Transcriptomic Analysis Reveals Sixteen Potential Genes Associated with the Successful Differentiation of Antibody-Secreting Cells through the Utilization of Unfolded Protein Response Mechanisms in Robust Responders to the Influenza Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Haemagglutination Inhibition Assay
2.3. Transcriptomic Profiling and Differential Gene Expression Analysis
3. Results
3.1. Vaccination Immunogenicity
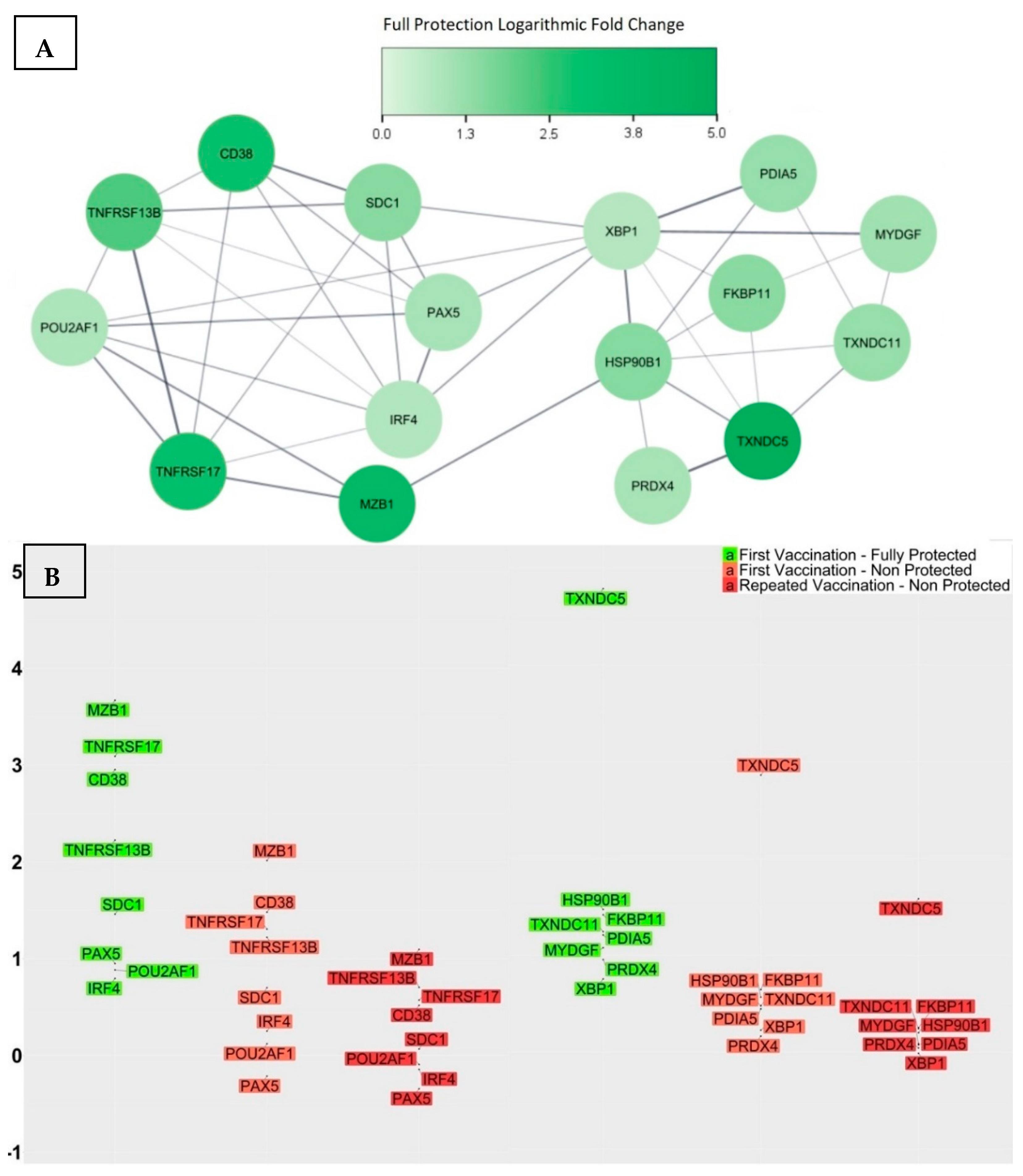
3.2. Transcriptional Signatures of Fully Protected Participants
3.3. Genes with Significant Variations in log2-Fold Change between the Fully Protected Group and the Non-Fully Protected Groups
- CD38 was up-regulated within the fully protected group, with an average log2-fold change of 2.95. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 1.64, 1.36, and 1.15, respectively. Moreover, the significant differences in the average log2-fold changes, when comparing the fully protected group to the partially, weakly, and non-protected groups, as indicated by the calculated confidence intervals, were 1.31 [0.33, 2.27] (adjusted p-value = 1.5 × 10−3), 1.59 [0.68, 2.47] (adjusted p-value = 1.1 × 10−5), and 1.8 (0.89, 2.69) (adjusted p-value = 1.1 × 10−6), respectively. CD38, also referred to as CADPR1, is a multifunctional protein expressed on the surface of B cells in healthy individuals. It acts as both a receptor and a multifunctional enzyme, and one of its key roles is to catalyse the synthesis and hydrolysis of a general calcium messenger molecule cyclic ADP-ribose (cADPR) [45]. Recent research has revealed that CD38 has a strong connection with CD19 in inactive B cells, as well as with the immunoglobulin M (IgM) B cell receptor when it is in an engaged state, suggesting a modulatory effect on B cell activation upon antigen recognition by regulating CD19 [46]. Furthermore, researchers used CD19, CD38, and CD138 to classify different plasma cell subsets in the bone marrow of human subjects, and their findings revealed that the CD19−CD38++CD138+ subset was morphologically distinct and exclusively contained plasma cells that targeted viral antigens, which the subjects had not encountered for over four decades as a result of durable immunisation [47]; consequently, elevated levels of CD38 expression may indicate the effective production of LLPCs.
- SDC1 was up-regulated within the fully protected group, with an average log2-fold change of 1.46. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.79, 0.73, and 0.48, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.67 [0.12, 1.2] (adjusted p-value = 3.9 × 10−3), 0.73 [0.23, 1.22] (adjusted p-value = 4.6 × 10−4), and 0.98 [0.47, 1.47] (adjusted p-value = 1.6 × 10−6), respectively. SDC1, also referred to as the CD138 antigen, encodes a heparan sulphate glycoprotein. SDC1 is a member of the syndecan proteoglycan family, which mediates cell signalling, cell binding, and cell migration. CD138 serves as a cell surface marker for normal B cells and is expressed at varying levels throughout different stages of B cell differentiation [48]. A higher expression of CD138 indicates the presence of LLPCs. Notably, the expression of CD138 on ASCs leads to increased levels of heparan sulphate, known for its ability to bind pro-survival cytokines like IL-6 and APRIL, to protect ASCs from apoptosis, and to promote longevity [49].
- MZB1 was up-regulated and exhibited a remarkable increase in expression level within the fully protected group, with an average log2-fold change of 3.66. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 2.36, 2.04, and 1.7, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 1.3 [0.24, 2.37] (adjusted p-value = 3.4 × 10−3), 1.62 [0.64, 2.61] (adjusted p-value = 3.6 × 10−5), and 1.96 [0.97, 2.95] (adjusted p-value = 1.4 × 10−6), respectively. MZB1, also referred to as pERp1, encodes the marginal zone B and the B1 cell specific protein. It is found within the endoplasmic reticulum (ER) as a component of the binding immunoglobulin protein (BiP) chaperone complex. MZB1 belongs to the canopy (CNPY) family of ER resident saposin-like proteins, and it has a saposin fold, with unique sequence extensions that are not present in other saposin proteins [50]. During the process of the B cell to plasma cell differentiation, MZB1 is significantly up-regulated, and analyses demonstrated that MZB1 is an unusual type of resident ER protein that specifically assists immunoglobulin biosynthesis. Knowing this, plasma cells have the ability to produce and release huge amounts of immunoglobulin molecules that undergo assembly and oxidative folding within the ER [51]. Other studies revealed that the deletion of MZB1 adversely affects humoral immune responses and the secretion of antibodies in plasma cells that naturally undergo ER stress [52]. In addition, the retention of ASCs in the bone marrow and their maturation into plasma cells requires the involvement of a cell surface molecule known as very late antigen 4 (VLA4). Interestingly, ASCs deficient in the co-chaperone MZB1, which is essential for VLA4 activation, showed an impaired ability to migrate and home in the bone marrow [53].
- TNFRSF17 was up-regulated within the fully protected group, with an average log2-fold change of 3.09. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 1.63, 1.3, and 1.06, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 1.46 [0.25, 2.67] (adjusted p-value = 4.6 × 10−3), 1.79 [0.67, 2.9] (adjusted p-value = 8.2 × 10−5), and 2.03 [0.9, 3.16] (adjusted p-value = 3.1 × 10−5), respectively. TNFRSF17, also referred to as BCMA or TNFRSF13A, encodes the tumour necrosis factor (TNF) receptor superfamily member 17 protein. It is a non-glycosylated integral membrane protein that is preferentially expressed in mature B lymphocytes. TNFRSF17 interacts with TNF receptor-associated factors TRAF1, TRAF2, and TRAF3, leading to the activation of nuclear factor kappa B (NF-κB), elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase [54]. B-cell activating antigen (BCMA), transmembrane activator and CAML interactor (TACI), and B-cell activating factor receptor (BAFFR) serve as receptors for the B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), establishing a complex network that plays a crucial role in the progression of humoral immunity. More specifically, the functional activity of the BCMA receptor aids in promoting the survival of LLPCs [55,56,57,58]. The investigation into the genetic knockout of TNFRSF17 yielded noteworthy findings, specifically a substantial reduction in ASCs in the bone marrow when compared to wild-type controls, occurring 6–8 weeks after immunisation. Nevertheless, germinal centre responses and early antigen-specific serum IgM and IgG levels remained within normal ranges, indicating that the primary impact of losing BCMA affected the LLPCs [58].
- TNFRSF13B was up-regulated within the fully protected group, with an average log2-fold change of 2.22. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 1.17, 1.07, and 1.05, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 1.05 [0.04, 2.06] (adjusted p-value = 0.2 × 10−2), 1.15 [0.22, 2.1] (adjusted p-value = 4.2 × 10−3), and 1.17 [0.23, 2.13] (adjusted p-value = 4.1 × 10−3), respectively. TNFRSF13B, also referred to as TACI, encodes a lymphocyte-specific member of the TNF receptor superfamily. The TNF superfamily ligands BAFF and APRIL, along with their three receptors BAFFR, BCMA, and TACI, play significant roles in the immunological functions of the B cell arm of the immune system. BAFF-R specifically targets BAFF, while BCMA has a greater affinity for APRIL than BAFF. On the other hand, TACI is capable of binding both ligands with equal effectiveness. TACI facilitates NF-κB responses and triggers the process of immunoglobulin IgG and IgA class-switch recombination in B cells. In humans, TACI deficiency was found to manifest as an antibody deficiency syndrome [59,60,61,62,63].
- POU2AF1 was up-regulated within the fully protected group, with an average log2-fold change of 0.88. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.36, 0.18, and 0.05, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.52 [0.05, 1.0] (adjusted p-value = 0.1 × 10−2), 0.7 [0.26, 1.14] (adjusted p-value = 8.4 × 10−5), and 0.83 [0.39, 1.28] (adjusted p-value = 3.2 × 10−6), respectively. POU2AF1, also referred to as B cell-specific coactivator (OCA-B) or OBF-1, encodes an octamer-binding factor protein. OCA-B polypeptide is the primary factor that activates immunoglobulin (Ig) promoters in B cells, working in conjunction with OCT-1 and OCT-2 binding proteins; it forms a binding complex that specifically targets octamer sites found in both promoters and enhancers. Its main role is to initiate the transcription process for Ig genes in B cells [64,65,66]. Furthermore, the transcriptional activator OCT-2, along with its cofactor OBF-1, serves as key regulatory factors for IL6 expression, driving the differentiation of activated CD4+ T cells into TFH cells. Therefore, OBF1 plays a role in the response of B cells to thymus-dependent antigens, being essential for the formation of germinal centres that are fundamental to the generation of high-affinity antibody-secreting cells [67,68,69].
- PAX5 was up-regulated within the fully protected group, with an average log2-fold change of 0.95. In contrast, it was down-regulated within the partially, weakly, and non-protected groups, with average log2-fold changes of −0.2, −0.05, and −0.25, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 1.15 [0.0, 2.31] (adjusted p-value = 2.3 × 10−2), 1.0 [−0.06, 2.07] (adjusted p-value = 3.5 × 10−2), and 1.2 [0.13, 2.28] (adjusted p-value = 9.8 × 10−3), respectively. PAX5, also referred to as B cell lineage-specific activator protein (BSAP), encodes a member of the paired box (PAX) family of transcription factors. PAX5 is a unique transcription factor that safeguards the B-lymphocyte lineage commitment and performs a dual role by activating B cell-specific genes and simultaneously repressing B cell-unspecific genes [70]. The pro-B cell stage signifies a B cell lineage commitment phase, wherein the rearrangement of heavy-chain genes takes place. Approximately 23% of all expression alterations observed during the transition from common lymphoid progenitors to committed pro-B cells can be attributed to PAX5-regulated genes, which identifies PAX5 as an essential regulator of the B cell developmental transition [71]. The targets of PAX5 activation include immune receptors, such as CD19 and CD21, as well as transcription factors’ interferon regulators, such as IRF4, IRF8, and BACH2 [72]. The PAX5 repressed genes control a wide range of biological functions, including cell communication, adhesion, migration, nuclear processes, and cellular metabolism as part of the process of B cell commitment [73]. For instance, PAX5 repression of the cohesin release factor WAPL in pro-B cells results in alterations to the chromosomal architecture, which facilitates the generation of a diverse antibody repertoire [74]. Studies also revealed that PAX5 plays a role in proliferation and immunoglobulin isotype switching in germinal centre B cells [75].
- IRF4 was up-regulated within the fully protected group, with an average log2-fold change of 0.8. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.26, 0.14, and 0.12, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.54 [0.0, 1.07] (adjusted p-value = 2.5 × 10−2), 0.65 [0.16, 1.15] (adjusted p-value = 1.8 × 10−3), and 0.67 [0.16, 1.18] (adjusted p-value = 9.9 × 10−4), respectively. IRF4 encodes a transcription factor belonging to the IRF (interferon regulatory factor) family of transcription factors, characterized by a specific DNA-binding domain and the ability to bind to regulatory elements in promoters of interferon-inducible genes [76]. IRF4 is required for the generation of germinal centre B cells by inducing the expression of key germinal centre genes, including BCL6 and AICDA. IRF4 also induces BLIMP1, which handles the transition from a germinal centre B cell gene expression program to that of a plasma cell. This multifunctional nature of IRF4 implies its involvement in a multifaceted regulatory network, wherein its expression levels play an additional role; lower IRF4 expression levels appear to facilitate the progression of the germinal centre pathway and higher expression levels promote the differentiation of plasma cells [77]. Investigating the involvement of IRF4 in post-germinal centre B cell development revealed that IRF4 plays an important role in the differentiation of plasma cells and the process of class switch recombination (CSR). The conditional deletion of IRF4 in germinal centre B cells lacked post-germinal centre plasma cells and the inability to differentiate memory B cells into plasma cells, which highlights the significance of IRF4 as a pivotal transcriptional regulator in the development of plasma cells [78].
- TXNDC5 was up-regulated and exhibited a remarkable increase in expression level within the fully protected group, with an average log2-fold change of 4.79. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 3.11, 2.74, and 2.43, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 1.68 [0.31, 3.03] (adjusted p-value = 4.6 × 10−3), 2.05 [0.79, 3.3] (adjusted p-value = 5.1 × 10−5), and 2.36 [1.09, 3.61] (adjusted p-value = 5.6 × 10−6), respectively. TXNDC5, also referred to as PDIA15 or ERp46, encodes the thioredoxin domain-containing protein 5 and a member of the protein disulfide isomerase (PDI) family. It plays a role in the formation and rearrangement of disulfide bonds for proper protein folding. In addition, TXNDC5 functions as a molecular chaperone, regulating the synthesis of abnormal proteins and maintaining cellular homeostasis. TXNDC5 possesses various biological functions, including anti-oxidation, the promotion of angiogenesis, cellular inflammation, and energy metabolism [79,80].
- TXNDC11 was up-regulated within the fully protected group, with an average log2-fold change of 1.24. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.7, 0.51, and 0.4, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.54 [0.03, 1.06] (adjusted p-value = 1.5 × 10−2), 0.73 [0.26, 1.21] (adjusted p-value = 1.7 × 10−4), and 0.84 [0.37, 1.32] (adjusted p-value = 1.1 × 10−5), respectively. TXNDC11, also referred to as EFP1, encodes the thioredoxin domain-containing protein 11. TXNDC11 is specifically induced by ER stress and is regulated by the IRE1–XBP1 pathway. This pathway is a major component of the unfolded protein response (UPR) signalling pathway, which plays a significant role in maintaining cellular homeostasis [81].
- HSP90B1 was up-regulated within the fully protected group, with an average log2-fold change of 1.47. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.71, 0.67, and 0.44, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.76 [0.18, 1.33] (adjusted p-value = 2.4 × 10−3), 0.8 [0.27, 1.33] (adjusted p-value = 1.6 × 10−4), and 1.03 [0.49, 1.56] (adjusted p-value = 1.8 × 10−6), respectively. HSP90B1, also referred to as GRP94, encodes heat shock protein 90 beta family member 1. It is an important ER molecular chaperone that plays a role in the UPR pathway and enhances the functioning of B cells by chaperoning Toll-like Receptors (TLRs) and integrins. The chaperone function of the HSP90B1 gene is of great significance in protein physiology, as well as in the processing and transportation of secreted proteins [82].
- FKBP11 was up-regulated within the fully protected group, with an average log2-fold change of 1.41. In contrast, the partially, weakly, and non-protected groups demonstrated lower average log2-fold changes of 0.74, 0.72, and 0.51, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.67 [0.13, 1.22] (adjusted p-value = 3.5 × 10−3), 0.69 [0.19, 1.19] (adjusted p-value = 1.1 × 10−3), and 0.9 [0.39, 1.41] (adjusted p-value = 8.6 × 10−6), respectively. FKBP11, also referred to as FKBP19, encodes FK506-binding protein 11. It is a member of the FKBP family of peptidyl-prolyl cis/trans isomerases, which accelerate the folding of proteins during protein synthesis. Recent studies have discovered that the differentiation of B cells into plasma cells is accompanied by the induction of FKBP11 expression, suggesting its potential role as a catalyst for antibody folding in plasma cells. In particular, the upregulation of FKBP11 expression has been observed to correlate with the induction of ER stress as part of the UPR pathway and in a manner that is dependent on the X-box-binding protein 1 (XBP1) [83].
- PDIA5 was up-regulated within the fully protected group, with an average log2-fold change of 1.2. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.55, 0.54, and 0.32, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.65 [0.06, 1.25] (adjusted p-value = 1.1 × 10−2), 0.66 [0.12, 1.21] (adjusted p-value = 4.7 × 10−3), and 0.88 [0.33, 1.44] (adjusted p-value = 3.2 × 10−4), respectively. PDIA5, also referred to as PDIR, encodes disulfide isomerase A5 protein. It is a member of the disulfide isomerase (PDI) family of ER proteins, which catalyse protein folding and thiol–disulfide interchange reactions. The domain organization of PDIR is atypical and different from other well-known members of the PDI family. PDIR stands out as the sole PDI consisting of one N-terminal non-catalytic domain and three catalytic domains. Upon analysing the crystal structure of the non-catalytic domain of human PDIR, it became apparent that this domain serves as the primary binding site for the major ER chaperone calreticulin [84].
- MYDGF was up-regulated within the fully protected group, with an average log2-fold change of 1.07. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.53, 0.51, and 0.34, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.54 [0.05, 1.05] (adjusted p-value = 1.2 × 10−2), 0.56 [0.11, 1.03] (adjusted p-value = 3.7 × 10−3), and 0.73 [0.27, 1.19] (adjusted p-value = 5.1 × 10−4), respectively. MYDGF, also referred to as C19orf10 or IL-25, encodes paracrine-acting protein. It is highly conserved throughout evolution and can be found in various cellular compartments, including the ER, Golgi apparatus, and extracellular space [85]. MYDGF is produced by mucosal epithelial cells, and when it is overly expressed, it promotes eosinophilia and triggers the production of TH2-type cytokines [86]. Furthermore, it has been observed to activate the conventional pathways of the NF-κB through the phosphorylation of NF-κBp65 in germinal centre B cells [87].
- PRDX4 was up-regulated within the fully protected group, with an average log2-fold change of 0.95. In contrast, the partially, weakly, and non-protected groups demonstrated lower average log2-fold changes of 0.33, 0.29, and 0.12, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.62 [0.13, 1.11] (adjusted p-value = 3.7 × 10−3), 0.66 [0.21, 1.11] (adjusted p-value = 8.9 × 10−4), and 0.83 [0.38, 1.29] (adjusted p-value = 6.2 × 10−6), respectively. PRDX4, also referred to as AOE372, encodes an antioxidant enzyme and belongs to the peroxiredoxin family. It is located in the ER and plays a role in safeguarding against oxidative stress by detoxifying cellular peroxides [88]. However, the function of this protein goes beyond eliminating peroxide; it also promotes oxidative protein folding through the oxidation of PDI [89]. Furthermore, PRDX4 defines a reduction–oxidation pathway that specifically regulates the activity of NF-κB by modulating the phosphorylation of IκB-alpha in the cytoplasm [90].
- XBP1 was up-regulated within the fully protected group, with an average log2-fold change of 0.75. In contrast, the partially, weakly, and non-protected groups demonstrated statically significant reductions, with average log2-fold changes of 0.22, 0.31, and 0.14, respectively. The significant differences in the average log2-fold changes, when comparing the fully protected group and the non-fully protected groups, were 0.53 [0.07, 0.99] (adjusted p-value = 7.7× 10−3), 0.44 [0.02, 0.87] (adjusted p-value = 1.6 × 10−2), and 0.61 [0.18, 1.04] (adjusted p-value = 7.9 × 10−4), respectively. It is a basic-region leucine zipper protein in the CREB/ATF (cyclic AMP response element binding protein/activating transcription factor) family of transcription factors. XBP1 functions as a transcription factor during ER stress by facilitating the UPR pathway triggered by accumulated misfolded proteins [91], and it has an additional function in protecting cells against oxidative stress [92]. Studies have revealed that the specific signals responsible for triggering plasma cell differentiation and the UPR pathway operate in synchronization through XBP1 to promote successful terminal B-cell differentiation [93,94]. The activation of XBP1 is important to maintain the optimal differentiation, functioning, and survival of LLPCs with high secretory activity. However, recent studies revealed that the absence of XBP-1 only leads to a reduction, rather than a total cessation of antibody secretion in plasma cells [95].
3.4. Genes with Significant Variations in log2-Fold Change between Participants Receiving the Vaccine for the First Time and Those Repeatedly Vaccinated
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothberg, M.B.; Haessler, S.D.; Brown, R.B. Complications of viral influenza. Am. J. Med. 2008, 121, 258–264. [Google Scholar] [CrossRef]
- Buchy, P.; Selim, B. Who and when to vaccinate against influenza. Int. J. Infect. Dis. 2020, 93, 375–387. [Google Scholar] [CrossRef]
- Manzoli, L.; Schioppa, F.; Boccia, A.; Villari, P. The efficacy of influenza vaccine for healthy children. A meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr. Infect. Dis. J. 2007, 26, 97–106. [Google Scholar] [CrossRef]
- Jefferson, T.; Rivetti, A.; Harnden, A.; Di Pietrantonj, C.; Demicheli, V. Vaccines for preventing influenza in health children. Cochrane Database Syst. Rev. 2008, 2, CD004879. [Google Scholar]
- Negri, E.; Colombo, C.; Giordano, L.; Groth, L.; Apolone, G.; Vecchia, C.L. Influenza vaccine in health children: A meta-analysis. Vaccine 2005, 23, 2851–2861. [Google Scholar] [CrossRef]
- Villari, P.; Manzoli, L.; Boccia, A. Methodological quality of studies and patient age as major sources of variation in efficacy estimates of influenza vaccination in healthy adults: A meta-analysis. Vaccine 2004, 22, 3475–3486. [Google Scholar] [CrossRef]
- Beyer, W.E.P. Heterogeneity of case definitions used in vaccine effectiveness studies—And its impact on meta-analysis. Vaccine 2006, 24, 6602–6604. [Google Scholar] [CrossRef]
- Jefferson, T.O.; Rivetti, D.; Di Pietrantonj, C.; Rivetti, A.; Demicheli, V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2007, 2, CD001269. [Google Scholar]
- Govaert, T.M.; Thijs, C.T.; Masurel, N.; Sprenger, M.J.; Dinant, G.J.; Knottnerus, J.A. The efficacy of influenza vaccination in elderly individuals. A randomized doubleblind placebo-controlled trial. JAMA 1994, 272, 1661–1665. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in development and application of influenza vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef] [PubMed]
- Soema, P.C.; Kompier, R.; Amorij, J.P.; Kersten, G.F. Current and next generation influenza vaccines: Formulation and production strategies. Eur. J. Pharm. Biopharm. 2015, 94, 251–263. [Google Scholar] [CrossRef]
- Krammer, F.; Fouchier, R.A.; Eichelberger, M.C.; Webby, R.J.; Shaw-Saliba, K.; Wan, H.; Wilson, P.C.; Compans, R.W.; Skountzou, I.; Monto, A.S. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 2018, 9, e02332-17. [Google Scholar] [CrossRef]
- Sangster, M.Y.; Nguyen, P.Q.; Topham, D.J. Role of memory B cells in hemagglutinin-specific antibody production following human influenza A virus infection. Pathogens 2019, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Hannoun, C.; Megas, F.; Piercy, J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004, 103, 133–138. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.I.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.E.; Heiberger, R.M. Analysis of variance; designed experiments. In Statistical Models in S; Routledge: London, UK, 2017; pp. 145–193. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, S.M.; Roth, A.; Santos, A.; Von Mering, C.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D. B cell responses: Cell interaction dynamics and decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef]
- Cyster, J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 2010, 11, 989–996. [Google Scholar] [CrossRef]
- Leu, C.M.; Davis, R.S.; Gartland, L.A.; Fine, W.D.; Cooper, M.D. FcRH1: An activation coreceptor on human B cells. Blood 2005, 105, 1121–1126. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Kolhatkar, N.S.; Thouvenel, C.; Khim, S.; Rawlings, D.J. CD4+ T cells and CD40 participate in selection and homeostasis of peripheral B cells. J. Immunol. 2014, 193, 3492–3502. [Google Scholar] [CrossRef]
- MacLennan, I.C.; Toellner, K.M.; Cunningham, A.F.; Serre, K.; Sze, D.M.Y.; Zúñiga, E.; Cook, M.C.; Vinuesa, C.G. Extrafollicular antibody responses. Immunol. Rev. 2003, 194, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T follicular helper cell biology: A decade of discovery and diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Kerfoot, S.M.; Yaari, G.; Patel, J.R.; Johnson, K.L.; Gonzalez, D.G.; Kleinstein, S.H.; Haberman, A.M. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 2011, 34, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal center B cell dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N. Affinity enhancement of antibodies: How low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shlomchik, M.J. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, A.D.; Shulman, Z.; Nussenzweig, M.C. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 2014, 509, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Tas, J.M.J.; Mesin, L.; Pasqual, G.; Targ, S.; Jacobsen, J.T.; Mano, Y.M.; Chen, C.S.; Weill, J.-C.; Reynaud, C.-A.; Browne, E.P.; et al. Visualizing antibody affinity maturation in germinal centers. Science 2016, 351, 1048–1054. [Google Scholar] [CrossRef]
- Heesters, B.A.; Myers, R.C.; Carroll, M.C. Follicular dendritic cells: Dynamic antigen libraries. Nat. Rev. Immunol. 2014, 14, 495–504. [Google Scholar] [CrossRef]
- Lindhout, E.; Mevissen, M.L.C.M.; Kwekkeboom, J.; Tager, J.M.; De Groot, C. Direct evidence that human follicular dendritic cells (FDC) rescue germinal centre B cells from death by apoptosis. Clin. Exp. Immunol. 1993, 91, 330–336. [Google Scholar] [CrossRef]
- Chevrier, S.; Kratina, T.; Emslie, D.; Tarlinton, D.M.; Corcoran, L.M. IL4 and IL21 cooperate to induce the high Bcl6 protein level required for germinal center formation. Immunol. Cell Biol. 2017, 95, 925–932. [Google Scholar] [CrossRef]
- Shlomchik, M.J.; Luo, W.; Weisel, F. Linking signaling and selection in the germinal center. Immunol. Rev. 2019, 288, 49–63. [Google Scholar] [CrossRef]
- Shlomchik, M.J.; Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 2012, 247, 52–63. [Google Scholar] [CrossRef]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Slifka, M.K.; Matloubian, M.; Ahmed, R. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 1995, 69, 1895–1902. [Google Scholar] [CrossRef]
- Slifka, M.K.; Antia, R.; Whitmire, J.K.; Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 1998, 8, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Bonaud, A.; Lemos, J.P.; Espéli, M.; Balabanian, K. Hematopoietic multipotent progenitors and plasma cells: Neighbors or roommates in the mouse bone marrow ecosystem? Front. Immunol. 2021, 12, 658535. [Google Scholar] [CrossRef] [PubMed]
- Taillardet, M.; Haffar, G.; Mondière, P.; Asensio, M.-J.; Gheit, H.; Burdin, N.; Defrance, T.; Genestier, L. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood J. Am. Soc. Hematol. 2009, 114, 4432–4440. [Google Scholar] [CrossRef]
- Kometani, K.; Nakagawa, R.; Shinnakasu, R.; Kaji, T.; Rybouchkin, A.; Moriyama, S.; Furukawa, K.; Koseki, H.; Takemori, T.; Kurosaki, T. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity 2013, 39, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef]
- Liu, Q.; Kriksunov, I.A.; Jiang, H.; Graeff, R.; Lin, H.; Lee, H.C.; Hao, Q. Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38. Chem. Biol. 2008, 15, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Camponeschi, A.; Kläsener, K.; Sundell, T.; Lundqvist, C.; Manna, P.T.; Ayoubzadeh, N.; Sundqvist, M.; Thorarinsdottir, K.; Gatto, M.; Visentini, M.; et al. Human CD38 regulates B cell antigen receptor dynamic organization in normal and malignant B cells. J. Exp. Med. 2022, 219, e20220201. [Google Scholar] [CrossRef] [PubMed]
- Halliley, J.L.; Tipton, C.M.; Liesveld, J.; Rosenberg, A.F.; Darce, J.; Gregoretti, I.V.; Popova, L.; Kaminiski, D.; Fucile, C.F.; Albizua, I.; et al. Long-lived plasma cells are contained within the CD19− CD38hiCD138+ subset in human bone marrow. Immunity 2015, 43, 132–145. [Google Scholar] [CrossRef]
- Garimilla, S.; Nguyen, D.C.; Halliley, J.L.; Tipton, C.; Rosenberg, A.F.; Fucile, C.F.; Saney, C.L.; Kyu, S.; Kaminski, D.; Lee, F.E.H.; et al. Differential transcriptome and development of human peripheral plasma cell subsets. JCI Insight 2019, 4, e126732. [Google Scholar] [CrossRef]
- McCarron, M.J.; Park, P.W.; Fooksman, D.R. CD138 mediates selection of mature plasma cells by regulating their survival. Blood J. Am. Soc. Hematol. 2017, 129, 2749–2759. [Google Scholar] [CrossRef]
- Sowa, S.T.; Moilanen, A.; Biterova, E.; Saaranen, M.J.; Lehtiö, L.; Ruddock, L.W. High-resolution Crystal Structure of Human pERp1, A Saposin-like Protein Involved in IgA, IgM and Integrin Maturation in the Endoplasmic Reticulum. J. Mol. Biol. 2021, 433, 166826. [Google Scholar] [CrossRef]
- Shimizu, Y.; Meunier, L.; Hendershot, L.M. pERp1 is significantly up-regulated during plasma cell differentiation and contributes to the oxidative folding of immunoglobulin. Proc. Natl. Acad. Sci. USA 2009, 106, 17013–17018. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Andreani, V.; Kapoor, T.; Herp, S.; Flach, H.; Duchniewicz, M.; Grosschedl, R. MZB1 is a GRP94 cochaperone that enables proper immunoglobulin heavy chain biosynthesis upon ER stress. Genes Dev. 2014, 28, 1165–1178. [Google Scholar] [CrossRef]
- Andreani, V.; Ramamoorthy, S.; Pandey, A.; Lupar, E.; Nutt, S.L.; Lämmermann, T.; Grosschedl, R. Cochaperone Mzb1 is a key effector of Blimp1 in plasma cell differentiation and β1-integrin function. Proc. Natl. Acad. Sci. USA 2018, 115, E9630–E9639. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, A.; Roussel, J.; Bourgeade, M.-F.; Rogier, E.; Madry, C.; Inoue, J.; Devergne, O.; Tsapis, A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-κB, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000, 165, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.S.; Schneider, P.; Kalled, S.L.; Wang, L.; Lefevre, E.A.; Cachero, T.G.; MacKay, F.; Bixler, S.A.; Zafari, M.; Liu, Z.-Y.; et al. BAFF binds to the tumor necrosis factor receptor–like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J. Exp. Med. 2000, 192, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Salzer, U.; Jennings, S.; Grimbacher, B. To switch or not to switch–the opposing roles of TACI in terminal B cell differentiation. Eur. J. Immunol. 2007, 37, 17–20. [Google Scholar] [CrossRef]
- Coquery, C.M.; Erickson, L.D. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit. Rev. Immunol. 2012, 32, 287–305. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.-L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Hymowitz, S.G.; Patel, D.R.; Wallweber, H.J.; Runyon, S.; Yan, M.; Yin, J.; Shriver, S.K.; Gordon, N.C.; Pan, B.; Starovasnik, M.A.; et al. Structures of APRIL-receptor complexes: Like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J. Biol. Chem. 2005, 280, 7218–7227. [Google Scholar] [CrossRef]
- Bossen, C.; Schneider, P. BAFF, APRIL and their receptors: Structure, function and signaling. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2006; Volume 18, pp. 263–275. [Google Scholar]
- Yu, G.; Boone, T.; Delaney, J.; Hawkins, N.; Kelley, M.; Ramakrishnan, M.; McCabe, S.; Qiu, W.-R.; Kornuc, M.; Xia, X.-Z.; et al. APRIL and TALL-1 and receptors BCMA and TACI: System for regulating humoral immunity. Nat. Immunol. 2000, 1, 252–256. [Google Scholar] [CrossRef]
- Wu, Y.; Bressette, D.; Carrell, J.A.; Kaufman, T.; Feng, P.; Taylor, K.; Gan, Y.; Cho, Y.H.; Garcia, A.D.; Gollatz, E.; et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J. Biol. Chem. 2000, 275, 35478–35485. [Google Scholar] [CrossRef]
- He, B.; Santamaria, R.; Xu, W.; Cols, M.; Chen, K.; Puga, I.; Shan, M.; Xiong, H.; Bussel, J.B.; Cerutti, A.; et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010, 11, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Strubin, M.; Newell, J.W.; Matthias, P. OBF-1, a novel B cell-specific coactivator that stimulates immunoglobulin promoter activity through association with octamer-binding proteins. Cell 1995, 80, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Roeder, R.G. Cloning, functional characterization, and mechanism of action of the B-cell-specific transcriptional coactivator OCA-B. Mol. Cell. Biol. 1995, 15, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Siegel, R.; Kim, U.; Roeder, R.G. Direct interactions of OCA-B and TFII-I regulate immunoglobulin heavy-chain gene transcription by facilitating enhancer-promoter communication. Mol. Cell 2011, 42, 342–355. [Google Scholar] [CrossRef]
- Karnowski, A.; Chevrier, S.; Belz, G.T.; Mount, A.; Emslie, D.; D’costa, K.; Tarlinton, D.M.; Kallies, A.; Corcoran, L.M. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J. Exp. Med. 2012, 209, 2049–2064. [Google Scholar] [CrossRef]
- Schubart, D.B.; Rolink, A.; Kosco-Vilbois, M.H.; Botteri, F.; Matthias, P. B-cell-specif ic coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature 1996, 383, 538–542. [Google Scholar] [CrossRef]
- Corcoran, L.M.; Hasbold, J.; Dietrich, W.; Hawkins, E.; Kallies, A.; Nutt, S.L.; Tarlinton, D.M.; Matthias, P.; Hodgkin, P.D. Differential requirement for OBF-1 during antibody-secreting cell differentiation. J. Exp. Med. 2005, 201, 1385–1396. [Google Scholar] [CrossRef]
- Nutt, S.L.; Heavey, B.; Rolink, A.G.; Busslinger, M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 1999, 401, 556–562. [Google Scholar] [CrossRef]
- Revilla-I-Domingo, R.; Bilic, I.; Vilagos, B.; Tagoh, H.; Ebert, A.; Tamir, I.M.; Smeenk, L.; Trupke, J.; Sommer, A.; Jaritz, M.; et al. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 2012, 31, 3130–3146. [Google Scholar] [CrossRef]
- Schebesta, A.; McManus, S.; Salvagiotto, G.; Delogu, A.; Busslinger, G.A.; Busslinger, M. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 2007, 27, 49–63. [Google Scholar] [CrossRef]
- Delogu, A.; Schebesta, A.; Sun, Q.; Aschenbrenner, K.; Perlot, T.; Busslinger, M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity 2006, 24, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.; Ebert, A.; Jaritz, M.; Wutz, G.; Nagasaka, K.; Tagoh, H.; Kostanova-Poliakova, D.; Schindler, K.; Sun, Q.; Bönelt, P.; et al. Wapl repression by Pax5 promotes V gene recombination by Igh loop extrusion. Nature 2020, 584, 142–147. [Google Scholar] [CrossRef]
- Max, E.E.; Wakatsuki, Y.; Neurath, M.F.; Strober, W. The role of BSAP in immunoglobulin isotype switching and B-cell proliferation. In Mechanisms in B-Cell Neoplasia 1994; Springer: Berlin/Heidelberg, Germany, 1995; pp. 449–458. [Google Scholar]
- Falini, B.; Fizzotti, M.; Pucciarini, A.; Bigerna, B.; Marafioti, T.; Gambacorta, M.; Pacini, R.; Alunni, C.; Natali–Tanci, L.; Stein, H.; et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood J. Am. Soc. Hematol. 2000, 95, 2084–2092. [Google Scholar] [CrossRef]
- Ochiai, K.; Maienschein-Cline, M.; Simonetti, G.; Chen, J.; Rosenthal, R.; Brink, R.; Chong, A.S.; Klein, U.; Dinner, A.R.; Sciammas, R.; et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 2013, 38, 918–929. [Google Scholar] [CrossRef]
- Klein, U.; Casola, S.; Cattoretti, G.; Shen, Q.; Lia, M.; Mo, T.; Ludwig, T.; Rajewsky, K.; Dalla-Favera, R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 2006, 7, 773–782. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Chang, X. The role and mechanism of TXNDC5 in diseases. Eur. J. Med. Res. 2022, 27, 145. [Google Scholar] [CrossRef]
- Pace, P.E.; Peskin, A.V.; Han, M.H.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidized peroxiredoxin 2 interacts with the protein disulfide-isomerase ERp46. Biochem. J. 2013, 453, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Murase, R.; Yamamoto, A.; Hirata, Y.; Oh-Hashi, K. Expression analysis and functional characterization of thioredoxin domain-containing protein 11. Mol. Biol. Rep. 2022, 49, 10541–10556. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood J. Am. Soc. Hematol. 2008, 112, 1223–1230. [Google Scholar] [CrossRef]
- Preisendörfer, S.; Ishikawa, Y.; Hennen, E.; Winklmeier, S.; Schupp, J.C.; Knüppel, L.; Fernandez, I.E.; Binzenhöfer, L.; Flatley, A.; Juan-Guardela, B.M.; et al. FK506-binding protein 11 is a novel plasma cell-specific antibody folding catalyst with increased expression in idiopathic pulmonary fibrosis. Cells 2022, 11, 1341. [Google Scholar] [CrossRef]
- Vinaik, R.; Kozlov, G.; Gehring, K. Structure of the non-catalytic domain of the protein disulfide isomerase-related protein (PDIR) reveals function in protein binding. PLoS ONE 2013, 8, e62021. [Google Scholar] [CrossRef]
- Bortnov, V.; Annis, D.S.; Fogerty, F.J.; Barretto, K.T.; Turton, K.B.; Mosher, D.F. Myeloid-derived growth factor is a resident endoplasmic reticulum protein. J. Biol. Chem. 2018, 293, 13166–13175. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Ponzoni, M.; Doglioni, C.; Pistoia, V. IL-17 superfamily cytokines modulate normal germinal center B cell migration. J. Leucoc. Biol. 2016, 100, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Tavender, T.J.; Sheppard, A.M.; Bulleid, N.J. Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme forming oligomeric complexes in human cells. Biochem. J. 2008, 411, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, K.; Wang, X.E.; Wang, X.; Wang, C.C. A novel reaction of peroxiredoxin 4 towards substrates in oxidative protein folding. PLoS ONE 2014, 9, e105529. [Google Scholar] [CrossRef]
- Jin, D.Y.; Chae, H.Z.; Rhee, S.G.; Jeang, K.T. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J. Biol. Chem. 1997, 272, 30952–30961. [Google Scholar] [CrossRef]
- Acosta-Alvear, D.; Zhou, Y.; Blais, A.; Tsikitis, M.; Lents, N.H.; Arias, C.; Lennon, C.J.; Kluger, Y.; Dynlacht, B.D. XBP1 controls diverse cell type-and condition-specific transcriptional regulatory networks. Mol. Cell 2007, 27, 53–66. [Google Scholar] [CrossRef]
- Liu, Y.; Adachi, M.; Zhao, S.; Hareyama, M.; Koong, A.C.; Luo, D.; A Rando, T.; Imai, K.; Shinomura, Y. Preventing oxidative stress: A new role for XBP1. Cell Death Differ. 2009, 16, 847–857. [Google Scholar] [CrossRef]
- Reimold, A.M.; Iwakoshi, N.N.; Manis, J.; Vallabhajosyula, P.; Szomolanyi-Tsuda, E.; Gravallese, E.M.; Friend, D.; Grusby, M.J.; Alt, F.; Glimcher, L.H. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001, 412, 300–307. [Google Scholar] [CrossRef]
- Iwakoshi, N.N.; Lee, A.H.; Glimcher, L.H. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol. Rev. 2003, 194, 29–38. [Google Scholar] [CrossRef]
- Taubenheim, N.; Tarlinton, D.M.; Crawford, S.; Corcoran, L.M.; Hodgkin, P.D.; Nutt, S.L. High rate of antibody secretion is not integral to plasma cell differentiation as revealed by XBP-1 deficiency. J. Immunol. 2012, 189, 3328–3338. [Google Scholar] [CrossRef]
- Kaufman, R.J.; Scheuner, D.; Schröder, M.; Shen, X.; Lee, K.; Liu, C.Y.; Arnold, S.M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002, 3, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Welsh, J.P.; Swartz, J.R. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc. Natl. Acad. Sci. USA 2014, 111, 125–130. [Google Scholar] [CrossRef]
- Eichelberger, M.C.; Monto, A.S. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. J. Infect. Dis. 2019, 219 (Suppl. S1), S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Mezhenskaya, D.; Isakova-Sivak, I.; Rudenko, L. M2e-based universal influenza vaccines: A historical overview and new approaches to development. J. Biomed. Sci. 2019, 26, 1–15. [Google Scholar] [CrossRef]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Pengo, N.; Scolari, M.; Oliva, L.; Milan, E.; Mainoldi, F.; Raimondi, A.; Fagioli, C.; Merlini, A.; Mariani, E.; Pasqualetto, E.; et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 2013, 14, 298–305. [Google Scholar] [CrossRef]
- Cassese, G.; Arce, S.; Hauser, A.E.; Lehnert, K.; Moewes, B.; Mostarac, M.; Muehlinghaus, G.; Szyska, M.; Radbruch, A.; Manz, R.A. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 2003, 171, 1684–1690. [Google Scholar] [CrossRef]
- Kawano, M.M.; Mihara, K.; Huang, N.; Tsujimoto, T.; Kuramoto, A. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood 1995, 85, 487–494. [Google Scholar] [CrossRef]
- Belnoue, E.; Pihlgren, M.; McGaha, T.L.; Tougne, C.; Rochat, A.-F.; Bossen, C.; Schneider, P.; Huard, B.; Lambert, P.-H.; Siegrist, C.-A. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood J. Am. Soc. Hematol. 2008, 111, 2755–2764. [Google Scholar] [CrossRef]
- Hargreaves, D.C.; Hyman, P.L.; Lu, T.T.; Ngo, V.N.; Bidgol, A.; Suzuki, G.; Zou, Y.-R.; Littman, D.R.; Cyster, J.G. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 2001, 194, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wols, H.A.M.; Underhill, G.H.; Kansas, G.S.; Witte, P.L. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol. 2002, 169, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, C.H.; Utley, A.; Carlson, L.M.; Farren, M.R.; Murray, M.; Russell, L.M.; Nair, J.R.; Yang, Z.; Brady, W.; Garrett-Sinha, L.A.; et al. CD28 promotes plasma cell survival, sustained antibody responses, and BLIMP-1 upregulation through its distal PYAP proline motif. J. Immunol. 2015, 194, 4717–4728. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, S.; Genton, C.; Kallies, A.; Karnowski, A.; Otten, L.A.; Malissen, B.; Malissen, M.; Botto, M.; Corcoran, L.M.; Nutt, S.L.; et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc. Natl. Acad. Sci. USA 2009, 106, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Liu, G.J.; Jaritz, M.; Wöhner, M.; Agerer, B.; Bergthaler, A.; Malin, S.G.; Busslinger, M. Repression of the B cell identity factor Pax5 is not required for plasma cell development. J. Exp. Med. 2020, 217, e20200147. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.-M.; et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011, 12, 786–795. [Google Scholar] [CrossRef]
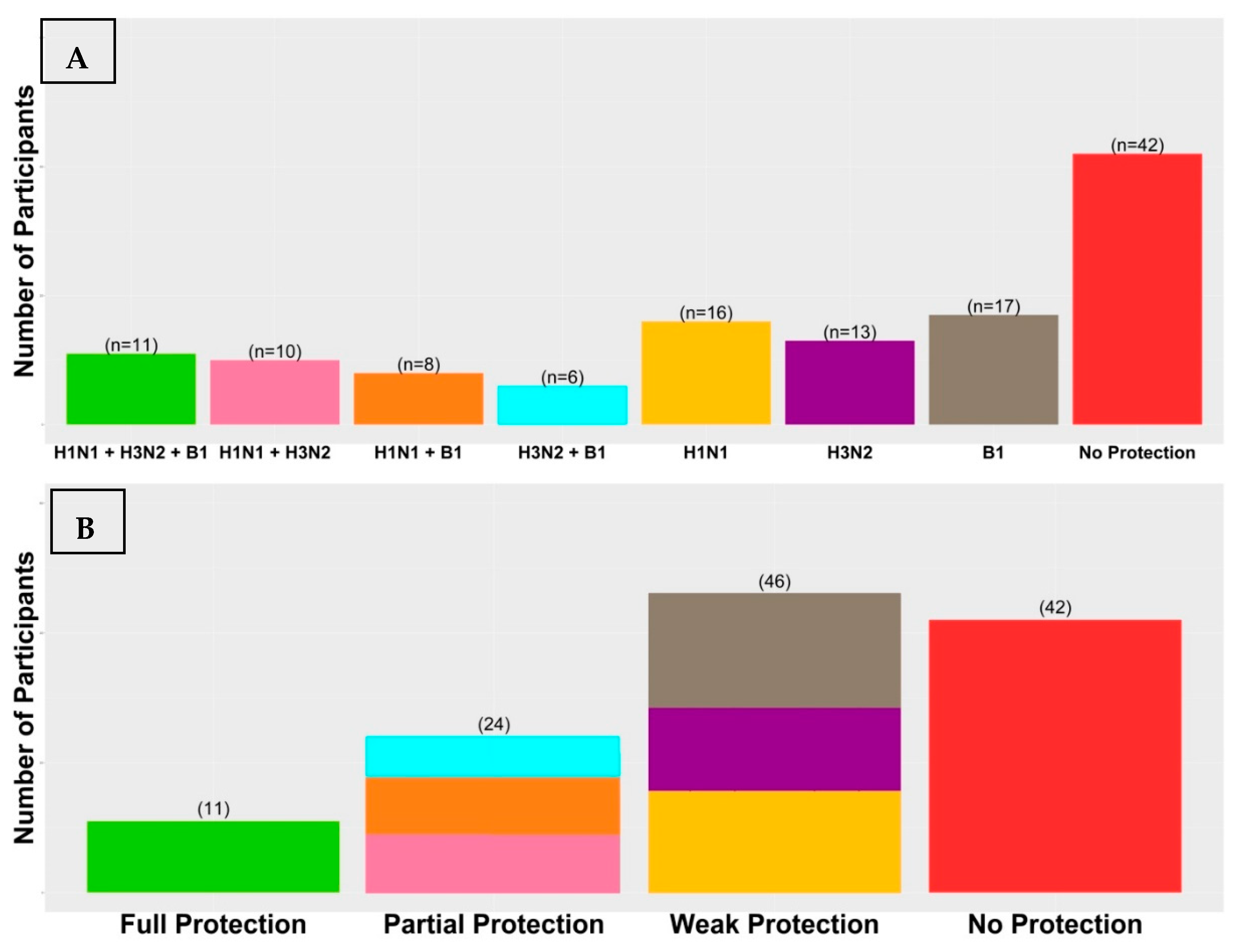
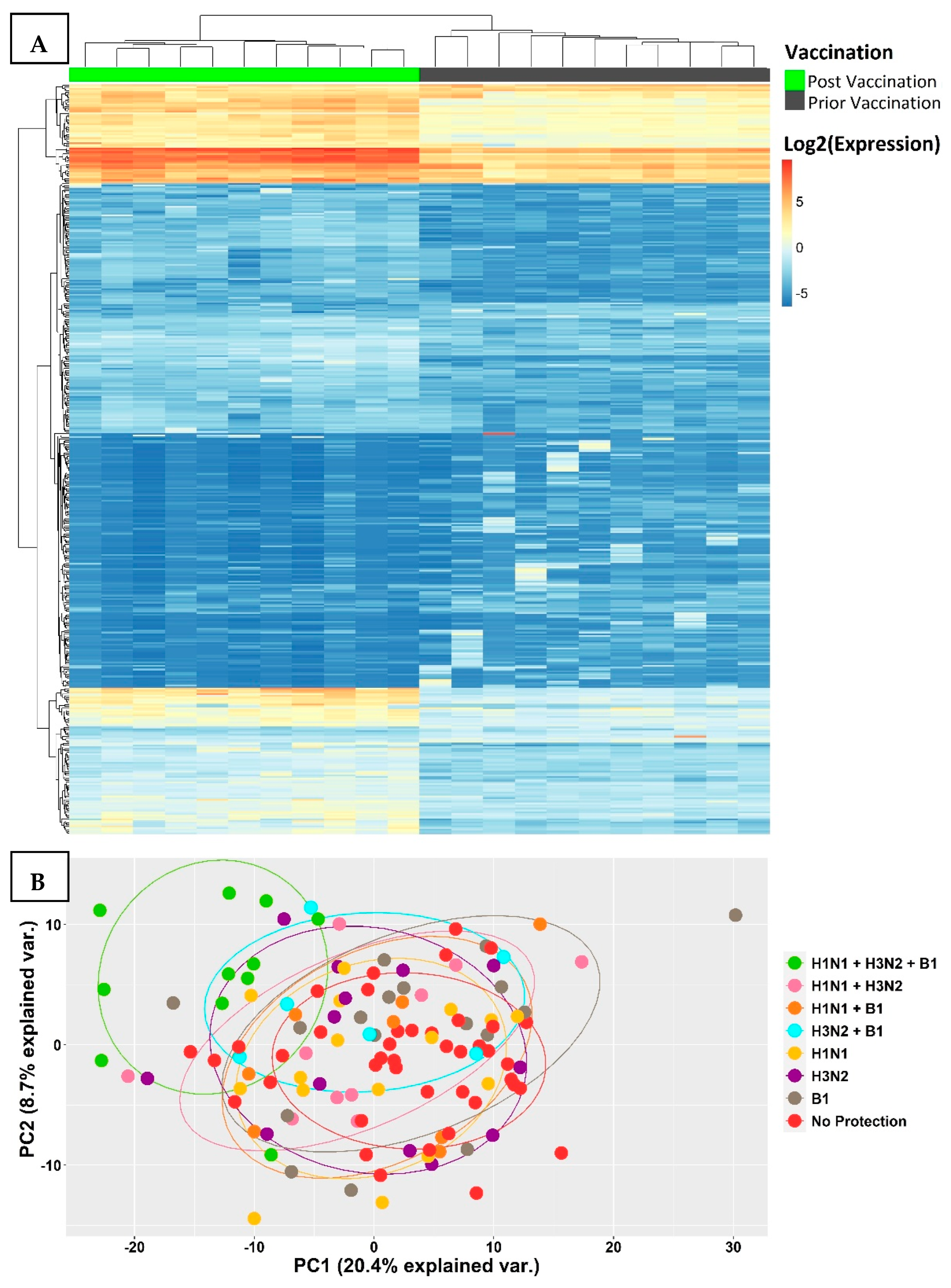
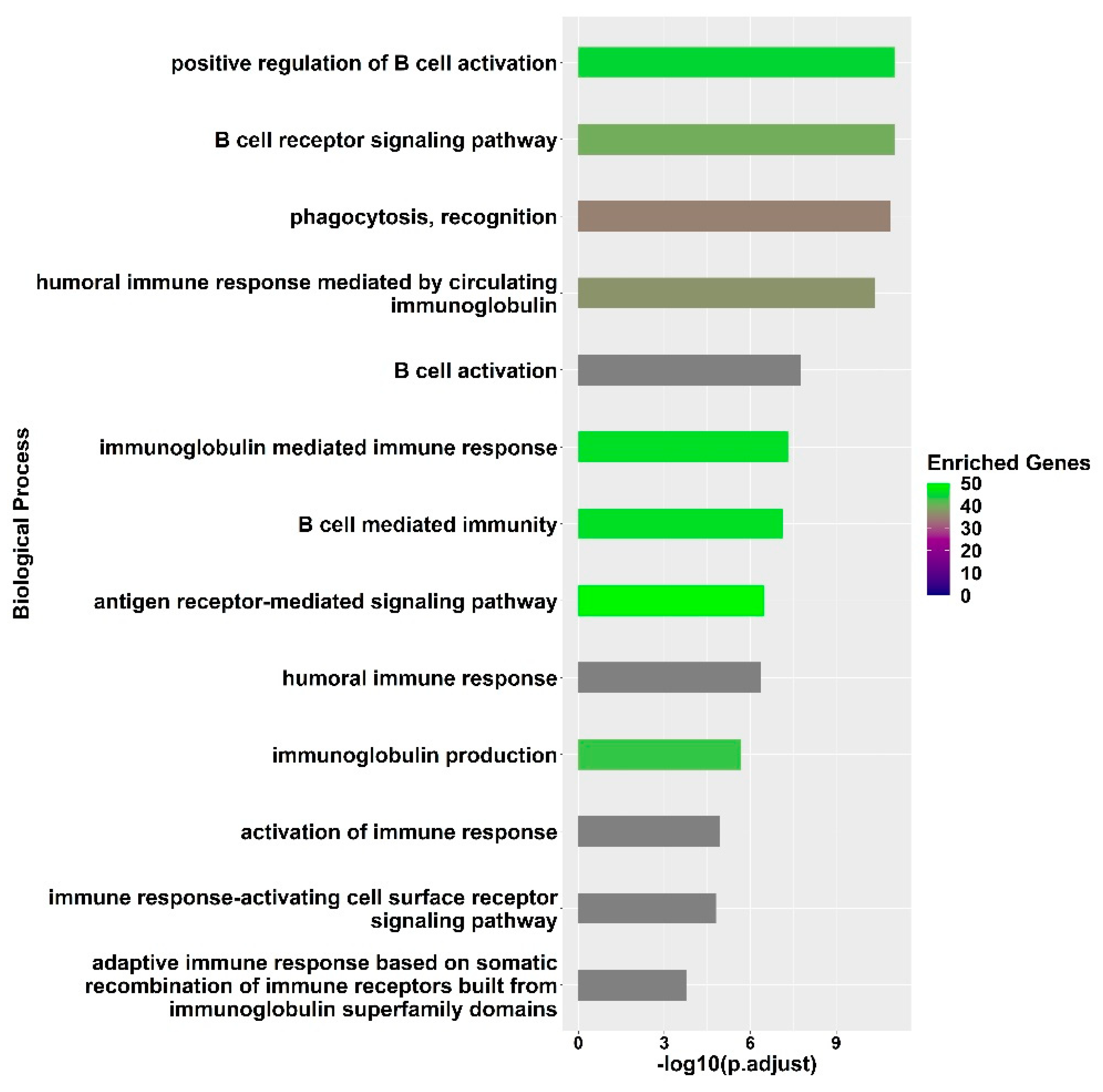
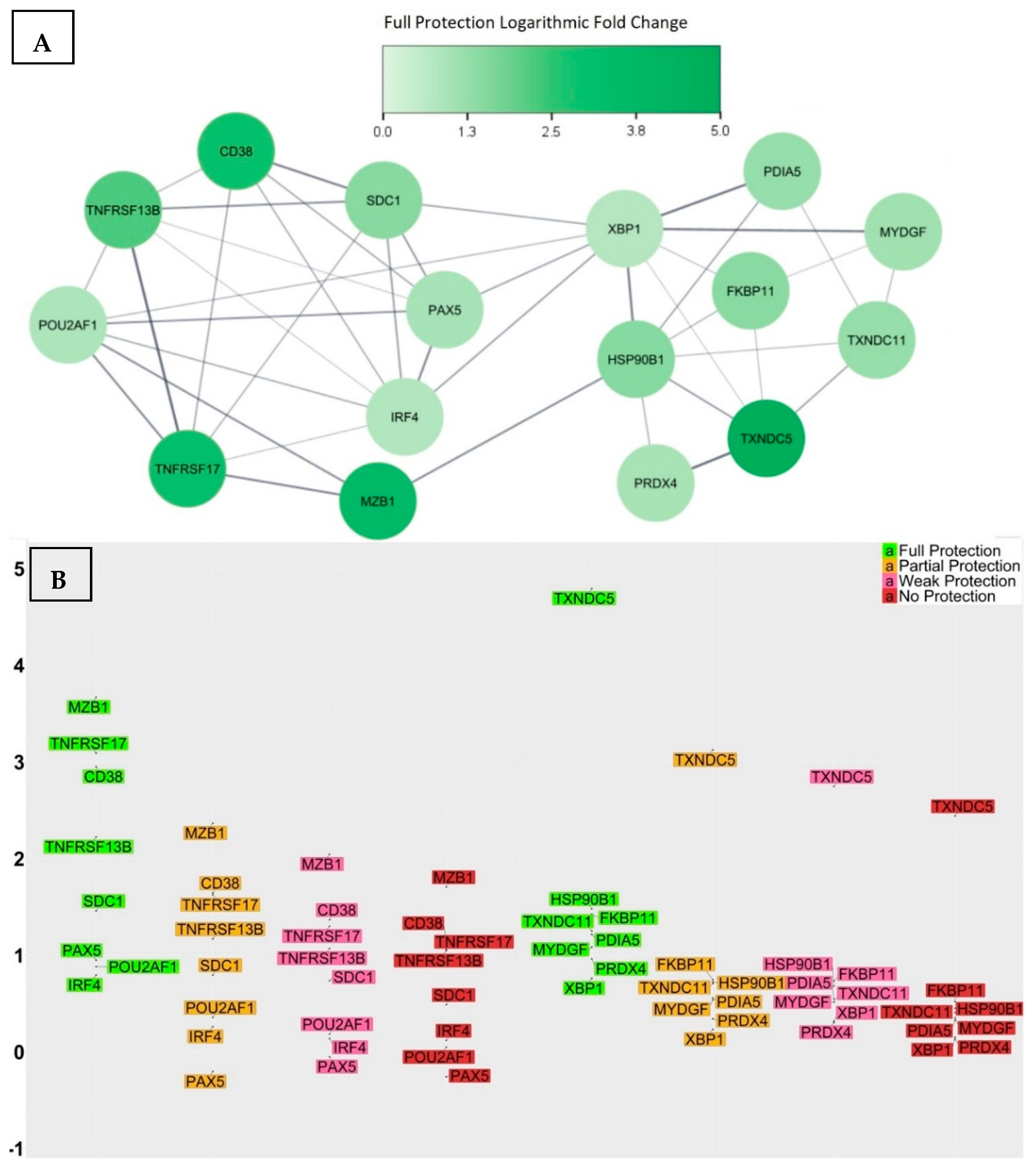
| Seroprotected Participants | H1N1 | H3N2 | B1 |
|---|---|---|---|
| Day 0 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Day 7 | 54 (43.9%) | 47 (38.21%) | 49 (39.83%) |
| Day 90 | 68 (55.28%) | 76 (61.78%) | 91 (73.98%) |
| Neither Day 7 nor Day 90 (non-responders) | 46 (37.4%) | 40 (32.52%) | 25 (20.33%) |
| Only Day 7 (short-term responders) | 9 (7.32%) | 7 (5.69%) | 7 (5.69%) |
| Only Day 90 (late responders) | 23 (18.7%) | 36 (29.27%) | 49 (39.83%) |
| Day 7 and Day 90 (ideal responders) | 45 (36.58%) | 40 (32.52%) | 42 (34.15%) |
| Seroprotected Participants | First Vaccine (91 Participants) | Repeated Vaccine (32 Participants) | ||||
|---|---|---|---|---|---|---|
| H1N1 | H3N2 | B1 | H1N1 | H3N2 | B1 | |
| Day 0 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Day 7 | 42 (46.15%) | 34 (37.36%) | 38 (41.76%) | 12 (37.5%) | 13 (40.62%) | 11 (34.38%) |
| Day 90 | 59 (64.84%) | 61 (67.03%) | 71 (78.02%) | 9 (28.13%) | 15 (46.88%) | 20 (62.5%) |
| Neither Day 7 nor Day 90 (non-responders) | 28 (30.77%) | 26 (28.57%) | 16 (17.58%) | 18 (56.25%) | 14 (43.75%) | 9 (28.16%) |
| Only Day 7 (short-term responders) | 4 (4.4%) | 4 (4.4%) | 4 (4.4%) | 5 (15.62%) | 3 (9.37%) | 3 (9.37%) |
| Only Day 90 (late responders) | 21 (23.08%) | 31 (34.07%) | 37 (40.66%) | 2 (6.25%) | 5 (15.62%) | 12 (37.5%) |
| Day 7 and Day 90 (ideal responders) | 38 (41.76%) | 30 (32.97%) | 34 (37.36%) | 7 (21.88%) | 10 (31.25%) | 8 (25.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawfik, A.; Kawaguchi, T.; Takahashi, M.; Setoh, K.; Yamaguchi, I.; Tabara, Y.; Van Steen, K.; Sakuntabhai, A.; Matsuda, F. Transcriptomic Analysis Reveals Sixteen Potential Genes Associated with the Successful Differentiation of Antibody-Secreting Cells through the Utilization of Unfolded Protein Response Mechanisms in Robust Responders to the Influenza Vaccine. Vaccines 2024, 12, 136. https://doi.org/10.3390/vaccines12020136
Tawfik A, Kawaguchi T, Takahashi M, Setoh K, Yamaguchi I, Tabara Y, Van Steen K, Sakuntabhai A, Matsuda F. Transcriptomic Analysis Reveals Sixteen Potential Genes Associated with the Successful Differentiation of Antibody-Secreting Cells through the Utilization of Unfolded Protein Response Mechanisms in Robust Responders to the Influenza Vaccine. Vaccines. 2024; 12(2):136. https://doi.org/10.3390/vaccines12020136
Chicago/Turabian StyleTawfik, Ahmed, Takahisa Kawaguchi, Meiko Takahashi, Kazuya Setoh, Izumi Yamaguchi, Yasuharu Tabara, Kristel Van Steen, Anavaj Sakuntabhai, and Fumihiko Matsuda. 2024. "Transcriptomic Analysis Reveals Sixteen Potential Genes Associated with the Successful Differentiation of Antibody-Secreting Cells through the Utilization of Unfolded Protein Response Mechanisms in Robust Responders to the Influenza Vaccine" Vaccines 12, no. 2: 136. https://doi.org/10.3390/vaccines12020136
APA StyleTawfik, A., Kawaguchi, T., Takahashi, M., Setoh, K., Yamaguchi, I., Tabara, Y., Van Steen, K., Sakuntabhai, A., & Matsuda, F. (2024). Transcriptomic Analysis Reveals Sixteen Potential Genes Associated with the Successful Differentiation of Antibody-Secreting Cells through the Utilization of Unfolded Protein Response Mechanisms in Robust Responders to the Influenza Vaccine. Vaccines, 12(2), 136. https://doi.org/10.3390/vaccines12020136






