Development, Pre-Clinical Safety, and Immune Profile of RENOVAC—A Dimer RBD-Based Anti-Coronavirus Subunit Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine DevelopmentProcess
2.1.1. Protein Designing, Expression and Purification
2.1.2. Formulation, Filling, and QC Analysis
2.2. Subject Details and Ethical Aspects of the Study
2.2.1. Laboratory Animals
2.2.2. GLP and Ethics Statement
2.3. Toxicity Study in Sprague Dawley Rats
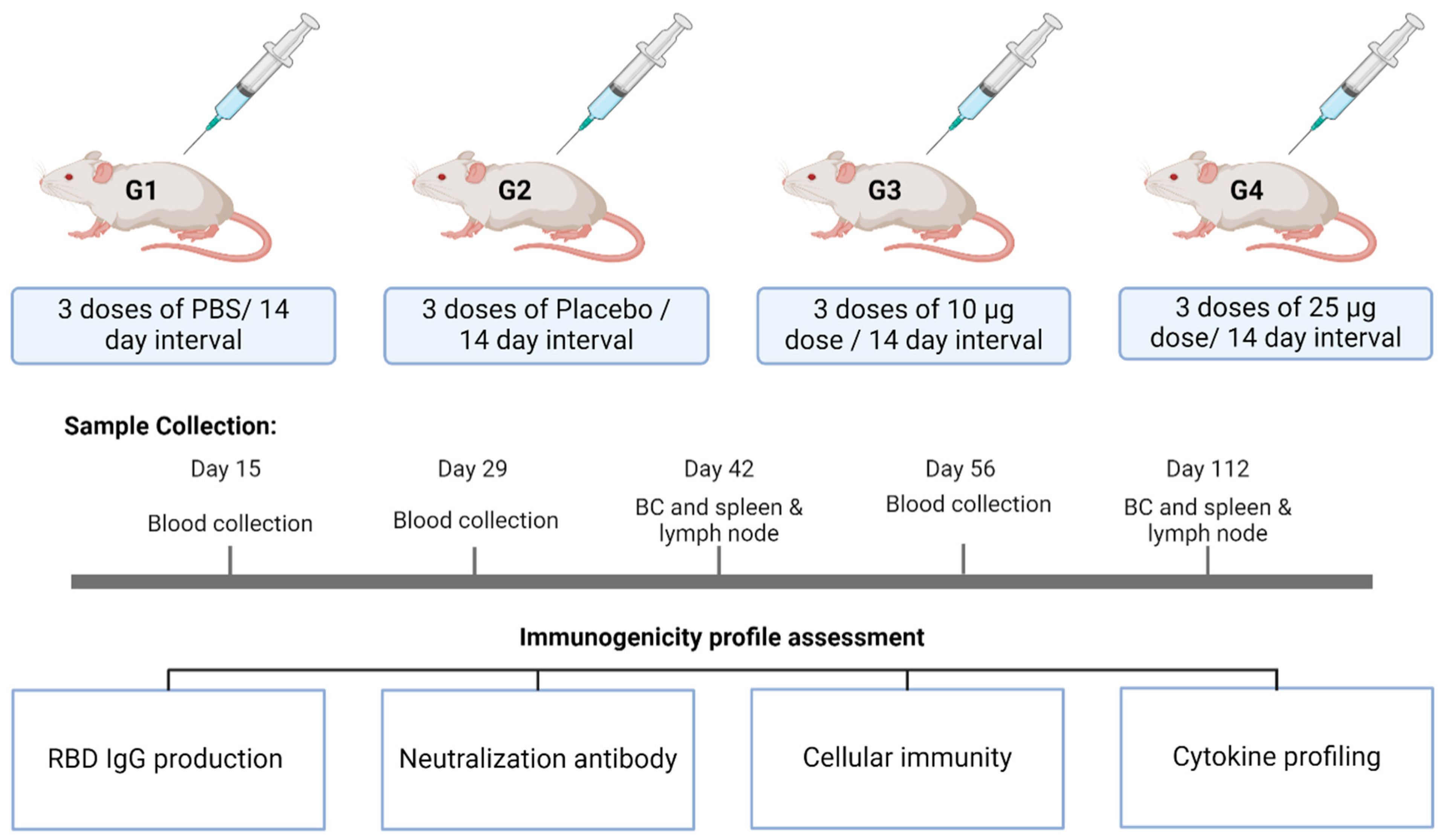
2.4. Immunogenicity Studies in Mice
2.4.1. Study Design
2.4.2. Assessment of Anti-RBD IgG Production
2.4.3. Neutralization Antibody Titer Assessment
2.4.4. Cellular Immunity Assessment in Spleen and Lymph Node
2.4.5. Cytokine Profile Assessment by Multiplexing
2.5. Data Analysis
3. Results and Discussions
3.1. Vaccine Development
3.2. Toxicity Study
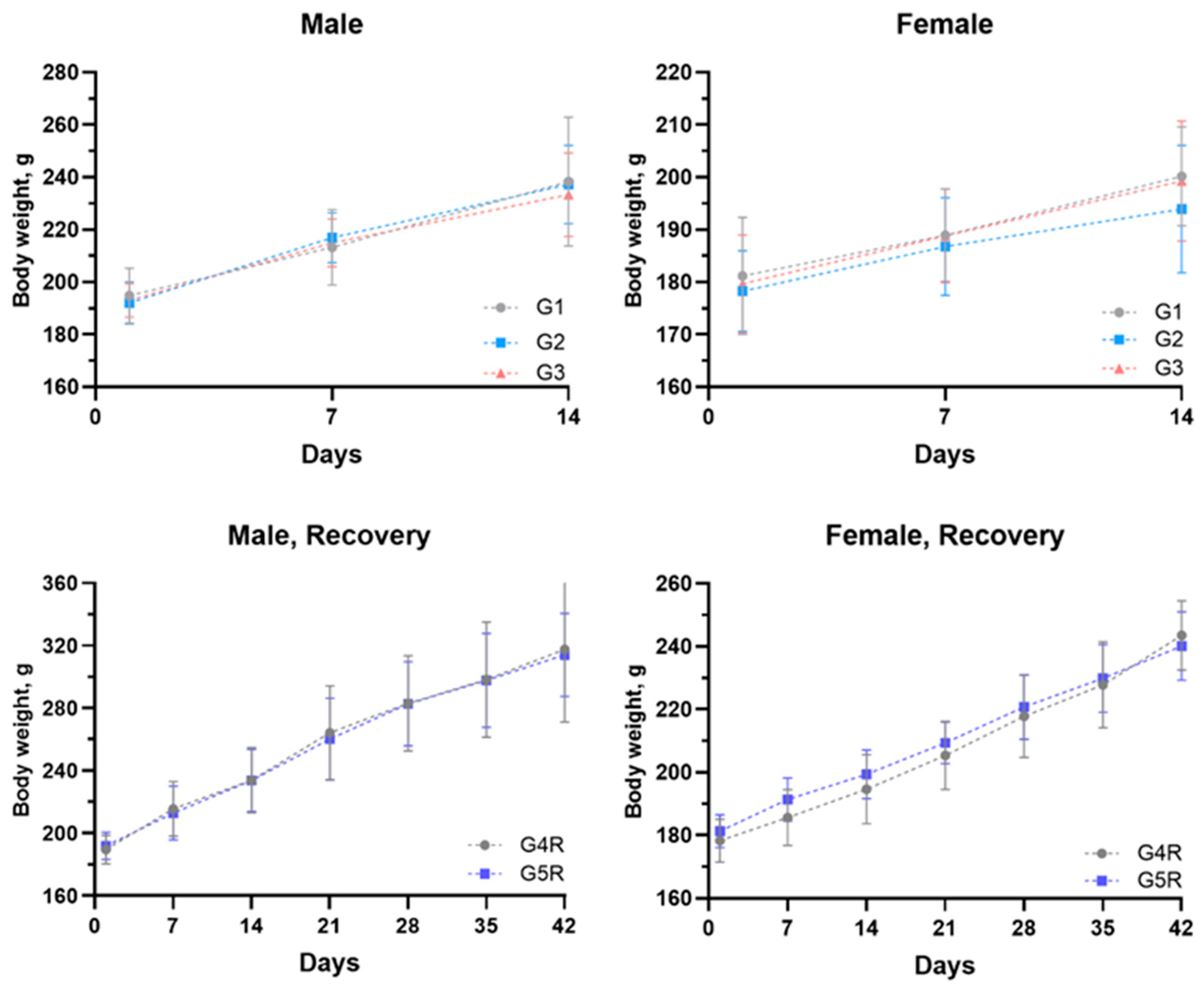
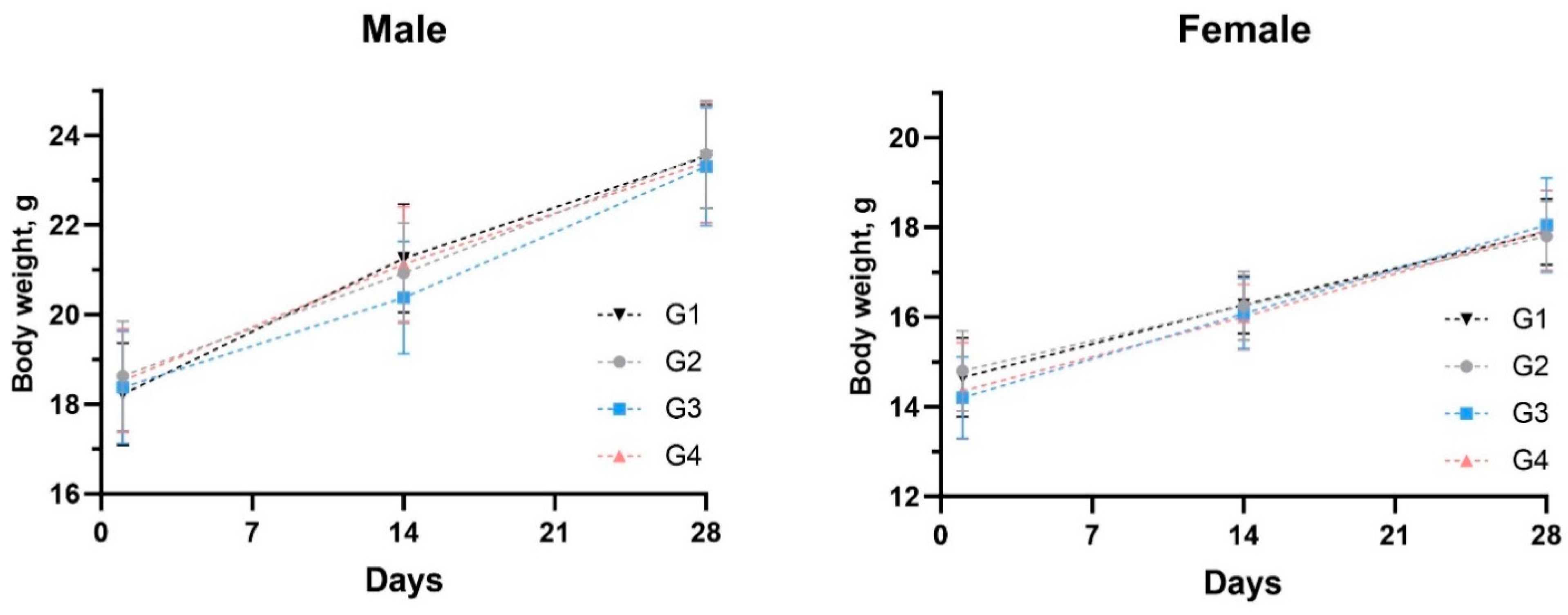
3.2.1. Body Weights and Feed Consumption
3.2.2. Hematology
3.2.3. Clinical Chemistry
3.2.4. Urine Analysis and Gross Pathology
3.2.5. Organ Weights
3.2.6. Histopathology
3.3. Immunogenicity Study in Mice
3.3.1. Mice Safety and Body Weight Transformation
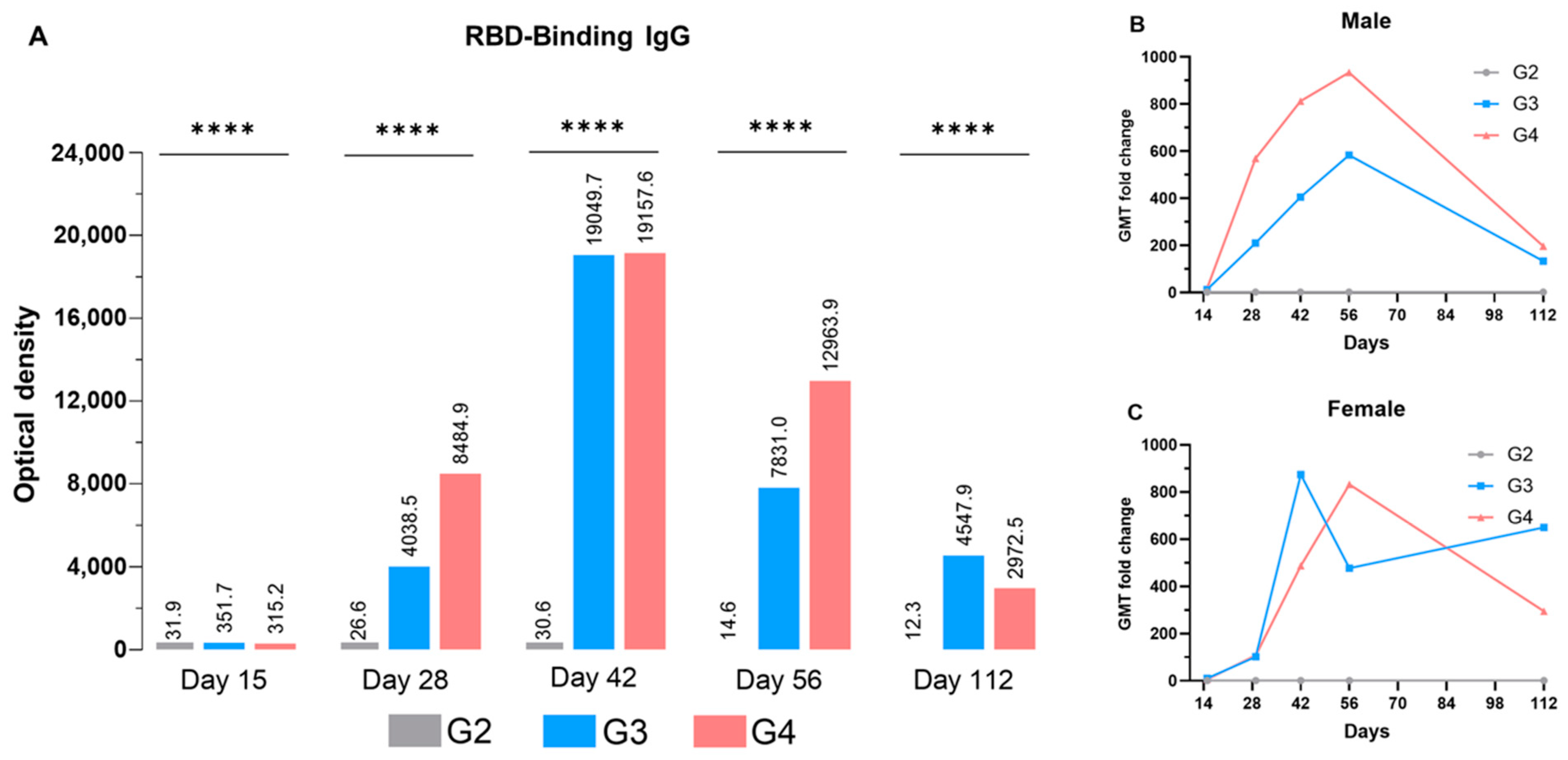
3.3.2. Anti-RBD IgG Production
3.3.3. Neutralization Antibody
3.3.4. Cellular Immunity
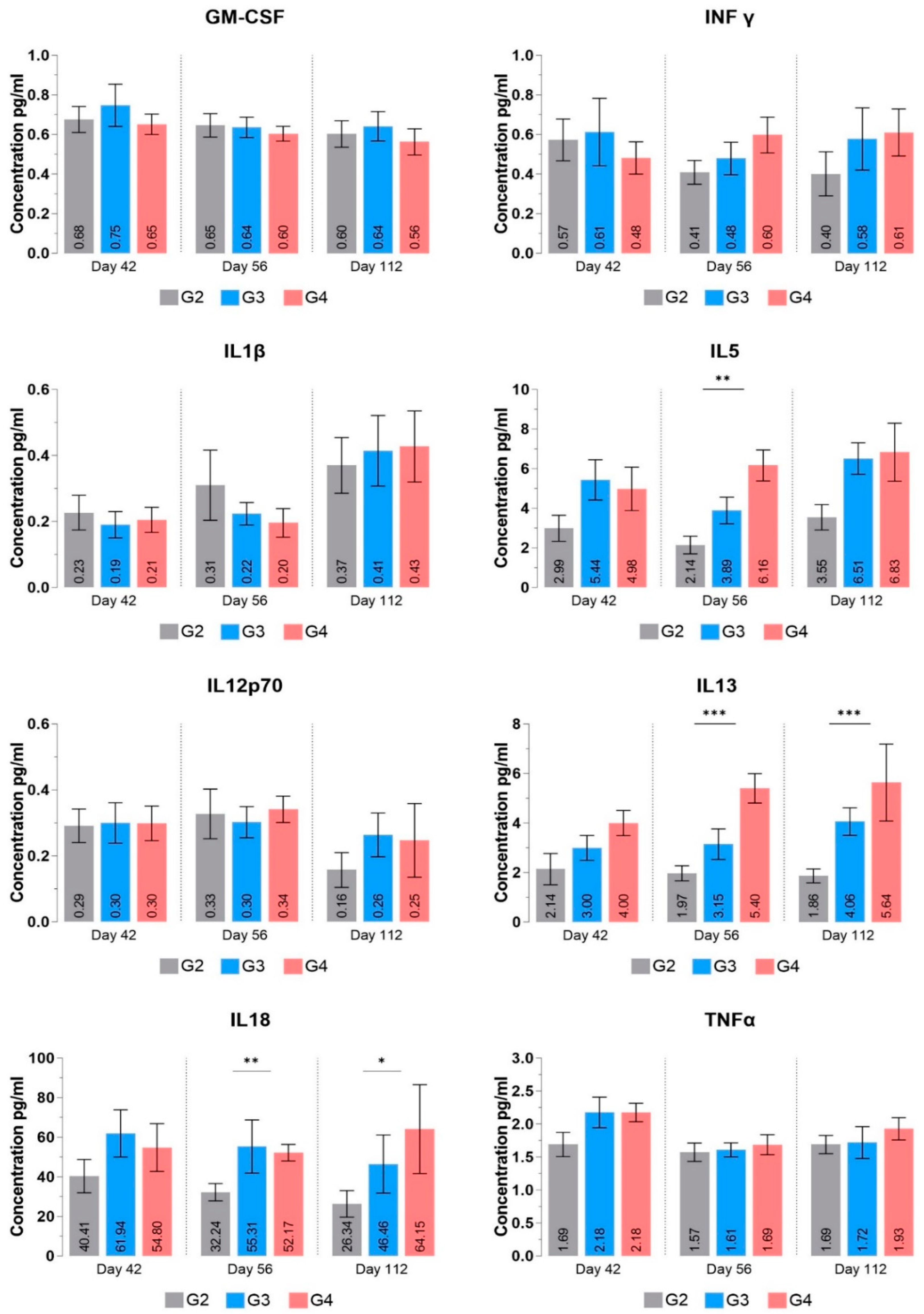
3.3.5. Cytokine Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Cases. WHO COVID-19 Dashboard. Datadot. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 23 February 2024).
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.; Chang, H. Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects. Front. Immunol. 2022, 13, 843928. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 23 February 2024).
- Sheikhshahrokh, A.; Ranjbar, R.; Saeidi, E.; Dehkordi, F.S.; Heiat, M.; Ghasemi-Dehkordi, P.; Goodarzi, H. Frontier Therapeutics and Vaccine Strategies for SARS-CoV-2 (COVID-19): A Review. Iran. J. Public Health 2020, 49, 18–29. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Dai, L.; Zheng, T.; Xu, K.; Han, Y.; Xu, L.; Huang, E.; An, Y.; Cheng, Y.; Li, S.; Liu, M.; et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722–733.e11. [Google Scholar] [CrossRef]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer–Based Covid-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef]
- Turdikulova, S.; Dalimova, D.; Abdullaev, A.; Muminov, M.; Ruyue, W.; Pengfei, L.; Musabaev, E.; Yusupaliev, B.; Kurbanov, B.; Abdurakhmonov, I.Y. COVID-19 Response in Uzbekistan: From RT-PCR Test System to the Clinical Trial of Subunit Vaccine. In COVID-19 Vaccines—Current State and Perspectives; Abdurakhmonov, P.I.Y., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Cell-PLoc: A Package of Web Servers for Predicting Subcellular Localization of Proteins in Various Organisms. Nature Protocols. Available online: https://www.nature.com/articles/nprot.2007.494 (accessed on 24 March 2023).
- Gibco. For transfection of ExpiCHO-S™ Cells in a defined, serum-free medium. In ExpiCHO™ Expression System User Guide; MAN0014337; Thermo Fisher Scientific: Carlsbad, CA, USA, 2018. [Google Scholar]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Shimizu, S. CHAPTER 32—Routes of Administration. In The Laboratory Mouse; Hedrich, H.J., Bullock, G., Eds.; Academic Press: Cambridge, MA, USA, 2004; pp. 527–542. [Google Scholar]
- Zhang, N.; Zheng, B.-J.; Lu, L.; Zhou, Y.; Jiang, S.; Du, L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015, 17, 123–134. [Google Scholar] [CrossRef]
- Mathew, S.; Faheem, M.; Hassain, N.A.; Benslimane, F.M.; Al Thani, A.A.; Zaraket, H.; Yassine, H.M. Platforms exploited for SARS-CoV-2 vaccine development. Vaccines 2020, 9, 11. [Google Scholar] [CrossRef]
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A Comprehensive Review of the Protein Subunit Vaccines Against COVID-19. Front. Microbiol. 2022, 13, 927306. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.J.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Lugovik, I.; Makarova, M. Toxicological studies. Reference intervals of mass coefficients of internal organs in a sample of 1000 rats. Lab. Zhivotnye Dlya Nauchnych Issled. (Lab. Anim. Sci.) 2021, 1, 3–11. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure-function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- King, R.L.; Kurtin, P.J. 7—Small B-Cell Lymphomas. In Hematopathology, 3rd ed.; Hsi, E.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 213–270.e3. [Google Scholar]
- Ahire, E.D.; Kshirsagar, S.J. Immune responses induced by different vaccine platforms against coronavirus disease-19. Explor. Immunol. 2021, 1, 243–257. [Google Scholar] [CrossRef]
- Bayani, F.; Hashkavaei, N.S.; Arjmand, S.; Rezaei, S.; Uskoković, V.; Alijanianzadeh, M.; Uversky, V.N.; Siadat, S.O.R.; Mozaffari-Jovin, S.; Sefidbakht, Y. An overview of the vaccine platforms to combat COVID-19 with a focus on the subunit vaccines. Prog. Biophys. Mol. Biol. 2023, 178, 32–49. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Moderbacher, C.R.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.-S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF Activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]
- Hildeman, D.A.; Zhu, Y.; Mitchell, T.C.; Kappler, J.; Marrack, P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 2002, 14, 354–359. [Google Scholar] [CrossRef]
- Melo, A.K.G.; Milby, K.M.; Caparroz, A.L.M.A.; Pinto, A.C.P.N.; Santos, R.R.P.; Rocha, A.P.; Ferreira, G.A.; Souza, V.A.; Valadares, L.D.A.; Vieira, R.M.R.A.; et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS ONE 2021, 16, e0253894. [Google Scholar] [CrossRef]
- Farshi, E. Cytokine Storm Response to COVID-19 Vaccinations. J. Cytokine Biol. 2021, 5, 2. [Google Scholar]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Verardi, P.H.; Legrand, F.A.; Chan, K.S.; Peng, Y.; Jones, L.A.; Yilma, T.D. IL-18 Expression Results in a Recombinant Vaccinia Virus That Is Highly Attenuated and Immunogenic. J. Interf. Cytokine Res. 2014, 34, 169–178. [Google Scholar] [CrossRef]
- Hidalgo-Gajardo, A.; Gutiérrez, N.; Lamazares, E.; Espinoza, F.; Escobar-Riquelme, F.; Leiva, M.J.; Villavicencio, C.; Mena-Ulecia, K.; Montesino, R.; Altamirano, C.; et al. Co-Formulation of Recombinant Porcine IL-18 Enhances the Onset of Immune Response in a New Lawsonia intracellularis Vaccine. Vaccines 2023, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.J.; Rudnick, K.A.; McCarthy, S.G.; Mateo, L.R.S.; Harris, M.C.; McCauley, C.; Snyder, L.A. Interleukin-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine 2006, 24, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Wolf, N.; Lavelle, E.C. A Guide to IL-1 family cytokines in adjuvanticity. FEBS J. 2018, 285, 2377–2401. [Google Scholar] [CrossRef]
- Baba, Y.; Ripley, B.; Kishimoto, T.; Kurosaki, T. Cytokine Regulation of B Cell Activation and Differentiation. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 244–252. [Google Scholar]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B 2011, 87, 463–485. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S.; Moro, K. Type 2 innate immune responses and the natural helper cell. Immunology 2011, 132, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S. Natural Helper Cells and TH2-Type Innate Immunity. Cornea 2012, 31 (Suppl. S1), S20–S24. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Jacob, C.O.; Aiso, S.; Michie, S.A.; McDevitt, H.O.; Acha-Orbea, H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): Similarities between TNF-alpha and interleukin 1. Proc. Natl. Acad. Sci. USA 1990, 87, 968–972. [Google Scholar] [CrossRef]
- Wu, A.J.; Hua, H.; Munson, S.H.; McDevitt, H.O. Tumor necrosis factor-α regulation of CD4+C25+ T cell levels in NOD mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12287–12292. [Google Scholar] [CrossRef]
- Gordon, C.; Ranges, G.E.; Greenspan, J.S.; Wofsy, D. Chronic therapy with recombinant tumor necrosis factor-α in autoimmune NZB/NZW F1 mice. Clin. Immunol. Immunopathol. 1989, 52, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Hill, A.V.S. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793–798. [Google Scholar] [CrossRef] [PubMed]





| RENOVAC: Placebo | RENOVAC: 10 µg | RENOVAC: 25 µg | |
|---|---|---|---|
| Active ingredient | 0 | 10 µg | 25 µg |
| 20× Alum adjuvant | 25 µL | 25 µL | 25 µL |
| PBS buffer, pH-7.4 | Up to 0.5 mL | Up to 0.5 mL | Up to 0.5 mL |
| Study Groups | G1 | G2 | G3 | G4 Recovery | G5 Recovery |
|---|---|---|---|---|---|
| Treatment | placebo | 10 µg | 25 µg | placebo | 25 µg |
| Administration | Day 1 and Day 8 | ||||
| Monitoring Parameters | mortality, clinical signs, local tolerance, detailed clinical examination, body weight, ophthalmoscopic examination, and feed consumption | ||||
| Euthanasia | Day 15 | Day 43 | |||
| Clinical Pathology | hematology, clinical chemistry, urinalysis, gross pathology, organ weights, and histopathological examination | ||||
| Tube No. | Antibody Staining | Ab: Cell Suspension |
|---|---|---|
| 1 | Unstained | - |
| 2 | Armenian Hamster IgG isotype control, APC | 1:100 |
| 3 | CD3e monoclonal antibody, APC | 1:100 |
| 4 | Rat IgG2b kappa isotype control, FITC | 1:100 |
| 5 | CD4 monoclonal antibody, FITC | 1:100 |
| 6 | Rat IgG2a kappa isotype control, PE | 1:100 |
| 7 | CD8a monoclonal antibody, PE | 1:100 |
| 8 | Mix of CD3-APC/CD4-FITC/CD8-PE | 1:100 each |
| Parameters | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4R | G5R | G1 | G2 | G3 | G4R | G5R | ||
| WBC (103/µL) | Mean | 15.97 | 16.13 | 19.02 | 13.68 | 10.08 | 12.63 | 12.43 | 12.50 | 7.07 | 8.12 |
| SD | 3.72 | 6.43 | 5.06 | 3.80 | 3.09 | 4.16 | 4.16 | 5.67 | 1.78 | 1.06 | |
| RBC (106/µL) | Mean | 7.36 | 7.30 | 7.31 | 8.09 | 8.54 | 6.61 | 6.59 | 6.98 | 7.37 | 7.37 |
| SD | 0.49 | 0.42 | 0.39 | 0.59 | 0.59 | 0.43 | 0.21 | 0.43 | 0.49 | 0.17 | |
| HGB (g/dL) | Mean | 12.65 | 13.07 | 12.62 | 13.38 | 13.92 | 12.05 | 12.50 | 12.55 | 12.52 | 12.92 |
| SD | 0.52 | 0.45 | 0.52 | 0.97 | 1.08 | 0.61 | 0.50 | 0.82 | 0.74 | 0.36 | |
| HCT (%) | Mean | 39.68 | 40.35 | 39.27 | 42.90 | 44.95 | 35.55 | 36.17 | 37.12 | 39.13 | 39.33 |
| SD | 1.61 | 2.02 | 1.83 | 3.11 | 3.83 | 1.82 | 1.32 | 2.36 | 2.43 | 0.96 | |
| MCV (fL) | Mean | 54.02 | 55.33 | 53.77 | 53.05 | 52.65 | 53.85 | 54.87 | 53.20 | 53.15 | 53.37 |
| SD | 1.86 | 1.81 | 1.80 | 1.22 | 2.08 | 1.09 | 1.05 | 1.18 | 0.43 | 0.55 | |
| MCH (pg) | Mean | 17.23 | 17.95 | 17.28 | 16.53 | 16.28 | 18.25 | 18.97 * | 18.00 | 17.03 | 17.53 |
| SD | 0.78 | 0.92 | 0.71 | 0.60 | 0.69 | 0.40 | 0.39 | 0.67 | 0.52 | 0.50 | |
| MCHC (g/dL) | Mean | 31.90 | 32.40 | 32.13 | 31.20 | 30.98 | 33.88 | 34.55 | 33.80 | 32.00 | 32.87 |
| SD | 0.37 | 0.77 | 0.49 | 0.53 | 0.43 | 0.43 | 0.27 | 0.73 | 0.81 | 0.70 | |
| PLT (103/µL) | Mean | 1067.3 | 969.3 | 999.1 | 801.0 | 944.3 | 1023.6 | 978.0 | 957.3 | 870.1 | 858.5 |
| SD | 156.36 | 103.71 | 156.21 | 160.22 | 123.38 | 121.65 | 116.44 | 181.60 | 86.62 | 120.65 | |
| RETICS (%) | Mean | 0.97 | 0.80 | 1.00 | 1.00 | 0.93 | 1.10 | 1.10 | 1.07 | 0.90 | 0.93 |
| SD | 0.29 | 0.25 | 0.36 | 0.28 | 0.33 | 0.37 | 0.27 | 0.33 | 0.37 | 0.24 | |
| Parameters | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4R | G5R | G1 | G2 | G3 | G4R | G5R | ||
| Neutrophils | Mean | 22.33 | 24.00 | 27.50 * | 23.17 | 24.33 | 24.67 | 23.50 | 25.67 | 25.50 | 25.33 |
| SD | 2.25 | 2.83 | 3.62 | 3.37 | 2.16 | 3.67 | 2.81 | 3.01 | 2.66 | 1.63 | |
| Lymphocytes | Mean | 76.17 | 74.33 | 71.33 * | 75.67 | 74.17 | 74.00 | 75.00 | 73.00 | 73.17 | 73.17 |
| SD | 1.94 | 2.73 | 3.27 | 3.61 | 2.99 | 4.20 | 2.37 | 3.10 | 2.79 | 2.32 | |
| Monocytes | Mean | 0.67 | 0.83 | 0.50 | 0.50 | 0.67 | 0.50 | 0.50 | 0.50 | 0.67 | 0.67 |
| SD | 0.82 | 0.75 | 0.84 | 0.84 | 0.82 | 0.84 | 0.55 | 0.84 | 0.82 | 0.82 | |
| Eosinophils | Mean | 0.83 | 0.83 | 0.67 | 0.67 | 0.83 | 0.83 | 1.00 | 0.83 | 0.67 | 0.83 |
| SD | 0.75 | 0.75 | 0.82 | 0.82 | 0.75 | 0.75 | 0.89 | 0.75 | 0.82 | 0.98 | |
| Basophils | Mean | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SD | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Parameters | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4R | G5R | G1 | G2 | G3 | G4R | G5R | ||
| GPT (U/L) | Mean | 55.58 | 58.53 | 59.88 | 52.10 | 36.03 * | 53.23 | 59.12 | 51.88 | 49.37 | 43.72 |
| SD | 13.29 | 7.35 | 10.67 | 10.41 | 5.10 | 10.38 | 9.93 | 7.52 | 9.10 | 8.09 | |
| GOT (U/L) | Mean | 104.73 | 97.38 | 111.77 | 84.57 | 91.60 | 83.33 | 99.13 | 121.07 * | 93.15 | 108.17 |
| SD | 14.69 | 26.71 | 21.05 | 8.00 | 12.21 | 11.98 | 17.58 | 39.14 | 12.69 | 24.16 | |
| ALP (U/L) | Mean | 341.83 | 509.83 * | 453.67 | 388.17 | 313.67 | 251.83 | 262.17 | 328.67 | 220.50 | 239.67 |
| SD | 26.83 | 148.68 | 100.77 | 106.15 | 70.60 | 65.24 | 73.20 | 54.77 | 93.13 | 71.01 | |
| BUL (mg/dL) | Mean | 34.47 | 29.22 * | 34.12 | 27.55 | 36.05 * | 32.23 | 34.68 | 36.22 | 34.52 | 37.68 |
| SD | 4.81 | 1.35 | 3.99 | 4.63 | 3.18 | 6.15 | 4.28 | 6.66 | 2.41 | 6.08 | |
| CREAT (mg/dL) | Mean | 0.58 | 0.55 | 0.60 | 0.62 | 0.62 | 0.57 | 0.61 | 0.60 | 0.62 | 0.64 |
| SD | 0.04 | 0.07 | 0.05 | 0.05 | 0.05 | 0.06 | 0.04 | 0.07 | 0.03 | 0.03 | |
| GLU (mg/dL) | Mean | 97.10 | 107.78 | 115.25 | 142.62 | 115.28 | 101.52 | 104.13 | 113.83 | 91.87 | 111.92 * |
| SD | 8.16 | 22.64 | 17.58 | 30.00 | 13.56 | 14.11 | 10.85 | 17.70 | 13.04 | 6.72 | |
| CHOLE (mg/dL) | Mean | 60.50 | 65.50 | 64.33 | 53.83 | 53.00 | 71.00 | 71.67 | 66.50 * | 76.17 | 71.83 |
| SD | 3.73 | 5.61 | 9.93 | 8.66 | 5.90 | 12.49 | 11.22 | 6.50 | 10.21 | 7.94 | |
| Ca (mg/dL) | Mean | 9.75 | 9.68 | 9.98 | 9.45 | 9.48 | 9.68 | 9.82 | 9.78 | 9.60 | 9.72 |
| SD | 0.41 | 0.31 | 0.43 | 0.52 | 0.56 | 0.38 | 0.45 | 0.44 | 0.24 | 0.33 | |
| ALB (g/dL) | Mean | 2.41 | 2.33 | 2.38 | 2.26 | 2.42 | 2.47 | 2.51 | 2.56 | 2.78 | 2.65 |
| SD | 0.04 | 0.14 | 0.10 | 0.12 | 0.13 | 0.14 | 0.10 | 0.15 | 0.14 | 0.22 | |
| PRO (g/dL) | Mean | 5.98 | 5.85 | 6.14 | 5.90 | 5.99 | 6.21 | 6.05 | 6.10 | 6.60 | 6.64 |
| SD | 0.29 | 0.46 | 0.24 | 0.35 | 0.44 | 0.29 | 0.33 | 0.37 | 0.27 | 0.25 | |
| TRIG (mg/dL) | Mean | 89.52 | 67.75 | 71.12 | 59.17 | 39.07 * | 56.87 | 50.98 | 45.95 | 33.45 | 29.53 |
| SD | 19.77 | 12.65 | 26.33 | 13.56 | 15.78 | 19.36 | 22.28 | 15.21 | 8.32 | 9.18 | |
| BIT (mg/dL) | Mean | 0.13 | 0.10 | 0.11 | 0.14 | 0.13 | 0.11 | 0.11 | 0.10 | 0.10 | 0.09 |
| SD | 0.05 | 0.01 | 0.02 | 0.04 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | |
| ALB/GLO | Mean | 0.68 | 0.67 | 0.64 | 0.62 | 0.68 * | 0.66 | 0.71 | 0.73 | 0.73 | 0.67 |
| SD | 0.06 | 0.06 | 0.06 | 0.04 | 0.05 | 0.06 | 0.06 | 0.06 | 0.03 | 0.08 | |
| CBUN (mg/dL) | Mean | 16.08 | 13.63 * | 15.92 | 12.86 | 16.82 * | 15.04 | 16.19 | 16.90 | 16.11 | 17.59 |
| SD | 2.25 | 0.63 | 1.86 | 2.16 | 1.48 | 2.87 | 2.00 | 3.11 | 1.12 | 2.84 | |
| GLO (g/dL) | Mean | 3.57 | 3.52 | 3.76 | 3.64 | 3.57 | 3.75 | 3.54 | 3.54 | 3.82 | 3.99 |
| SD | 0.31 | 0.38 | 0.24 | 0.28 | 0.33 | 0.27 | 0.28 | 0.29 | 0.15 | 0.21 | |
| Na (mmol/L) | Mean | 138.63 | 135.38 | 137.27 | 138.28 | 139.35 | 135.50 | 139.65 | 138.98 | 139.30 | 138.65 |
| SD | 0.55 | 7.45 | 0.56 | 1.02 | 1.63 | 7.19 | 1.14 | 1.25 | 0.72 | 0.81 | |
| K (mmol/L) | Mean | 4.68 | 4.65 | 4.68 | 4.43 | 4.66 | 4.32 | 4.39 | 4.55 | 4.48 | 3.91 * |
| SD | 0.25 | 0.45 | 0.31 | 0.32 | 0.32 | 0.48 | 0.13 | 0.57 | 0.28 | 0.12 | |
| Cl (mmol/L) | Mean | 100.88 | 105.28 | 100.27 | 101.77 | 102.05 | 105.18 | 101.55 | 101.47 | 103.13 | 100.77 * |
| SD | 1.17 | 9.77 | 0.98 | 0.57 | 1.12 | 8.57 | 0.95 | 1.08 | 0.74 | 1.00 | |
| Organs/Groups | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4R | G5R | G1 | G2 | G3 | G4R | G5R | ||
| Liver | Mean | 4.43 | 4.27 | 4.44 | 4.58 | 4.01 * | 4.03 | 4.10 | 4.09 | 4.21 | 4.42 |
| SD | 0.50 | 0.13 | 0.25 | 0.46 | 0.19 | 0.41 | 0.45 | 0.20 | 0.72 | 0.54 | |
| Spleen | Mean | 0.33 | 0.32 | 0.39 | 0.29 | 0.26 | 0.33 | 0.33 | 0.31 | 0.30 | 0.32 |
| SD | 0.05 | 0.09 | 0.11 | 0.06 | 0.07 | 0.10 | 0.08 | 0.11 | 0.08 | 0.05 | |
| Heart | Mean | 0.37 | 0.37 | 0.36 | 0.36 | 0.35 | 0.35 | 0.40 * | 0.38 | 0.37 | 0.38 |
| SD | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | |
| Thymus | Mean | 0.12 | 0.10 | 0.11 | 0.10 | 0.11 | 0.15 | 0.14 | 0.16 | 0.13 | 0.18 * |
| SD | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.05 | 0.02 | 0.05 | 0.02 | 0.03 | |
| Kidneys | Mean | 0.87 | 0.81 | 0.84 | 1.00 | 0.91 | 0.78 | 0.81 | 0.79 | 0.94 | 1.09 |
| SD | 0.13 | 0.06 | 0.06 | 0.11 | 0.12 | 0.07 | 0.06 | 0.06 | 0.07 | 0.19 | |
| Adrenals | Mean | 0.013 | 0.010 | 0.014 | 0.017 | 0.013 | 0.024 | 0.028 | 0.021 | 0.032 | 0.034 |
| SD | 0.004 | 0.003 | 0.003 | 0.004 | 0.004 | 0.005 | 0.006 | 0.007 | 0.005 | 0.011 | |
| Testes/Ovaries | Mean | 1.23 | 1.15 | 1.16 | 1.07 | 1.02 | 0.028 | 0.033 | 0.030 | 0.034 | 0.032 |
| SD | 0.12 | 0.10 | 0.06 | 0.14 | 0.09 | 0.002 | 0.009 | 0.005 | 0.009 | 0.006 | |
| Brain | Mean | 0.79 | 0.72 | 0.76 | 0.72 | 0.67 | 0.88 | 0.95 * | 0.91 | 0.89 | 0.93 |
| SD | 0.07 | 0.04 | 0.07 | 0.13 | 0.07 | 0.06 | 0.04 | 0.02 | 0.09 | 0.16 | |
| Sex | Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | G1 | G2 | G3 | G4 R | G5 R | G1 | G2 | G3 | G4 R | G5 R |
| Spleen | 0/6 | 0/6 | 2/6 | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muminov, M.; Tsiferova, N.; Pshenichnov, E.; Ermatova, K.; Charishnikova, O.; Abdullaev, A.; Levitskaya, Y.; Dalimova, D.; MVS, S.; Tomar, G.; et al. Development, Pre-Clinical Safety, and Immune Profile of RENOVAC—A Dimer RBD-Based Anti-Coronavirus Subunit Vaccine. Vaccines 2024, 12, 1420. https://doi.org/10.3390/vaccines12121420
Muminov M, Tsiferova N, Pshenichnov E, Ermatova K, Charishnikova O, Abdullaev A, Levitskaya Y, Dalimova D, MVS S, Tomar G, et al. Development, Pre-Clinical Safety, and Immune Profile of RENOVAC—A Dimer RBD-Based Anti-Coronavirus Subunit Vaccine. Vaccines. 2024; 12(12):1420. https://doi.org/10.3390/vaccines12121420
Chicago/Turabian StyleMuminov, Muzaffar, Nargiza Tsiferova, Egor Pshenichnov, Khusnora Ermatova, Oksana Charishnikova, Alisher Abdullaev, Yuliya Levitskaya, Dilbar Dalimova, Sandhya MVS, Geetanjali Tomar, and et al. 2024. "Development, Pre-Clinical Safety, and Immune Profile of RENOVAC—A Dimer RBD-Based Anti-Coronavirus Subunit Vaccine" Vaccines 12, no. 12: 1420. https://doi.org/10.3390/vaccines12121420
APA StyleMuminov, M., Tsiferova, N., Pshenichnov, E., Ermatova, K., Charishnikova, O., Abdullaev, A., Levitskaya, Y., Dalimova, D., MVS, S., Tomar, G., Dewle, A., Choudhari, P., Wangikar, A., Jadhav, A., Mule, M., Wangikar, P., Abdurakhmonov, I., & Turdikulova, S. (2024). Development, Pre-Clinical Safety, and Immune Profile of RENOVAC—A Dimer RBD-Based Anti-Coronavirus Subunit Vaccine. Vaccines, 12(12), 1420. https://doi.org/10.3390/vaccines12121420






