Platform Technology in Global Vaccine Regulation: Development, Applications, and Regulatory Strategies with Insights from China
Abstract
1. Introduction
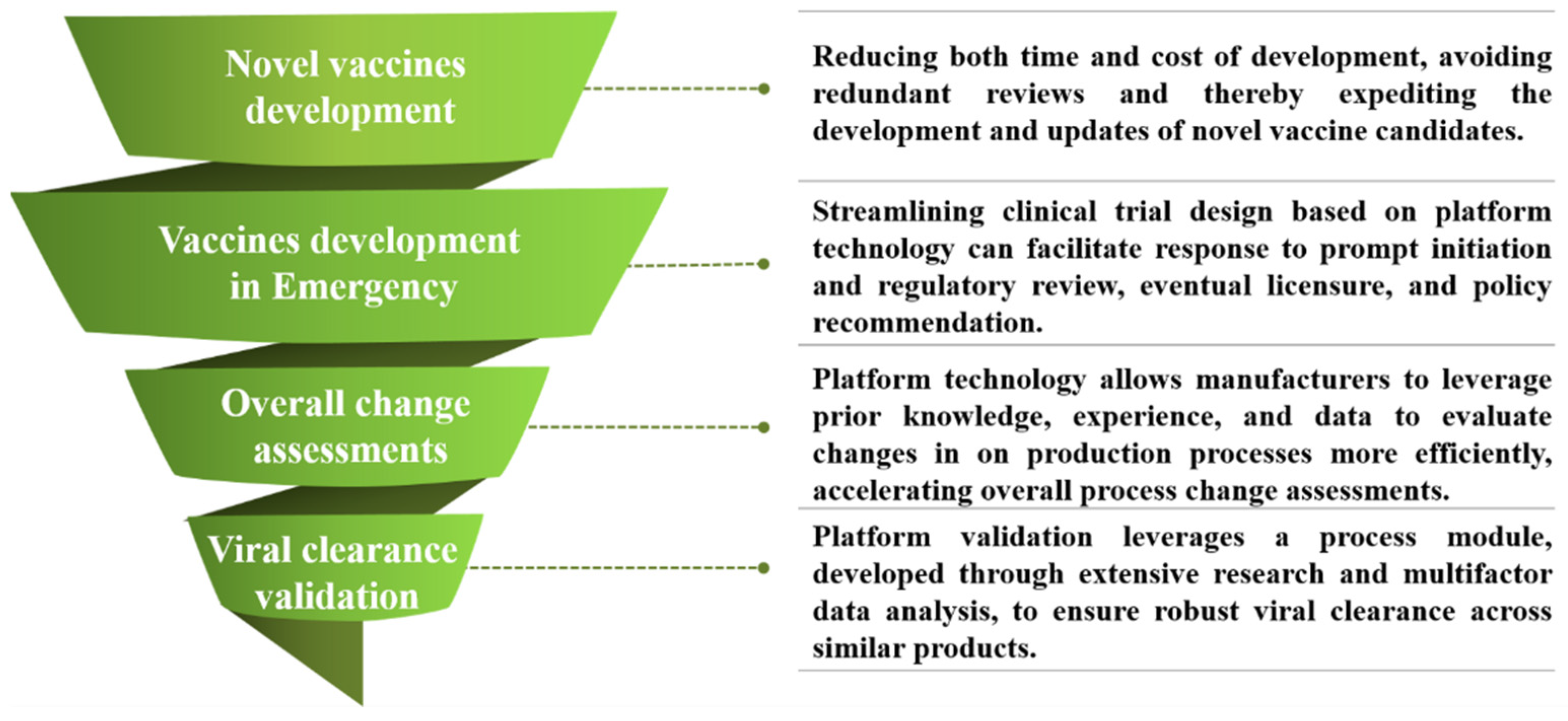
2. Application and Evaluation of Platform Technology
2.1. Streamlining Chemistry, Manufacturing, and Controls (CMCs) Validation and Research
2.2. Exemption of Non-Clinical/Clinical Data and Pre-Approval Inspection
2.3. Simplified Submission Dossier Through Cross-Referencing
3. Global Regulatory Processes and Technical Guidelines
3.1. Global Regulatory Advancements in Platform Technology
3.2. Challenges and Regional Variations in Establishing Regulatory Approach for Platform Technology
4. China’s Experience with Regulating Platform Technology
4.1. Viral Clearance Validation
4.2. Change Risk Assessment and Validation
4.3. Development and Approval of Emergency Vaccines
4.4. Regulatory Approaches for Conventional and Innovative Vaccines
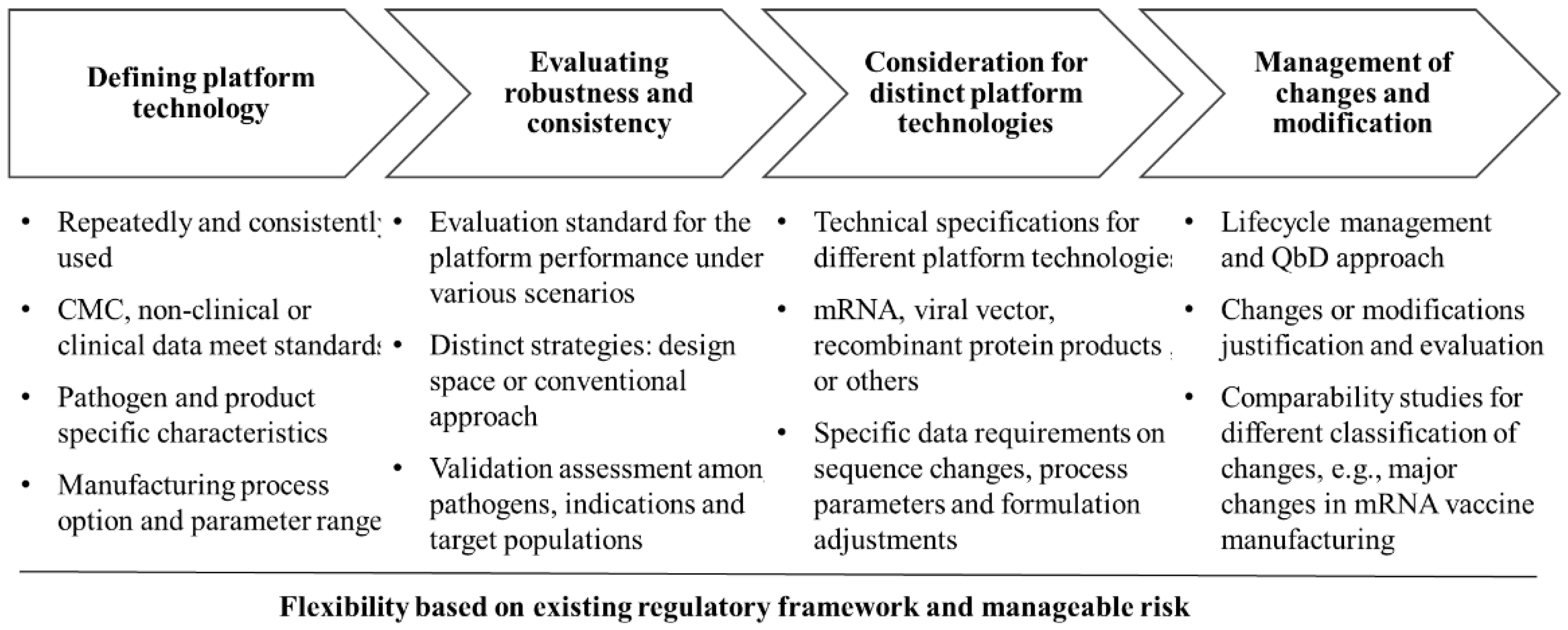
5. Challenges in the Platform Regulatory Approach
5.1. Defining Platform Technology
5.2. Evaluating Robustness and Consistency
5.3. Navigating Distinct Challenges of Different Technical Routes
5.4. Managing Changes and Updates to Platform Technology
5.5. Leveraging Emerging Technologies to Address Regulatory Challenges
6. Implications of Platform Technology for Pathogen X
6.1. Preparing for Future Pandemic Scenarios
6.2. Leveraging Platform Technology for Rapid Response: Challenges and Considerations
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO TRS1039 Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations; World Health Organization: Geneva, Switzerland, 2024.
- European Medicines Agency. EMA’s Guideline on Data Requirements for Vaccine Platform Technology Master Files (vPTMF) for Veterinarians; European Medicines Agency: Amsterdam, The Netherlands, 2024.
- U.S. Food and Drug Administration. FDA Platform Technology Designation Program for Drug Development Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2024.
- Center for Drug Evaluation of NMPA. Guideline for Chemistry, Manufacturing, and Controls Changes to Biological Investigational Medicinal Products in Clinical Trials (Trial); Center for Drug Evaluation of NMPA: Beijing, China, 2024.
- Rappuoli, R.; Alter, G.; Pulendran, B. Transforming vaccinology. Cell 2024, 187, 5171–5194. [Google Scholar] [CrossRef] [PubMed]
- Farlow, A.; Torreele, E.; Gray, G.; Ruxrungtham, K.; Rees, H.; Prasad, S.; Gomez, C.; Sall, A.; Magalhães, J.; Olliaro, P.; et al. The future of epidemic and pandemic vaccines to serve global public health needs. Vaccines 2023, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO TRS1011 Guidelines on the Quality, Safety and Efficacy of Ebola Vaccines; World Health Organization: Geneva, Switzerland, 2024.
- Niazi, S.K. The United States Food and Drug Administration’s Platform Technology Designation to Expedite the Development of Drugs. Pharmaceutics 2024, 16, 918. [Google Scholar] [CrossRef]
- World Health Organization. WHO Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness; World Health Organization: Geneva, Switzerland, 2024.
- Center for Drug Evaluation of NMPA. Technical Guidelines for Virus Clearance Process Platform Validation of Therapeutic Recombinant Biotechnology Products at the CTA Stage (Trial); Center for Drug Evaluation of NMPA: Beijing, China, 2024.
- O’Donnell, K.; Kartoglu, U. QRM, Knowledge Management, and the Importance of ICH Q9(R1). Pharm. Technol. 2024, 48, 20+. [Google Scholar]
- World Health Organization. Statement on the Outcomes of the ICMRA-WHO Joint Workshop on COVID-19 Vaccines Strain Change; World Health Organization: Geneva, Switzerland, 2024.
- International Coalition of Medicines Regulatory Authorities. Report from the ICMRA/WHO Workshop on: Global Perspectives on COVID-19 Vaccines Strain Update Alignment on Timing and Data Requirements; International Coalition of Medicines Regulatory Authorities: Tokyo, Japan, 2024. [Google Scholar]
- European Medicines Agency. COVID-19 Vaccine Strain Updates: Global Regulators Agree on Timing and Data Requirements; European Medicines Agency: Amsterdam, The Netherlands, 2024.
- Bollaerts, K.; Wyndham-Thomas, C.; Miller, E.; Izurieta, H.S.; Black, S.; Andrews, N.; Rubbrecht, M.; Van Heuverswyn, F.; Neels, P. The Role of Real-World Evidence for Regulatory and Public Health Decision-Making for Accelerated Vaccine Deployment—A Meeting Report. Biologicals 2024, 85, 101750. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, M.; He, W. COVID-19 Vaccine: Recent Advancements and Future Prospects. MedComm 2024, 5, e646. [Google Scholar] [CrossRef]
- Skritt, J.H.; Tucek-Szabo, C.; Sutton, B.; Nolan, T. The Platform Technology Approach to mRNA Product Development and Regulation. Vaccines 2024, 12, 528. [Google Scholar] [CrossRef]
- European Federation of Pharmaceutical Industries and Associations. Position on the European Health Emergency Preparedness and Response Authority (HERA); European Federation of Pharmaceutical Industries and Associations: Brussels, Belgium, 2024. [Google Scholar]
- Francis, M.J. Considerations for Rapid Development and Licensing of Conventional and Platform Technology Veterinary Vaccines. Avian Pathol. 2022, 51, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Entrican, G.; Francis, M.J. Applications of Platform Technologies in Veterinary Vaccinology and the Benefits for One Health. Vaccine 2022, 40, 2833–2840. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Q11 Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2024. [Google Scholar]
- Kalinke, U.; Barouch, D.H.; Rizzi, R.; Lagkadinou, E.; Türeci, Ö.; Pather, S.; Neels, P. Clinical Development and Approval of COVID-19 Vaccines. Expert Rev. Vaccines 2022, 21, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation of NMPA. Technical Guidelines for Clinical Trials of Human Papillomavirus (HPV) Vaccines (Trial); National Medical Products Administration: Beijing, China, 2023.
- Shirley, M. 20Valent Pneumococcal Conjugate Vaccine: Pediatric First Approval. Pediatr. Drugs 2023, 25, 613–619. [Google Scholar] [CrossRef]
- Gupta, A.; Rudra, A.; Reed, K.; Langer, R.; Anderson, D.G. Advanced Technologies for the Development of Infectious Disease Vaccines. Nat. Rev. Drug Discov. 2024, 23, 914–938. [Google Scholar] [CrossRef] [PubMed]
- Heaton, P.M. Challenges of Developing Novel Vaccines With Particular Global Health Importance. Front. Immunol. 2020, 11, 517290. [Google Scholar] [CrossRef]
- Daniel, S.; Kis, Z.; Kontoravdi, C.; Shah, N. Quality by Design for enabling RNA platform production processes. Trends Biotechnol. 2022, 40, 1213–1228. [Google Scholar] [CrossRef]
- Nag, K.; Sarker, M.E.H.; Kumar, S.; Khan, H.; Chakraborty, S.; Islam, M.J.; Baray, J.C.; Khan, M.R.; Mahmud, A.; Barman, U.; et al. DoE-derived continuous and robust process for manufacturing of pharmaceutical-grade wide-range LNPs for RNA-vaccine/drug delivery. Sci. Rep. 2022, 12, 9394. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Guideline on Influenza Vaccines Non-Clinical and Clinical Module; European Medicines Agency: Amsterdam, The Netherlands, 2016. [Google Scholar]
- U.S. Food and Drug Administration. Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023.
- European Medicines Agency. COVID-19: Lessons Learned from the Joint Report-Response to Public Health Emergency; European Medicines Agency: Amsterdam, The Netherlands, 2024.
- European Medicines Agency. Initiatives for Acceleration of Development, Support, and Evaluation Procedures for COVID-19 Treatments and Vaccines; European Medicines Agency: Amsterdam, The Netherlands, 2024.
- Josefsberg, J.O.; Buckland, B. Vaccine Process Technology. Biotechnol. Bioeng. 2012, 109, 1443–1460. [Google Scholar] [CrossRef]
- Buckland, B.; Sanyal, G.; Ranheim, T.; Pollard, D.; Searles, J.A.; Behrens, S.; Pluschkell, S.; Josefsberg, J.; Roberts, C.J. Vaccine Process Technology—A Decade of Progress. Biotechnol. Bioeng. 2024, 121, 2604–2635. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Kheirvari, M.; Liu, H.; Tumban, E. Virus-like Particle Vaccines and Platforms for Vaccine Development. Viruses 2023, 15, 1109. [Google Scholar] [CrossRef]
- Syed, Y.Y. Respiratory Syncytial Virus Prefusion F Subunit Vaccine: First Approval of a Maternal Vaccine to Protect Infants. Paediatr. Drugs 2023, 25, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Monslow, M.A.; Elbashir, S.; Sullivan, N.L.; Thiriot, D.S.; Ahl, P.; Smith, J.; Miller, E.; Cook, J.; Cosmi, S.; Thoryk, E.; et al. Immunogenicity Generated by mRNA Vaccine Encoding VZV gE Antigen is Comparable to Adjuvanted Subunit Vaccine and Better Than Live Attenuated Vaccine in Nonhuman Primates. Vaccine 2020, 38, 5793–5802. [Google Scholar] [CrossRef]
- Rai, C.I.; Kuo, T.H.; Chen, Y.C. Novel Administration Routes, Delivery Vectors, and Application of Vaccines Based on Biotechnologies: A Review. Vaccines 2024, 12, 1002. [Google Scholar] [CrossRef]
- Pardi, N.; Krammer, F. mRNA Vaccines for Infectious Diseases—Advances, Challenges and Opportunities. Nat. Rev. Drug Discov. 2024, 23, 838–861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. Research Advances on the Stability of mRNA Vaccines. Viruses 2023, 15, 668. [Google Scholar] [CrossRef]
- Elkashif, A.; Alhashimi, M.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Adenoviral Vector-Based Platforms for Developing Effective Vaccines to Combat Respiratory Viral Infections. Clin. Transl. Immunol. 2021, 10, e1345. [Google Scholar] [CrossRef]
- Ampa, C.; Clenet, D.; Halpern, J.; Le Palaire, L.; Krishna, M.; McGoldrick, M.; Bilanin, M.; Pattnaik, P.; Pelt, R.; Restrepo, S. Case Studies on Changes and Proposed Process Development Approaches Reflecting Applicability of PDA Technical Report No. 89 Strategies for Vaccine Development and Lifecycle Management. PDA J. Pharm. Sci. Technol. 2024, 78, 012976. [Google Scholar] [CrossRef]
- Lipa, M.J.; Greene, A.; Calnan, N. Knowledge Management as a Pharmaceutical Quality System Enabler: How Enhanced Knowledge Transfer Can Help Close the ICH Q10 to ICH Q12 Gap. PDA J. Pharm. Sci. Technol. 2021, 75, 64–90. [Google Scholar] [CrossRef]
- Barone, P.W.; Keumurian, F.J.; Neufeld, C.; Koenigsberg, A.; Kiss, R.; Leung, J.; Wiebe, M.; Ait-Belkacem, R.; Tabrizi, C.A.; Barbirato, C.; et al. Historical Evaluation of the In Vivo Adventitious Virus Test and Its Potential for Replacement with Next Generation Sequencing (NGS). Biologicals 2023, 81, 101661. [Google Scholar] [CrossRef] [PubMed]
- Vignali, V.; Hines, P.A.; Cruz, A.G.; Ziętek, B.; Herold, R. Health Horizons: Future Trends and Technologies from the European Medicines Agency’s Horizon Scanning Collaborations. Front. Med. 2022, 9, 1064003. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.Y.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D Cell Culture Models: Drug Pharmacokinetics, Safety Assessment, and Regulatory Consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.M.; Gressard, H.; Li, X.; Magagnoli, C.; Moriconi, A.; Stranges, D.; Strodiot, L.; Tello Soto, M.; Zwierzyna, M.; Campa, C. CMC Strategies and Advanced Technologies for Vaccine Development to Boost Acceleration and Pandemic Preparedness. Vaccines 2023, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- King, D.F.; Groves, H.; Weller, C.; Jones, I.; Cramer, J.P.; Gilbert, P.B.; Goldblatt, D.; Gruber, M.F.; Kampmann, B.; Maïga, D.; et al. Realising the Potential of Correlates of Protection for Vaccine Development, Licensure and Use: Short Summary. NPJ Vaccines 2024, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; He, J.; Su, J.; Ou, Z.; Liu, G.; Fu, R.; Shou, Q.; Zheng, M.; Group, T.; Luxembourg, A.; et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in Chinese females aged 9 to 26 years: A phase 3, open-label, immunobridging study. Vaccine 2021, 39, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Cassetti, M.C.; Pierson, T.C.; Patterson, L.J.; Bok, K.; DeRocco, A.J.; Deschamps, A.M.; Graham, B.S.; Erbelding, E.J.; Fauci, A.S. Prototype Pathogen Approach for Vaccine and Monoclonal Antibody Development: A Critical Component of the NIAID Plan for Pandemic Preparedness. J. Infect. Dis. 2023, 227, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ebola Trial Candidate Vaccines Arrive in Uganda in Record 79 Days After Outbreak Declared; World Health Organization: Geneva, Switzerland, 2022.
- Albakri, K.; Al-Hajali, M.; Saleh, O.; Alkhalil, A.M.; Mohd, A.B.; Samain, C.A.; Abuasad, N.N.; Hasan, H.; Khaity, A.; Farahat, R.A. Marburg Virus Disease Treatments and Vaccines: Recent Gaps and Implications. Ann. Med. Surg. 2023, 85, 328–330. [Google Scholar] [CrossRef]
- Rousculp, M.D.; Hollis, K.; Ziemiecki, R.; Odom, D.; Marchese, A.M.; Montazeri, M.; Odak, S.; Jackson, L.; Beyhaghi, H.; Toback, S. Reactogenicity Differences between Adjuvanted, Protein-Based and Messenger Ribonucleic Acid (mRNA)-Based COVID-19 Vaccines. Vaccines 2024, 12, 802. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
| Guideline | Name | Definition | Platform Technology Instructional Description | Release Date |
|---|---|---|---|---|
| ICH Q11 Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) | Platform manufacturing | Platform manufacturing is the approach of developing a production strategy for a new drug starting from manufacturing processes similar to those used by the same applicant to manufacture other drugs of the same type (e.g., as in the production of monoclonal antibodies using predefined host cell, cell culture, and purification processes, for which there already exists considerable experience). | A technical guideline to drug development that introduces the macro concept of platform manufacturing as a development tool to support process development | May 2012 |
| ICH Q5A(R2) Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin | Platform validation | Platform validation is defined as the use of prior knowledge, including in-house experience with viral reduction data from other products, to claim a reduction factor for a new similar product, according to current understanding. | A technical guideline for virus safety evaluation, which clarifies the concept and general principles of platform validation, and provides specific cases for different processes | November 2023 |
| WHO TRS1011 Guidelines on the Quality, Safety and Efficacy of Ebola Vaccines | Platform technology | Platform technology is a production technology in which different viral vectored vaccines are produced by incorporating heterologous genes for different proteins into an identical viral vector backbone. | A technical guideline supports the development and licensing of an Ebola vaccine, which discusses opportunities to accelerate vaccine development and product availability based on “platform technology” during a public health emergency, the minimum dataset for CMC, non-clinical, and clinical research and evaluation required are highlighted. | July 2018 |
| WHO TRS1039 Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations | Platform technology | Platform technology is a group of technologies used as a base upon which other applications, processes or technologies are developed. In the context of mRNA vaccines, a given manufacturer might have one or more platforms on which they will develop vaccines (or therapeutics) against various diseases or pathogen strains. | A technical guideline for mRNA vaccines, which outlines prerequisites for platform technology in process development, stability, and clinical and non-clinical studies and explains the considerations for reducing non-clinical studies (abbreviated nonclinical program) under different circumstances and using immunobridging data to demonstrate protective efficacy during public health emergencies. | October 2021 |
| EMA’s Guideline on Data Requirements for Vaccine Platform Technology Master Files (vPTMFs) for Veterinarians | Platform technology | In practice, a vaccine platform is a manufacturing process that relies on a single vector or expression system (“backbone carrier”) and a standard process for inserting a gene or genes of interest into the system to generate different recombinant master seeds, master sequences or constructs, which are then used to produce a vaccine. The gene of interest may consist of one or more complete or partial gene sequences. The active substance/s obtained is blended with adjuvants and/or excipients for the different target species to manufacture finished products with certain defined properties. | Guideline on data requirements for vPTMF, which outlines the data to be included in the vPTMF, as well as the data requirements for initial submission and re-evaluation. | January 2022 |
| FDA Platform Technology Designation Program for Drug Development | Platform technology | Platform technology is a well-understood and reproducible technology, which can include a nucleic acid sequence, molecular structure, mechanism of action, delivery method, vector, or a combination of any such technologies that the Secretary determines to be appropriate, that the sponsor demonstrates (1) is incorporated in or used by a drug and is essential to the structure or function of such drug; (2) can be adapted for, incorporated into, or used by, more than one drug sharing common structural elements; and (3) facilitates the manufacture or development of more than one drug through a standardized production or manufacturing process or processes. | A procedural guide for platform technology, which describes the scope of platform technology designation, application time and procedure, etc. | May 2024 |
| CDE, NMPA Technical Guidelines for Virus Clearance Process Platform Validation of Therapeutic Recombinant Biotechnology Products at the CTA stage (Trial) | Platform validation | Platform validation refers to a study method evaluating the virus clearance performance of specific process steps in the manufacturing process of other similar in-house products based on the internal experience of virus clearance process validation studies of similar products in combination with external knowledge appropriately. | A technical guideline for virus clearance process platform validation, including a clear description of how to establish a platform validation and the requirements for data establishment | January 2024 |
| CDE, NMPA Guideline for Chemistry, Manufacturing, and Controls Changes to Biological Investigational Medicinal Products in Clinical Trials (Trial) | Platform technology | Platform technology is an existing technology, or group of technologies, applied to the development and/or production of similar products by a given manufacturer whose previous products might have been marketed or received clinical trial approval. A platform would be considered when the elements of the scaffold/skeleton (which may include nucleic acid vector, virus vector, protein backbone, etc.) or adjuvant, the manufacturing processes, CQAs and compliance GMP are essentially unchanged. It can be regarded as a platform. The experience and knowledge gained, data generated (manufacturing, control, and stability) and the method validation, non-clinical and clinical data, etc. can all be used as supportive data for the more rapid assessment and development of a new candidate product that fits within the boundaries of the platform. | Technical guidelines for CMC changes, which explain the scientific strategy and considerations for using platform technology to accelerate the development and evaluation of CMC changes for biological products in clinical trials. | June 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jin, S.; Guo, S.; Yang, D.; Sai, W.; Qiu, X.; Zhao, X.; Wang, L.; Wang, T.; Li, M. Platform Technology in Global Vaccine Regulation: Development, Applications, and Regulatory Strategies with Insights from China. Vaccines 2024, 12, 1436. https://doi.org/10.3390/vaccines12121436
Li X, Jin S, Guo S, Yang D, Sai W, Qiu X, Zhao X, Wang L, Wang T, Li M. Platform Technology in Global Vaccine Regulation: Development, Applications, and Regulatory Strategies with Insights from China. Vaccines. 2024; 12(12):1436. https://doi.org/10.3390/vaccines12121436
Chicago/Turabian StyleLi, Xiaojing, Su Jin, Shuyang Guo, Dan Yang, Wenbo Sai, Xiao Qiu, Xin Zhao, Lan Wang, Tao Wang, and Min Li. 2024. "Platform Technology in Global Vaccine Regulation: Development, Applications, and Regulatory Strategies with Insights from China" Vaccines 12, no. 12: 1436. https://doi.org/10.3390/vaccines12121436
APA StyleLi, X., Jin, S., Guo, S., Yang, D., Sai, W., Qiu, X., Zhao, X., Wang, L., Wang, T., & Li, M. (2024). Platform Technology in Global Vaccine Regulation: Development, Applications, and Regulatory Strategies with Insights from China. Vaccines, 12(12), 1436. https://doi.org/10.3390/vaccines12121436





