Novel Multi-Antigen Orf-Virus-Derived Vaccine Elicits Protective Anti-SARS-CoV-2 Response in Monovalent and Bivalent Formats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Production
2.2. Humoral Response against SARS-CoV-2 Beta RBD
2.3. Humoral Response against Orf Virus Protein F1L (ORFV059)
2.4. Detection and Quantification of Strain-Specific Neutralizing Antibodies
2.5. Flow Cytometry Analysis of Prime-2-CoV_Beta-Stimulated Human PBMCs
2.6. Luminex Assay
2.7. Intracellular Cytokine Staining (ICS) Assay
2.8. Animal Experimentation
2.8.1. Mouse Experimentation
Ethical Statement
Effect of Anti-Spike Pre-Existing Immunity on Response to Prime-2-CoV Vaccination
2.8.2. Hamster Experimentation
Ethical Statement
Immunization
Challenge
Sampling Post-Challenge
Viral Load in the Respiratory Tract
Histopathology
2.9. Statistical Analysis
3. Results
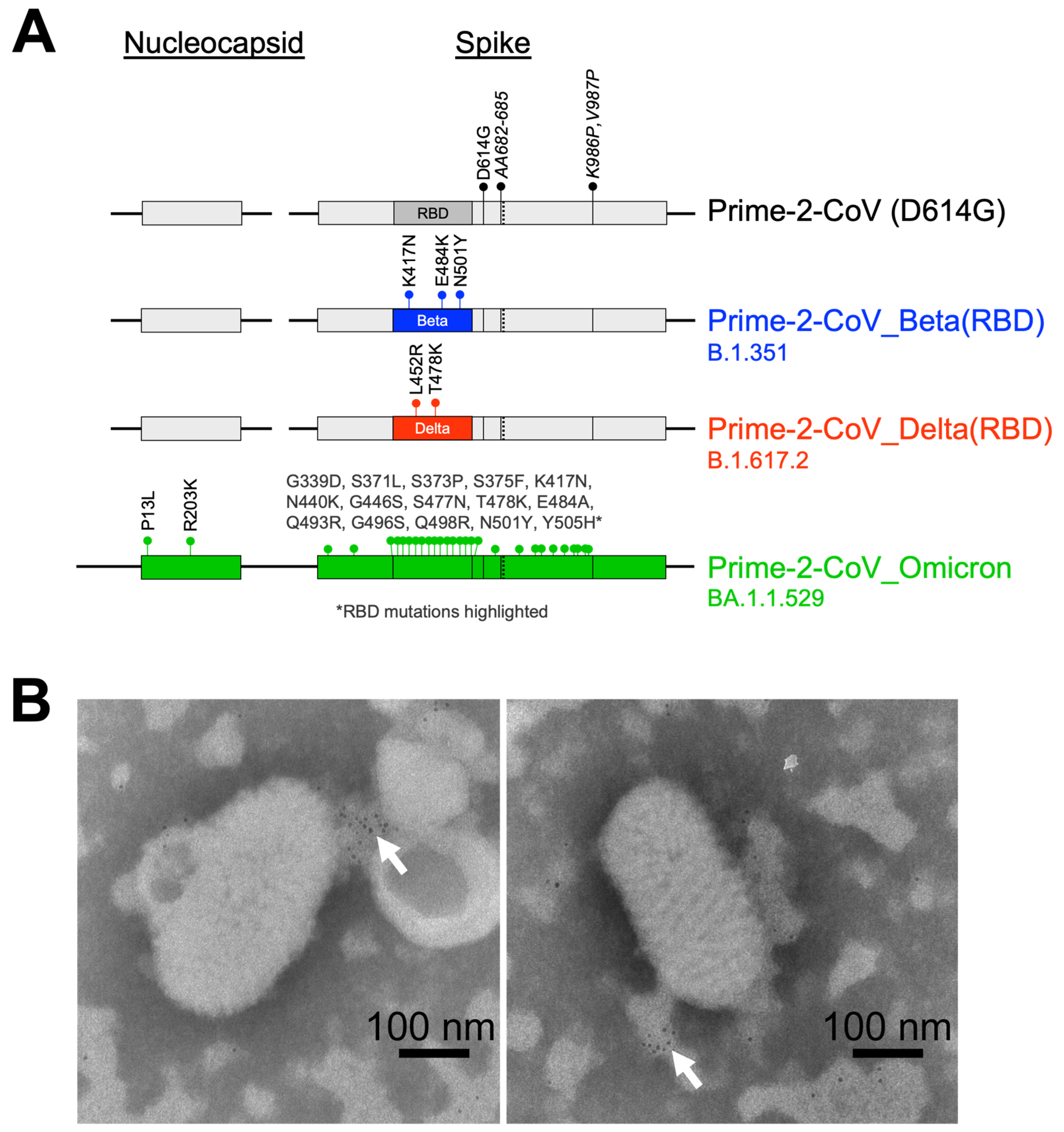
3.1. Prime-2-CoV
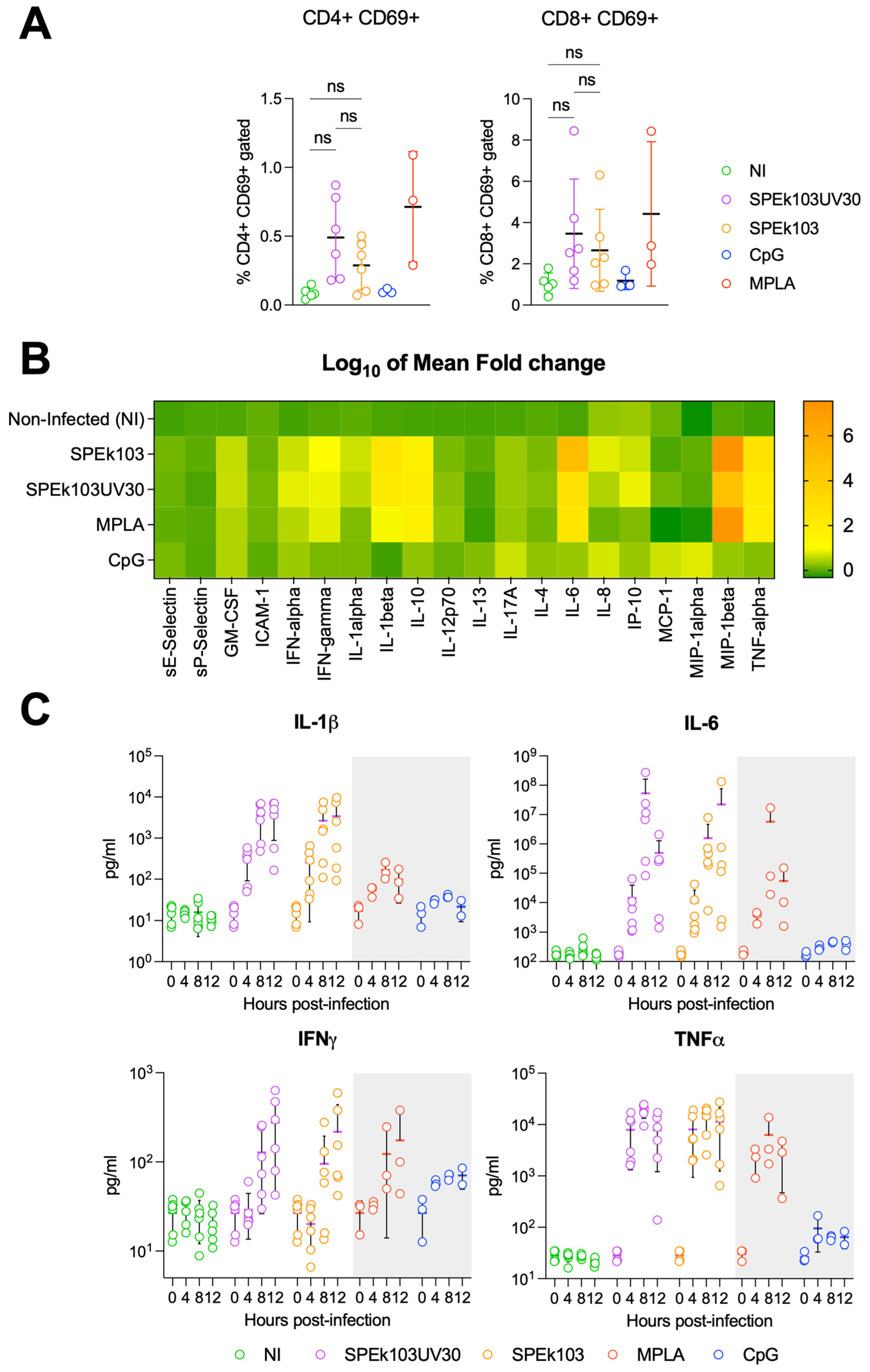
3.2. Prime-2-CoV_Beta Infection of Human PBMC
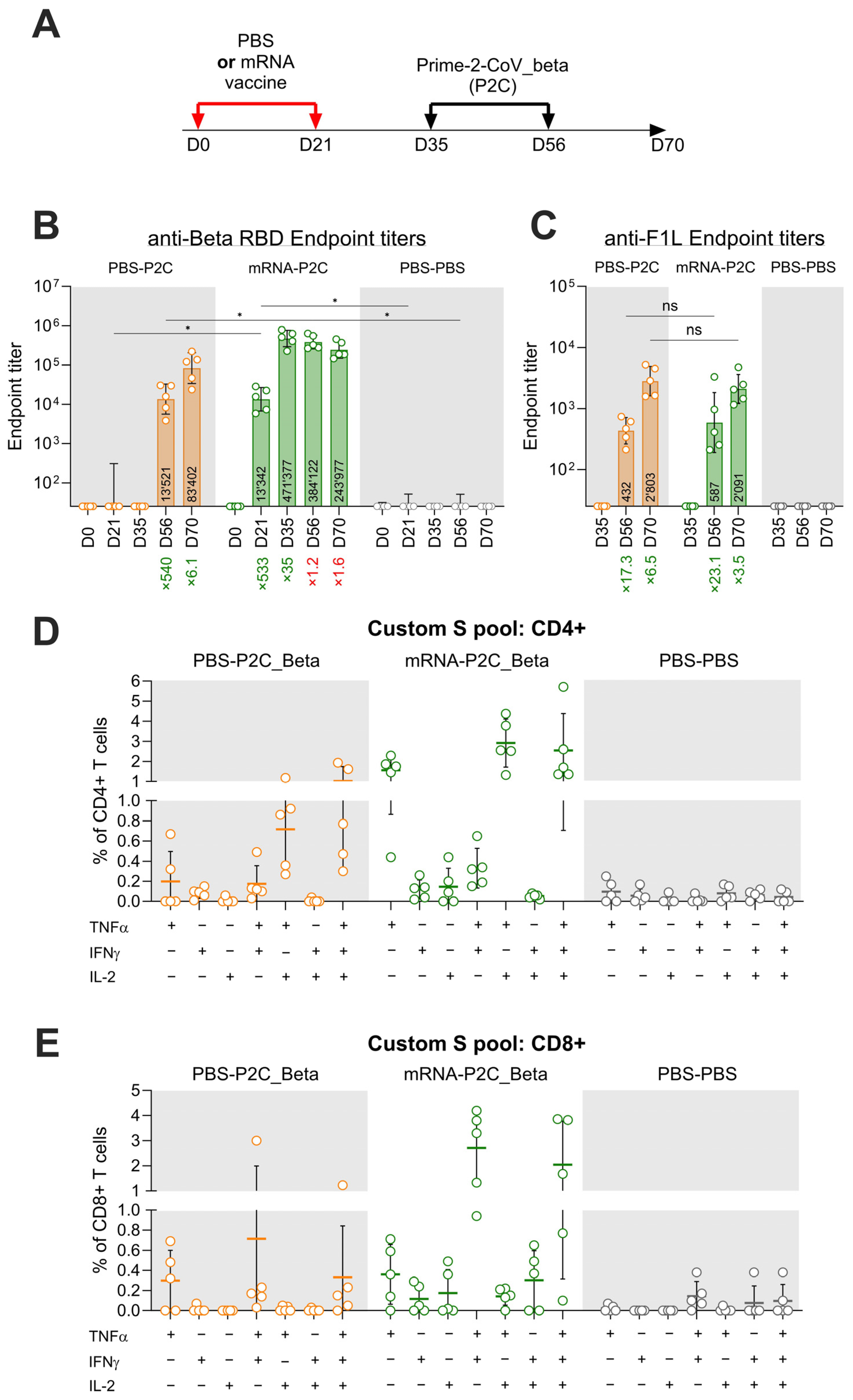
3.3. Effect of Pre-Existing Immunity on Prime-2-CoV_Beta Immunogenicity in Mice
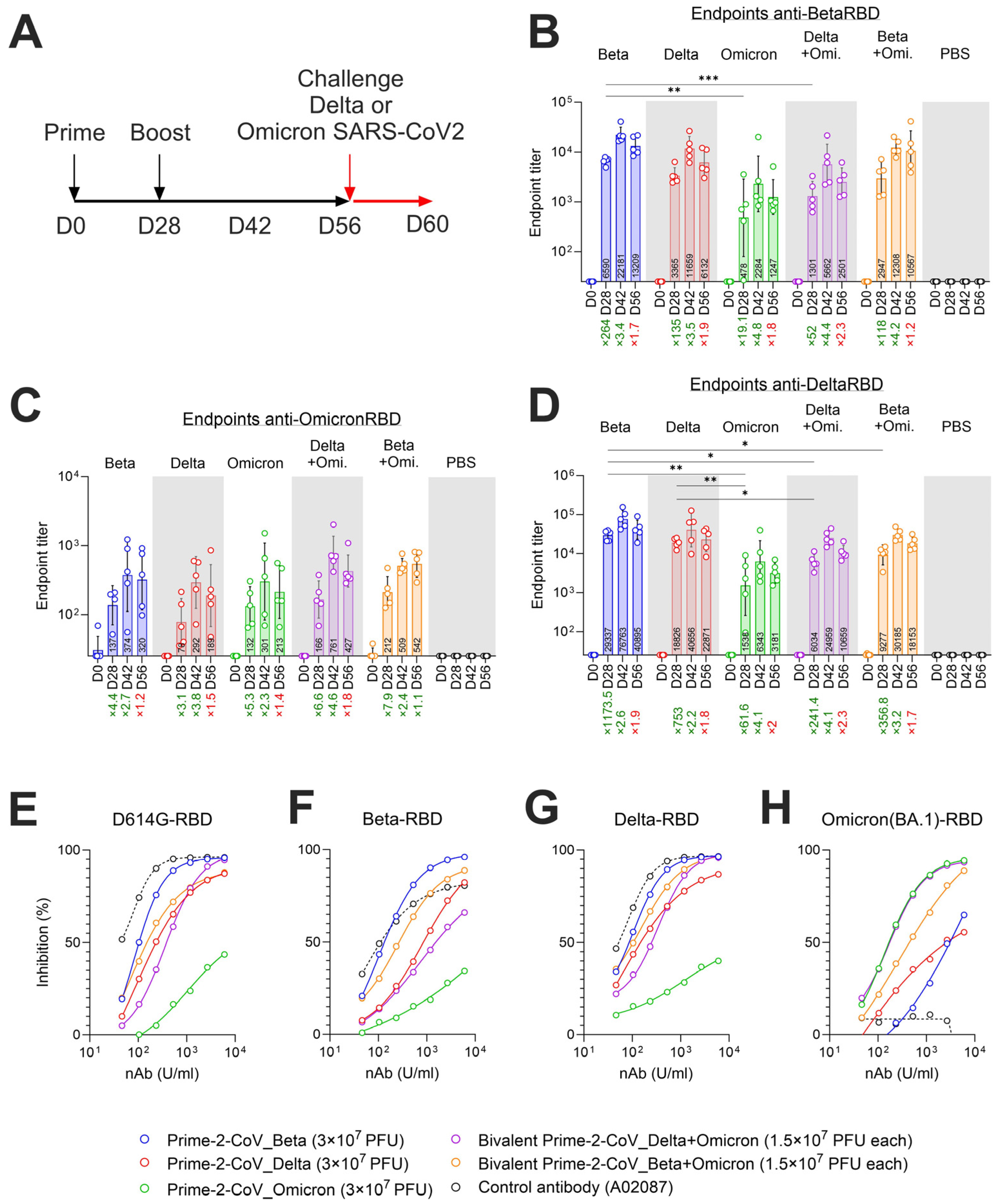
3.4. Prime-2-CoV_Beta Protects Hamsters against Severe Disease as Effectively as Monovalent and Bivalent Injections
3.5. Monovalent and Bivalent Prime-2-CoV Vaccinations Elicit RBD-Specific Binding and Neutralizing Antibodies in Hamsters
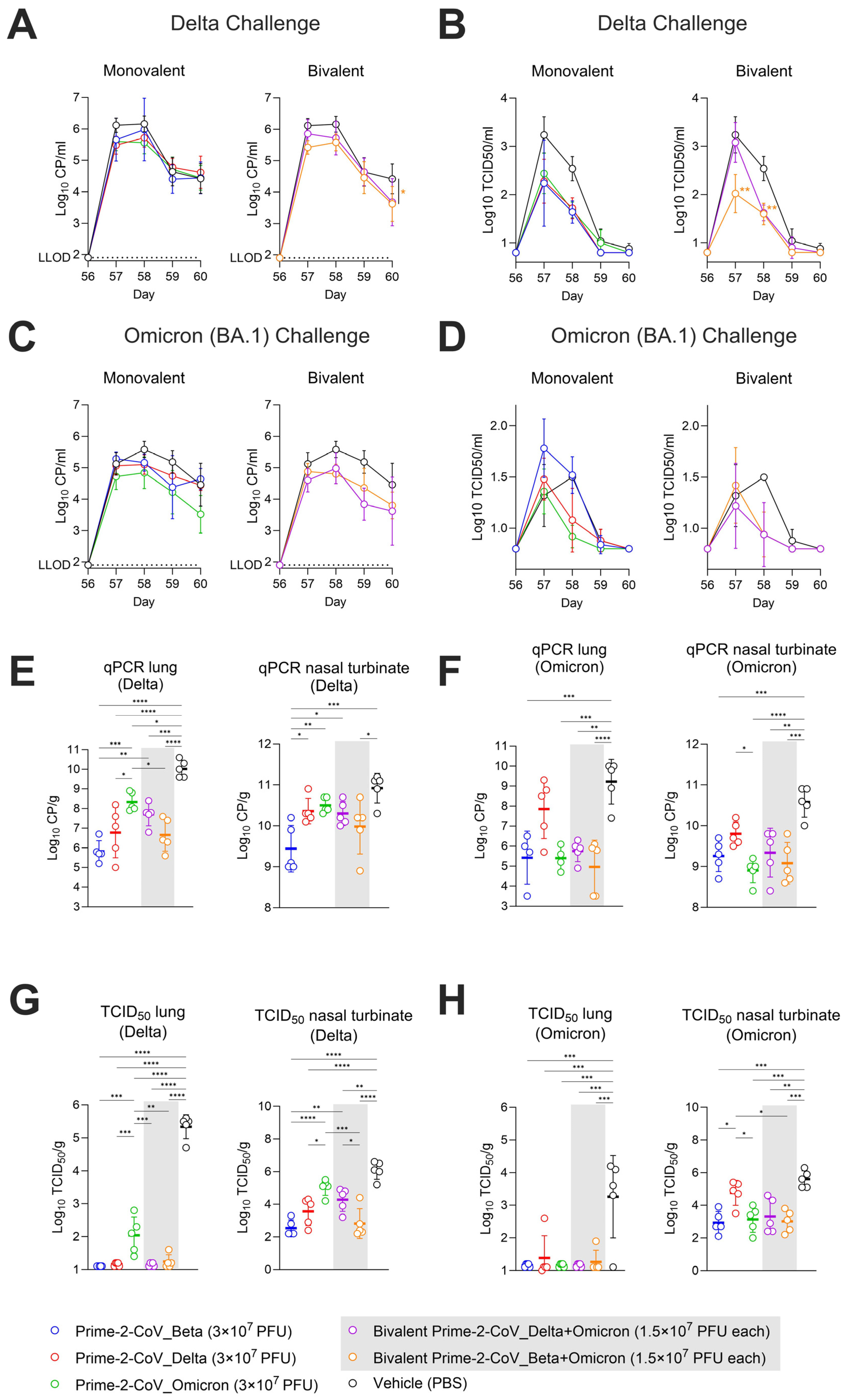
3.6. Monovalent and Bivalent Prime-2-CoV Vaccine Candidates Lower Viral Loads in Hamster Airways after SARS-CoV-2 Challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rziha, H.J.; Buttner, M.; Muller, M.; Salomon, F.; Reguzova, A.; Laible, D.; Amann, R. Genomic Characterization of Orf Virus Strain D1701-V (Parapoxvirus) and Development of Novel Sites for Multiple Transgene Expression. Viruses 2019, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.; Rohde, J.; Wulle, U.; Conlee, D.; Raue, R.; Martinon, O.; Rziha, H.J. A new rabies vaccine based on a recombinant Orf Virus (parapoxvirus) expressing the rabies virus glycoprotein. J. Virol. 2013, 87, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Reguzova, A.; Ghosh, M.; Muller, M.; Rziha, H.J.; Amann, R. Orf Virus-Based Vaccine Vector D1701-V Induces Strong CD8+ T Cell Response against the Transgene but Not against ORFV-Derived Epitopes. Vaccines 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Reguzova, A.; Fischer, N.; Muller, M.; Salomon, F.; Jaenisch, T.; Amann, R. A Novel Orf Virus D1701-VrV-Based Dengue Virus (DENV) Vaccine Candidate Expressing HLA-Specific T Cell Epitopes: A Proof-of-Concept Study. Biomedicines 2021, 9, 1862. [Google Scholar] [CrossRef] [PubMed]
- Reguzova, A.; Sigle, M.; Pagallies, F.; Salomon, F.; Rziha, H.J.; Bittner-Schrader, Z.; Verstrepen, B.; Böszörményi, K.; Verschoor, E.; Elbers, K.; et al. A Novel Multi-Antigenic Parapoxvirus-Based Vaccine Demonstrates Efficacy in Protecting Hamsters and Non-Human Primates against SARS-CoV-2 Challenge. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Sy, L.S.; Tubert, J.E.; Luo, Y.; Qiu, S.; Lee, G.S.; Bruxvoort, K.J.; Ku, J.H.; Florea, A.; et al. mRNA-1273 bivalent (original and Omicron) COVID-19 vaccine effectiveness against COVID-19 outcomes in the United States. Nat. Commun. 2023, 14, 5851. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Zhao, K.; Du, L.; Wang, X.; He, W.; Gao, F.; Song, D.; Guan, J. Orf Virus DNA prime-protein boost strategy is superior to adenovirus-based vaccination in mice and sheep. Front. Immunol. 2023, 14, 1077938. [Google Scholar] [CrossRef]
- Amanat, F.; Strohmeier, S.; Rathnasinghe, R.; Schotsaert, M.; Coughlan, L.; Garcia-Sastre, A.; Krammer, F. Introduction of Two Prolines and Removal of the Polybasic Cleavage Site Lead to Higher Efficacy of a Recombinant Spike-Based SARS-CoV-2 Vaccine in the Mouse Model. mBio 2021, 12, e02648-20. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. bioRxiv 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef]
- Muller, M.; Reguzova, A.; Loffler, M.W.; Amann, R. Orf Virus-Based Vectors Preferentially Target Professional Antigen-Presenting Cells, Activate the STING Pathway and Induce Strong Antigen-Specific T Cell Responses. Front. Immunol. 2022, 13, 873351. [Google Scholar] [CrossRef]
- Fleming, S.B.; Mercer, A.A. Genus Parapoxvirus. In Poxviruses; Mercer, A.A., Schmidt, A., Weber, O., Eds.; Birkhäuser: Basel, Switzerland, 2007; pp. 127–165. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Iida, S.; Iwatsuki-Horimoto, K.; Maemura, T.; Kiso, M.; Scheaffer, S.M.; Darling, T.L.; Joshi, A.; Loeber, S.; Singh, G.; et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 2022, 603, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Foo, C.S.; Zhang, X.; Lemmens, V.; Maes, P.; Slechten, B.; Raymenants, J.; Andre, E.; Weynand, B.; Dallmeier, K.; et al. The omicron (B.1.1.529) SARS-CoV-2 variant of concern does not readily infect Syrian hamsters. Antivir. Res. 2022, 198, 105253. [Google Scholar] [CrossRef]
- Schutze, N.; Raue, R.; Buttner, M.; Alber, G. Inactivated parapoxvirus ovis activates canine blood phagocytes and T lymphocytes. Vet. Microbiol. 2009, 137, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Fachinger, V.; Schlapp, T.; Strube, W.; Schmeer, N.; Saalmuller, A. Poxvirus-induced immunostimulating effects on porcine leukocytes. J. Virol. 2000, 74, 7943–7951. [Google Scholar] [CrossRef]
- Buttner, M.; Czerny, C.P.; Lehner, K.H.; Wertz, K. Interferon induction in peripheral blood mononuclear leukocytes of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet. Immunol. Immunopathol. 1995, 46, 237–250. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Feng, S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 2022, 22, 1131–1141. [Google Scholar] [CrossRef]
- Hannawi, S.; Saifeldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Liu, D.; Yan, L.; Xie, L. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C, in adults previously vaccinated with mRNA vaccine: A randomized, double-blind, placebo-controlled phase 1/2 clinical trial. EBioMedicine 2023, 87, 104386. [Google Scholar] [CrossRef]
- Das, S.; Singh, J.; Shaman, H.; Singh, B.; Anantharaj, A.; Sharanabasava, P.; Pandey, R.; Lodha, R.; Pandey, A.K.; Medigeshi, G.R. Pre-existing antibody levels negatively correlate with antibody titers after a single dose of BBV152 vaccination. Nat. Commun. 2022, 13, 3451. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burri, D.J.; Renz, L.; Mueller, M.; Pagallies, F.; Klinkhardt, U.; Amann, R.; Derouazi, M. Novel Multi-Antigen Orf-Virus-Derived Vaccine Elicits Protective Anti-SARS-CoV-2 Response in Monovalent and Bivalent Formats. Vaccines 2024, 12, 490. https://doi.org/10.3390/vaccines12050490
Burri DJ, Renz L, Mueller M, Pagallies F, Klinkhardt U, Amann R, Derouazi M. Novel Multi-Antigen Orf-Virus-Derived Vaccine Elicits Protective Anti-SARS-CoV-2 Response in Monovalent and Bivalent Formats. Vaccines. 2024; 12(5):490. https://doi.org/10.3390/vaccines12050490
Chicago/Turabian StyleBurri, Dominique Julien, Louis Renz, Melanie Mueller, Felix Pagallies, Ute Klinkhardt, Ralf Amann, and Madiha Derouazi. 2024. "Novel Multi-Antigen Orf-Virus-Derived Vaccine Elicits Protective Anti-SARS-CoV-2 Response in Monovalent and Bivalent Formats" Vaccines 12, no. 5: 490. https://doi.org/10.3390/vaccines12050490
APA StyleBurri, D. J., Renz, L., Mueller, M., Pagallies, F., Klinkhardt, U., Amann, R., & Derouazi, M. (2024). Novel Multi-Antigen Orf-Virus-Derived Vaccine Elicits Protective Anti-SARS-CoV-2 Response in Monovalent and Bivalent Formats. Vaccines, 12(5), 490. https://doi.org/10.3390/vaccines12050490





