Raising Epidemiological Awareness: Assessment of Measles/MMR Susceptibility in Highly Vaccinated Clusters within the Hungarian and Croatian Population—A Sero-Surveillance Analysis
Abstract
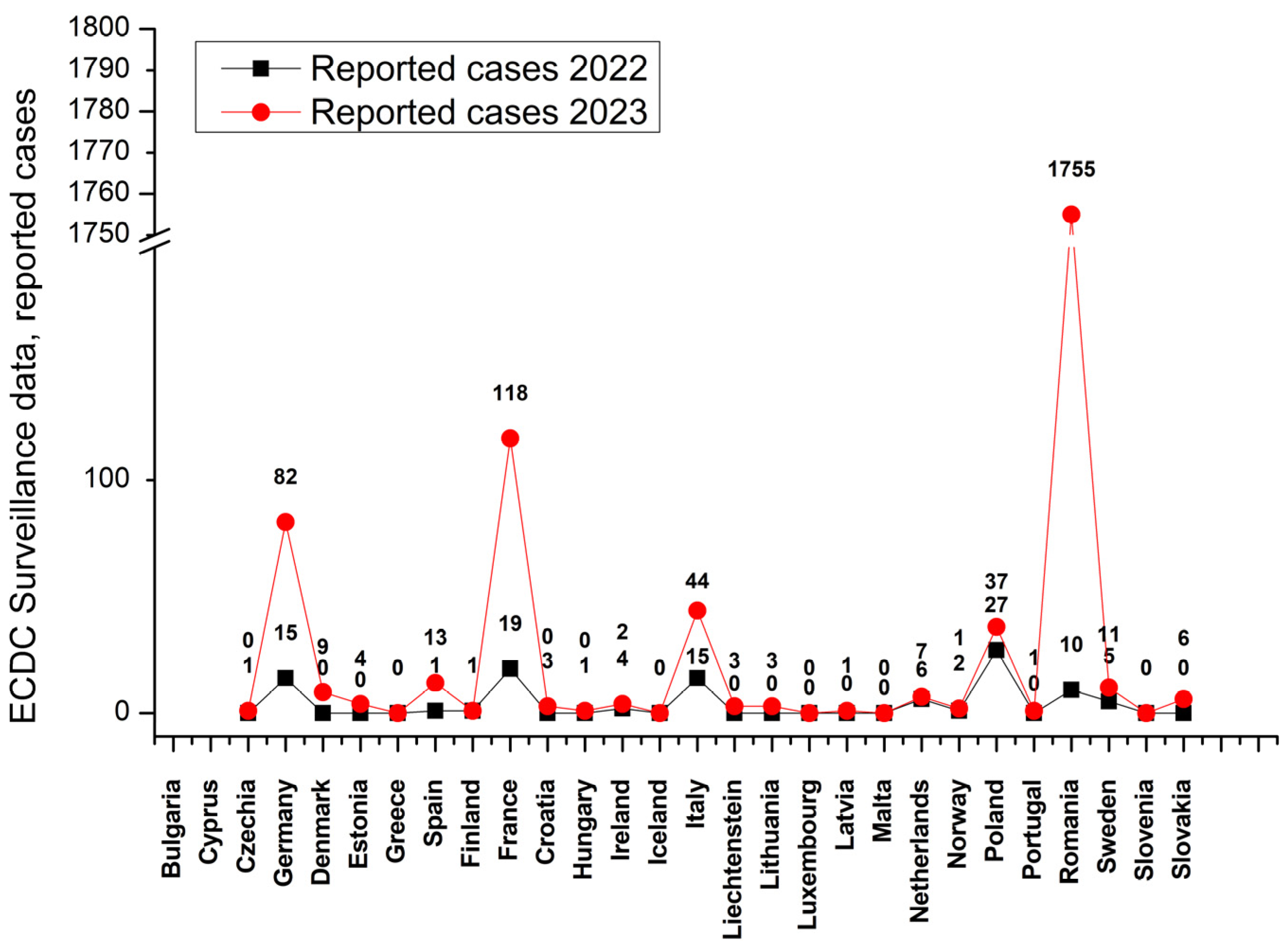
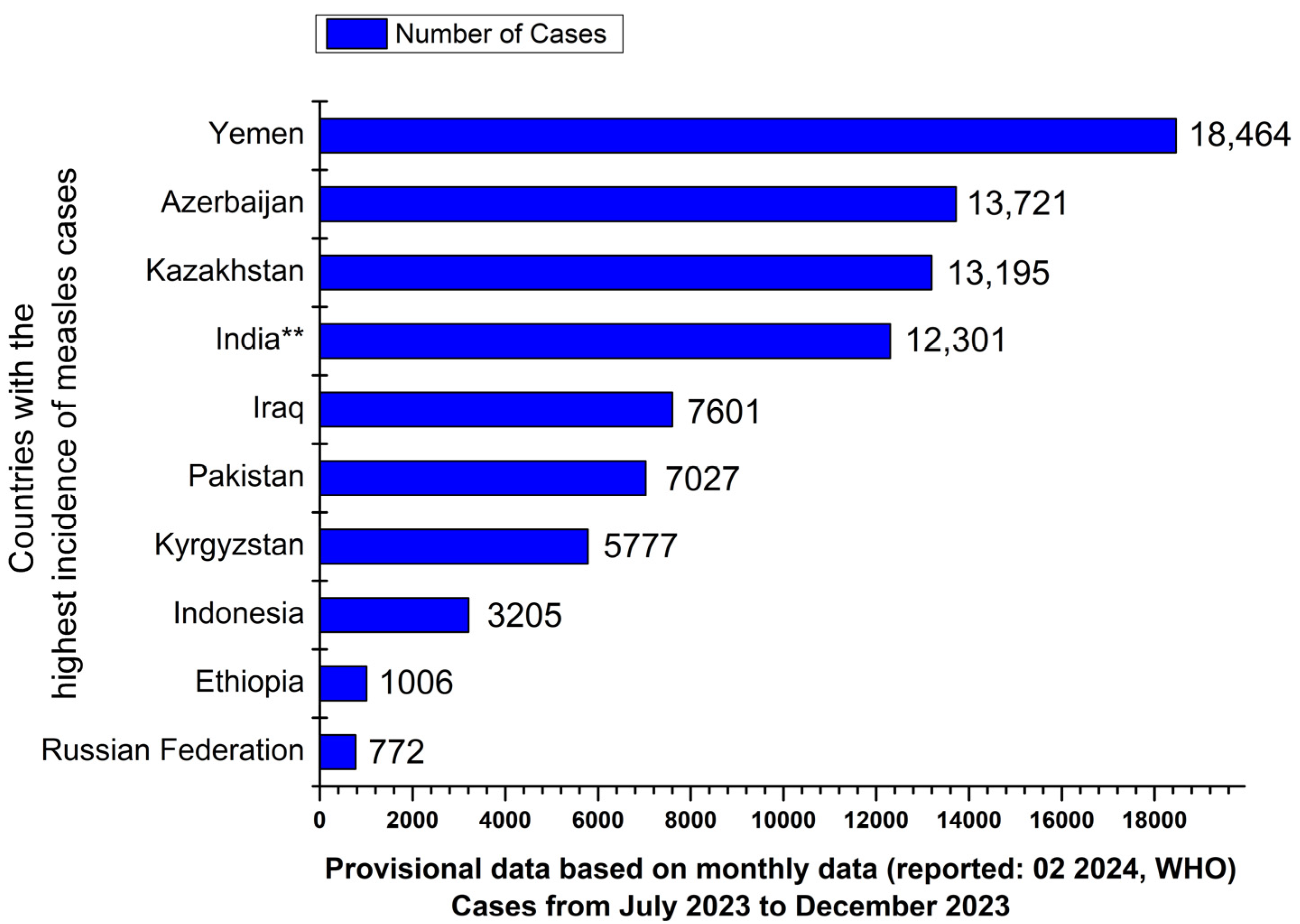
1. Introduction
2. Materials and Methods
2.1. Human Serum Samples
2.2. ImmunoSerological Meassurment of Human Serum Samples
2.3. Methods of Result Evaluation
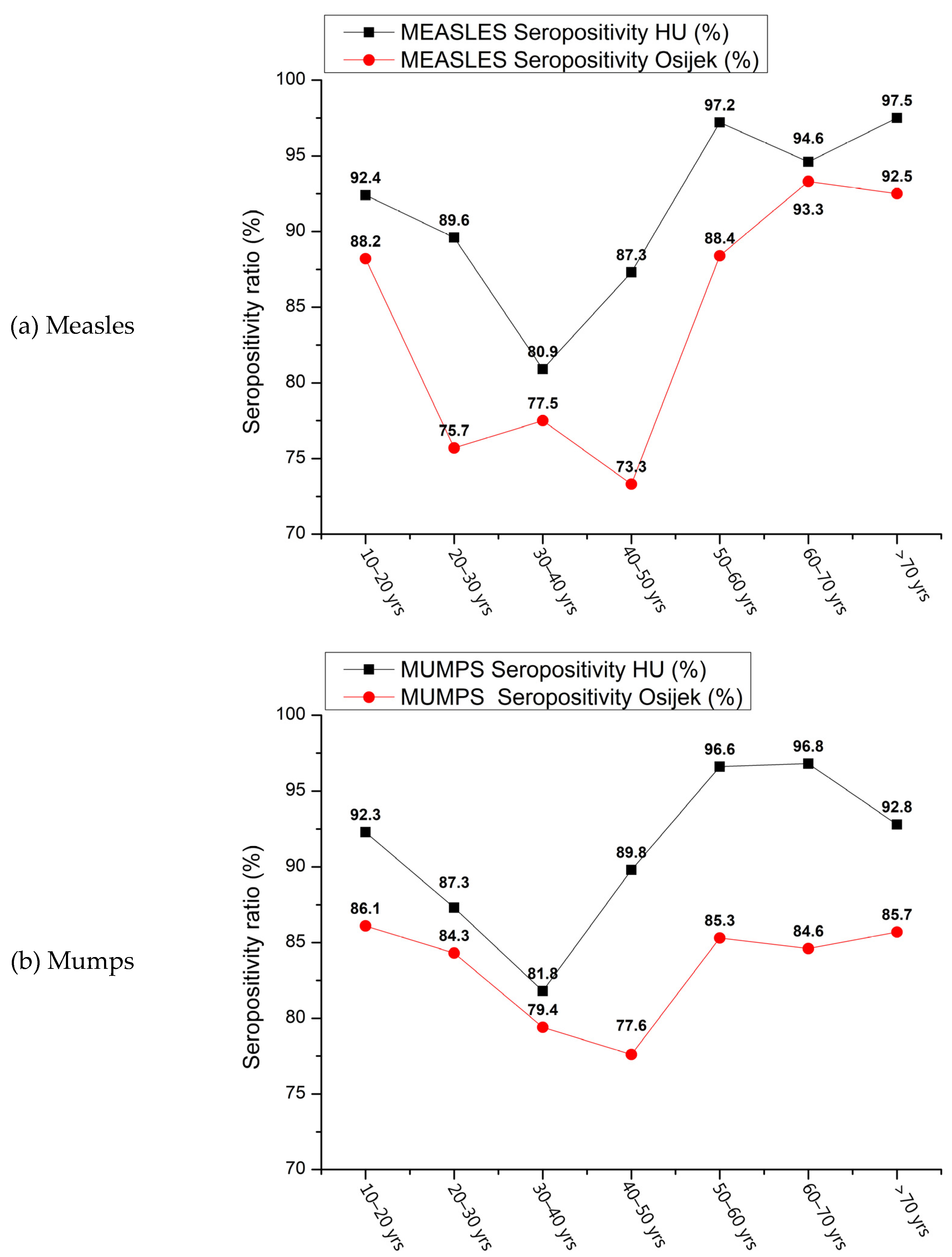
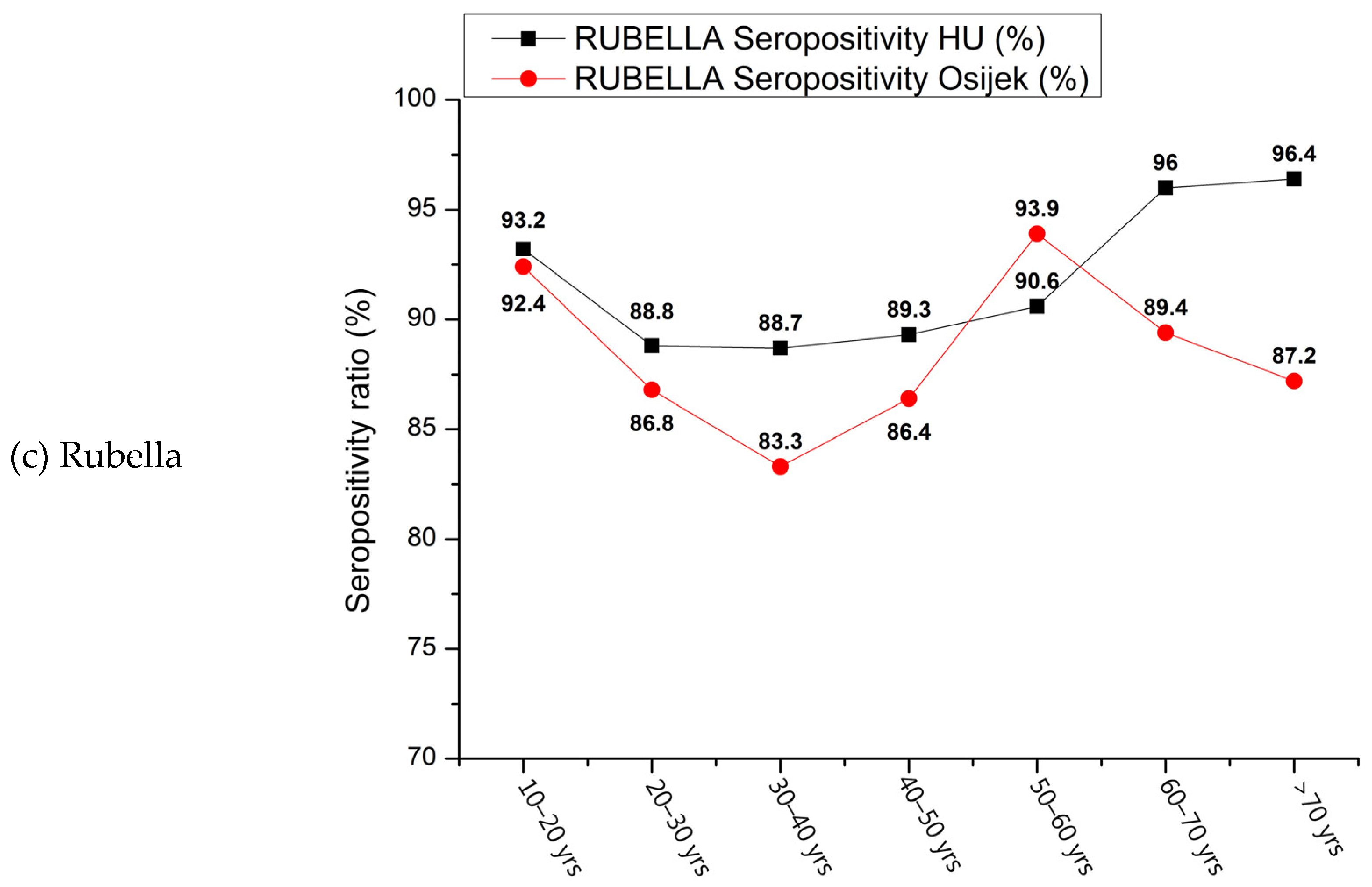
3. Results
4. Discussion
5. Conclusions
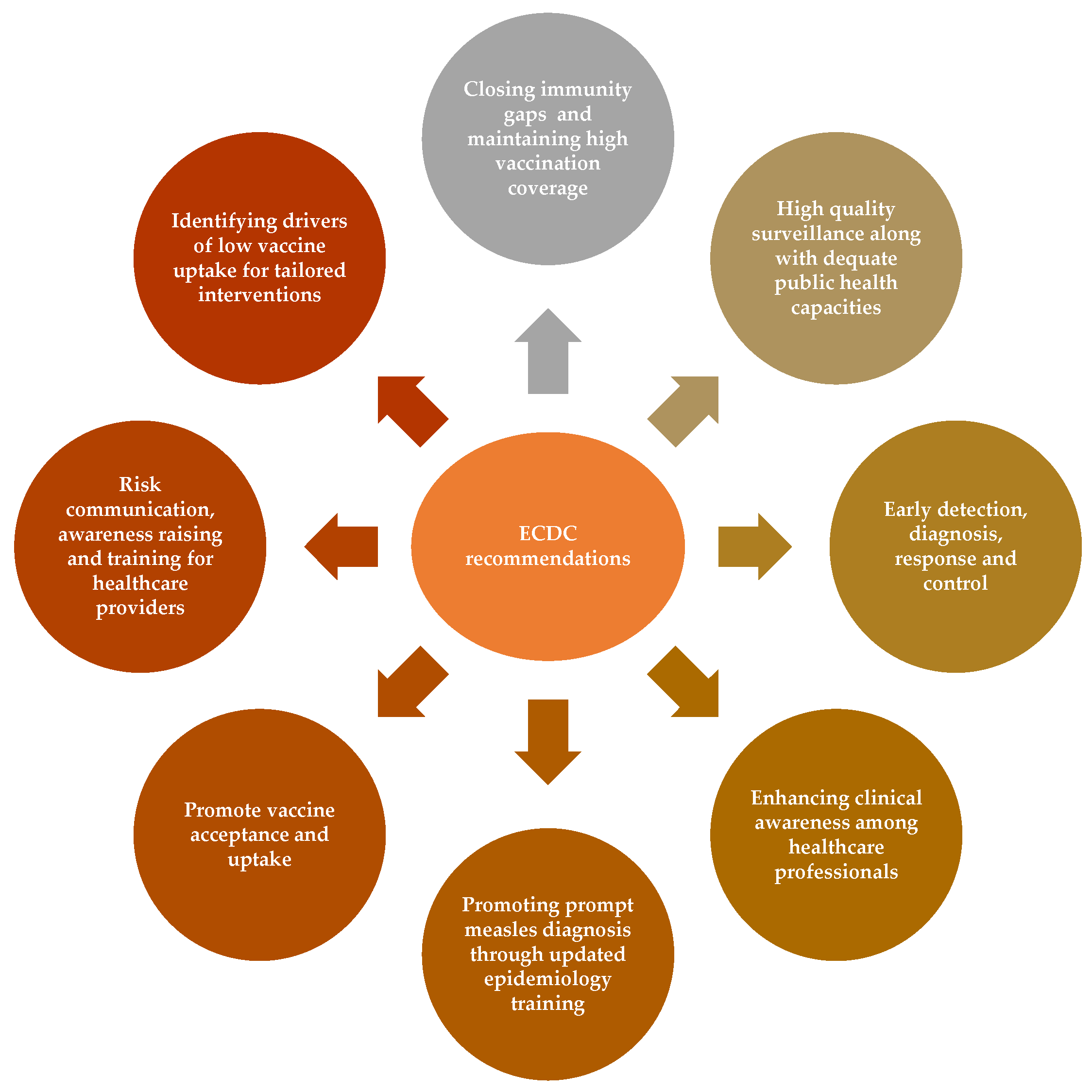
6. Implications of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rechel, B.; Mladovsky, P.; Ingleby, D.; Mackenbach, J.P.; McKee, M. Migration and Health in an Increasingly Diverse Europe. Lancet 2013, 381, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Metcalf, C.J.E.; Cutts, F.T.; Grenfell, B.T. Impact on Epidemic Measles of Vaccination Campaigns Triggered by Disease Outbreaks or Serosurveys: A Modeling Study. PLoS Med. 2016, 13, e1002144. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, P.; Poletti, P.; Stella, M.; Lepri, B.; Merler, S.; de Domenico, M. Heterogeneity in Social and Epidemiological Factors Determines the Risk of Measles Outbreaks. Proc. Natl. Acad. Sci. USA 2020, 117, 30118–30125. [Google Scholar] [CrossRef]
- Padovese, V.; Egidi, A.M.; Fenech, T.M.; Connor, M.P.; Didero, D.; Costanzo, G.; Mirisola, C. Migration and Determinants of Health: Clinical Epidemiological Characteristics of Migrants in Malta (2010–2011). J. Public Health 2014, 36, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Blitz, B.K.; D’Angelo, A.; Kofman, E.; Montagna, N. Health Challenges in Refugee Reception: Dateline Europe 2016. Int. J. Environ. Res. Public Health 2017, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Thomas, S.D.M. Displacement and Health. Br. Med. Bull. 2004, 69, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Kyprianidou, M.; Fakonti, G.; Tzira, E.; Pylli, M.; Giannakou, K. Associations between Socio-Demographic Characteristics and Maternal Attitudes towards Childhood Vaccination in Cyprus—A Cross-Sectional Survey. COVID 2023, 3, 1042–1051. [Google Scholar] [CrossRef]
- Abubakar, I.; Aldridge, R.W.; Devakumar, D.; Orcutt, M.; Burns, R.; Barreto, M.L.; Dhavan, P.; Fouad, F.M.; Groce, N.; Guo, Y.; et al. The UCL-Lancet Commission on Migration and Health: The Health of a World on the Move. Lancet 2018, 392, 2606–2654. [Google Scholar] [CrossRef]
- Islam, T.; Mandal, S.; Chouhan, P.; Bosetti, P.; Poletti, P.; Stella, M.; Lepri, B.; Merler, S.; de Domenico, M. Influence of Socio-Demographic Factors on Coverage of Full Vaccination among Children Aged 12–23 Months: A Study in Indian Context (2015–2016). Proc. Natl. Acad. Sci. USA 2020, 17, 5226–5234. [Google Scholar] [CrossRef]
- Bambra, C.; Riordan, R.; Ford, J.; Matthews, F. The COVID-19 Pandemic and Health Inequalities. J. Epidemiol. Commun. Health 2020, 74, 964–968. [Google Scholar] [CrossRef]
- Perry, M.; Akbari, A.; Cottrell, S.; Gravenor, M.B.; Roberts, R.; Lyons, R.A.; Bedston, S.; Torabi, F.; Griffiths, L. Inequalities in Coverage of COVID-19 Vaccination: A Population Register Based Cross-Sectional Study in Wales, UK. Vaccine 2021, 39, 6256–6261. [Google Scholar] [CrossRef] [PubMed]
- Sacre, A.; Bambra, C.; Wildman, J.M.; Thomson, K.; Bennett, N.; Sowden, S.; Todd, A. Socioeconomic Inequalities in Vaccine Uptake: A Global Umbrella Review. PLoS ONE 2023, 18, e0294688. [Google Scholar] [CrossRef]
- WHO COVID-19 Pandemic Fuels Largest Continued Backslide in Vaccinations in Three Decades. Available online: https://www.who.int/news/item/15-07-2022-covid-19-pandemic-fuels-largest-continued-backslide-in-vaccinations-in-three-decades (accessed on 25 April 2024).
- Laajaj, R.; Webb, D.; Aristizabal, D.; Behrentz, E.; Bernal, R.; Buitrago, G.; Cucunubá, Z.; de la Hoz, F.; Gaviria, A.; Hernández, L.J.; et al. Understanding How Socioeconomic Inequalities Drive Inequalities in COVID-19 Infections. Sci. Rep. 2022, 12, 8269. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E. Background and Introduction: UK Experiences of Health Measured, and Monitored. In Health Inequalities: Critical Perspectives; Oxford Academic: Oxford, UK, 2015; pp. 1–21. ISBN 9780198703358. [Google Scholar]
- Rainey, J.J.; Watkins, M.; Ryman, T.K.; Sandhu, P.; Bo, A.; Banerjee, K. Reasons Related to Non-Vaccination and under-Vaccination of Children in Low and Middle Income Countries: Findings from a Systematic Review of the Published Literature, 1999–2009. Vaccine 2011, 29, 8215–8221. [Google Scholar] [CrossRef]
- Antai, D. Inequitable Childhood Immunization Uptake in Nigeria: A Multilevel Analysis of Individual and Contextual Determinants. BMC Infect. Dis. 2009, 9, 181. [Google Scholar] [CrossRef]
- Sia, D.; Fournier, P.; Kobiané, J.F.; Sondo, B.K. Rates of Coverage and Determinants of Complete Vaccination of Children in Rural Areas of Burkina Faso (1998–2003). BMC Public Health 2009, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, J.; Gerver, S.M.; Gill, M.; Cooper, E.; Manikam, L.; Ward, H. The Global Effect of Maternal Education on Complete Childhood Vaccination: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2017, 17, 801. [Google Scholar] [CrossRef]
- Vikram, K.; Vanneman, R.; Desai, S. Linkages between Maternal Education and Childhood Immunization in India. Soc. Sci. Med. 2012, 75, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Paunio, M.; Hedman, K.; Davidkin, I.; Valle, M.; Heinonen, O.P.; Leinikki, P.; Salmi, A.; Peltola, H. Secondary Measles Vaccine Failures Identified by Measurement of IgG Avidity: High Occurrence among Teenagers Vaccinated at a Young Age. Epidemiol. Infect. 2000, 124, 263–271. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Are the Objectives Proposed by the Who for Routine Measles Vaccination Coverage and Population Measles Immunity Sufficient to Achieve Measles Elimination from Europe? Vaccines 2020, 8, 218. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Low Percentages of Measles Vaccination Coverage with Two Doses of Vaccine and Low Herd Immunity Levels Explain Measles Incidence and Persistence of Measles in the European Union in 2017–2018. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.G.; Meekison, W.G.; Arcand, T.A.; Schechter, M.T. The Role of Secondary Vaccine Failures in Measles Outbreaks. Am. J. Public Health 1989, 79, 475–478. [Google Scholar] [CrossRef]
- Plotkin, S.A. Failures of Protection by Measles Vaccine. J. Pediatr. 1973, 82, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Zahraei, S.M.; Izadi, S.; Mokhtari-Azad, T. Factors Affecting the Seroconversion Rate of 12-Month-Old Babies after the First Injection of Measles Vaccine in the Southeast of Iran. Hum. Vaccines Immunother. 2016, 12, 3118–3124. [Google Scholar] [CrossRef]
- Bahl, S.; Khanal, S.; Sangal, L.; Tabassum, S.; Ungchusak, K.; Andrus, J. Measles and Rubella Elimination: Protecting Children through Immunization in South-East Asia Region (SEAR). Lancet Reg. Health Southeast Asia 2023, 18, 100303. [Google Scholar] [CrossRef] [PubMed]
- Plans-Rubió, P. Why Does Measles Persist in Europe? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The Basic Reproduction Number (R0) of Measles: A Systematic Review. Lancet. Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef]
- Duncan, C.J.; Duncan, S.R.; Scott, S. The Dynamics of Measles Epidemics. Theor. Popul. Biol. 1997, 52, 155–163. [Google Scholar] [CrossRef]
- Berche, P. History of Measles. Press. Medicale 2022, 51, 104149. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Dénes, A. Stability and Threshold Dynamics in a Seasonal Mathematical Model for Measles Outbreaks with Double-Dose Vaccination. Mathematics 2023, 11, 1791. [Google Scholar] [CrossRef]
- Microbiology, A.S. Measles Vaccination and Infection: Questions and Misconceptions. Available online: https://asm.org/articles/2019/july/measles-vaccination-and-infection-questions-and-mi (accessed on 21 March 2024).
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning Immunity and Re-Emergence of Measles and Mumps in the Vaccine Era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Böröcz, K.; Samardžić, S.; Drenjančević, I.; Markovics, Á.; Berki, T.; Németh, P. Dynamic Features of Herd Immunity: Similarities in Age-Specific Anti-Measles Seroprevalence Data between Two Countries of Different Epidemiological History. J. Clin. Med. 2022, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 23 March 2024).
- ECDC. THREAT ASSESSMENT BRIEF Measles on the Rise in the EU/EEA: Considerations for Public Health Response Epidemiological Situation Global Situation; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- World Health Organization. A 30-Fold Rise of Measles Cases in 2023 in the WHO European Region Warrants Urgent Action. Available online: https://www.who.int/europe/news/item/14-12-2023-a-30-fold-rise-of-measles-cases-in-2023-in-the-who-european-region-warrants-urgent-action (accessed on 20 April 2024).
- WHO; European Region Measles and Rubella Monthly Update—WHO European Region—March 2023. Available online: https://cdn.who.int/media/docs/librariesprovider2/euro-health-topics/vaccines-and-immunization/eur_mr_monthly-_update_en_march-2023.pdf?sfvrsn=ddc18e02_2&download=true (accessed on 20 April 2024).
- WHO. Immunization Analysis and Insights. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/immunization-coverage (accessed on 20 April 2024).
- Parums, D.V. Editorial: Global Health Concerns as Vaccine-Preventable Infections Including SARS-CoV-2 (JN.1), Influenza, Respiratory Syncytial Virus (RSV), and Measles Continue to Rise. Med. Sci. Monit. 2024, 30, e943911. [Google Scholar] [CrossRef] [PubMed]
- CDC Measles (Rubeola) Measles Cases and Outbreaks|CDC. Available online: https://www.cdc.gov/measles/cases-outbreaks.html#print (accessed on 20 April 2024).
- Mathis, A.D.; Raines, K.; Masters, N.B.; Filardo, T.D.; Kim, G.; Crooke, S.N.; Bankamp, B.; Rota, P.A.; Sugerman, D.E. Measles—United States, 1 January 2020–28 March 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.B.; Qureshi, K. Resurgence of Measles in Europe: A Systematic Review on Parental Attitudes and Beliefs of Measles Vaccine. J. Epidemiol. Glob. Health 2020, 10, 46–58. [Google Scholar] [CrossRef]
- Novilla, M.L.B.; Goates, M.C.; Redelfs, A.H.; Quenzer, M.; Novilla, L.K.B.; Leffler, T.; Holt, C.A.; Doria, R.B.; Dang, M.T.; Hewitt, M.; et al. Why Parents Say No to Having Their Children Vaccinated against Measles: A Systematic Review of the Social Determinants of Parental Perceptions on MMR Vaccine Hesitancy. Vaccines 2023, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Califf, R. Is Vaccination Approaching a Dangerous Tipping Point? JAMA 2024, 331, 283–284. [Google Scholar] [CrossRef]
- Brumbaugh, K.; Ornelas, I.J.; Casas, F.R.; Mokdad, A.H. Achieving Equity in Childhood Vaccination: A Mixed-Methods Study of Immunization Programs, Policies, and Coverage in 3 US States. J. Public Health Manag. Pract. 2024, 30, E31–E40. [Google Scholar] [CrossRef]
- Parums, D.V. A Review of the Resurgence of Measles, a Vaccine-Preventable Disease, as Current Concerns Contrast with Past Hopes for Measles Elimination. Med. Sci. Monit. 2024, 30, e944436. [Google Scholar] [CrossRef]
- Tanne, J.H. Measles in the US: Philadelphia Reports Outbreak and Travellers through DC Airports Warned of Possible Exposure. BMJ 2024, 384, q111. [Google Scholar] [CrossRef]
- Phadke, V.K.; Bednarczyk, R.A.; Salmon, D.A.; Omer, S.B. Association between Vaccine Refusal and Vaccine-Preventable Diseases in the United States A Review of Measles and Pertussis. JAMA J. Am. Med. Assoc. 2016, 315, 1149–1158. [Google Scholar] [CrossRef]
- Gardner, L.; Dong, E.; Khan, K.; Sarkar, S. Persistence of US Measles Risk Due to Vaccine Hesitancy and Outbreaks Abroad. Lancet Infect. Dis. 2020, 20, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. Measles Outbreaks in US and Abroad Prompt CDC Vaccination Alert. JAMA 2024. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO. Epidemiological Alert—Measles in the Region of the Americas—29 January 2024; PAHO/WHO: Washington, DC, USA, 2024; pp. 1–6. [Google Scholar]
- World Health Organization (WHO). Strengthening Response to Measles Outbreak in Ukraine. Available online: https://www.who.int/europe/news/item/22-01-2020-strengthening-response-to-measles-outbreak-in-ukraine (accessed on 12 April 2020).
- Wadman, M. Measles Epidemic in Ukraine Drove Troubling European Year. Science 2019, 363, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Orsini, D.; Martini, M. Measles: A New Danger for Ukraine’s Children! The Need for an Effective and Timely Vaccination Prevention Campaign for an Insidious Disease That Comes from Afar. J. Prev. Med. Hyg. 2023, 64, E204–E208. [Google Scholar] [CrossRef] [PubMed]
- Debate, V. Measles, War, and Health-Care Reforms in Ukraine. Lancet 2018, 392, 711. [Google Scholar]
- Holt, E. Experts Warn over Potential for Measles in Ukraine. Lancet 2023, 401, 719. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Smiyan, O.; Popov, S.; Petrashenko, V.; Zaitsev, I.; Redko, O.; Zahorodnii, M.; Kasyan, S. Child Health Care System in Ukraine. Turkish Arch. Pediatr. 2020, 55, S98–S104. [Google Scholar] [CrossRef]
- Angelo, K.M.; Gastañaduy, P.A.; Walker, A.T.; Patel, M.; Reef, S.; Lee, C.V.; Nemhauser, J. Spread of Measles in Europe and Implications for US Travelers. Pediatrics 2019, 144, e20190414. [Google Scholar] [CrossRef]
- Vojtek, I.; Larson, H.; Plotkin, S.; Van Damme, P. Evolving Measles Status and Immunization Policy Development in Six European Countries. Hum. Vaccin. Immunother. 2022, 18, 2031776. [Google Scholar] [CrossRef]
- Global Health Infectious Diseases Travel Fast and Far. Available online: https://www.cdc.gov/globalhealth/security/ghsa5year/cdc-5-years-ghsa.html (accessed on 20 April 2024).
- Lambach, P.; Orenstein, W.; Silal, S.; Sbarra, A.N.; Koh, M.; Aggarwal, R.; Farooqui, H.H.; Flasche, S.; Hogan, A.; Kim, S.Y.; et al. Report from the World Health Organization’s Immunization and Vaccines Related Implementation Research Advisory Committee (IVIR-AC) Meeting, Geneva, 11–13 September 2023. Vaccine 2024, 42, 1424–1434. [Google Scholar] [CrossRef]
- CDC. CDC—Global Immunization—Global Measles and Rubella 2023. Available online: https://www.cdc.gov/globalhealth/measles/index.html (accessed on 20 April 2024).
- CDC. Global Measles Outbreaks. Available online: https://www.cdc.gov/globalhealth/measles/data/global-measles-outbreaks.html (accessed on 31 January 2021).
- Böröcz, K.; Csizmadia, Z.; Markovics, Á.; Farkas, N.; Najbauer, J.; Berki, T.; Németh, P. Application of a Fast and Cost-Effective “three-in-One” MMR ELISA as a Tool for Surveying Anti-MMR Humoral Immunity: The Hungarian Experience. Epidemiol. Infect. 2020, 148, e17. [Google Scholar] [CrossRef]
- Böröcz, K.; Csizmadia, Z.; Markovics, Á.; Mészáros, V.; Farkas, K.; Telek, V.; Varga, V.; Maloba, G.O.; Bodó, K.; Najbauer, J.; et al. Development of a Robust and Standardized Immunoserological Assay for Detection of Anti-Measles IgG Antibodies in Human Sera. J. Immunol. Methods 2019, 464, 1–8. [Google Scholar] [CrossRef]
- Edmunds, W.J.; Gay, N.J.; Kretzschmar, M.; Pebody, R.G.; Wachmann, H. The Pre-Vaccination Epidemiology of Measles, Mumps and Rubella in Europe: Implications for Modelling Studies. Epidemiol. Infect. 2000, 125, 635–650. [Google Scholar] [CrossRef]
- Böröcz, K.; Simon, D.; Erdő-Bonyár, S.; Kovács, K.T.; Tuba, É.; Czirják, L.; Németh, P.; Berki, T. Relationship between Natural and Infection-Induced Antibodies in Systemic Autoimmune Diseases (SAD): SLE, SSc and RA. Clin. Exp. Immunol. 2021, 203, 32–40. [Google Scholar] [CrossRef]
- Szinger, D.; Berki, T.; Németh, P.; Erdo-Bonyar, S.; Simon, D.; Drenjančević, I.; Samardzic, S.; Zelić, M.; Sikora, M.; Požgain, A.; et al. Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation. Int. J. Mol. Sci. 2023, 24, 14961. [Google Scholar] [CrossRef]
- Böröcz, K.; Markovics, Á.; Zsuzsanna, C.; Joseph, N.; Timea, B.; Németh, P. Imported Infections versus Herd Immunity Gaps; a Didactic Demonstration of Compartment Models through the Example of a Minor Measles Outbreak In. Southeast. Eur. Med. J. 2021, 5, 1–16. [Google Scholar]
- Agócs, M.M.; Markowitz, L.E.; Straub, I.; Dömök, I. The 1988-1989 Measles Epidemic in Hungary: Assessment of Vaccine Failure. Int. J. Epidemiol. 1992, 21, 1007–1013. [Google Scholar] [CrossRef]
- International Notes Measles—Hungary. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001472.htm (accessed on 3 May 2018).
- Lengyel, G.; Marossy, A.; Ánosi, N.; Farkas, S.L.; Kele, B.; Nemes-Nikodém, É.; Szentgyörgyi, V.; Kopcsó, I.; Mátyus, M. Screening of More than 2000 Hungarian Healthcare Workers’ Anti-Measles Antibody Level: Results and Possible Population-Level Consequences. Epidemiol. Infect. 2019, 147, e7. [Google Scholar] [CrossRef]
- Rigó, Z.; Szomor, K.; Nagy, O.; Takács, M. Are We Protected? Imported Measles—On the Way to Eradication. Acta Microbiol. Immunol. Hung. 2012, 59, 119–129. [Google Scholar] [CrossRef]
- Drenjančević, I.; Samardžić, S.; Stupin, A.; Borocz, K.; Nemeth, P.; Berki, T. Measles Vaccination and Outbreaks in Croatia from 2001 to 2019; A Comparative Study to Other European Countries. Int. J. Environ. Res. Public Health 2022, 19, 4140. [Google Scholar] [CrossRef]
- Borčić, B.; Mažuran, R.; Kaić, B. Immunity to Measles in the Croatian Population. Infect. Dis. 2003, 18, 1079–1083. [Google Scholar]
- Kaić, B. Impact of Vaccination on Vaccine-Preventable Disease Burden in Croatia. Period. Biol. 2012, 114, 141–147. [Google Scholar]
- Brzovic, M.; Juretic, K.B.; Jurcev-Savicevic, A.; Mihojevic, L.; Nonkovic, D.; Rizvan, P.; Petrovic, M.V.; Tonkic, M.; Kaic, B.; Babic-Erceg, A.; et al. Measles Cases in Split-Dalmatia County (a Croatian Tourist Region), in May–July 2019: Outbreak Report and Lessons Learnt. Eur. J. Public Health 2022, 32, 948–954. [Google Scholar] [CrossRef]
- Lin, W.H.W.; Moran, E.; Adams, R.J.; Sievers, R.E.; Hauer, D.; Godin, S.; Griffin, D.E. A Durable Protective Immune Response to Wild-Type Measles Virus Infection of Macaques Is Due to Viral Replication and Spread in Lymphoid Tissues. Sci. Transl. Med. 2020, 12, eaax7799. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Thomas, A.; Larrabee, B.R.; Rubin, S.; Poland, G.A. Differential Durability of Immune Responses to Measles and Mumps Following MMR Vaccination. Vaccine 2019, 37, 1775–1784. [Google Scholar] [CrossRef]
- Takeda, M.; Tahara, M.; Nagata, N.; Seki, F. Wild-Type Measles Virus Is Intrinsically Dual-Tropic. Front. Microbiol. 2012, 2, 279. [Google Scholar] [CrossRef]
- Gonçalves, G.; Frade, J.; Nunes, C.; Mesquita, J.R.; Nascimento, M.S.J. Persistence of Measles Antibodies, Following Changes in the Recommended Age for the Second Dose of MMR-Vaccine in Portugal. Vaccine 2015, 33, 5057–5063. [Google Scholar] [CrossRef]
- Seagle, E.E.; Bednarczyk, R.A.; Hill, T.; Fiebelkorn, A.P.; Hickman, C.J.; Icenogle, J.P.; Belongia, E.A.; McLean, H.Q. Measles, Mumps, and Rubella Antibody Patterns of Persistence and Rate of Decline Following the Second Dose of the MMR Vaccine. Vaccine 2018, 36, 818–826. [Google Scholar] [CrossRef]
- Norrby, E.; Gollmar, Y. Appearance and Persistence of Antibodies against Different Virus Components after Regular Measles Infections. Infect. Immun. 1972, 6, 240–247. [Google Scholar] [CrossRef]
- MMWR. Weekly MMWR Publications|MMWR. Available online: https://www.cdc.gov/mmwr/publications/index.html (accessed on 5 May 2019).
- Molnár, Z.; Szomor, K.; Huszti, G.; Ozsvárné Csepregi, É. Local Mumps Outbreak in Hungary, 2007. Wkly. Releases 2007, 12, 3167. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe Measles and Rubella Elimination Country Profile: Hungary. Available online: https://iris.who.int/bitstream/handle/10665/337778/WHO-EURO-2020-1420-41170-55982-eng.pdf?sequence=2 (accessed on 20 April 2024).
- Bakašun, V. Mumps in the Region of Rijeka, Croatia. Eur. J. Epidemiol. 1997, 13, 117–119. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Vaccine Scheduler|ECDC. Eur. Cent. Dis. Prev. Control 2021. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=8&SelectedCountryIdByDisease=-1 (accessed on 20 April 2024).
- VACSATC. Hungary Vacsatc Vaccine Safety, Public Aspects—MMR Vaccination against Measles (Morbilli), Mumps (Epidemic Parotitis), and Rubella (German Measles). Available online: https://www.cdc.gov/vaccines/vpd/mmr/public/index.html (accessed on 20 April 2024).
- Drago, M. Ikic Reviews of Infectious Diseases 5 (1983): I–Vii. Rev. Infect. Dis. 1983, 5, 558–563. Available online: http://www.Jstor.Org/Stable/4453136 (accessed on 20 April 2024).
- GlaxoSmithKline. Priorix Product Monograph; GSK plc: Brentford, UK; Available online: https://ca.gsk.com/media/6254/priorix.pdf (accessed on 20 April 2024).
- Monograph, P. M-M-RVaxPro PRODUCT MONOGRAPH. 2023. Available online: https://www.medicines.org.uk/emc/files/pil.6307.pdf (accessed on 20 April 2024).
- Pannuti, C.S.; Morello, R.J.; De Moraes, J.C.; Curti, S.P.; Afonso, A.M.S.; Camargo, M.C.C.; De Souza, V.A.U.F. Identification of Primary and Secondary Measles Vaccine Failures by Measurement of Immunoglobulin G Avidity in Measles Cases during the 1997 São Paulo Epidemic. Clin. Diagn. Lab. Immunol. 2004, 11, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, G.; Ánosi, N.; Marossy, A.; Mátyus, M.; Bosnyákovits, T.; Orosz, L. Az Európai És a Magyarországi Kanyaróhelyzet Összefoglalása És Tanulságai. Orv. Hetil. 2019, 160, 767–773. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; De Nitto, S.; Germinario, C.; Tafuri, S. Long-Term Immunogenicity after Measles Vaccine vs. Wild Infection: An Italian Retrospective Cohort Study. Hum. Vaccines Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Kula, T.; Leng, Y.; Li, M.; De Vries, R.D.; Knip, M.; Siljander, H.; Rewers, M.; Choy, D.F.; Wilson, M.S.; et al. Measles Virus Infection Diminishes Preexistingantibodies That Offer Protection Fromother Pathogens. Sci. Viral Immunol. 2019, 606, 599–606. [Google Scholar]
- Kurata, T.; Kanbayashi, D.; Egawa, K.; Kinoshita, M.; Yoshida, H.; Miyazono, M.; Motomura, K. A Measles Outbreak from an Index Case with Immunologically Confirmed Secondary Vaccine Failure. Vaccine 2020, 38, 1467–1475. [Google Scholar] [CrossRef]
- Gallone, M.S.; Germinario, C.; Larocca, A.; Tafuri, S. Long Time Immunogenicity of Measles Vaccine in the Vaccination Era: An Open Question. Hum. Vaccines Immunother. 2017, 13, 117–119. [Google Scholar] [CrossRef]
- Holzmann, H.; Hengel, H.; Tenbusch, M.; Doerr, H.W. Eradication of Measles: Remaining Challenges. Med. Microbiol. Immunol. 2016, 205, 201–208. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Complex Correlates of Protection after Vaccination. Clin. Infect. Dis. 2013, 56, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Gilbert, P.B. Nomenclature for Immune Correlates of Protection after Vaccination. Clin. Infect. Dis. 2012, 54, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Basagaña, X.; Pedersen, M.; Barrera-Gómez, J.; Gehring, U.; Giorgis-Allemand, L.; Hoek, G.; Stafoggia, M.; Nieuwenhuijsen, M.J.; Brunekreef, B.; Slama, R. Analysis of Multicentre Epidemiological Studies: Contrasting Fixed or Random Effects Modelling and Meta-Analysis. Int. J. Epidemiol. 2018, 47, 1343–1354. [Google Scholar] [CrossRef]
- Arah, O.A. Bias Analysis for Uncontrolled Confounding in the Health Sciences. Annu. Rev. Public Health 2017, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.B.; Vanderweele, T.J. Methods to Address Confounding and Other Biases in Meta-Analyses: Review and Recommendations. Annu. Rev. Public Health 2022, 43, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Remes Lenicov, F.; Fink, N.E. Ethical Issues in the Use of Leftover Samples and Associated Personal Data Obtained from Diagnostic Laboratories. Clin. Chim. Acta 2023, 548, 117442. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, N.; Ico, L.C.; Sheridan, S.L.; Tanesi, M.Y.; Santos, C.G.; Barreto, I.; Gomes, N.; Oakley, T.; Draper, A.D.K.; Fancourt, N.S.S.; et al. The Use of Residual Serum Samples to Perform Serological Surveillance of Severe Acute Respiratory Syndrome Coronavirus 2 in Dili and Regional Areas of Timor-Leste. Trans. R. Soc. Trop. Med. Hyg. 2023, 117, 313–315. [Google Scholar] [CrossRef]
- Bonelli, F.; Sarasini, A.; Zierold, C.; Calleri, M.; Bonetti, A.; Vismara, C.; Blocki, F.A.; Pallavicini, L.; Chinali, A.; Campisi, D.; et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J. Clin. Microbiol. 2020, 58, 10–128. [Google Scholar] [CrossRef]

| 10–20 yrs | 20–30 yrs | 30–40 yrs | 40–50 yrs | 50–60 yrs | 60–70 yrs | ≥70 yrs | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| Measles | Hungary (Pécs) | 682 | 517 | 313 | 383 | 248 | 257 | 280 | 2680 |
| Croatia (Osijek) | 143 | 279 | 359 | 307 | 291 | 253 | 132 | 1764 | |
| Mumps | Hungary (Pécs) | 220 | 266 | 159 | 205 | 116 | 122 | 111 | 1199 |
| Croatia (Osijek) | 143 | 279 | 359 | 307 | 291 | 253 | 132 | 1764 | |
| Rubella | Hungary (Pécs) | 220 | 266 | 159 | 205 | 116 | 122 | 111 | 1199 |
| Croatia (Osijek) | 143 | 279 | 359 | 307 | 291 | 253 | 132 | 1764 |
| AGE (Years) | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | 60–70 | ≥70 | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Measles | Hungary (Pécs) | Sample numbers per age group | 682 | 517 | 313 | 383 | 248 | 257 | 280 | 2680 |
| Number of seronegative samples | 52 | 54 | 60 | 49 | 7 | 14 | 7 | 284.39 | ||
| Croatia (Osijek) | Sample numbers per age group | 143 | 279 | 359 | 307 | 291 | 253 | 132 | 1764 | |
| Number of seronegative samples | 17 | 68 | 81 | 82 | 34 | 17 | 10 | 309 | ||
| Mumps | Hungary (Pécs) | Sample numbers per age group | 220 | 266 | 159 | 205 | 116 | 122 | 111 | 1199 |
| Number of seronegative samples | 17 | 34 | 29 | 21 | 4 | 4 | 8 | 117 | ||
| Croatia (Osijek) | Sample numbers per age group | 143 | 279 | 359 | 307 | 291 | 253 | 132 | 1764 | |
| Number of seronegative samples | 20 | 44 | 74 | 69 | 43 | 39 | 19 | 308 | ||
| Rubella | Hungary (Pécs) | Sample numbers per age group | 220 | 266 | 159 | 205 | 116 | 122 | 111 | 1199 |
| Number of seronegative samples | 15 | 30 | 18 | 22 | 11 | 5 | 4 | 105 | ||
| Croatia (Osijek) | Sample numbers per age group | 143 | 279 | 359 | 307 | 291 | 253 | 132 | 1764 | |
| Number of seronegative samples | 11 | 37 | 60 | 42 | 18 | 27 | 17 | 212 |
| Year/Period of Vaccination | Who Received Vaccinations This Year, and What Were the Underlying Rationales for Their Administration? |
|---|---|
| Prior to 1969 | Patients who have not received vaccinations are susceptible to wild-type infections or have been through a wild-type virus infection. In 1969, the measles vaccine was introduced in Hungary, utilizing the live, attenuated Leningrad-16 strain manufactured in the Soviet Union. |
| 1969–1977 | Between 1969 and 1974, a single dose of the measles vaccine was administered during widespread campaigns to individuals aged 9–27 months. Initially, the recommended age for vaccination was 10 months, until it was adjusted to 14 months in 1978. After an initial decline in the incidence rate, notable epidemics emerged, predominantly among unvaccinated children aged 6 to 9 years, during the period spanning 1973–1974. Following the epidemic of 1980–81, individuals born from 1973 to 1977, who would have been vaccinated at 10 months, were given a revaccination. The 1988–89 epidemic predominantly affected individuals aged 17–21 years, who were prioritized for vaccination during the early phases of the vaccination program in Hungary. Subsequently, starting in 1989, children were routinely revaccinated at the age of 11 with the monovalent measles vaccine according to a structured schedule. As a result, the earliest recipients of this 11-year reminder vaccination were born in 1978. Consequently, the cohort born between 1969 and 1977 represents the final group not included in the official vaccination schedule to receive a reminder vaccine at age 11. |
| 1978–1987 | These are the first individuals who benefited from the reminder monovalent measles vaccine at the age of 11. In 1999, the administration of the trivalent vaccine was started in Hungary; consequently, those who received the first trivalent vaccine in 1999 were born in 1988. |
| 1988–1990 | In 1989, the rubella vaccine was introduced, coinciding with the initiation of the monovalent measles reminder vaccination at the age of 11. The following year, in 1990, the measles–rubella bivalent vaccines were introduced. |
| 1991–1995 | The initiation of the initial vaccine administration at 14 months of age persisted from 1978 until 1991. In 1991, the measles–mumps–rubella (MMR) trivalent vaccine was introduced. Subsequently, in 1992, the MMR vaccine was administered at 15 months of age. The MERCK MMR II, featuring the Enders’ Edmonston strain (live, attenuated), was introduced in 1996. |
| 1996–1998 | In 1996, the MERCK MMR II, incorporating the Enders’ Edmonston strain (live, attenuated), was introduced. In 1999, a shift occurred from the monovalent measles vaccine to the measles–mumps–rubella (MMR) revaccination. This transition coincided with the introduction of GSK PLUSERIX, featuring the Measles Schwarz Strain. |
| 1999–2002 | In 1999, the GSK PLUSERIX vaccine, containing the Measles Schwarz Strain, was introduced. Subsequently, in 2003, the GSK PRIORIX vaccine was introduced. |
| 2003 | In 2003, the GSK PRIORIX vaccine, containing attenuated Schwarz Measles, was introduced. |
| 2004–2005 | During the years 2004 to 2005, the MERCK MMR II vaccine was administered. |
| 2006–2010 | From 2006 to 2010, during a five-year tender period, the GSK PRIORIX vaccine containing attenuated Schwarz Measles was utilized. |
| After 2011 | Starting in 2011, a Sanofi-MSD product, MMRvaxPro, containing the live attenuated Measles virus Enders’ Edmonston strain, has been employed for both the initial vaccination and revaccination of children. Meanwhile, GSK PRIORIX remains available on the market and is predominantly utilized for vaccination in adulthood. |
| Vaccination Period (Years) | Who Received Vaccinations This Year, and What Were the Underlying Rationales for Their Administration? |
|---|---|
| …–1968/69 | 1968: The measles vaccine was incorporated into the national childhood vaccination schedule [90]. During the initial phases of vaccine implementation, individuals with a history of prior measles infection were generally not targeted for vaccination. Diagnosis primarily depended on medical history and clinical presentation, leading to the possibility that some children were not immunized due to underrecognition of past infections. |
| 1968–1969 | The live measles vaccine was cultivated in human diploid cells (WI-38) at the Institute of Immunology of Zagreb from a further-attenuated Edmonston–Zagreb strain originally propagated in tissue culture in chick embryos. The Edmonston–Zagreb strain of measles virus is a further-attenuated Edmonston–Enders strain that has undergone 19 passages in human diploid cells (WI-38), including three plaquings [92]. Based on contemporary data, post-immunization reactions induced by the Edmonston–Zagreb vaccine were categorized as mild. The incidence of individuals experiencing fever exceeding 38 °C was less than 2%. Additionally, a fourfold rise in antibody titers among the seronegative cohort exceeded 90% [92]. |
| 1969 | The implementation of large-scale measles vaccination initiatives began in the former Yugoslavia in 1969, utilizing a monovalent measles vaccine for both primary and booster doses. Children born between 1965 and 1967 who had not contracted the measles virus (MeV) were targeted for vaccination. Additionally, children attending first grade during the 1968/69 school year (typically aged 6 or 7, born in 1962 or 1963) and who remained free from measles infection were included in the vaccination campaign. Immunization efforts extended to infants in their eleventh month of life. Furthermore, children scheduled for vaccination in 1968 (those born in 1966), as well as subsequent cohorts, including second-grade students (aged 7 or 8, born in 1961–1962), and those in childcare facilities who missed vaccination opportunities due to various reasons, were also prioritized for immunization. |
| 1970 | Children born between 1963 and 1968 who had not been previously exposed to measles and had not undergone any vaccination were administered immunization, except for those designated to receive the third dose of the DTaP (Diphtheria, Tetanus, Pertussis) vaccine. Additionally, vaccination was provided to children in the fourth grade of elementary school during the 1969/70 academic year (aged 9 or 10; born in 1959 or 1960), who had not encountered the measles virus and had not yet received vaccination. Furthermore, infants in their eleventh month of life were administered vaccination following the continuous protocol. |
| 1973 | Primary vaccination was administered to children at one year of age, with the additional inclusion of the rubella component. |
| 1974 | The mumps component of the vaccine was added |
| 1975 | 1975: The rubella vaccine introduced in the national childhood vaccination schedule [90]. In 1975, children older than one year who followed a consistent vaccination schedule were set to receive their initial vaccination. Moreover, children born in 1973 eligible for targeted vaccination campaigns, excluding those awaiting their third DTaP dose, were designated to receive their first vaccination. Additionally, children over one year of age enrolled in preschool facilities who had not yet been vaccinated were also scheduled for their initial vaccination. Furthermore, children born in 1971 and those entering first grade in the 1974/75 academic year were also slated to receive their initial vaccination. |
| In 1976, the MMR trivalent vaccine was officially integrated into the national childhood vaccination schedule, replacing single-antigen vaccines for the first dose and introducing a mumps vaccination program. Additionally, a rubella catch-up vaccination program for 14-year-old girls was initiated in the same year [90]. In 1976, the Institute of Immunology in Zagreb introduced a trivalent measles–mumps–rubella vaccine, replacing the monovalent vaccine used for the initial dose. As a result, children received their first trivalent vaccinations against measles, mumps, and rubella (MMR) through ongoing vaccination protocols beginning after their first year of life since that time. Under the campaign vaccination approach, all children born in 1974, except those set to receive the third dose of DTaP (Diphtheria, Tetanus, Pertussis) during that timeframe, received their first vaccinations against measles, mumps, and rubella (MMR). Additionally, girls in the eighth grade of elementary school (born in 1963 or 1962) received their initial rubella vaccination. Furthermore, children entering first grade during the 1975/76 school year (aged 6 or 7, born in 1970 or 1969) received the measles vaccination. | |
| 1994 | In 1994, a second dose of MMR (MMR2) was introduced at 7 years of age, replacing the single-antigen vaccines for the second dose [90]. Since 1994, the trivalent vaccine of the Institute of Immunology in Zagreb has been routinely utilized for the administration of the second dose as well. |
| 1996 | Children who, for any reason, did not receive their initial MMR vaccination remained eligible for vaccination up to the age of 14. Additionally, all girls attending eighth grade during the 1996/97 academic year (aged 13 or 14, born in 1983 or 1982) received the rubella vaccination. The present regulations prohibit exemptions from vaccination for individuals who have previously experienced measles, mumps, or rubella infections. |
| 1997 | Since 1997, it had been recommended to administer MMR2 at 12 years of age [90]. The timing for revaccination, initially slated for administration during the first grade of elementary school, had been adjusted to take place in the sixth grade. |
| 1999 | In 1999, the recommendation for MMR2 was reverted back to 7 years of age [90]. |
| 2008–2009 | PRIORIX (GlaxoSmithKline), a live attenuated combined vaccine against measles, mumps, and rubella, is recommended for active immunization against these infections. PRIORIX is a lyophilized mixed preparation of the attenuated Schwarz measles, RIT4385 mumps (derived from the Jeryl Lynn strain) and Wistar RA 27/3 rubella strains of viruses, separately obtained by propagation either in chick embryo tissue cultures (mumps and measles) or MRC5 human diploid cells (rubella). In pediatric settings, a single dose is typically advised for children, either on or shortly after their first birthday. Older children lacking documented evidence of prior vaccination should also receive the vaccine [93]. |
| 2009 | Due to adverse events caused by the mumps component of the national ‘MoPaRU’ (MMR) vaccine (produced by the Institute of Immunology in Zagreb), which occurred after the first dose of the vaccine, this vaccine was replaced for the first dose by another producer in 2009. (Due to the discontinuation of its production in 2011, this vaccine was replaced by another, also for the second dose.) |
| 2010 | The aforementioned PRIORIX (GlaxoSmithKline) and M-M-RVaxPro (Merck Sharp & Dohme) are two commercially available vaccines used to confer protection against measles, mumps and rubella in individuals aged 12 months or older. M-M-RVaxPro may be administered to infants between 9 and 12 months of age under specific circumstances [94]. This vaccine contains live attenuated strains of measles virus (Enders’ Edmonston strain), mumps virus (Jeryl Lynn [Level B] strain) and rubella virus (Wistar RA 27/3 strain) [94]. In addition to these commercial products, the national vaccine “MoPaRU” (MMR), produced by the Institute of Immunology in Zagreb, remained in use until 2011. |
| 2011–2014 | PRIORIX (GlaxoSmithKline) and M-M-RVaxPro (Merck Sharp & Dohme) |
| 2015–… | PRIORIX (GlaxoSmithKline) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szinger, D.; Berki, T.; Drenjančević, I.; Samardzic, S.; Zelić, M.; Sikora, M.; Požgain, A.; Markovics, Á.; Farkas, N.; Németh, P.; et al. Raising Epidemiological Awareness: Assessment of Measles/MMR Susceptibility in Highly Vaccinated Clusters within the Hungarian and Croatian Population—A Sero-Surveillance Analysis. Vaccines 2024, 12, 486. https://doi.org/10.3390/vaccines12050486
Szinger D, Berki T, Drenjančević I, Samardzic S, Zelić M, Sikora M, Požgain A, Markovics Á, Farkas N, Németh P, et al. Raising Epidemiological Awareness: Assessment of Measles/MMR Susceptibility in Highly Vaccinated Clusters within the Hungarian and Croatian Population—A Sero-Surveillance Analysis. Vaccines. 2024; 12(5):486. https://doi.org/10.3390/vaccines12050486
Chicago/Turabian StyleSzinger, Dávid, Timea Berki, Ines Drenjančević, Senka Samardzic, Marija Zelić, Magdalena Sikora, Arlen Požgain, Ákos Markovics, Nelli Farkas, Péter Németh, and et al. 2024. "Raising Epidemiological Awareness: Assessment of Measles/MMR Susceptibility in Highly Vaccinated Clusters within the Hungarian and Croatian Population—A Sero-Surveillance Analysis" Vaccines 12, no. 5: 486. https://doi.org/10.3390/vaccines12050486
APA StyleSzinger, D., Berki, T., Drenjančević, I., Samardzic, S., Zelić, M., Sikora, M., Požgain, A., Markovics, Á., Farkas, N., Németh, P., & Böröcz, K. (2024). Raising Epidemiological Awareness: Assessment of Measles/MMR Susceptibility in Highly Vaccinated Clusters within the Hungarian and Croatian Population—A Sero-Surveillance Analysis. Vaccines, 12(5), 486. https://doi.org/10.3390/vaccines12050486






