zhang, T.; Wang, T.T.; Zhu, J. RNA Sensors of the Innate Immune System and Their Detection of Pathogens. IUBMB Life 2017, 69, 297–304. [Google Scholar] [CrossRef]
- Snoeck, J.; Chiers, K.; Tam, Y.; Sanders, N.N.; Garmyn, A. Evaluation of a Self-Amplifying MRNA Reporter Vaccine in Explant Models of Broiler Chickens. Poult. Sci. 2023, 102, 103078. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, B.; Yao, J.; Ruan, W. A New H9 Influenza Virus MRNA Vaccine Elicits Robust Protective Immunity against Infection. Vaccine 2023, 41, 2905–2913. [Google Scholar] [CrossRef]
- Hein, R.; Koopman, R.; García, M.; Armour, N.; Dunn, J.R.; Barbosa, T.; Martinez, A. Review of Poultry Recombinant Vector Vaccines. Avian Dis. 2021, 65, 438–452. [Google Scholar] [CrossRef]
- Swayne, D.E.; Beck, J.R.; Kinney, N. Failure of a Recombinant Fowl Poxvirus Vaccine Containing an Avian Influenza Hemagglutinin Gene to Provide Consistent Protection against Influenza in Chickens Preimmunized with a Fowl Pox Vaccine. Avian Dis. 2000, 44, 132. [Google Scholar] [CrossRef]
- Vagnozzi, A.; Zavala, G.; Riblet, S.M.; Mundt, A.; García, M. Protection Induced by Commercially Available Live-Attenuated and Recombinant Viral Vector Vaccines against Infectious Laryngotracheitis Virus in Broiler Chickens. Avian Pathol. 2012, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, H.S.; Ellakany, H.F.; Elbestawy, A.R.; Setta, A. The Combined Use of RHVT-H5 and RHVT-F Vector Vaccines in the Hatchery Enhances Immunity against Highly Pathogenic Avian Influenza H5N1 and Velogenic Newcastle Disease Viral Infections in Commercial Chickens. Poult. Sci. J. 2018, 6, 165–171. [Google Scholar] [CrossRef]
- Williams, C.J.; Hopkins, B.A. Field Evaluation of the Accuracy of Vaccine Deposition by Two Different Commercially Available in Ovo Injection Systems. Poult. Sci. 2011, 90, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Gergen, L.; Cook, S.; Ledesma, B.; Cress, W.; Higuchi, D.; Counts, D.; Cruz-Coy, J.; Crouch, C.; Davis, P.; Tarpey, I.; et al. A Double Recombinant Herpes Virus of Turkeys for the Protection of Chickens against Newcastle, Infectious Laryngotracheitis and Marek’s Diseases. Avian Pathol. 2019, 48, 45–56. [Google Scholar] [CrossRef]
- Huang, Z.; Elankumaran, S.; Panda, A.; Samal, S. Recombinant Newcastle Disease Virus as a Vaccine Vector. Poult. Sci. 2003, 82, 899–906. [Google Scholar] [CrossRef]
- Zhao, H.; Peeters, B.P.H. Recombinant Newcastle Disease Virus as a Viral Vector: Effect of Genomic Location of Foreign Gene on Gene Expression and Virus Replication. J. Gen. Virol. 2003, 84, 781–788. [Google Scholar] [CrossRef]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine Delivery Using Nanoparticles. Front. Cell Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Calderon-Nieva, D.; Goonewardene, K.B.; Gomis, S.; Foldvari, M. Veterinary Vaccine Nanotechnology: Pulmonary and Nasal Delivery in Livestock Animals. Drug Deliv. Transl. Res. 2017, 7, 558–570. [Google Scholar] [CrossRef]
- Zhao, K.; Rong, G.; Hao, Y.; Yu, L.; Kang, H.; Wang, X.; Wang, X.; Jin, Z.; Ren, Z.; Li, Z. IgA Response and Protection Following Nasal Vaccination of Chickens with Newcastle Disease Virus DNA Vaccine Nanoencapsulated with Ag@SiO2 Hollow Nanoparticles. Sci. Rep. 2016, 6, 25720. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan Solution Enhances Both Humoral and Cell-Mediated Immune Responses to Subcutaneous Vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.A.; Irza, A.V.; Chvala, I.A.; Frolov, S.F.; Drygin, V.V.; Kapczynski, D.R. Adjuvant Effects of Chitosan and Calcium Phosphate Particles in an Inactivated Newcastle Disease Vaccine. Avian Dis. 2014, 58, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-Adjuvanted Salmonella Subunit Nanoparticle Vaccine for Poultry Delivered through Drinking Water and Feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Muyldermans, S.; Lauwereys, M. Unique Single-Domain Antigen Binding Fragments Derived from Naturally Occurring Camel Heavy-Chain Antibodies. J. Mol. Recognit. 1999, 12, 131–140. [Google Scholar] [CrossRef]
- Loutet, S.A.; Cheung, S.; Zaytsoff, S.; Hofacre, C.; Jones, M.K.; Van Petegem, F.; Abnousi, H. Research Note: Clostridium Perfringens NetB and CnaA Neutralizing Nanobodies in Feed Reduce the Incidence of Poultry Necrotic Enteritis. Poult. Sci. 2024, 103, 103578. [Google Scholar] [CrossRef]
- Fan, W.; Ji, P.; Sun, X.; Kong, M.; Zhou, N.; Zhang, Q.; Wang, Y.; Liu, Q.; Li, X.; Zhou, E.M.; et al. Screening and Identification of Nucleocapsid Protein-Nanobodies That Inhibited Newcastle Disease Virus Replication in DF-1 Cells. Front. Microbiol. 2022, 13, 956561. [Google Scholar] [CrossRef]
- Schat, K.A.; Kaspers, B.; Kaiser, P. Avian Immunology; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123969651. [Google Scholar]
- Peebles, E.D. In Ovo Applications in Poultry: A Review. Poult. Sci. 2018, 97, 2322–2338. [Google Scholar] [CrossRef]
- Toro, H.; Tang, D.C.; Suarez, D.L.; Sylte, M.J.; Pfeiffer, J.; Van Kampen, K.R. Protective Avian Influenza in Ovo Vaccination with Non-Replicating Human Adenovirus Vector. Vaccine 2007, 25, 2886–2891. [Google Scholar] [CrossRef]
- Elliott, K.E.C.; Branton, S.L.; Evans, J.D.; Gerard, P.D.; Peebles, E.D. Layer Chicken Embryo Survival to Hatch When Administered an in Ovo Vaccination of Strain F Mycoplasma Gallisepticum and Locations of Bacteria Prevalence in the Newly Hatched Chick. Poult. Sci. 2017, 96, 3879–3884. [Google Scholar] [CrossRef]
- Wakenell, P.S.; Bryan, T.; Schaeffer, J.; Avakian, A.; Williams, C.; Whitfill, C. Effect of in Ovo Vaccine Delivery Route on Herpesvirus of Turkeys/SB-1 Efficacy and Viremia. Avian Dis. 2002, 46, 274–280. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.J.; Montiel, E. Practical Aspects of Poultry Vaccination. In Avian Immunology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 469–488. ISBN 9780128190715. [Google Scholar]
- Albanese, G.A.; Tensa, L.R.; Aston, E.J.; Hilt, D.A.; Jordan, B.J. Evaluation of a Coccidia Vaccine Using Spray and Gel Applications. Poult. Sci. 2018, 97, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Pottenger, S.; Watts, A.; Wedley, A.; Jopson, S.; Darby, A.C.; Wigley, P. Timing and Delivery Route Effects of Cecal Microbiome Transplants on Salmonella Typhimurium Infections in Chickens: Potential for in-Hatchery Delivery of Microbial Interventions. Anim. Microbiome 2023, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Gentry, R.F.; Braune, M.O. Prevention of Virus Inactivation During Drinking Water Vaccination of Poultry. Poult. Sci. 1972, 51, 1450–1456. [Google Scholar] [CrossRef]
- Gharaibeh, S.; Mahmoud, K.; Al-Natour, M. Field Evaluation of Maternal Antibody Transfer to a Group of Pathogens in Meat-Type Chickens. Poult. Sci. 2008, 87, 1550–1555. [Google Scholar] [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef]
- Ekino, S.; Suginohara, K.; Uranot, T.; Fujii, H.; Matsuno, K.; Kotani, M. The Bursa of Fabricius: A Trapping Site for Environmental Antigens. Immunology 1985, 55, 405–410. [Google Scholar]
- Ifrah, M.E.; Perelman, B.; Finger, A.; Uni, Z. The Role of the Bursa of Fabricius in the Immune Response to Vaccinal Antigens and the Development of Immune Tolerance in Chicks (Gallus domesticus) Vaccinated at a Very Young Age. Poult. Sci. 2017, 96, 51–57. [Google Scholar] [CrossRef]
- Hughes, C.L.; Henderson, D.C. Induction of Avian Immunological Responsiveness Following Cloacal Drinking of Immunogen. Immunol. Investig. 1977, 6, 195–206. [Google Scholar] [CrossRef]
- Abaza, M.; ElBorai, I.; Eissa, A.; Zyan, K. Comparative Immunopathological Study of Various Intra-Cloacal Infectious Bursal Disease Vaccine Doses. Benha Vet. Med. J. 2020, 39, 142–146. [Google Scholar] [CrossRef]
- Abaza, M.A.; Elboraay, E.M.; Saad, A.E.; Zayan, K.A. Assessment of the Role of Intracloacal Inoculation of Live Infectious Bursal Disease Vaccine in Breaking through Maternally Derived Antibodies. Avian Pathol. 2020, 49, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Gaghan, C.; Gorrell, K.; Taha-Abdelaziz, K.; Sharif, S.; Kulkarni, R.R. Intracloacal Inoculation of Broiler Chickens with Clostridium Perfringens Strains: Evaluation of Necrotic Enteritis Disease Development and Lymphoid Immune Responses. Microorganisms 2023, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.C.; Coulter, A.R. Adjuvants—A Classification and Review of Their Modes of Action. Vaccine 1997, 15, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Herbert, W.J. The Mode of Action of Mineral-Oil Emulsion Adjuvants on Antibody Production in Mice. Immunology 1968, 14, 301–318. [Google Scholar]
- Freund, J.; Casals, J.; Hosmer, E.P. Sensitization and Antibody Formation after Injection of Tubercle bacilli and Paraffin Oil. Proc. Soc. Exp. Biol. Med. 1937, 37, 509–513. [Google Scholar] [CrossRef]
- Leenaars, P.P.A.M.; Hendriksen, C.F.M.; Angulo, A.F.; Koedam, M.A.; Claassen, E. Evaluation of Several Adjuvants as Alternatives to the Use of Freund’s Adjuvant in Rabbits. Vet. Immunol. Immunopathol. 1994, 40, 225–241. [Google Scholar] [CrossRef]
- Arous, J.B.; Deville, S.; Pal, J.K.; Baksi, S.; Bertrand, F.; Dupuis, L. Reduction of Newcastle Disease Vaccine Dose Using a Novel Adjuvant for Cellular Immune Response in Poultry. Procedia Vaccinol. 2013, 7, 28–33. [Google Scholar] [CrossRef]
- Jang, S.I.; Lillehoj, H.S.; Lee, S.H.; Lee, K.W.; Park, M.S.; Bauchan, G.R.; Lillehoj, E.P.; Bertrand, F.; Dupuis, L.; Deville, S. Immunoenhancing Effects of MontanideTM ISA Oil-Based Adjuvants on Recombinant Coccidia Antigen Vaccination against Eimeria Acervulina Infection. Vet. Parasitol. 2010, 172, 221–228. [Google Scholar] [CrossRef]
- Chada, K.E.; Forshee, R.; Golding, H.; Anderson, S.; Yang, H. A Systematic Review and Meta-Analysis of Cross-Reactivity of Antibodies Induced by Oil-in-Water Emulsion Adjuvanted Influenza H5N1 Virus Monovalent Vaccines. Vaccine 2017, 35, 3162–3170. [Google Scholar] [CrossRef]
- Banzhoff, A.; Gasparini, R.; Laghi-Pasini, F.; Staniscia, T.; Durando, P.; Montomoli, E.; Capecchi, P.; di Giovanni, P.; Sticchi, L.; Gentile, C.; et al. MF59®-Adjuvanted H5N1 Vaccine Induces Immunologic Memory and Heterotypic Antibody Responses in Non-Elderly and Elderly Adults. PLoS ONE 2009, 4, e4384. [Google Scholar] [CrossRef]
- Barnett, P.V.; Pullen, L.; Williams, L.; Doel, T.R. International Bank for Foot-and-Mouth Disease Vaccine: Assessment of Montanide ISA 25 and ISA 206, Two Commercially Available Oil Adjuvants. Vaccine 1996, 14, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Hussain, I.; Mahmood, M.S.; Khan, A. Preparation and Evaluation of Montanide ISA 206 Adjuvanted Bacterin of Borrelia Anserina in Laying Chickens. J. Appl. Poult. Res. 2013, 22, 196–203. [Google Scholar] [CrossRef]
- Gupta, S.K.; Deb, R.; Dey, S.; Chellappa, M.M. Toll-like Receptor-Based Adjuvants: Enhancing the Immune Response to Vaccines against Infectious Diseases of Chicken. Expert. Rev. Vaccines 2014, 13, 909–925. [Google Scholar] [CrossRef]
- Temperley, N.D.; Berlin, S.; Paton, I.R.; Griffin, D.K.; Burt, D.W. Evolution of the Chicken Toll-like Receptor Gene Family: A Story of Gene Gain and Gene Loss. BMC Genom. 2008, 9, 62. [Google Scholar] [CrossRef]
- Chaung, H.C.; Cheng, L.T.; Hung, L.H.; Tsai, P.C.; Skountzou, I.; Wang, B.; Compans, R.W.; Lien, Y.Y. Salmonella Flagellin Enhances Mucosal Immunity of Avian Influenza Vaccine in Chickens. Vet. Microbiol. 2012, 157, 69–77. [Google Scholar] [CrossRef]
- Liang, J.; Fu, J.; Kang, H.; Lin, J.; Yu, Q.; Yang, Q. Comparison of 3 Kinds of Toll-like Receptor Ligands for Inactivated Avian H5N1 Influenza Virus Intranasal Immunization in Chicken. Poult. Sci. 2013, 92, 2651–2660. [Google Scholar] [CrossRef]
- Bhadouriya, S.; Sharma, B.K.; Kakker, N.K.; Chhabra, R. Toll like Receptors and Cytokines as Immunostimulatory Adjuvants in Poultry Vaccines: Current Status and Future Trends. Worlds Poult. Sci. J. 2019, 75, 417–427. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Li, J.; Cao, T.; Tian, X.; Zhou, F. Enhancement of Mucosal Immune Responses by Intranasal Co-Delivery of Newcastle Disease Vaccine plus CpG Oligonucleotide in SPF Chickens in Vivo. Res. Vet. Sci. 2008, 85, 495–502. [Google Scholar] [CrossRef]
- He, H.; Lowry, V.K.; Swaggerty, C.L.; Ferro, P.J.; Kogut, M.H. In Vitro Activation of Chicken Leukocytes and in Vivo Protection against Salmonella Enteritidis Organ Invasion and Peritoneal S. Enteritidis Infection-Induced Mortality in Neonatal Chickens by Immunostimulatory CpG Oligodeoxynucleotide. FEMS Immunol. Med. Microbiol. 2005, 43, 81–89. [Google Scholar] [CrossRef]
- Barjesteh, N.; Shojadoost, B.; Brisbin, J.T.; Emam, M.; Hodgins, D.C.; Nagy, É.; Sharif, S. Reduction of Avian Influenza Virus Shedding by Administration of Toll-like Receptor Ligands to Chickens. Vaccine 2015, 33, 4843–4849. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.-H.; Tsai, P.-C.; Wang, C.-H.; Li, S.-L.; Huang, C.-C.; Lien, Y.-Y.; Chaung, H.-C. Immunoadjuvant Efficacy of Plasmids with Multiple Copies of a CpG Motif Coadministrated with Avian Influenza Vaccine in Chickens. Vaccine 2011, 29, 4668–4675. [Google Scholar] [CrossRef] [PubMed]
- Alqazlan, N.; Astill, J.; Raj, S.; Sharif, S. Strategies for Enhancing Immunity against Avian Influenza Virus in Chickens: A Review. Avian Pathol. 2022, 51, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.E.C.J.; Weining, K.C.; Nuijten, P.; Rijke, E.O.; Staeheli, P. Immunoadjuvant Activities of E. coli- and Plasmid-Expressed Recombinant Chicken IFN-α/β, IFN-γ and IL-1β in 1-Day- and 3-Week-Old Chickens. Vaccine 2000, 18, 2147–2154. [Google Scholar] [CrossRef]
- Gan, L.; Tian, Y.; Zhao, Y.; Shan, X.; Zhou, W.; Xia, B.-B.; Chen, J.; Wang, M.-L.; Zhao, J. Enhancing Immunogenicity and Protective Efficacy of Inactivated Avian Influenza H9N2vaccine with Recombinant Chicken IFN-α in Chicken. Vet. Microbiol. 2019, 234, 77–82. [Google Scholar] [CrossRef]
- Karaca, K.; Sharma, J.M.; Winslow, B.J.; Junker, D.E.; Reddy, S.; Cochran, M.; McMillen, J. Recombinant Fowlpox Viruses Coexpressing Chicken Type I IFN and Newcastle Disease Virus HN and F Genes: Influence of IFN on Protective Efficacy and Humoral Responses of Chickens Following in Ovo or Post-Hatch Administration of Recombinant Viruses. Vaccine 1998, 16, 1496–1503. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Q.; Zhang, X.; Yang, Q. Co-Administration of Inactivated Avian Influenza Virus with CpG or RIL-2 Strongly Enhances the Local Immune Response after Intranasal Immunization in Chicken. Vaccine 2009, 27, 5628–5632. [Google Scholar] [CrossRef]
- Yang, Y.; Leggat, D.; Herbert, A.; Roberts, P.C.; Sundick, R.S. A Novel Method to Incorporate Bioactive Cytokines as Adjuvants on the Surface of Virus Particles. J. Interferon Cytokine Res. 2009, 29, 9–22. [Google Scholar] [CrossRef]
- Lim, K.-L.; Jazayeri, S.D.; Yeap, S.K.; Alitheen, N.B.M.; Bejo, M.H.; Ideris, A.; Omar, A.R. Co-Administration of Avian Influenza Virus H5 Plasmid DNA with Chicken IL-15 and IL-18 Enhanced Chickens Immune Responses. BMC Vet. Res. 2012, 8, 132. [Google Scholar] [CrossRef]
- Melgoza-González, E.A.; Bustamante-Córdova, L.; Hernández, J. Recent Advances in Antigen Targeting to Antigen-Presenting Cells in Veterinary Medicine. Front. Immunol. 2023, 14, 1080238. [Google Scholar] [CrossRef]
- Chen, P.; Liu, X.; Sun, Y.; Zhou, P.; Wang, Y.; Zhang, Y. Dendritic Cell Targeted Vaccines: Recent Progresses and Challenges. Hum. Vaccin. Immunother. 2016, 12, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Longet, S.; Lundahl, M.L.E.; Lavelle, E.C. Targeted Strategies for Mucosal Vaccination. Bioconjug Chem. 2018, 29, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ju, X.; Silveira, P.A.; Abadir, E.; Hsu, W.-H.; Hart, D.N.J.; Clark, G.J. CD83: Activation Marker for Antigen Presenting Cells and Its Therapeutic Potential. Front. Immunol. 2019, 10, 01312. [Google Scholar] [CrossRef] [PubMed]
- Sun, P. Structural Recognition of Immunoglobulins by Fcγ Receptors. In Antibody Fc: Linking Adaptive and Innate Immunity; Academic Press: Cambridge, MA, USA, 2014; pp. 131–144. [Google Scholar]
- Shrestha, A.; Sadeyen, J.-R.; Lukosaityte, D.; Chang, P.; Van Hulten, M.; Iqbal, M. Targeting Haemagglutinin Antigen of Avian Influenza Virus to Chicken Immune Cell Receptors Dec205 and CD11c Induces Differential Immune-Potentiating Responses. Vaccines 2021, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Meeuws, R.; Sadeyen, J.-R.; Chang, P.; Van Hulten, M.; Iqbal, M. Haemagglutinin Antigen Selectively Targeted to Chicken CD83 Overcomes Interference from Maternally Derived Antibodies in Chickens. NPJ Vaccines 2022, 7, 33. [Google Scholar] [CrossRef]
- Shrestha, A.; Sadeyen, J.-R.; Lukosaityte, D.; Chang, P.; Smith, A.; Van Hulten, M.; Iqbal, M. Selectively Targeting Haemagglutinin Antigen to Chicken CD83 Receptor Induces Faster and Stronger Immunity against Avian Influenza. NPJ Vaccines 2021, 6, 90. [Google Scholar] [CrossRef]
- Chou, W.K.; Chen, C.H.; Vuong, C.N.; Abi-Ghanem, D.; Waghela, S.D.; Mwangi, W.; Bielke, L.R.; Hargis, B.M.; Berghman, L.R. Significant Mucosal SIgA Production after a Single Oral or Parenteral Administration Using in Vivo CD40 Targeting in the Chicken. Res. Vet. Sci. 2016, 108, 112–115. [Google Scholar] [CrossRef]
- Yin, G.; Lin, Q.; Qiu, J.; Qin, M.; Tang, X.; Suo, X.; Huang, Z.; Liu, X. Immunogenicity and Protective Efficacy of an Eimeria Vaccine Candidate Based on Eimeria tenella Immune Mapped Protein 1 and Chicken CD40 Ligand. Vet. Parasitol. 2015, 210, 19–24. [Google Scholar] [CrossRef]
- Pecora, A.; Malacari, D.A.; Perez Aguirreburualde, M.S.; Bellido, D.; Nuñez, M.C.; Dus Santos, M.J.; Escribano, J.M.; Wigdorovitz, A. Development of an APC-Targeted Multivalent E2-Based Vaccine against Bovine Viral Diarrhea Virus Types 1 and 2. Vaccine 2015, 33, 5163–5171. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral Treatment of Chickens with Lactobacilli Influences Elicitation of Immune Responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Anderson, R.C.; Harvey, R.B.; Genovese, K.J.; Kennedy, C.N.; Venn, D.W.; Nisbet, D.J. Probiotics, Prebiotics and Competitive Exclusion for Prophylaxis against Bacterial Disease. Anim. Health Res. Rev. 2008, 9, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of Lactobacilli on Cytokine Expression by Chicken Spleen and Cecal Tonsil Cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096.e19. [Google Scholar] [CrossRef] [PubMed]
- Casteleyn, C.; Doom, M.; Lambrechts, E.; Van den Broeck, W.; Simoens, P.; Cornillie, P. Locations of Gut-Associated Lymphoid Tissue in the 3-Month-Old Chicken: A Review. Avian Pathol. 2010, 39, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. Biomed. Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef]
- Shojadoost, B.; Kulkarni, R.R.; Brisbin, J.T.; Quinteiro-Filho, W.; Alkie, T.N.; Sharif, S. Interactions between Lactobacilli and Chicken Macrophages Induce Antiviral Responses against Avian Influenza Virus. Res. Vet. Sci. 2019, 125, 441–450. [Google Scholar] [CrossRef]
- Alqazlan, N.; Astill, J.; Taha-Abdelaziz, K.; Nagy, É.; Bridle, B.; Sharif, S. Probiotic Lactobacilli Enhance Immunogenicity of an Inactivated H9N2 Influenza Virus Vaccine in Chickens. Viral Immunol. 2021, 34, 86–95. [Google Scholar] [CrossRef]
- Lee, J.E.; Kye, Y.-C.; Park, S.-M.; Shim, B.-S.; Yoo, S.; Hwang, E.; Kim, H.; Kim, S.-J.; Han, S.H.; Park, T.S.; et al. Bacillus Subtilis Spores as Adjuvants against Avian Influenza H9N2 Induce Antigen-Specific Antibody and T Cell Responses in White Leghorn Chickens. Vet. Res. 2020, 51, 68. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Alizadeh, M.; Astill, J.; Alqazlan, N.; Matsuyama-Kato, A.; Shojadoost, B.; Taha-Abdelaziz, K.; Sharif, S. Effects of Administration of Probiotic Lactobacilli on Immunity Conferred by the Herpesvirus of Turkeys Vaccine against Challenge with a Very Virulent Marek’s Disease Virus in Chickens. Vaccine 2021, 39, 2424–2433. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.E.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The Simultaneous Administration of a Probiotic or Prebiotic with Live Salmonella Vaccine Improves Growth Performance and Reduces Fecal Shedding of the Bacterium in Salmonella-Challenged Broilers. Animals 2020, 10, 70. [Google Scholar] [CrossRef]
- Yousaf, A.; Anwar, H. Effect of GutcareTM and Enterogermina® on Humoral Response of Avian Influenza Immunization in Broilers. Int. J. Vet. Sci. Anim. Husb. 2019, 4, 19–23. [Google Scholar]
- Wu, P.; Lu, J.; Feng, L.; Wu, H.; Zhang, X.; Mei, M.; Hou, J.; Liu, X.; Tang, Y. Antigen-Sparing and Enhanced Efficacy of Multivalent Vaccines Adjuvanted with Immunopotentiators in Chickens. Front. Microbiol. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.A.; Huggins, M.B.; Orbell, S.J.; Mawditt, K.; Cavanagh, D. Infectious Bronchitis Virus Vaccine Interferes with the Replication of Avian Pneumovirus Vaccine in Domestic Fowl. Avian Pathol. 2001, 30, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.R.; Dimitrov, K.M.; Miller, P.J.; Garcia, M.; Turner-Alston, K.; Brown, A.; Hartman, A. Evaluation of Protective Efficacy When Combining Turkey Herpesvirus–Vector Vaccines. Avian Dis. 2018, 63, 75. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A Multivalent Nucleoside-Modified MRNA Vaccine against All Known Influenza Virus Subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
| Pathogen | Biological Agent | Type of Vaccine | Further Information | Delivery Method | Adjuvant Used | Veterinary Medicine (VM) Number |
|---|---|---|---|---|---|---|
| Avian encephalomyelitis virus | Virus | Live attenuated | Attenuation method unspecified. Strain = 1143 Calnek | Drinking water | None specified | 00879/5005 |
| Live attenuated | Attenuation method unspecified. Strain = 1143 Calnek | Drinking water | None specified | 01708/4281 | ||
| Live attenuated | Attenuation method unspecified. Strain = AE-6710 Calnek | Drinking water | None specified | 42058/5134 | ||
| Live (FPV viral vector) | Genetically attenuated. Strain = 1143 Calnek | Wing web | None specified | 15052/5029 | ||
| Avian influenza virus (H5N2) | Virus | Inactivated | Inactivation method unspecified. Strain = A/duck/Potsdam/1402/86 | Subcutaneous, intramuscular | Mineral oil for water-in-oil emulsion | 01708/5044 |
| Avian metapneumovirus | Virus | Live attenuated | Attenuation method unspecified. Strain = CRR126 | Oculonasal, eyedrop, spray | None specified | 15052/4086 |
| Live attenuated | Attenuation method unspecified. Strain = PL21 | Water, spray | None specified | 08327/5008 | ||
| Inactivated | β-propiolactone inactivated. Strain = But 1 #8544 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5105 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = But 1 #8544 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5083 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = But 1 #8544 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5093 | ||
| Live attenuated | Attenuation method unspecified. Strain = 11/94 | Oculonasal, spray | None specified | 01708/4505 | ||
| Inactivated | Inactivation method unspecified. Strain = VCO3 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/3023 | ||
| Inactivated | Inactivation method unspecified. Strain = VCO3 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5022 | ||
| Inactivated | Inactivation method unspecified. Strain = VCO3 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5025 | ||
| Avian reovirus | Virus | Inactivated | Formalin inactivated. Strain = 1733 and 2408 | Intramuscular, subcutaneous | Mineral oil for water-in-oil emulsion | 01708/4329 |
| Chicken anaemia virus | Virus | Live | Apathogenic strain (for adult birds). Strain = Cux-1 | Drinking water | None specified | 00879/5042 |
| Live attenuated | Attenuation method unspecified. Strain = 26P4 | Intramuscular, subcutaneous | dl-α-tocopherol acetate | 01708/4322 | ||
| Coccidia (Eimeria) | Parasite | Live attenuated | Attenuation method unspecified. Strain = 044, 013, 007, 004 | In ovo | None specified | 17533/5014 |
| Live | Precocious strain (for adult birds). Strain = RA-3+20, MCK+10, Jormit 3+9, Rt 3+15 | Spray, feed, drinking water | None specified | 30282/5018 | ||
| Live | Precocious strain (for adult birds). Strain = mednec 3+8, roy 3+28 | Spray, feed, drinking water | None specified | 30282/4032 | ||
| Live | Precocious strain (for adult birds). Strain = HP, CP, MFP | Spray, feed, drinking water | None specified | 01708/5101 | ||
| Live | Precocious strain (for adult birds). Strain = HP, CP, MFP | Spray, feed, drinking water | None specified | 01708/4572 | ||
| E. coli | Bacteria | Live attenuated | Genetically modified. Strain = O78-EC34195 | Spray, drinking water | None specified | 42058/5046 |
| Inactivated | Inactivation method unspecified. F11-antigen (fimbrial antigen), FT-antigen (flagellar toxin antigen) | Intramuscular, subcutaneous | Mineral oil for water-in-oil emulsion | 01708/5085 | ||
| Egg drop syndrome (EDS) | Virus | Inactivated | Inactivation method unspecified. Strain = V127 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5012 |
| Inactivated | Inactivation method unspecified. Strain = V127 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5025 | ||
| Inactivated | Inactivation method unspecified. Strain = BC14 | Intramuscular, subcutaneous | Mineral oil for water-in-oil emulsion | 01708/4275 | ||
| Inactivated | β-propiolactone inactivated. Strain = BC14 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5083 | ||
| Fowlpox virus (FPV) | Virus | Live viral vector | Genetically attenuated. Strain = rFP-LT, expressing fusion protein gene and encapsidation protein gene of ILT | Wing web | None specified | 15052/5029 |
| Live viral vector | Genetically attenuated. Strain = rFP-LT, expressing fusion protein gene and encapsidation protein gene of ILT | Wing web | None specified | 15052/5028 | ||
| Infectious bronchitis virus (IBV) | Virus | Live attenuated | Attenuation method unspecified. Strain = V-173/11 | Spray, oculonasal, drinking water | None specified | 43676/4005 |
| Live attenuated | Attenuation method unspecified. Strain = H-20 | Spray, oculonasal, drinking water | None specified | 43676/4002 | ||
| Live attenuated | Attenuation method unspecified. Strain = 1/96 | Spray, oculonasal, drinking water | None specified | 15052/5043 | ||
| Live attenuated | Attenuation method unspecified. Strain = B-48 | Spray | None specified | 15052/4088 | ||
| Inactivated | Inactivation method unspecified. Strain = Mass41 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5012 | ||
| Inactivated | Inactivation method unspecified. Strain = Mass41 | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5025 | ||
| Live attenuated | Attenuation Method Unspecified. Strain = CR88121 | Spray | None specified | 08327/4265 | ||
| Live attenuated | Attenuation Method Unspecified. Strain = H120 | Spray | None specified | 08327/5005 | ||
| Inactivated | Inactivation method unspecified. Strain = M41 | Intramuscular, subcutaneous | Mineral oil for water-in-oil emulsion | 01708/4275 | ||
| Live attenuated | Attenuation method unspecified. Strain = 4–91 | Spray, intraocular/nasal, drinking water | None specified | 01708/5043 | ||
| Live attenuated | Attenuation method unspecified. Strain = Ma5 | Spray, intraocular/nasal, drinking water | None specified | 01708/4283 | ||
| Live attenuated | Attenuation method unspecified. Strain = D388 | Spray, intraocular/nasal | None specified | 01708/5042 | ||
| Live attenuated | Attenuation method unspecified. Strain = Ma5 | Spray, intraocular/nasal, drinking water | None specified | 01708/4315 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = M41, 249 g | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5093 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = M41, 249 g | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5083 | ||
| Live attenuated | Attenuation method Unspecified. Strain = H120 | Spray, intraocular/nasal, drinking water | None specified | 42058/5121 | ||
| Live attenuated | Attenuation method unspecified. Strain = H120, D274 | Spray, drinking water | None specified | 42058/4103 | ||
| Live attenuated | Attenuation method unspecified. Strain = L1148 | Spray | None specified | 42058/5103 | ||
| Live attenuated | Attenuation method unspecified. Strain = Ma1263, Ak3168 | Spray | None specified | 42058/5140 | ||
| Infectious bursal disease (IBD) | Virus | Live attenuated | Attenuation method unspecified. Strain = LC75 | Drinking water | None specified | 00879/4187 |
| Live attenuated | Attenuation method unspecified. Strain = G6 | Drinking water | None specified | 43676/5000 | ||
| Live attenuated | Attenuation method unspecified. Strain = G61, Winterfield 2512 | Drinking water | None specified | 15052/4059 | ||
| Live attenuated | Attenuation method unspecified. Strain = Winterfield 2512 | In ovo, subcutaneous | None specified | 15052/4030 | ||
| Live attenuated | Attenuation method unspecified. Strain = S706 | Spray, drinking water | None specified | 08327/4192 | ||
| Live attenuated | Attenuation method unspecified. Strain = S706 | Drinking water | None specified | 08327/4323 | ||
| Live attenuated | Attenuation method unspecified. Strain = 1052 | In ovo, subcutaneous | None specified | 17533/5005 | ||
| Live attenuated | Attenuation method unspecified. Strain = CH/80 | Drinking water | None specified | 17533/5017 | ||
| Live attenuated | Attenuation method unspecified. Strain = GM97 | Drinking water | None specified | 17533/4002 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, IBDV, and ILT vaccination | In ovo, subcutaneous | None specified | 01708/5082 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, NDV and IBDV vaccination | In ovo, subcutaneous | None specified | 01708/5040 | ||
| Live attenuated | Attenuation method unspecified. Strain = G61, Winterfield 2512 | In ovo, subcutaneous | None specified | 15052/4154 | ||
| Live attenuated | Attenuation method unspecified. Strain = 228E | Drinking water | None specified | 01708/4333 | ||
| Live attenuated | Attenuation method unspecified. Strain = D78 | Spray, intraocular/nasal, drinking water | None specified | 01708/4237 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = D78 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5093 | ||
| Live attenuated | Attenuation method unspecified. Strain = SYZA26 | Subcutaneous | None specified | 15052/5032 | ||
| Live attenuated | Attenuation method unspecified. Strain = V877 | Drinking water | None specified | 42058/5138 | ||
| Live attenuated | Attenuation method unspecified. Strain = Lukert | Spray, drinking water | None specified | 42058/5137 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV and IBDV | In ovo, subcutaneous | None specified | 04491/5043 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, NDV, and IBDV | In ovo, subcutaneous | None specified | 15052/5001 | ||
| Live (HVT viral vector) | Genetic recombination-HVT vector for MDV and IBDV | In ovo, subcutaneous | None specified | 04491/5060 | ||
| Infectious laryngotracheitis (ILT) | Virus | Live (HVT viral vector) | Genetic recombination, HVT vector, gD and gl glycoproteins. Strain = 138 | Subcutaneous | None specified | 01708/5039 |
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, IBDV, and ILT vaccination | In ovo, subcutaneous | None specified | 01708/5082 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, NDV, and ILT vaccination | In ovo, subcutaneous | None specified | 01708/5041 | ||
| Live attenuated | Attenuation method unspecified. Strain = Salsbury 146 | Intraocular | None specified | 42058/4106 | ||
| Live (FPV viral vector) | Genetically attenuated. Strain = rFP-LT, expressing fusion protein gene and encapsidation protein gene of ILT | Wing web | None specified | 15052/5029 | ||
| Live (FPV viral vector) | Genetically attenuated. Strain = rFP-LT, expressing fusion protein gene and encapsidation protein gene of ILT | Wing web | None specified | 15052/5028 | ||
| Marek’s disease virus (MDV) | Virus | Live (HVT viral vector) | Genetic recombination-HVT vector for MDV vaccination. Strain = FC 126 | In ovo, subcutaneous | None specified | 15052/4089 |
| Live attenuated | Attenuation method unspecified. Strain = CVI-988 | Subcutaneous | None specified | 15052/4151 | ||
| Live attenuated | Attenuation method unspecified. Strain = CVI-988 | Subcutaneous, intramuscular | None specified | 01708/4294 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV vaccination. Strain = FC 126, CVI-988 | In ovo, subcutaneous | None specified | 01708/4354 | ||
| Live attenuated | Attenuation method unspecified. Strain = CVI-988 | Intramuscular | None specified | 42058/4107 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV vaccination. Strain = FC 126, CVI-988 | Subcutaneous, intramuscular | None specified | 42058/4108 | ||
| Live attenuated | Attenuation method unspecified. Strain = RN1250 | Subcutaneous | None specified | 04491/5042 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV and IBDV | In ovo, subcutaneous | None specified | 04491/5043 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, IBDV, and ILT vaccination. | In ovo, subcutaneous | None specified | 01708/5082 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, NDV, and IBDV vaccination. | In ovo, subcutaneous | None specified | 01708/5040 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, NDV, and ILT vaccination. | In ovo, subcutaneous | None specified | 01708/5041 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV, NDV, and IBDV. | In ovo, subcutaneous | None specified | 15052/5001 | ||
| Live (HVT viral vector) | Genetic recombination, HVT vector for MDV and IBDV. | In ovo, subcutaneous | None specified | 04491/5060 | ||
| Live | Apathogenic strain (for adult birds). Strain = FC126 | Intramuscular, subcutaneous | None specified | 01708/4289 | ||
| Mycoplasma gallisepticum; Mycoplasma synovia | Bacteria | Inactivated | Inactivation method unspecified. Strain = MS-NEV1, MS-NEV2 | Subcutaneous | Mineral oil for water-in-oil emulsion | 43877/5001 |
| Inactivated | Inactivation method unspecified. Strain = MG-NEV40, MG-NEV45 | Subcutaneous | Mineral oil for water-in-oil emulsion | 43877/5000 | ||
| Live attenuated | Attenuation method unspecified. Strain = MS-H | Intraocular | None specified | 42983/5000 | ||
| Live attenuated | Attenuation method unspecified. Strain = MG 6/85 | Spray | None specified | 01708/5103 | ||
| Live attenuated | Attenuation method unspecified. Strain = MS1 | Spray | None specified | 01708/4607 | ||
| Live attenuated | Attenuation method unspecified. Strain = VG/GA-AVINEW 5.5 | Intraocular | None specified | 08327/5043 | ||
| Newcastle disease (NDV) | Virus | Live attenuated | Attenuation method unspecified. Strain = 13–1 | Intraocular, spray, drinking water | None specified | 00879/5035 |
| Live lentogenic | Strain = Hitchner B1 | Intraocular/nasal, spray, drinking water | None specified | 43676/4003 | ||
| Live lentogenic | Strain = La Sota | Intraocular/nasal, spray, drinking water | None specified | 43676/4000 | ||
| Inactivated | Inactivation method unspecified. Strain = Ulster 2C | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5012 | ||
| Inactivated | Inactivation method unspecified. Strain = Ulster 2C | Intramuscular | Mineral oil for water-in-oil emulsion | 08327/5025 | ||
| Live (HVT Viral Vector) | Genetic recombination, HVT vector for MDV, NDV, and IBDV vaccination. | In ovo, subcutaneous | None specified | 01708/5040 | ||
| Live (HVT Viral Vector) | Genetic recombination, HVT vector for MDV, NDV, and ILT vaccination. | In ovo, subcutaneous | None specified | 01708/5041 | ||
| Inactivated | Inactivation method unspecified. Strain = Clone 30 | Intramuscular, subcutaneous | Mineral oil for water-in-oil emulsion | 01708/4275 | ||
| Live attenuated | Attenuation method unspecified. Strain = Clone 30 | Spray, intraocular/nasal, drinking water | None specified | 01708/4315 | ||
| Live attenuated | Attenuation method unspecified. Strain = C2 | Spray, intraocular/nasal | None specified | 01708/5088 | ||
| Live attenuated | Attenuation method unspecified. Strain = Clone 30 | Spray, intraocular/nasal, drinking water | None specified | 01708/4276 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = Clone 30 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5093 | ||
| Inactivated | Formaldehyde or β-propiolactone inactivated. Strain = Clone 30 | Intramuscular | Mineral oil for water-in-oil emulsion | 01708/5083 | ||
| Live attenuated | Attenuation method unspecified. Strain = sm24/Rif12/Ssq, Nal2/Rif9/Rtt | Drinking water | None specified | 00879/4188 | ||
| Salmonella (enteritidis, typhimurium, and infantis) | Bacteria | Live attenuated | Attenuation method unspecified. Strain = sm24/Rif2/Ssq, | Drinking water | None specified | 00879/4189 |
| Live attenuated | Attenuation method unspecified. Strain = Nal2/Rif9/Rtt | Drinking water | None specified | 00879/5037 | ||
| Live attenuated | Genetically attenuated. Strain = 441/014 | Drinking water | None specified | 15052/5045 | ||
| Inactivated | Inactivation method unspecified. Strain = PT4, DT104, S03499-06 | Intramuscular | Aluminium hydroxide | 01708/5073 | ||
| Inactivated | Inactivation method unspecified. Strain = PT4, DT104 | Intramuscular | Aluminium hydroxide | 01708/5114 | ||
| Live attenuated | Attenuation method unspecified. Strain = ST CAL 10 Sm+/Rif+/Ssq- | Drinking water | None specified | 20634/5002 | ||
| Live attenuated | Attenuation method unspecified. Strain = ST CAL 16 Str+/Rif+/Enr- | Drinking water | None specified | 20634/5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodman-Harris, O.; Rollier, C.S.; Iqbal, M. Approaches to Enhance the Potency of Vaccines in Chickens. Vaccines 2024, 12, 1337. https://doi.org/10.3390/vaccines12121337
Bodman-Harris O, Rollier CS, Iqbal M. Approaches to Enhance the Potency of Vaccines in Chickens. Vaccines. 2024; 12(12):1337. https://doi.org/10.3390/vaccines12121337
Chicago/Turabian StyleBodman-Harris, Oenone, Christine S. Rollier, and Munir Iqbal. 2024. "Approaches to Enhance the Potency of Vaccines in Chickens" Vaccines 12, no. 12: 1337. https://doi.org/10.3390/vaccines12121337
APA StyleBodman-Harris, O., Rollier, C. S., & Iqbal, M. (2024). Approaches to Enhance the Potency of Vaccines in Chickens. Vaccines, 12(12), 1337. https://doi.org/10.3390/vaccines12121337







