Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030
Abstract
:1. Introduction
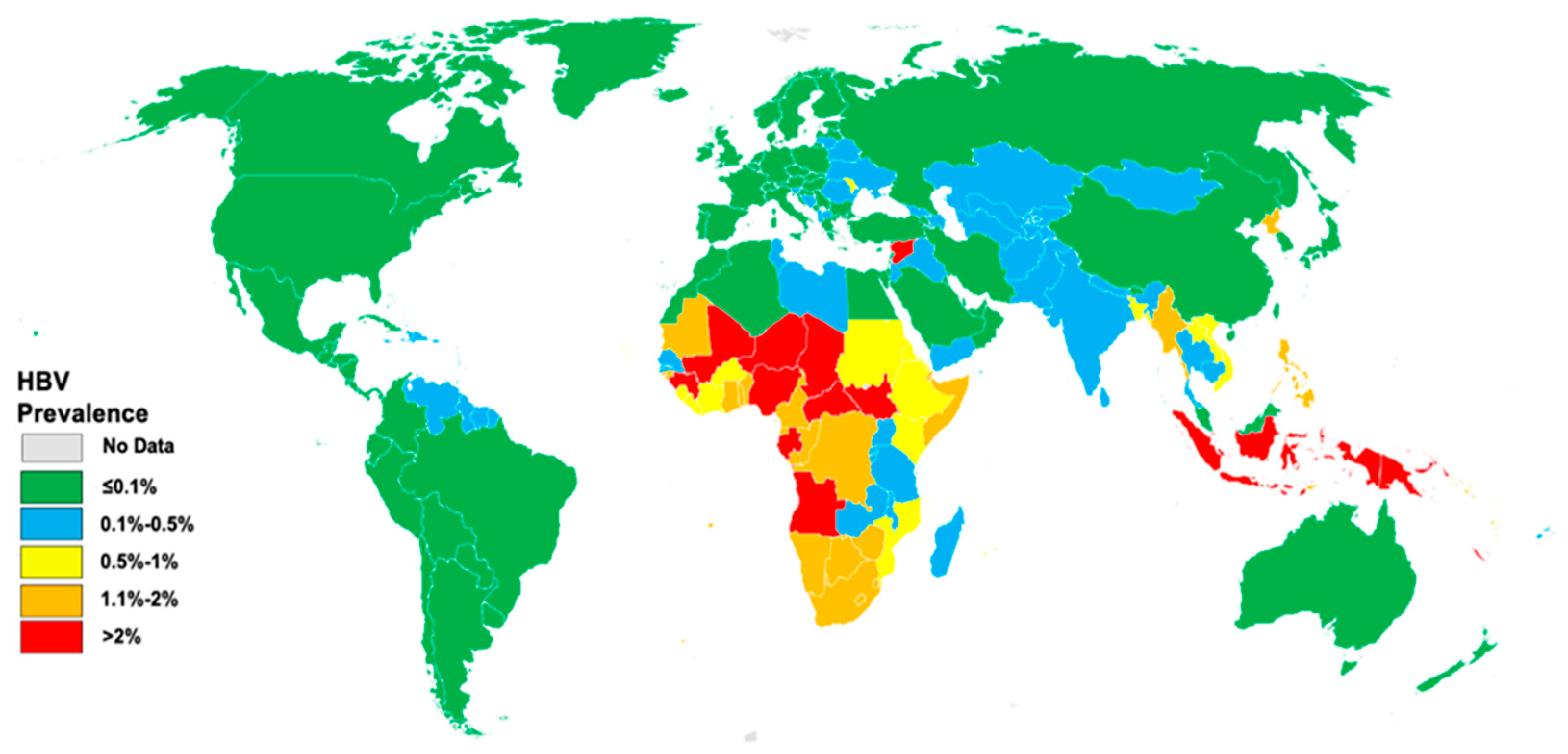
1.1. Overview of Hepatitis B and Its Global Impact
1.2. Significance of Achieving HBV Elimination by 2030
- Infant vaccination: Administering the three-dose HBV vaccine to infants.
- Preventing mother-to-child transmission (MTCT): Using either the HBV birth dose vaccine or alternative approaches.
- Ensuring blood and injection safety: Implementing protocols to minimize transmission through unsafe medical practices.
- Harm reduction: Implementing strategies to reduce transmission among high-risk groups like intravenous drug users.
2. Methodology
3. Hepatitis B Vaccines: Evolution and Effectiveness
3.1. From Plasma to Recombinant: A Paradigm Shift
3.2. The Efficacy and Safety of HBV Vaccination
3.3. Concerns with HBV Vaccination
3.3.1. Durability of the Immune Response
3.3.2. Vaccine Escape Mutants
3.3.3. Impact of HBV Genotypic Variability
3.3.4. Vaccine Non-Responders
3.4. Vaccine for Healthcare Workers
3.5. Cost-Effectiveness of HBV Vaccination
3.6. Recent Advances in HBV Vaccination
3.6.1. Improving Vaccine Immunogenicity
3.6.2. Improving Vaccine Coverage
3.6.3. Use of Alternative Routes of Vaccination
3.6.4. Therapeutic HBV Vaccines
3.6.5. AI Applications
3.7. The Effectiveness of the HBV Vaccine in Different Populations
3.7.1. Vaccination for High-Risk Populations
Pre-Exposure High-Risk Groups
Patients with End-Stage Kidney Diseases
Post-Renal Transplant Patients
Men Having Sex with Men (MSM)
HIV Patients
Patients with Chronic Liver Disease
Post-Liver Transplant Patients
Mother-to-Child Transmission (MTCT) Settings
3.7.2. HBV Mass Vaccination
The Effect on Chronic HBV Infection
The Effect on Diseases Related to Acute HBV Infection
The Effect on Diseases Related to Chronic HBV Infection
The Effect on Hepatocellular Carcinoma (HCC)
4. The Global Impact of HBV Vaccination Programs: Successes and Challenges
5. Progress towards Achieving the WHO Targets for 2030 HBV Elimination: 2016–2023
5.1. The Impact of the HBV Vaccination
5.2. The Feasibility of HBV Elimination by 2030
5.3. The Effect of COVID-19 on the 2030 Elimination Plan
5.4. Key Advancements in Achieving the 2030 Elimination Targets
5.5. Strategies for Achieving HBV Elimination by 2030
6. The Road Ahead: Sustaining Progress beyond 2030
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Locarnini, S.; Hatzakis, A.; Chen, D.S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62 (Suppl. S1), S76–S86. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Flaxman, A.D.; Naghavi, M.; Fitzmaurice, C.; Vos, T.; Abubakar, I.; Abu-Raddad, L.J.; Assadi, R.; Bhala, N.; Cowie, B.; et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet 2016, 388, 1081–1088. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, P.J.; Chen, D.S. Recent Advances in the Research of Hepatitis B Virus-Related Hepatocellular Carcinoma: Epidemiologic and Molecular Biological Aspects. Adv. Cancer Res. 2010, 108, 21–72. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Lok, A.S.F. Chronic hepatitis B. N. Engl. J. Med. 2002, 346, 1682–1683. [Google Scholar] [CrossRef]
- Ibrahim, S.; El Din, S.; Bazzal, I. Antibody level after hepatitis-B vaccination in hemodialysis patients: Impact of dialysis adequacy, chronic inflammation, local endemicity and nutritional status. J. Natl. Med. Assoc. 2006, 98, 1953. [Google Scholar]
- Venook, A.P.; Papandreou, C.; Furuse, J.; Ladrón de Guevara, L. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010, 15 (Suppl. S4), 5–13. [Google Scholar] [CrossRef]
- Stevens, C.E.; Toy, P.T.; Tong, M.J.; Taylor, P.E.; Vyas, G.N.; Nair, P.V.; Gudavalli, M.; Krugman, S. Perinatal Hepatitis B Virus Transmission in the United States: Prevention by Passive-Active Immunization. JAMA 1985, 253, 1740–1745. [Google Scholar] [CrossRef]
- Stevens, C.E.; Beasley, R.P.; Tsui, J.; Lee, W.C. Vertical transmission of hepatitis B antigen in Taiwan. N. Engl. J. Med. 1975, 292, 771–774. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategies on HIV, Viral Hepatitis and the Sexually Transmitted Infections; World Health Organization: Geneva, Switzerland, 2021; pp. 2022–2030. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 16 December 2023).
- Razavi-Shearer, D.; Gamkrelidze, I.; Pan, C.; Jia, J.; Berg, T.; Gray, R.; Lim, Y.S.; Chen, C.J.; Ocama, P.; Desalegn, H.; et al. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [Google Scholar] [CrossRef]
- Jaquet, A.; Muula, G.; Ekouevi, D.K.; Wandeler, G. Elimination of Viral Hepatitis in Low and Middle-Income Countries: Epidemiological Research Gaps. Curr. Epidemiol. Rep. 2021, 8, 89–96. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, X.; Xu, Y.; Yang, L.; Zhang, Y.; Shen, Q. Association Between Income and Hepatitis B Seroprevalence: A Systematic Review and Meta-analysis. Hepat. Mon. 2020, 20, 104675. [Google Scholar] [CrossRef]
- Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. Available online: https://www.who.int/publications/i/item/WHO-HIV-2016.06 (accessed on 16 January 2024).
- Solomon-Rakiep, T.; Olivier, J.; Amponsah-Dacosta, E. Weak Adoption and Performance of Hepatitis B Birth-Dose Vaccination Programs in Africa: Time to Consider Systems Complexity?—A Scoping Review. Trop. Med. Infect. Dis. 2023, 8, 474. [Google Scholar] [CrossRef]
- Howell, J.; Seaman, C.; Wallace, J.; Xiao, Y.; Scott, N.; Davies, J.; De Santis, T.; Adda, D.; El-Sayed, M.; Feld, J.J.; et al. Pathway to global elimination of hepatitis B: HBV cure is just the first step. Hepatology 2023, 78, 976. [Google Scholar] [CrossRef]
- McCulloch, K.; Romero, N.; MacLachlan, J.; Allard, N.; Cowie, B. Modeling Progress Toward Elimination of Hepatitis B in Australia. Hepatology 2020, 71, 1170–1181. [Google Scholar] [CrossRef]
- Cox, A.L.; El-Sayed, M.H.; Kao, J.H.; Lazarus, J.V.; Lemoine, M.; Lok, A.S.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef]
- Waheed, Y.; Siddiq, M.; Jamil, Z.; Najmi, M.H. Hepatitis elimination by 2030: Progress and challenges. World J. Gastroenterol. 2018, 24, 4959. [Google Scholar] [CrossRef]
- Elsharkawy, A.; Samir, R.; Abdallah, M.; Hassany, M.; El-Kassas, M. The implications of the COVID-19 pandemic on hepatitis B and C elimination programs in Egypt: Current situation and future perspective. Egypt. Liver J. 2023, 13, 50. [Google Scholar] [CrossRef]
- CDC. Global Viral Hepatitis: Millions of People are Affected. Available online: https://www.cdc.gov/hepatitis/global/index.htm (accessed on 23 December 2023).
- Chen, S.; Mao, W.; Guo, L.; Zhang, J.; Tang, S. Combating hepatitis B and C by 2030: Achievements, gaps, and options for actions in China. BMJ Glob Health 2020, 5, 2306. [Google Scholar] [CrossRef]
- Cui, F.; Blach, S.; Manzengo Mingiedi, C.; Gonzalez, M.A.; Sabry Alaama, A.; Mozalevskis, A.; Séguy, N.; Rewari, B.B.; Chan, P.L.; Le, L.-V.; et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol. Hepatol. 2023, 8, 332–342. [Google Scholar] [CrossRef]
- Regional Consultation on the Global Health Sector Strategies on HIV, Hepatitis and STIs, 2022–2030. EMHJ 2021, 27, 941–942. [CrossRef]
- Akbar, S.M.F.; Al-Mahtab, M.; Khan, S.; Yoshida, O.; Hiasa, Y. Elimination of Hepatitis by 2030: Present Realities and Future Projections. Infect. Dis. Immun. 2022, 2, 3–8. [Google Scholar] [CrossRef]
- Luo, Z.; Li, L.; Ruan, B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int. J. Infect. Dis. 2012, 16, e82–e88. [Google Scholar] [CrossRef]
- Chitnis, A.S.; Wong, R.J. Review of Updated Hepatitis B Vaccine Recommendations and Potential Challenges with Implementation. Gastroenterol. Hepatol. 2022, 18, 447. [Google Scholar]
- Kao, J.H.; Chen, D.S. Global control of hepatitis B virus infection. Lancet Infect. Dis. 2002, 2, 395–403. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.; Zhou, Y.H. Hepatitis B vaccine development and implementation. Hum. Vaccin. Immunother. 2020, 16, 1533–1544. [Google Scholar] [CrossRef]
- Van Damme, P. Long-term Protection After Hepatitis B Vaccine. J. Infect. Dis. 2016, 214, 1–3. [Google Scholar] [CrossRef]
- Hilleman, M.R.; McAleer, W.J.; Buynak, E.B.; McLean, A.A. The preparation and safety of hepatitis B vaccine. J. Infect. 1983, 7, 3–8. [Google Scholar] [CrossRef]
- Francis, D.P.; Feorino, P.M.; Mcdougal, S.; Warfield, D.; Getchell, J.; Cabradilla, C.; Tong, M.; Miller, W.J.; Schultz, L.D.; Bailey, F.J.; et al. The Safety of the Hepatitis B Vaccine: Inactivation of the AIDS Virus During Routine Vaccine Manufacture. JAMA 1986, 256, 869–872. [Google Scholar] [CrossRef]
- Stevens, C.E.; Taylor, P.E.; Tong, M.J.; Toy, P.T.; Vyas, G.N.; Nair, P.V.; Weissman, J.Y.; Krugman, S. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA 1987, 257, 2612–2616. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG Motifs in Bacterial DNA and Their Immune Effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Davidson, M.; Krugman, S. Immunogenicity of recombinant yeast hepatitis B vaccine. Lancet 1985, 325, 108–109. [Google Scholar] [CrossRef]
- Jilg, W.; Schmidt, M.; Zoulek, G.; Lorbeer, B.; Wilske, B.; Deinhardt, F. Clinical evaluation of a recombinant hepatitis B Vaccine. Lancet 1984, 324, 1174–1175. [Google Scholar] [CrossRef]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S4), S343. [Google Scholar] [CrossRef]
- Janssen, J.M.; Heyward, W.L.; Martin, J.T.; Janssen, R.S. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease and type 2 diabetes mellitus. Vaccine 2015, 33, 833–837. [Google Scholar] [CrossRef]
- Janssen, R.S.; Mangoo-Karim, R.; Pergola, P.E.; Girndt, M.; Namini, H.; Rahman, S.; Bennett, S.R.; Heyward, W.L.; Martin, J.T. Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease. Vaccine 2013, 31, 5306–5313. [Google Scholar] [CrossRef] [PubMed]
- Sablan, B.P.; Kim, D.J.; Barzaga, N.G.; Chow, W.C.; Cho, M.; Ahn, S.H.; Hwang, S.G.; Lee, J.H.; Namini, H.; Heyward, W.L. Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine 2012, 30, 2689–2696. [Google Scholar] [CrossRef]
- Hristian, C.; Onfavreux, C.; Uissa, A.S.; Atricia, P.; Addier, S.; Alérie, V.; Ourdès, B.; Andra, S.; Ukusic, V.; Clerosis, S.; et al. Vaccinations and the Risk of Relapse in Multiple Sclerosis. N. Engl. J. Med. 2001, 344, 319–326. [Google Scholar] [CrossRef]
- Hernán, M.A.; Jick, S.S.; Olek, M.J.; Jick, H. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: A prospective study. Neurology 2004, 63, 838–842. [Google Scholar] [CrossRef]
- Jack, A.D.; Hall, A.J.; Maine, N.; Mendy, M.; Whittle, H.C. What level of hepatitis B antibody is protective? J. Infect. Dis. 1999, 179, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Poorolajal, J.; Mahmoodi, M.; Majdzadeh, R.; Nasseri-Moghaddam, S.; Haghdoost, A.; Fotouhi, A. Long-term protection provided by hepatitis B vaccine and need for booster dose: A meta-analysis. Vaccine 2010, 28, 623–631. [Google Scholar] [CrossRef]
- Hepatitis B Vaccines: WHO Position Paper. July 2017. Available online: https://www.who.int/publications/i/item/WER9227 (accessed on 19 January 2024).
- Jilg, W.; Schmidt, M.; Deinhardt, F. Persistence of specific antibodies after hepatitis B vaccination. J. Hepatol. 1988, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Jilg, W.; Schmidt, M.; Deinhardt, F.; Zachoval, R. Hepatitis B vaccination: How long does protection last? Lancet 1984, 2, 458. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Jilg, W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 2006, 24, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Gara, N.; Abdalla, A.; Rivera, E.; Zhao, X.; Werner, J.M.; Liang, T.J.; Hoofnagle, J.H.; Rehermann, B.; Ghany, M.G. Durability of antibody response against hepatitis B virus in healthcare workers vaccinated as adults. Clin. Infect. Dis. 2014, 60, 505–513. [Google Scholar] [CrossRef]
- Meireles, L.C.; Marinho, R.T.; Van Damme, P. Three Decades of Hepatitis B Control with Vaccination. World J. Hepatol. 2015, 7, 2127–2132. [Google Scholar] [CrossRef]
- West, D.J.; Calandra, G.B. Vaccine Induced Immunologic Memory for Hepatitis B Surface Antigen: Implications for Policy on Booster Vaccination. Vaccine 1996, 14, 1019–1027. [Google Scholar] [CrossRef]
- Huang, L.I.M.; Chiang, B.L.; Lee, C.Y.; Lee, P.I.; Chi, W.K.; Chang, M.H. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology 1999, 29, 954–959. [Google Scholar] [CrossRef]
- McMahon, B.J.; Bruden, D.L.; Petersen, K.M.; Bulkow, L.R.; Parkinson, A.J.; Nainan, O.; Khristova, M.; Zanis, C.; Peters, H.; Margolis, H.S. Antibody Levels and Protection after Hepatitis B Vaccination: Results of a 15-Year Follow-Up. Ann. Intern. Med. 2005, 142, 333–341. [Google Scholar] [CrossRef]
- Plotkin, S.; Leuridan, E.; Van Damme, P. Hepatitis B and the need for a booster dose. Clin. Infect. Dis. 2011, 53, 68–75. [Google Scholar] [CrossRef]
- Banatvala, J.E.; Van Damme, P. Hepatitis B vaccine—Do we need boosters? J. Viral Hepat. 2003, 10, 1–6. [Google Scholar] [CrossRef]
- Hall, A.J. Boosters for hepatitis B vaccination? Need for an evidence-based policy. Hepatology 2010, 51, 1485–1486. [Google Scholar] [CrossRef]
- Ma, J.C.; Wu, Z.W.; Zhou, H.S.; Gao, Z.; Hao, Z.Y.; Jin, F.; Zhang, Y.H.; Li, M.J.; Wang, F.; Li, Q.; et al. Long-term protection at 20–31 years after primary vaccination with plasma-derived hepatitis B vaccine in a Chinese rural community. Hum. Vaccines Immunother. 2020, 16, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Jan, C.F.; Huang, K.C.; Chien, Y.C.; Greydanus, D.E.; Dele Davies, H.; Chiu, T.Y.; Huang, L.M.; Chen, C.J.; Chen, D.S. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology 2010, 51, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Saito, I. Hepatitis B booster vaccination for healthcare workers. Lancet 2000, 355, 1464. [Google Scholar] [CrossRef] [PubMed]
- Banatvala, J.; Van Damme, P.; Van Hattum, J. Boosters for hepatitis B. Lancet 2000, 356, 337–338. [Google Scholar] [CrossRef]
- Purdy, M.A. Hepatitis B virus S gene escape mutants. Asian J. Transfus. Sci. 2007, 1, 62. [Google Scholar] [CrossRef] [PubMed]
- Carman, W.F.; Karayiannis, P.; Waters, J.; Thomas, H.C.; Zanetti, A.R.; Manzillo, G.; Zuckerman, A.J. Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990, 336, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Chang, M.H.; Ni, Y.H.; Chen, H.L. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut 2004, 53, 1499–1503. [Google Scholar] [CrossRef]
- Kalinina, T.; Iwanski, A.; Will, H.; Sterneck, M. Deficiency in Virion Secretion and Decreased Stability of the Hepatitis B Virus Immune Escape Mutant G145R. Hepatology 2003, 38, 1274–1281. [Google Scholar] [CrossRef]
- Mele, A.; Tancredi, F.; Romanoó, L.; Giuseppone, A.; Colucci, M.; Sangiuolo, A.; Lecce, R.; Adamo, B.; Tosti, M.E.; Taliani, G.; et al. Effectiveness of hepatitis B vaccination in babies born to hepatitis B surface antigen-positive mothers in Italy. J. Infect. Dis. 2001, 184, 905–908. [Google Scholar] [CrossRef]
- Chen, D.S. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J. Hepatol. 2009, 50, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Cote, P.J.; Zanetti, A.R.; Miller, R.H.; Shapiro, M.; Gerin, J.; Purcell, R.H. Licensed recombinant hepatitis B vaccines protect chimpanzees against infection with the prototype surface gene mutant of hepatitis B virus. Hepatology 1999, 30, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.F. The Chimpanzee Model for Hepatitis B Virus Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a021469. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.; Gish, R. Chronic Hepatitis B Infection. Clin. Liver Dis. 2012, 16, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, Y. Cross-Protection of Hepatitis B Vaccination among Different Genotypes. Vaccines 2020, 8, 456. [Google Scholar] [CrossRef]
- Stramer, S.L.; Wend, U.; Candotti, D.; Foster, G.A.; Hollinger, F.B.; Dodd, R.Y.; Allain, J.-P.; Gerlich, W. Nucleic Acid Testing to Detect HBV Infection in Blood Donors. N. Engl. J. Med. 2011, 364, 236–247. [Google Scholar] [CrossRef]
- Li, T.; Fu, Y.; Allain, J.P.; Li, C. Chronic and occult hepatitis B virus infections in the vaccinated Chinese population. Ann. Blood 2017, 2, 4. [Google Scholar] [CrossRef]
- Zhu, H.L.; Li, X.; Li, J.; Zhang, Z.H. Genetic variation of occult hepatitis B virus infection. World J. Gastroenterol. 2016, 22, 3531. [Google Scholar] [CrossRef]
- Mu, S.C.; Lin, Y.M.; Jow, G.M.; Chen, B.F. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J. Hepatol. 2009, 50, 264–272. [Google Scholar] [CrossRef]
- Ni, Y.H.; Huang, L.M.; Chang, M.H.; Yen, C.J.; Lu, C.Y.; You, S.L.; Kao, J.H.; Lin, Y.C.; Chen, H.L.; Hsu, H.Y.; et al. Two Decades of Universal Hepatitis B Vaccination in Taiwan: Impact and Implication for Future Strategies. Gastroenterology 2007, 132, 1287–1293. [Google Scholar] [CrossRef]
- Yang, S.; Tian, G.; Cui, Y.; Ding, C.; Deng, M.; Yu, C.; Xu, K.; Ren, J.; Yao, J.; Li, Y.; et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci. Rep. 2016, 6, 27251. [Google Scholar] [CrossRef]
- Hepatitis B Foundation: Vaccine Non-Responders. Available online: https://www.hepb.org/prevention-and-diagnosis/vaccination/vaccine-non-responders/ (accessed on 20 January 2024).
- Corrao, G.; Calleri, M.; Zotti, M.; Barral, C.; Russo, R.; Garella, D.; Ruggenini, A.M. Immune response to anti-HBV vaccination: Study of conditioning factors. Epidemiology 1988, 4, 492–496. [Google Scholar] [CrossRef]
- Fisman, D.N.; Agrawal, D.; Leder, K. The Effect of Age on Immunologic Response to Recombinant Hepatitis B Vaccine: A Meta-analysis. Clin. Infect. Dis. 2002, 35, 1368–1375. [Google Scholar] [CrossRef]
- Launay, O. Safety and Immunogenicity of 4 Intramuscular Double Doses and 4 Intradermal Low Doses vs Standard Hepatitis B Vaccine Regimen in Adults with HIV-1. JAMA 2011, 305, 1432. [Google Scholar] [CrossRef] [PubMed]
- Senden, F.T. Response to intradermal hepatitis B vaccination: Differences between males and females? Vaccine 1990, 8, 612–613. [Google Scholar] [CrossRef]
- Shaw, F.E.; Baker, R.K.; Moorman, A.C.; Wood, K.C.; Fuhrer, J.; McCabe, R.E.; Holmberg, S.D.; HIV Outpatient Study (HOPS) Investigators. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989, 7, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Tedaldi, E.M.; Baker, R.K.; Moorman, A.C.; Wood, K.C.; Fuhrer, J.; McCabe, R.E.; Holmberg, S.D.; HIV Outpatient Study (HOPS) Investigators. Hepatitis A and B Vaccination Practices for Ambulatory Patients Infected with HIV. Clin. Infect. Dis. 2004, 38, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Van Loveren, H.; Van Amsterdam, J.G.; Vandebriel, R.J.; Kimman, T.G.; Rümke, H.C.; Steerenberg, P.S.; Vos, J.G. Vaccine-induced antibody responses as parameters of the influence of endogenous and environmental factors. Environ. Health Perspect. 2001, 109, 757–764. [Google Scholar] [CrossRef]
- Winter, A.; Follett, E.; Mcintyre, J.; Stewart, J.; Symington, I. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine 1994, 12, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Mounier, M.; Hessel, L.; Michel, J.P.; Gualde, N.; Dubois, F.; Barin, F.; Goudeau, A. Hepatitis-B vaccination in the elderly. J. Infect. Dis. 1984, 149, 1019. [Google Scholar] [CrossRef]
- de Rave, S.; Heijtink, R.A.; Bakker-Bendik, M.; Boot, J.; Schalm, S.W. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine 1994, 12, 532–534. [Google Scholar] [CrossRef]
- McNulty, C.A.M.; Bowen, J.K.; Williams, A.J. Hepatitis B vaccination in predialysis chronic renal failure patients a comparison of two vaccination schedules. Vaccine 2005, 23, 4142–4147. [Google Scholar] [CrossRef]
- DaRoza, G.; Loewen, A.; Djurdjev, O.; Love, J.; Kempston, C.; Burnett, S.; Kiaii, M.; Taylor, P.A.; Levin,, A. Stage of Chronic Kidney Disease Predicts Seroconversion after Hepatitis B Immunization: Earlier is Better. Am. J. Kidney Dis. 2003, 42, 1184–1192. [Google Scholar] [CrossRef]
- De Mattos, A.A.; Gomes, E.B.; Tovo, C.V.; Alexandre, C.O.P.; Remião, J.O.D.R. Hepatitis B vaccine efficacy in patients with chronic liver disease by hepatitis C virus. Arq. Gastroenterol. 2004, 41, 180–184. [Google Scholar] [CrossRef]
- Gutierrez Domingo, I.; Pascasio Acevedo, J.M.; Alcalde Vargas, A.; Ramos Cuadra, A.; Ferrer Ríos, M.T.; Sousa Martin, J.M.; Sayago Mota, M.; Giráldez Gallego, A.; Suárez Artacho, G. Response to vaccination against hepatitis B virus with a schedule of four 40-μg doses in cirrhotic patients evaluated for liver transplantation: Factors associated with a response. Transplant. Proc. 2012, 44, 1499–1501. [Google Scholar] [CrossRef]
- Overton, E.T.; Sungkanuparph, S.; Powderly, W.G.; Seyfried, W.; Groger, R.K.; Aberg, J.A. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin. Infect. Dis. 2005, 41, 1045–1048. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Mengoli, C.; Serpelloni, G.; Lanza, A.; Gomma, M.; Nardi, S.; Rimondo, C.; Bricolo, F.; Consolaro, S.; Trevisan, M.T.; et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine 2009, 27, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Messa, P.; Martin, P. Hepatitis B virus vaccine in chronic kidney disease: Improved immunogenicity by adjuvants? A meta-analysis of randomized trials. Vaccine 2012, 30, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Walayat, S.; Ahmed, Z.; Martin, D.; Puli, S.; Cashman, M.; Dhillon, S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J. Hepatol. 2015, 7, 2503–2509. [Google Scholar] [CrossRef]
- Vesikari, T.; Finn, A.; Van Damme, P.; Leroux-Roels, I.; Leroux-Roels, G.; Segall, N.; Toma, A.; Vallieres, G.; Aronson, R.; Reich, D.; et al. Immunogenicity and Safety of a 3-Antigen Hepatitis B Vaccine vs a Single-Antigen Hepatitis B Vaccine: A Phase 3 Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2128652. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Davoulou, P.; Konstantakis, C.; Thomopoulos, K.; Triantos, C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev. Med. Virol. 2017, 27, e1942. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, M.; Lionetti, E.; Gennaro, A.; Lanzafame, A.; Arrigo, T.; Salpietro, C.; La Rosa, M.; Leonardi, S. Hepatitis B vaccine by intradermal route in non responder patients: An update. World J. Gastroenterol. WJG 2014, 20, 10383. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, M.; Lionetti, E.; Gennaro, A.; Lanzafame, A.; Arrigo, T.; Salpietro, C.; La Rosa, M.; Leonardi, S. HBV Vaccines: Advances and Development. Vaccines 2023, 11, 1862. [Google Scholar] [CrossRef]
- Prüss-Üstün, A.; Rapiti, E.; Hutin, Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am. J. Ind. Med. 2005, 48, 482–490. [Google Scholar] [CrossRef]
- Batra, V.; Goswami, A.; Dadhich, S.; Kothari, D.; Bhargava, N. Hepatitis B immunization in healthcare workers. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 276. [Google Scholar]
- ·Chahal, H.S.; Peters, M.G.; Harris, A.M.; McCabe, D.; Volberding, P.; Kahn, J.G. Cost-effectiveness of Hepatitis B Virus Infection Screening and Treatment or Vaccination in 6 High-risk Populations in the United States. Open Forum Infect. Dis. 2019, 6, ofy353. [Google Scholar] [CrossRef]
- Talbird, S.E.; Anderson, S.A.; Nossov, M.; Beattie, N.; Rak, A.T.; Diaz-Mitoma, F. Cost-effectiveness of a 3-antigen versus single-antigen vaccine for the prevention of hepatitis B in adults in the United States. Vaccine 2023, 41, 3506–3517. [Google Scholar] [CrossRef]
- Mokhtari, A.M.; Barouni, M.; Moghadami, M.; Hassanzadeh, J.; Dewey, R.S.; Mirahmadizadeh, A. Evaluating the cost-effectiveness of universal hepatitis B virus vaccination in Iran: A Markov model analysis. Hum. Vaccines Immunother. 2021, 17, 1825–1833. [Google Scholar] [CrossRef]
- Lu, S.Q.; McGhee, S.M.; Xie, X.; Cheng, J.; Fielding, R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine 2013, 31, 1864–1869. [Google Scholar] [CrossRef]
- Woo, G.; Tomlinson, G.; Yim, C.; Lilly, L.; Therapondos, G.; Wong, D.K.H.; Ungar, W.; Einarson, T.R.; Sherman, M.; Heathcote, J.E.; et al. Health state utilities and quality of life in patients with hepatitis B. Can. J. Gastroenterol. 2012, 26, 445–451. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Gay, N.; Edmunds, W.J.; Ramsay, M. Economic evaluation of infant and adolescent hepatitis B vaccination in the UK. Vaccine 2011, 29, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.J.; Estes, C.; Razavi-Shearer, D.; Sahdra, K.; Lipton, N.; Shah, H.; Capraru, C.; Janssen, H.L.A.; Razavi, H.; Feld, J.J. Cost-effectiveness modelling of birth and infant dose vaccination against hepatitis B virus in Ontario from 2020 to 2050. Can. Med. Assoc. Open Access J. 2023, 11, E24–E32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, M.; Hutton, D.W.; Wagner, A.L.; Yao, Y.; Zhu, W.; Cao, L.; Tang, S.; Pan, J.; Wang, Y. Impact of the national hepatitis B immunization program in China: A modeling study. Infect. Dis. Poverty 2022, 11, 106. [Google Scholar] [CrossRef]
- Janssen, R.S.; Bruxvoort, K.; Jacobsen, S.J.; Slezak, J.; David, C.; Hyer, R.; Poland, G.A. Considerations for estimating real-world outcomes and value in vaccination: A case study with adult hepatitis B virus vaccination. Vaccine 2021, 39, 5666–5672. [Google Scholar] [CrossRef]
- Bahrami, A.A.; Bandehpour, M.; Kazemi, B.; Bozorgmehr, M.; Mosaffa, N.; Chegeni, R. Assessment of a poly-epitope candidate vaccine against Hepatitis B, C, and poliovirus in interaction with monocyte-derived dendritic cells: An ex-vivo study. Hum. Immunol. 2020, 81, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Michaelides, K.; Prasanna, M.; Badhan, R.; Mohammed, A.U.R.; Walters, A.; Howard, M.K.; Dulal, P.; Al-Khattawi, A. Single administration vaccines: Delivery challenges, in vivo performance, and translational considerations. Expert. Rev. Vaccines 2023, 22, 579–595. [Google Scholar] [CrossRef]
- Davitt, C.J.H.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef]
- Scaramuzzi, K.; Tanaka, G.D.; Neto, F.M.; Garcia, P.R.A.F.; Gabrili, J.J.M.; Oliveira, D.C.A.; Tambourgi, D.V.; Mussalem, J.S.; Paixão-Cavalcante, D.; D’Azeredo Orlando, M.T.; et al. Nanostructured SBA-15 silica: An effective protective vehicle to oral hepatitis B vaccine immunization. Nanomedicine 2016, 12, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Tomar, J.; Tonnis, W.F.; Patil, H.P.; de Boer, A.H.; Hagedoorn, P.; Vanbever, R.; Frijlink, H.W.; Hinrichs, W.L.J. Pulmonary immunization: Deposition site is of minor relevance for influenza vaccination but deep lung deposition is crucial for hepatitis B vaccination. Acta Pharm. Sin. B 2019, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Maini, M.K.; Pallett, L.J. Defective T-cell immunity in hepatitis B virus infection: Why therapeutic vaccination needs a helping hand. Lancet Gastroenterol. Hepatol. 2018, 3, 192–202. [Google Scholar] [CrossRef]
- Burton, A.R.; Pallett, L.J.; McCoy, L.E.; Suveizdyte, K.; Amin, O.E.; Swadling, L.; Alberts, E.; Davidson, B.R.; Kennedy, P.T.; Gill, U.S.; et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Investig. 2018, 128, 4588–4603. [Google Scholar] [CrossRef] [PubMed]
- Horng, J.-H.; Lin, W.-H.; Wu, C.-R.; Lin, Y.-Y.; Wu, L.-L.; Chen, D.-S.; Chen, P.-J. HBV X protein-based therapeutic vaccine accelerates viral antigen clearance by mobilizing monocyte infiltration into the liver in HBV carrier mice. J. Biomed. Sci. 2020, 27, 70. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, X.; Wang, X.; Zheng, L.; Lan, S.; Jin, S.; Ou, Z.; Wu, J. In Silico Analysis of Epitope-Based Vaccine Candidates against Hepatitis B Virus Polymerase Protein. Viruses 2017, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, C.; Mohideen, S.P. Personalised Vaccination Prediction for Hepatitis B using Machine Learning. Ann. Rom. Soc. Cell Biol. 2021, 25, 10069–10088. [Google Scholar]
- Aevermann, B.D.; Shannon, C.P.; Novotny, M.; Ben-Othman, R.; Cai, B.; Zhang, Y.; Ye, J.C.; Kobor, M.S.; Gladish, N.; Lee, A.H.Y.; et al. Machine Learning-Based Single Cell and Integrative Analysis Reveals That Baseline mDC Predisposition Correlates with Hepatitis B Vaccine Antibody Response. Front. Immunol. 2021, 12, 690470. [Google Scholar] [CrossRef]
- Alter, M.J.; Hadler, S.C.; Margolis, H.S.; Alexander, W.J.; Hu, P.Y.; Judson, F.N.; Mares, A.; Miller, J.K.; Moyer, L.A. The Changing Epidemiology of Hepatitis B in the United States: Need for Alternative Vaccination Strategies. JAMA 1990, 263, 1218–1222. [Google Scholar] [CrossRef]
- Arevalo, J.A.; Washington, A.E. Cost-effectiveness of Prenatal Screening and Immunization for Hepatitis B Virus. JAMA 1988, 259, 365–369. [Google Scholar] [CrossRef]
- Chen, D.S.; Hsu, N.H.M.; Sung, J.L.; Hsu, T.C.; Hsu, S.T.; Kuo, Y.T.; Li, K.J.; Shih, Y.T. A Mass Vaccination Program in Taiwan Against Hepatitis B Virus Infection in Infants of Hepatitis B Surface Antigen—Carrier Mothers. JAMA 1987, 257, 2597–2603. [Google Scholar] [CrossRef]
- Van Damme, P.; Meheus, A.; Kane, M. Integration of Hepatitis B Vaccination into National Immunisation Programmes. BMJ 1997, 314, 1033. [Google Scholar] [CrossRef]
- Ruff, T.A.; Gertig, D.M.; Otto, B.F.; Gust, I.D.; Sutanto, A.; Soewarso, T.I.; Kandun, N.; Marschner, I.C.; Maynard, J.E. Lombok Hepatitis B Model Immunization Project: Toward universal infant hepatitis B immunization in Indonesia. J. Infect. Dis. 1995, 171, 290–296. [Google Scholar] [CrossRef]
- Fortuin, M.; Chotard, J.; Jack, A.D.; Maine, N.P.; Mendy, M.; Hall, A.J.; Inskip, H.M.; George, M.O.; Whittle, H.C. Efficacy of hepatitis B vaccine in the Gambian expanded programme on immunisation. Lancet 1993, 341, 1129–1132. [Google Scholar] [CrossRef]
- Acharya, S.K. Does the level of hepatitis B virus vaccination in health-care workers need improvement? J. Gastroenterol. Hepatol. 2008, 23, 1628–1631. [Google Scholar] [CrossRef]
- Crosnier, J.; Jungers, P.; Couroucé, A.M.; Laplanche, A.; Benhamou, E.; Degos, F.; Lacour, B.; Prunet, P.; Cerisier, Y.; Guesry, P. Randomised placebo-controlled trial of hepatitis b surface antigen vaccine in french haemodialysis units: II, haemodialysis patients. Lancet 1981, 317, 797–800. [Google Scholar] [CrossRef]
- Crosnier, J.; Jungers, P.; Couroucé, A.M.; Laplanche, A.; Benhamou, E.; Degos, F.; Lacour, B.; Prunet, P.; Cerisier, Y.; Guesry, P. Hepatitis B Vaccine in Medical Staff of Hemodialysis Units: Efficacy and Subtype Cross-Protection. N. Engl. J. Med. 1982, 307, 1481–1486. [Google Scholar] [CrossRef]
- Stevens, C.E.; Alter, H.J.; Taylor, P.E.; Zang, E.A.; Harley, E.J.; Szmuness, W. Hepatitis B Vaccine in Patients Receiving Hemodialysis: Immunogenicity and Efficacy. N. Engl. J. Med. 1984, 311, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.M.; Yap, D.Y.H.; Yip, T.P.S.; Hung, I.F.N.; Tang, S.C.W.; Chan, T.M. Vaccination in patients with chronic kidney disease—Review of current recommendations and recent advances. Nephrology 2021, 26, 5–11. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cerutti, R.; Dixit, V.; Ridruejo, E. Hepatitis B virus vaccine and chronic kidney disease. The advances. Nefrologia 2021, 41, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, A.E. Hepatitis B Vaccination in Chronic Kidney Disease: Review of Evidence in Non-Dialyzed Patients. Hepat. Mon. 2012, 12, 7359. [Google Scholar] [CrossRef]
- Ong, K.Y.; Wong, H.Y.; Khee, G.Y. What is the hepatitis B vaccination regimen in chronic kidney disease? Cleve Clin. J. Med. 2018, 85, 32–34. [Google Scholar] [CrossRef]
- Faser, G.M.; Ochana, N.; Fenyves, D.; Neumann, L.; Chazan, R.; Niv, Y.; Chaimovitch, S. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J. Hepatol. 1994, 21, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Udomkarnjananun, S.; Takkavatakarn, K.; Praditpornsilpa, K.; Nader, C.; Eiam-Ong, S.; Jaber, B.L.; Susantitaphong, P. Hepatitis B virus vaccine immune response and mortality in dialysis patients: A meta-analysis. J. Nephrol. 2020, 33, 343–354. [Google Scholar] [CrossRef]
- Kovacic, V.; Sain, M.; Vukman, V. Efficient haemodialysis improves the response to hepatitis B virus vaccination. Intervirology 2002, 45, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Asan, A.; Demirhan, H.; Sorkun, H.Ç.; Özkan, S.; Aydın, M.; Akın, D.; Tatar, B.; Çatak, B.; Şener, A.; Köse, Ş. Factors affecting responsiveness to hepatitis B immunization in dialysis patients. Int. Urol. Nephrol. 2017, 49, 1845–1850. [Google Scholar] [CrossRef]
- Ayub, M.A.; Bacci, M.R.; Fonseca, F.L.A.; Chehter, E.Z. Hemodialysis and hepatitis B vaccination: A challenge to physicians. Int. J. Gen. Med. 2014, 7, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, E.; Courouce, A.M.; Jungers, P.; Laplanche, A.; Degos, F.; Brangier, J.; Crosnier, J. Hepatitis B vaccine: Randomized trial of immunogenicity in hemodialysis patients. Clin. Nephrol. 1984, 21, 143–147. [Google Scholar] [PubMed]
- Bel’eed, K.; Wright, M.; Eadington, D.; Farr, M.; Sellars, L. Vaccination against hepatitis B infection in patients with end stage renal disease. Postgrad. Med. J. 2002, 78, 538–540. [Google Scholar] [CrossRef]
- Somi, M.H.; Hajipour, B. Improving Hepatitis B Vaccine Efficacy in End-Stage Renal Diseases Patients and Role of Adjuvants. ISRN Gastroenterol. 2012, 2012, 960413. [Google Scholar] [CrossRef]
- Friedrich, P.; Sattler, A.; Müller, K.; Nienen, M.; Reinke, P.; Babel, N. Comparing Humoral and Cellular Immune Response Against HBV Vaccine in Kidney Transplant Patients. Am. J. Transplant. 2015, 15, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, S.; Kolovou, K.; Sakellariou, S.; Boletis, J.N.; Delladetsima, I.K. Hepatitis B in renal transplant patients. World J. Hepatol. 2017, 9, 1054. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Factors affecting effectiveness of vaccination against hepatitis B virus in hemodialysis patients. World J. Gastroenterol. WJG 2014, 20, 12018. [Google Scholar] [CrossRef] [PubMed]
- Lefebure, A.F.; Verpooten, G.A.; Couttenye, M.M.; De, M.E. Broe Immunogenicity of a recombinant DNA hepatitis B vaccine in renal transplant patients. Vaccine 1993, 11, 397–399. [Google Scholar] [CrossRef]
- Fabrizi, F.; Martin, P.; Dixit, V.; Bunnapradist, S.; Dulai, G. Meta-analysis: The effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Aliment. Pharmacol. Ther. 2004, 20, 1053–1062. [Google Scholar] [CrossRef]
- Goilav, C.; Piot, P. Vaccination against hepatitis B in homosexual men: A review. Am. J. Med. 1989, 87, S21–S25. [Google Scholar] [CrossRef]
- Szmuness, W.; Stevens, C.E.; Harley, E.J.; Zang, E.A.; Oleszko, W.R.; William, D.C.; Sadovsky, R.; Morrison, J.M.; Kellner, A. Hepatitis B Vaccine: Demonstration of Efficacy in a Controlled Clinical Trial in a High-Risk Population in the United States. N. Engl. J. Med. 1980, 303, 833–841. [Google Scholar] [CrossRef]
- Odaka, N.; Eldred, L.; Cohn, S.; Muñoz, A.; Fields, H.A.; Fox, R.; Solomon, R.; Kaslow, R.; Polk, B.F. Comparative Immunogenicity of Plasma and Recombinant Hepatitis B Virus Vaccines in Homosexual Men. JAMA J. Am. Med. Assoc. 1988, 260, 3635–3637. [Google Scholar] [CrossRef]
- Francis, D.P.; Hadler, S.C.; Thompson, S.E.; Maynard, J.E.; Ostrow, D.G.; Altman, N.; Braff, E.H.; O’Malley, P.; Hawkins, D.; Judson, F.N.; et al. The prevention of hepatitis B with vaccine. Report of the centers for disease control multi-center efficacy trial among homosexual men. Ann. Intern. Med. 1982, 97, 362–366. [Google Scholar] [CrossRef]
- Vet, R.; de Wit, J.B.F.; Das, E. Factors associated with hepatitis B vaccination among men who have sex with men: A systematic review of published research. Int. J. STD AIDS 2015, 28, 534–542. [Google Scholar] [CrossRef]
- Francis, D.P.; Hadler, S.C.; Thompson, S.E.; Maynard, J.E.; Ostrow, D.G.; Altman, N.; Braff, E.H.; O’Malley, P.; Hawkins, D.; Judson, F.N. Assessment of the HBV vaccine response in a group of HIV-infected children in Morocco. BMC Public Health 2017, 17, 752. [Google Scholar] [CrossRef]
- Catherine, F.X.; Piroth, L. Hepatitis B virus vaccination in HIV-infected people: A review. Hum. Vaccin. Immunother. 2017, 13, 1304. [Google Scholar] [CrossRef]
- Pippi, F.; Bracciale, L.; Stolzuoli, L.; Giaccherini, R.; Montomoli, E.; Gentile, C.; Filetti, S.; De Luca, A.; Cellesi, C. Serological response to hepatitis B virus vaccine in HIV-infected children in Tanzania. HIV Med. 2008, 9, 519–525. [Google Scholar] [CrossRef]
- Zuin, G.; Principi, N.; Tornaghi, R.; Paccagnini, S.; Re, M.; Massironi, E.; Ragni, M.C. Impaired response to hepatitis B vaccine in HIV infected children. Vaccine 1992, 10, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Laurence, J.C. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am. J. Med. 2005, 118, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Paitoonpong, L.; Suankratay, C. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand. J. Infect. Dis. 2008, 40, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Rech-Medeiros, A.F.; dos Marcon, P.S.; do Tovo, C.V.; de Mattos, A.A. Evaluation of response to hepatitis B virus vaccine in adults with human immunodeficiency virus. Ann. Hepatol. 2019, 18, 725–729. [Google Scholar] [CrossRef]
- Mohareb, A.M.; Kim, A.Y. Hepatitis B Vaccination in People Living With HIV—If at First You Don’t Succeed, Try Again. JAMA Netw. Open 2021, 4, e2121281. [Google Scholar] [CrossRef]
- Kim, Y.; Loucks, J.; Shah, M. Efficacy of Hepatitis B Vaccine in Adults with Chronic Liver Disease. J. Pharm. Pract. 2022, 36, 839–844. [Google Scholar] [CrossRef]
- Roni, D.A.; Pathapati, R.M.; Kumar, A.S.; Nihal, L.; Sridhar, K.; Tumkur Rajashekar, S. Safety and efficacy of hepatitis B vaccination in cirrhosis of liver. Adv. Virol. 2013, 2013, 196704. [Google Scholar] [CrossRef]
- Reiss, G.; Keeffe, E.B. Hepatitis vaccination in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2004, 19, 715–727. [Google Scholar] [CrossRef]
- Horta, D.; Forné, M.; Agustí, A.; Raga, A.; Martín-Cardona, A.; Hernández-Soto, J.M.; Ruiz-Ramírez, P.; Esteve-Comas, M. Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice. Vaccines 2022, 10, 1323. [Google Scholar] [CrossRef]
- Hassnine, A.A.; Saber, M.A.; Fouad, Y.M.; Sarhan, H.; Elsayed, M.M.; Zaki, Z.M.; Abdelraheem, E.M.; Abdelhalim, S.M.; Elsayed, A.M. Clinical study on the efficacy of hepatitis B vaccination in hepatitis C virus related chronic liver diseases in Egypt. Virus Res. 2023, 323, 198953. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, C.; Roselle, G.A.; Lybecker, L.A.; Marshall, L.E.; Grossman, C.J.; Myre, S.A.; Weesner, R.E.; Morgan, D.D. Hepatitis B vaccination—Response of alcoholic with and without liver injury. Dig. Dis. Sci. 1988, 33, 263–269. [Google Scholar] [CrossRef]
- Degos, F.; Duhamel, G.; Brechot, C.; Nalpas, B.; Courouce, A.M.; Tron, F.; Berthelot, P. Hepatitis B vaccination in chronic alcoholics. J. Hepatol. 1986, 2, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Pascasio, J.M.; Colmenero, J. Prophylaxis and treatment in liver transplantation. VII Consensus Document of the Spanish Society of Liver Transplantation. Gastroenterol. Hepatol. (Engl. Ed.) 2020, 43, 169–177. [Google Scholar] [CrossRef]
- Takaki, A.; Yasunaka, T.; Yagi, T. Molecular Mechanisms to Control Post-Transplantation Hepatitis B Recurrence. Int. J. Mol. Sci. 2015, 16, 17494–17513. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.; Wu, G.Y. Prevention of HBV Recurrence after Liver Transplant: A Review. J. Clin. Transl. Hepatol. 2020, 8, 150. [Google Scholar] [CrossRef]
- Jiménez-Pérez, M.; González-Grande, R.; Torres, J.M.; Arjona, C.G.; Rando-Muñoz, F.J. Management of hepatitis B virus infection after liver transplantation. World J. Gastroenterol. 2015, 21, 12083. [Google Scholar] [CrossRef]
- Ishigami, M.; Honda, T.; Ishizu, Y.; Onishi, Y.; Kamei, H.; Hayashi, K.; Ogura, Y.; Hirooka, Y.; Goto, H. Frequent incidence of escape mutants after successful hepatitis B vaccine response and stopping of nucleos(t)ide analogues in liver transplant recipients. Liver Transplant. 2014, 20, 1211–1220. [Google Scholar] [CrossRef]
- Jo, H.S.; Khan, J.F.; Han, J.H.; Yu, Y.D.; Kim, D.S. Efficacy and Safety of Hepatitis B Virus Vaccination Following Hepatitis B Immunoglobulin Withdrawal After Liver Transplantation. Transplant. Proc. 2021, 53, 3016–3021. [Google Scholar] [CrossRef]
- Rodrigues, I.C.; De Da Silva, R.C.M.A.; De Felício, H.C.C.; Da Silva, R.F. New immunization schedule effectiveness against hepatitis B in liver transplantation patients. Arq. Gastroenterol. 2019, 56, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Chen, P.Y.; Saab, C.E.; Tong, M.J. The Management of Hepatitis B in Liver Transplant Recipients. Clin. Liver Dis. 2016, 20, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Global Hepatitis Report. 2017. Available online: https://www.who.int/publications/i/item/9789241565455 (accessed on 17 January 2024).
- Razavi-Shearer, D.; Razavi, H. Global prevalence of hepatitis B virus infection and prevention of mother-to-child transmission—Authors’ reply. Lancet Gastroenterol. Hepatol. 2018, 3, 599. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Jia, J. Prevention of Mother-to-Child Transmission of Hepatitis B Virus in the Western Pacific Region. Clin. Liver Dis. (Hoboken) 2021, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-H.; Chen, D.-S.; Hsu, H.-C.; Hsu, H.-Y.; Lee, C.-Y. Maternal transmission of hepatitis B virus in childhood hepatocellular carcinoma. Cancer 1989, 64, 2377–2380. [Google Scholar] [CrossRef]
- Stevens, C.E.; Toy, P.; Kamili, S.; Taylor, P.E.; Tong, M.J.; Xia, G.L.; Vyas, G.N. Eradicating hepatitis B virus: The critical role of preventing perinatal transmission. Biologicals 2017, 50, 3–19. [Google Scholar] [CrossRef]
- Assateerawatt, A.; Tanphaichitr, V.S.; Suvatte, V.; Yodthong, S. Immunogenicity and efficacy of a recombinant DNA hepatitis B vaccine, GenHevac B Pasteur in high risk neonates, school children and healthy adults. Asian Pac. J. Allergy Immunol. 1993, 11, 85–91. [Google Scholar]
- Poovorawan, Y.; Sanpavat, S.; Pongpunlert, W.; Chumdermpadetsuk, S.; Sentrakul, P.; Safary, A. Protective Efficacy of a Recombinant DNA Hepatitis B Vaccine in Neonates of HBe Antigen—Positive Mothers. JAMA 1989, 261, 3278–3281. [Google Scholar] [CrossRef]
- Milne, A.; West, D.J.; Van Chinh, D.; Moyes, C.D.; Poerschke, G. Field evaluation of the efficacy and immunogenicity of recombinant hepatitis B vaccine without HBIG in newborn Vietnamese infants. J. Med. Virol. 2002, 67, 327–333. [Google Scholar] [CrossRef]
- Tan, C.X.T.; Chan, S.M.; Lee, L.Y.; Ong, C.; Phua, K.B.; Aw, M.M.; Saw, S.; Lee, G.H.; Wong, F.; Thoon, K.C. Serologic Responses after Hepatitis B Vaccination in Preterm Infants Born to Hepatitis B Surface Antigen-Positive Mothers: Singapore Experience. Pediatr. Infect. Dis. J. 2017, 36, e208–e210. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Rui, Y.; Chen, J.; Chen, T.; Dai, Y.; Xu, B.; Hu, Y.; Chen, J.; Zhou, Y. Efficacy of the hepatitis B vaccine alone in the prevention of hepatitis B perinatal transmission in infants born to hepatitis B e antigen-negative carrier mothers. J. Virus Erad. 2022, 8, 100076. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Theamboonlers, A.; Vimolket, T.; Sinlaparatsamee, S.; Chaiear, K.; Siraprapasiri, T.; Khwanjaipanich, S.; Owatanapanich, S.; Hirsch, P.; Chunsuttiwat, S. Impact of hepatitis B immunisation as part of the EPI. Vaccine 2000, 19, 943–949. [Google Scholar] [CrossRef]
- Stevens, C.E.; Toy, P.T.; Taylor, P.E.; Lee, T.; Yip, H.Y. Prospects for Control of Hepatitis B Virus Infection: Implications of Childhood Vaccination and Long-term Protection. Pediatrics 1992, 90, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.M.; Chen, D.S.; Chuang, C.H.; Li, J.C.F.; Jwo, M.; Lee, C.C.; Li, H.C.; Cheng, S.H.; Wang, Y.F.; Wang, C.C.; et al. Efficacy of a Mass Hepatitis B Vaccination Program in Taiwan: Studies on 3464 Infants of Hepatitis B Surface Antigen—Carrier Mothers. JAMA 1988, 260, 2231–2235. [Google Scholar] [CrossRef]
- Burk, R.D.; Hwang, L.Y.; Ho, G.Y.F.; Shafritz, D.A.; Beasley, R.P. Outcome of perinatal hepatitis b virus exposure is dependent on maternal virus load. J. Infect. Dis. 1994, 170, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Belopolskaya, M.; Avrutin, V.; Firsov, S.; Yakovlev, A. HBsAg level and hepatitis B viral load correlation with focus on pregnancy. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 379. [Google Scholar]
- Wen, W.H.; Huang, C.W.; Chie, W.C.; Yeung, C.Y.; Zhao, L.L.; Lin, W.T.; Wu, J.F.; Ni, Y.H.; Hsu, H.Y.; Chang, M.H.; et al. Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection. Hepatology 2016, 64, 1451–1461. [Google Scholar] [CrossRef]
- Sun, K.X.; Li, J.; Zhu, F.C.; Liu, J.X.; Li, R.C.; Zhai, X.J.; Li, Y.P.; Chang, Z.J.; Nie, J.J.; Zhuang, H.A. A predictive value of quantitative HBsAg for serum HBV DNA level among HBeAg-positive pregnant women. Vaccine 2012, 30, 5335–5340. [Google Scholar] [CrossRef]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Pan, C.Q.; Duan, Z.; Dai, E.; Zhang, S.; Han, G.; Wang, Y.; Zhang, H.; Zou, H.; Zhu, B.; Zhao, W.; et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N. Engl. J. Med. 2016, 374, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis B Is Preventable with Safe and Effective Vaccines. Available online: https://www.who.int/southeastasia/activities/hepatitis-b-is-preventable-with-safe-and-effective-vaccines (accessed on 12 January 2024).
- Chang, M.H.; Chen, D.S. Prevention of Hepatitis B. Cold Spring Harb. Perspect. Med. 2015, 5, a021493. [Google Scholar] [CrossRef] [PubMed]
- Immunization Coverage. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 16 January 2024).
- Wait, S.; Chen, D.S. Towards the eradication of hepatitis B in Taiwan. Kaohsiung J. Med. Sci. 2012, 28, 1–9. [Google Scholar] [CrossRef]
- Chiang, C.J.; Yang, Y.W.; You, S.L.; Lai, M.S.; Chen, C.J. Thirty-Year Outcomes of the National Hepatitis B Immunization Program in Taiwan. JAMA 2013, 310, 974–976. [Google Scholar] [CrossRef]
- Ni, Y.H.; Chang, M.H.; Wu, J.F.; Hsu, H.Y.; Chen, H.L.; Chen, D.S. Minimization of hepatitis B infection by a 25-year universal vaccination program. J. Hepatol. 2012, 57, 730–735. [Google Scholar] [CrossRef]
- Hsu, H.M.; Lu, C.F.; Lee, S.C.; Lin, S.R.; Chen, D.S. Seroepidemiologic survey for hepatitis B virus infection in Taiwan: The effect of hepatitis B mass immunization. J. Infect. Dis. 1999, 179, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Moghadami, M.; Dadashpour, N.; Mokhtari, A.M.; Ebrahimi, M.; Mirahmadizadeh, A. The effectiveness of the national hepatitis B vaccination program 25 years after its introduction in Iran: A historical cohort study. Braz. J. Infect. Dis. 2019, 23, 419–426. [Google Scholar] [CrossRef]
- Childs, L.; Roesel, S.; Tohme, R.A. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992–2015. Vaccine 2018, 36, 6–14. [Google Scholar] [CrossRef]
- Chang, M.H. Hepatitis B virus infection. Semin. Fetal Neonatal Med. 2007, 12, 160–167. [Google Scholar] [CrossRef]
- Lolekha, S.; Warachit, B.; Hirunyachote, A.; Bowonkiratikachorn, P.; West, D.J.; Poerschke, G. Protective efficacy of hepatitis B vaccine without HBIG in infants of HBeAg-positive carrier mothers in Thailand. Vaccine 2002, 20, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.P.; Easterbrook, P.J.; McMahon, B.J. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin. Liver Dis. 2016, 20, 607. [Google Scholar] [CrossRef]
- CDC. Pinkbook: Hepatitis B. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html (accessed on 13 January 2024).
- Kao, J.H.; Hsu, H.M.; Shau, W.Y.; Chang, M.H.; Chen, D.S. Universal hepatitis B vaccination and the decreased mortality from fulminant hepatitis in infants in Taiwan. J. Pediatr. 2001, 139, 349–352. [Google Scholar] [CrossRef]
- Chen, H.L.; Chang, C.J.; Kong, M.S.; Huang, F.C.; Lee, H.C.; Lin, C.C.; Liu, C.C.; Lee, I.H.; Wu, T.C.; Wu, S.F.; et al. Pediatric fulminant hepatic failure in endemic areas of hepatitis B infection: 15 years after universal hepatitis B vaccination. Hepatology 2004, 39, 58–63. [Google Scholar] [CrossRef]
- Mele, A.; Tosti, M.E.; Mariano, A.; Pizzuti, R.; Ferro, A.; Borrini, B.; Zotti, C.; Lopalco, P.; Curtale, F.; Balocchini, E.; et al. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: Areas of improvement and emerging challenges. Clin. Infect. Dis. 2008, 46, 868–875. [Google Scholar] [CrossRef]
- Mele, A.; Mariano, A.; Tosti, M.E.; Stroffolini, T.; Pizzuti, R.; Gallo, G.; Ragni, P.; Zotti, C.; Lopalco, P.; Curtale, F.; et al. Acute hepatitis delta virus infection in Italy: Incidence and risk factors after the introduction of the universal anti-hepatitis B vaccination campaign. Clin. Infect. Dis. 2007, 44, e17–e24. [Google Scholar] [CrossRef]
- Goh, K.T. Prevention and control of hepatitis B virus infection in Singapore. Ann. Acad. Med. Singap. 1997, 26, 671–681. [Google Scholar] [PubMed]
- Bhimma, R.; Coovadia, H.M.; Adhikari, M.; Connolly, C.A. The impact of the hepatitis B virus vaccine on the incidence of hepatitis B virus-associated membranous nephropathy. Arch. Pediatr. Adolesc. Med. 2003, 157, 1025–1030. [Google Scholar] [CrossRef]
- Xu, H.; Sun, L.; Zhou, L.J.; Fang, L.J.; Sheng, F.Y.; Guo, Y.Q. The effect of hepatitis B vaccination on the incidence of childhood HBV-associated nephritis. Pediatr. Nephrol. 2003, 18, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.T.; Chang, M.H.; Lin, F.G.; Tsai, I.J.; Chang, Y.W.; Tsau, Y.K. Universal Hepatitis B Vaccination Reduces Childhood Hepatitis B Virus–Associated Membranous Nephropathy. Pediatrics 2011, 128, e600–e604. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-H.; Chen, C.-J.; Lai, M.-S.; Hsu, H.-M.; Wu, T.-C.; Kong, M.-S.; Liang, D.-C.; Shau, W.-Y.; Chen, D.-S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zi, X.; Zhu, Q.; Zheng, Y.; Wu, C.; Ren, H.; Liu, X.; Liu, Z.; Li, Y.; Pan, Q.; et al. Accelerating Decreases in the Incidences of Hepatocellular Carcinoma at a Younger Age in Shanghai Are Associated with Hepatitis B Virus Vaccination. Front. Oncol. 2022, 12, 855945. [Google Scholar] [CrossRef]
- Flores, J.E.; Thompson, A.J.; Ryan, M.; Howell, J. The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma. Vaccines 2022, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.A. Trend in incidence of hepatitis B virus infection during a decade of universal childhood hepatitis B vaccination in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Yang, J.Y.; Gong, J.; Li, Y.P.; Huang, Z.N.; Fang, K.X.; Xu, Z.Y.; Liu, C.B.; Zhao, K.; Zhuang, H. Efficacy of hepatitis B vaccination on hepatitis B prevention and on hepatocellular carcinoma. Zhonghua Liu Xing Bing Xue Za Zhi 2004, 25, 385–387. [Google Scholar] [PubMed]
- Lee, M.S.; Kim, D.H.; Kim, H.; Lee, H.S.; Kim, C.Y.; Park, T.S.; Yoo, K.Y.; Park, B.J.; Ahn, Y.O. Hepatitis B vaccination and reduced risk of primary liver cancer among male adults: A cohort study in Korea. Int. J. Epidemiol. 1998, 27, 316–319. [Google Scholar] [CrossRef]
- Sheena, B.S.; Hiebert, L.; Han, H.; Ippolito, H.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abbastabar, H.; Abdoli, A.; Abubaker Ali, H.; Adane, M.M.; et al. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.; Akmatov, M.K.; Krause, G. Hepatitis B vaccination timing: Results from demographic health surveys in 47 countries. Bull. World Health Organ. 2017, 95, 199. [Google Scholar] [CrossRef]
- Center for Global Development. Vaccine Introduction and Coverage in Gavi-Supported Countries 2015–2018: Implications for Gavi 5.0. Available online: https://www.cgdev.org/publication/vaccine-introduction-and-coverage-gavi-supported-countries-2015-2018-implications-gavi (accessed on 25 January 2024).
- BIGHAM, J. Implementing and Understanding Barriers to the Updated Hepatitis B Vaccination Recommendations and Guidance. Fam. Pract. Manag. 2023, 30, 29–32. [Google Scholar]
- Machmud, P.B.; Führer, A.; Gottschick, C.; Mikolajczyk, R. Barriers to and Facilitators of Hepatitis B Vaccination among the Adult Population in Indonesia: A Mixed Methods Study. Vaccines 2023, 11, 398. [Google Scholar] [CrossRef]
- Freeland, C.; Kanu, F.; Mohammed, Y.; Nwokoro, U.U.; Sandhu, H.; Ikwe, H.; Uba, B.; Asekun, A.; Akataobi, C.; Adewole, A.; et al. Barriers and facilitators to hepatitis B birth dose vaccination: Perspectives from healthcare providers and pregnant women accessing antenatal care in Nigeria. PLOS Global Public Health 2023, 3, e0001332. [Google Scholar] [CrossRef]
- Mohanty, P.; Jena, P.; Patnaik, L. Vaccination against Hepatitis B: A Scoping Review. Asian Pac. J. Cancer Prev. 2020, 21, 3453–3459. [Google Scholar] [CrossRef]
- Ali, A.S.; Hussein, N.A.; Elmi, E.O.H.; Ismail, A.M.; Abdi, M.M. Hepatitis B vaccination coverage and associated factors among medical students: A cross-sectional study in Bosaso, Somalia, 2021. BMC Public Health 2023, 23, 1060. [Google Scholar] [CrossRef]
- Smith, S.; Harmanci, H.; Hutin, Y.; Hess, S.; Bulterys, M.; Peck, R.; Rewari, B.; Mozalevskis, A.; Shibeshi, M.; Mumba, M.; et al. Global progress on the elimination of viral hepatitis as a major public health threat: An analysis of WHO Member State responses 2017. JHEP Rep 2019, 1, 81–89. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis, B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 16 January 2024).
- The Global Hepatitis Health Sector Strategy—Global 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442270/ (accessed on 29 January 2024).
- Thomas, D.L. Global Elimination of Chronic Hepatitis. N. Engl. J. Med. 2019, 380, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, W.; Jing, W.; Liu, M. Countdown to 2030: Eliminating hepatitis B disease, China. Bull. World Health Organ. 2019, 97, 230–238. [Google Scholar] [CrossRef]
- Razavi-Shearer, D.; Gamkrelidze, I.; Nguyen, M.H.; Chen, D.S.; Van Damme, P.; Abbas, Z.; Abdulla, M.; Abou Rached, A.; Adda, D.; Aho, I.; et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, S.; Shimakawa, Y.; Lemoine, M. Mother-to-child transmission of hepatitis B: What more needs to be done to eliminate it around the world? J. Viral Hepat. 2020, 27, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Schröeder, S.E.; Pedrana, A.; Scott, N.; Wilson, D.; Kuschel, C.; Aufegger, L.; Atun, R.; Baptista-Leite, R.; Butsashvili, M.; El-Sayed, M.; et al. Innovative strategies for the elimination of viral hepatitis at a national level: A country case series. Liver Int. 2019, 39, 1818–1836. [Google Scholar] [CrossRef] [PubMed]
- Tordrup, D.; Hutin, Y.; Stenberg, K.; Lauer, J.A.; Hutton, D.W.; Toy, M.; Scott, N.; Bulterys, M.; Ball, A.; Hirnschall, G.; et al. Additional resource needs for viral hepatitis elimination through universal health coverage: Projections in 67 low-income and middle-income countries, 2016–2030. Lancet Glob. Health 2019, 7, e1180–e1188. [Google Scholar] [CrossRef] [PubMed]
- Razavi, H.; Sanchez Gonzalez, Y.; Yuen, C.; Cornberg, M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020, 40, 522–529. [Google Scholar] [CrossRef]
- Palayew, A.; Razavi, H.; Hutchinson, S.J.; Cooke, G.S.; Lazarus, J.V. Do the most heavily burdened countries have the right policies to eliminate viral hepatitis B and C? Lancet Gastroenterol. Hepatol. 2020, 5, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Kondili, L.A.; Buti, M.; Riveiro-Barciela, M.; Maticic, M.; Negro, F.; Berg, T.; Craxì, A. Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey. JHEP Rep. 2022, 4, 100531. [Google Scholar] [CrossRef] [PubMed]
- Blach, S.; Kondili, L.A.; Aghemo, A.; Cai, Z.; Dugan, E.; Estes, C.; Gamkrelidze, I.; Ma, S.; Pawlotsky, J.M.; Razavi-Shearer, D.; et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 2021, 74, 31. [Google Scholar] [CrossRef] [PubMed]
- Sowah, L.; Chiou, C. Impact of Coronavirus Disease 2019 Pandemic on Viral Hepatitis Elimination: What Is the Price? AIDS Res. Hum. Retroviruses 2021, 37, 585. [Google Scholar] [CrossRef]
- Laury, J.; Hiebert, L.; Ward, J.W. Impact of COVID-19 Response on Hepatitis Prevention Care and Treatment: Results from Global Survey of Providers and Program Managers. Clin. Liver Dis. 2021, 17, 41–46. [Google Scholar] [CrossRef]
- Bertram, M.Y.; Edejer, T.T.T. Introduction to the Special Issue on ‘The World Health Organization Choosing Interventions That Are Cost-Effective (WHO-CHOICE) Update’. Int. J. Health Policy Manag. 2021, 10, 670–672. [Google Scholar] [CrossRef]
- Consolidated Strategic Information Guidelines for Viral Hepatitis Planning and Tracking Progress towards Elimination: Guidelines. Available online: https://www.who.int/publications/i/item/9789241515191 (accessed on 3 February 2024).
- New Cost-Effectiveness Updates from WHO-CHOICE. Available online: https://www.who.int/news-room/feature-stories/detail/new-cost-effectiveness-updates-from-who-choice (accessed on 3 February 2024).
- Hepatitis WPRO. Available online: https://www.who.int/westernpacific/health-topics/hepatitis#tab=tab_1 (accessed on 3 February 2024).
- Gavi, the Vaccine Alliance. From Rabies to Hepatitis: Gavi to Start Rolling out New Vaccines to Lower-Income Countries. Available online: https://www.gavi.org/vaccineswork/kickstarting-essential-immunisation-roll-outs (accessed on 16 January 2024).
- Lee, S.W.; Kwon, J.H.; Lee, H.L.; Yoo, S.H.; Nam, H.C.; Sung, P.S.; Nam, S.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: A large-scale, propensity score analysis. Gut 2020, 69, 1301–1308. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. Review article: Novel therapies for hepatitis B virus cure—Advances and perspectives. Aliment. Pharmacol. Ther. 2016, 44, 213–222. [Google Scholar] [CrossRef]
- Lee, S.W.; Kwon, J.H.; Lee, H.L.; Yoo, S.H.; Nam, H.C.; Sung, P.S.; Nam, S.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B—Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology 2019, 71, 1070–1092. [Google Scholar] [CrossRef]
- Polaris Observatory—CDA Foundation. Available online: https://cdafound.org/polaris/ (accessed on 2 February 2024).
- Coalition for Global Hepatitis Elimination. The Hepatitis Prevention, Control, and Elimination Program in Mongolia—Элэг бүтэн Монгол үндэсний хѳтѳлбѳр (National Program). Available online: https://www.globalhep.org/about/partner-programs/hepatitis-prevention-control-and-elimination-program-mongolia-eleg-buten (accessed on 3 February 2024).
- Find the Missing Millions—World Hepatitis Alliance. Available online: https://www.worldhepatitisalliance.org/find-the-missing-millions/ (accessed on 4 February 2024).
- Rajbhandari, R.; Chung, R.T. Screening for hepatitis B virus infection: A public health imperative. Ann. Intern. Med. 2014, 161, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Toumi, M.; Wallace, J.; Cohen, C.; Marshal, C.; Kitchen, H.; Macey, J.; Pegram, H.; Slagle, A.F.; Gish, R.G.; Ning, Q.; et al. Experience and impact of stigma in people with chronic hepatitis B: A qualitative study in Asia, Europe, and the United States. BMC Public Health 2024, 24, 611. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Chen, G.J.; Hung, C.C.; Yu, M.L. HCV Microelimination for High-risk Special Populations. J. Infect. Dis. 2023, 228 (Suppl. S3), S168–S179. [Google Scholar] [CrossRef]
- Abbas, Z.; Abbas, M. Challenges in Formulation and Implementation of Hepatitis B Elimination Programs. Cureus 2021, 13, e14657. [Google Scholar] [CrossRef]
- Lemoine, M.; Shimakawa, Y.; Njie, R.; Taal, M.; Ndow, G.; Chemin, I.; Ghosh, S.; Njai, H.F.; Jeng, A.; Sow, A.; et al. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: The Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. Lancet Glob. Health 2016, 4, e559–e567. [Google Scholar] [CrossRef]
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: Towards a cure. Nat. Rev. Drug Discov. 2019, 18, 827–844. [Google Scholar] [CrossRef]
- Howell, J.; Pedrana, A.; Schroeder, S.E.; Scott, N.; Aufegger, L.; Atun, R.; Baptista-Leite, R.; Hirnschall, G.; ‘t Hoen, E.; Hutchinson, S.J.; et al. A global investment framework for the elimination of hepatitis B. J. Hepatol. 2021, 74, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S. Fighting against viral hepatitis: Lessons from Taiwan. Hepatology 2011, 54, 381–392. [Google Scholar] [CrossRef] [PubMed]


| Target Area | Baseline 2015 | 2020 Targets | 2030 Targets |
|---|---|---|---|
| Impact targets | |||
| Incidence: New cases of CHB and CHC infections | Between 6 and 10 million infections are reduced to 0.9 million infections by 2030 (95% decline in HBV infections, 80% decline in HCV infections) | 30% reduction (equivalent to 1% prevalence of HBsAg among children) | 90% reduction (equivalent to 0.1% prevalence of HBsAg among children) |
| Mortality: HBV and HCV deaths | 1.4 million deaths reduced to less than 500,000 by 2030 (65% for both HBV and HCV) | 10% reduction | 65% reduction |
| Service coverage targets | |||
| HBV vaccination: childhood vaccine coverage (third dose coverage) | 82% in infants | 90% | 90% |
| Prevention of HBV MTCTs: HBV birth-dose vaccination coverage or other approach to prevent MTCTs | 38% | 50% | 90% |
| Blood safety | 39 countries do not routinely test all blood donations for transfusion transmissible infections, 89% of donations are screened in a quality-assured manner | All countries have hemovigilance systems in place to identify and quantify viral hepatitis transfusion transmission rates | Reduce rates of transmission by 99% compared with 2020 |
| Safe injections: percentage of injections administered with safety-engineered devices in and out of health facilities | 5% | 50% | 90% |
| Harm reduction: number of sterile needles and syringes provided per person who injects drugs per year | 20 | 200 | 300 |
| HBV and HCV diagnosis | <5% of chronic hepatitis infections diagnosed | 50% | 90% |
| HBV and HCV treatment | <1% receiving treatment | 5 million people receiving HBV treatment, 3 million people received HCV treatment | 80% of eligible persons with CHB infection treated, 80% of eligible persons with CHC infection treated |
| Maternal Screening | Infants Receive | Efficacy | Cost | Example | |
|---|---|---|---|---|---|
| Vaccine | HBIG | ||||
| Yes (HBsAg and then HBeAg) | Yes | HBeAg-positive mothers’ infants only | Higher | Higher | Taiwan |
| Yes (HBsAg only) | Yes | All HBsAg-positive mothers’ infants | Highest | Highest | US |
| Yes (HBeAg only) | Yes | HBeAg-positive mothers’ infants only (2 doses) | High | Highest | Japan |
| No | Yes | No | Modest | Low | Thailand |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Busafi, S.A.; Alwassief, A. Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030. Vaccines 2024, 12, 288. https://doi.org/10.3390/vaccines12030288
Al-Busafi SA, Alwassief A. Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030. Vaccines. 2024; 12(3):288. https://doi.org/10.3390/vaccines12030288
Chicago/Turabian StyleAl-Busafi, Said A., and Ahmed Alwassief. 2024. "Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030" Vaccines 12, no. 3: 288. https://doi.org/10.3390/vaccines12030288
APA StyleAl-Busafi, S. A., & Alwassief, A. (2024). Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030. Vaccines, 12(3), 288. https://doi.org/10.3390/vaccines12030288






