Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. SARS-CoV-2 mRNA Vaccination
2.1.2. Sample Preparation
2.2. Laboratory Analyses
2.2.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.2.2. Avidity Enzyme-Linked Immunosorbent Assay (Chaotrope ELISA)
2.3. Assay Data Analyses
3. Results
3.1. Sample Demographic Characteristics
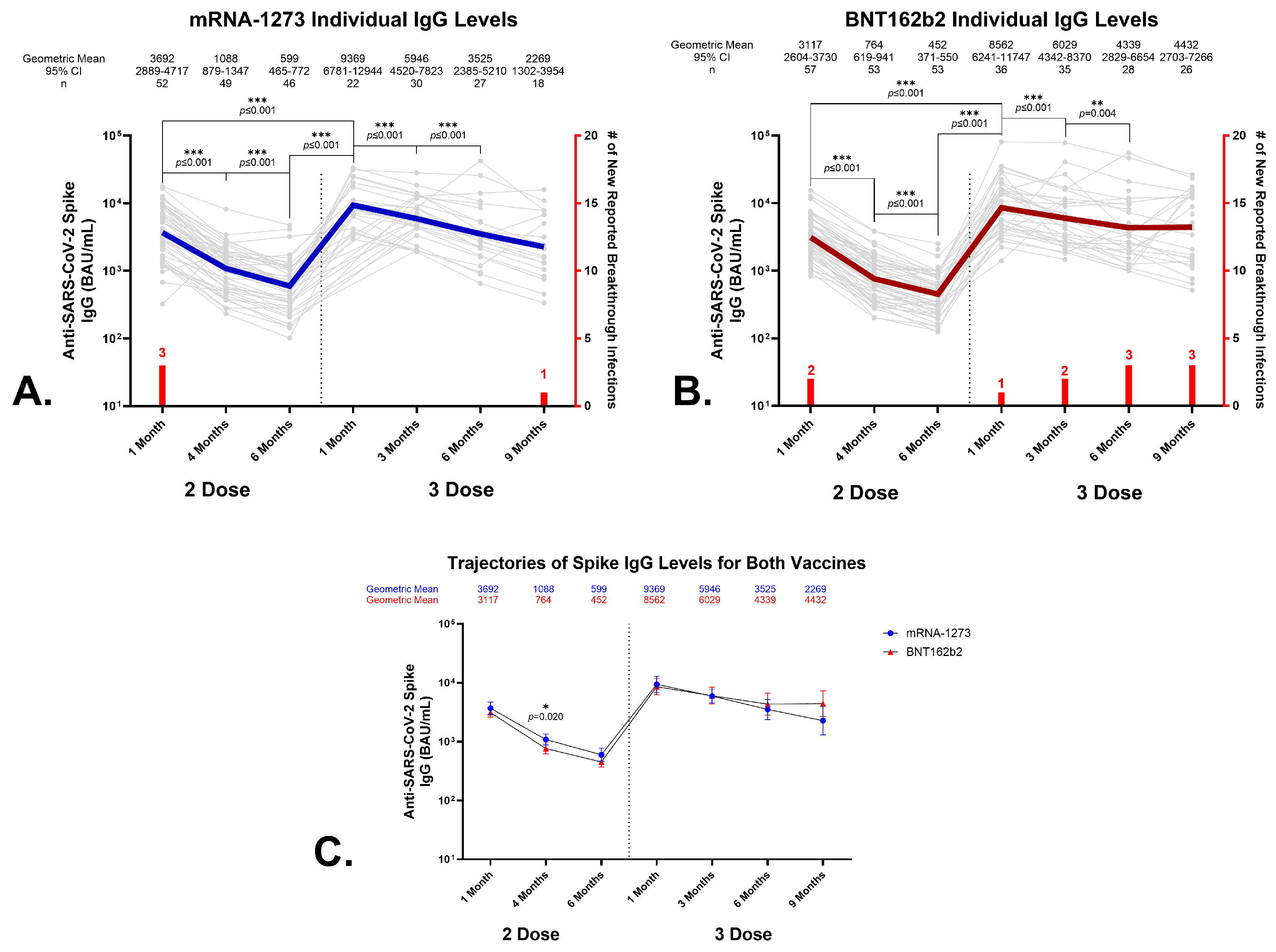
3.2. Antibody Responses to SARS-CoV-2 Spike Protein Following Primary Vaccination with BNT162b2 or mRNA-1273
3.3. Effects of a Homologous Third Dose on Circulating Anti-SARS-CoV-2 Spike IgG Levels
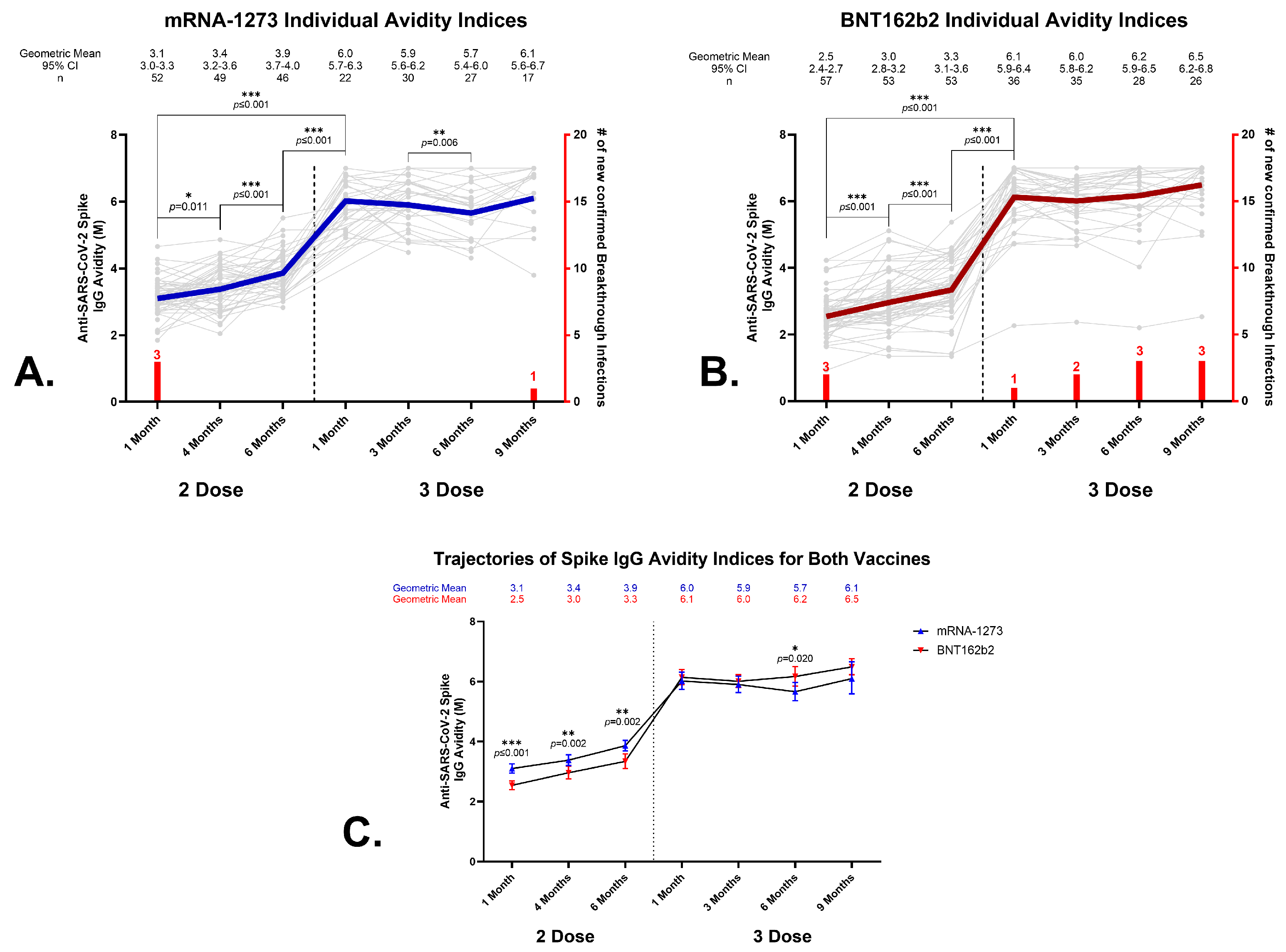
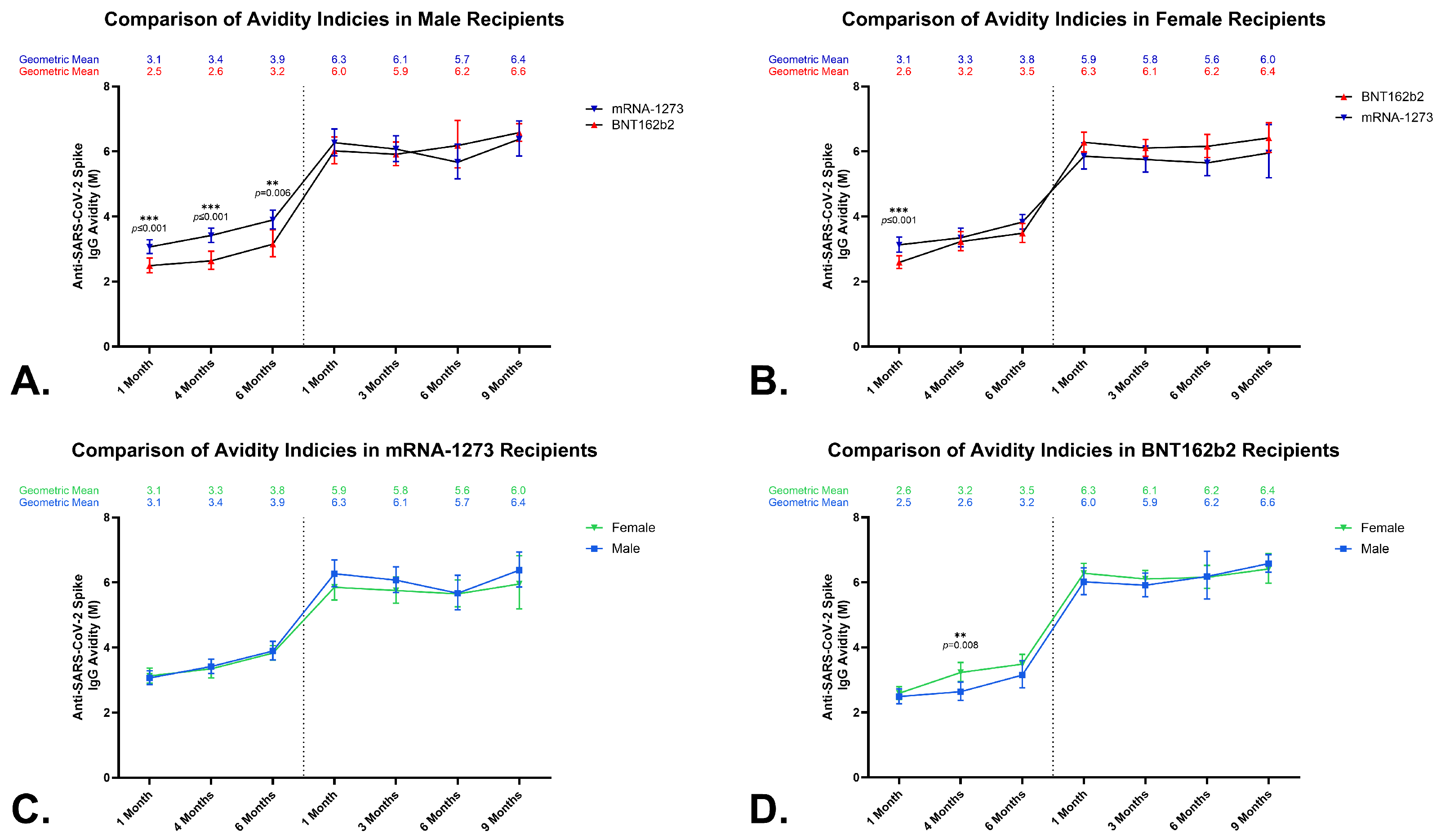
3.4. Anti-SARS-CoV-2 Spike IgG Avidity in Sera from BNT162b2 and mRNA-1273 Vaccine Recipients
3.5. Effects of a Homologous Third Dose on Anti-SARS-CoV-2 Spike IgG Avidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm. Res. 2021, 38, 473–478. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease 2019 (COVID-19) Situation Report—66; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- CDC. Where are Vaccines (Company and Brand) Administered Globally? Available online: https://covid.cdc.gov/covid-data-tracker/#global-vaccinations (accessed on 22 August 2023).
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Bertollini, R.; National Study Group for COVID-19 Vaccination. Waning mRNA-1273 Vaccine Effectiveness against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2022, 386, 1091–1093. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Kemp, T.J.; Bullock, J.; Bouk, A.; Metz, J.; Neish, A.; Cherry, J.; Lowy, D.R.; Pinto, L.A. SARS-CoV-2 IgG Spike antibody levels and avidity in natural infection or following vaccination with mRNA-1273 or BNT162b2 vaccines. Hum. Vaccin. Immunother. 2023, 2215677. [Google Scholar] [CrossRef] [PubMed]
- Budroni, S.; Buricchi, F.; Cavallone, A.; Bourguignon, P.; Caubet, M.; Dewar, V.; D’Oro, U.; Finco, O.; Garcon, N.; El Idrissi, M.; et al. Antibody avidity, persistence, and response to antigen recall: Comparison of vaccine adjuvants. NPJ Vaccines 2021, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- CDC. CDC Expands COVID-19 Booster Recommendations. Available online: https://www.cdc.gov/media/releases/2021/s1129-booster-recommendations.html (accessed on 22 August 2023).
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, F.; Marchi, S.; Leonardi, M.; Piu, P.; Lovreglio, P.; Decaro, N.; Buonvino, N.; Stufano, A.; Lorusso, E.; Bombardieri, E.; et al. Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination. J. Immunol. Res. 2022, 2022, 4813199. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Woudenberg, T.; Rosado, J.; Dyer, A.H.; Donnadieu, F.; Planas, D.; Bruel, T.; Schwartz, O.; Prazuck, T.; Velay, A.; et al. Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination. Viruses 2022, 14, 1491. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.M.; Haralambieva, I.H.; Warner, N.D.; Grill, D.E.; Quach, H.Q.; Kennedy, R.B. Longitudinal antibody titer, avidity, and neutralizing responses after SARS-CoV-2 infection. Heliyon 2022, 8, e11676. [Google Scholar] [CrossRef]
- Callaway, H.M.; Hastie, K.M.; Schendel, S.L.; Li, H.; Yu, X.; Shek, J.; Buck, T.; Hui, S.; Bedinger, D.; Troup, C.; et al. Bivalent intra-spike binding provides durability against emergent Omicron lineages: Results from a global consortium. Cell Rep. 2023, 42, 112014. [Google Scholar] [CrossRef] [PubMed]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P. Avidity in antibody effector functions and biotherapeutic drug design. Nat. Rev. Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef]
- Kemp, T.J.; Quesinberry, J.T.; Cherry, J.; Lowy, D.R.; Pinto, L.A. Selection, Characterization, Calibration, and Distribution of the U.S. Serology Standard for Anti-SARS-CoV-2 Antibody Detection. J. Clin. Microbiol. 2022, 60, e0099522. [Google Scholar] [CrossRef]
- Amanna, I.J.; Messaoudi, I.; Slifka, M.K. Protective immunity following vaccination: How is it defined? Hum. Vaccin. 2008, 4, 316–319. [Google Scholar] [CrossRef]
- Dobano, C.; Sanz, H.; Sorgho, H.; Dosoo, D.; Mpina, M.; Ubillos, I.; Aguilar, R.; Ford, T.; Diez-Padrisa, N.; Williams, N.A.; et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat. Commun. 2019, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Usinger, W.R.; Lucas, A.H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 1999, 67, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Kelly, D.F.; Yu, L.M.; Slack, M.P.; Booy, R.; Heath, P.T.; Siegrist, C.A.; Moxon, R.E.; Pollard, A.J. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin. Infect. Dis. 2008, 46, 186–192. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al Thani, A.A.; Yassine, H.M. Viral-Induced Enhanced Disease Illness. Front. Microbiol. 2018, 9, 2991. [Google Scholar] [CrossRef]
- Polack, F.P.; Hoffman, S.J.; Crujeiras, G.; Griffin, D.E. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat. Med. 2003, 9, 1209–1213. [Google Scholar] [CrossRef]
- Benner, S.E.; Patel, E.U.; Laeyendecker, O.; Pekosz, A.; Littlefield, K.; Eby, Y.; Fernandez, R.E.; Miller, J.; Kirby, C.S.; Keruly, M.; et al. SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors. J. Infect. Dis. 2020, 222, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Harthaller, T.; Falkensammer, B.; Bante, D.; Huber, M.; Schmitt, M.; Benainouna, H.; Rössler, A.; Fleischer, V.; von Laer, D.; Kimpel, J.; et al. Retained avidity despite reduced cross-binding and cross-neutralizing antibody levels to Omicron after SARS-COV-2 wild-type infection or mRNA double vaccination. Front. Immunol. 2023, 14, 1196988. [Google Scholar] [CrossRef] [PubMed]
- Đaković Rode, O.; Bodulić, K.; Zember, S.; Cetinić Balent, N.; Novokmet, A.; Čulo, M.; Rašić, Ž.; Mikulić, R.; Markotić, A. Decline of Anti-SARS-CoV-2 IgG Antibody Levels 6 Months after Complete BNT162b2 Vaccination in Healthcare Workers to Levels Observed Following the First Vaccine Dose. Vaccines 2022, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Nomura, H.; Shimono, N. Dynamics of anti-Spike IgG antibody level after the second BNT162b2 COVID-19 vaccination in health care workers. J. Infect. Chemother. 2022, 28, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.A.; Gerlovin, H.; Madenci, A.L.; Kurgansky, K.E.; Ferolito, B.R.; Figueroa Muniz, M.J.; Gagnon, D.R.; Gaziano, J.M.; Cho, K.; Casas, J.P.; et al. Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans. N. Engl. J. Med. 2022, 386, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xia, P.; Li, S.; Zhang, T.; Wang, T.T.; Zhu, J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life 2017, 69, 297–304. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Hyun, H.; Heo, J.Y.; Seo, Y.B.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Kim, H.J.; Choi, J.Y.; Lee, Y.J.; et al. Longitudinal immune kinetics of COVID-19 booster versus primary series vaccination: Insight into the annual vaccination strategy. Heliyon 2024, 10, e27211. [Google Scholar] [CrossRef]
- Zember, S.; Bodulić, K.; Balent, N.C.; Mikulić, R.; Markotić, A.; Đaković Rode, O. Slower Waning of Anti-SARS-CoV-2 IgG Levels Six Months after the Booster Dose Compared to Primary Vaccination. Vaccines 2022, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.J.; Hempel, H.A.; Pan, Y.; Roy, D.; Cherry, J.; Lowy, D.R.; Pinto, L.A. Assay Harmonization Study To Measure Immune Response to SARS-CoV-2 Infection and Vaccines: A Serology Methods Study. Microbiol. Spectr. 2023, 11, e0535322. [Google Scholar] [CrossRef]
- Keshavarz, B.; Richards, N.E.; Workman, L.J.; Patel, J.; Muehling, L.M.; Canderan, G.; Murphy, D.D.; Brovero, S.G.; Ailsworth, S.M.; Eschenbacher, W.H.; et al. Trajectory of IgG to SARS-CoV-2 After Vaccination with BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison with Natural Infection. Front. Immunol. 2022, 13, 850987. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Amano, M.; Uemura, Y.; Tsuchiya, K.; Matsushima, T.; Noda, K.; Shimizu, Y.; Fujiwara, A.; Takamatsu, Y.; Ichikawa, Y.; et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci. Rep. 2021, 11, 22848. [Google Scholar] [CrossRef]
- Triebelhorn, J.; Schneider, J.; Spinner, C.D.; Iakoubov, R.; Voit, F.; Wagner, L.; Erber, J.; Rothe, K.; Berthele, A.; Pernpeintner, V.; et al. Clinical and immunological outcomes of SARS-CoV-2-infected vaccine responders, vaccine non-responders, and unvaccinated patients evaluated for neutralizing monoclonal antibody treatment at a single German tertiary care center: A retrospective cohort study with prospective follow-up. Infection 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Golding, L.; Watts, A.W.; Shew, J.; Viñeta Paramo, M.; Mâsse, L.C.; Goldfarb, D.M.; Abu-Raya, B.; Lavoie, P.M. A Novel Anti-nucleocapsid Antibody Avidity Method for Identifying SARS-CoV-2 Reinfections. J. Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Sonnenberg, K.; Niedrig, M.; Lam, S.Y.; Pang, C.M.; Chan, K.M.; Ma, S.K.; Seto, W.H.; Peiris, J.S. Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection. Clin. Vaccine Immunol. 2007, 14, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N.; Sonnenberg, K.; Sidaway, F.; Shead, S.; Niedrig, M.; Steinhagen, K.; Horsman, G.B.; Drebot, M.A. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J. Clin. Microbiol. 2005, 43, 5873–5875. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Yamada, H.; Wada, S.; Tanimura, K.; Deguchi, M.; Uchida, A.; Nishikawa, A. Toxoplasma gondii IgG avidity for the diagnosis of primary infection in pregnant women: Comparison between chemiluminescent microparticle immunoassay and enzyme-linked immunosorbent assay. J. Infect. Chemother. 2023, 30, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, S.; de Assis, R.; Khalil, G.; Luu, M.K.; Jain, A.; Horvath, P.; Nakajima, R.; Palma, A.M.; Hoang, A.; Razzak, E.; et al. Analysis and comparison of SARS-CoV-2 variant antibodies and neutralizing activity for 6 months after a booster mRNA vaccine in a healthcare worker population. Front. Immunol. 2023, 14, 1166261. [Google Scholar] [CrossRef]
- Ailsworth, S.M.; Keshavarz, B.; Richards, N.E.; Workman, L.J.; Murphy, D.D.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster vaccination and comparison of BNT162b2 with mRNA-1273. Ann. Allergy Asthma Immunol. 2023, 130, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, T.; Arima, Y.; Muraoka, H.; Sato, A.; Oba, K.; Uehara, Y.; Arioka, H.; Yanai, H.; Kuramochi, J.; Ihara, G.; et al. Coronavirus Disease 19 (COVID-19) Vaccine Effectiveness Against Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection During Delta-Dominant and Omicron-Dominant Periods in Japan: A Multicenter Prospective Case-control Study (Factors Associated with SARS-CoV-2 Infection and the Effectiveness of COVID-19 Vaccines Study). Clin. Infect. Dis. 2023, 76, e108–e115. [Google Scholar] [CrossRef] [PubMed]
- Solante, R.; Alvarez-Moreno, C.; Burhan, E.; Chariyalertsak, S.; Chiu, N.C.; Chuenkitmongkol, S.; Dung, D.V.; Hwang, K.P.; Ortiz Ibarra, J.; Kiertiburanakul, S.; et al. Expert review of global real-world data on COVID-19 vaccine booster effectiveness and safety during the omicron-dominant phase of the pandemic. Expert Rev. Vaccines 2023, 22, 2143347. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults—United States, March–July 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
| Participants | BNT162b2 | mRNA-1273 |
|---|---|---|
| Number (n) | 57 | 52 |
| Mean Age (Years) | 47 | 44 |
| Age Min–Max (Years) | 23–66 | 23–67 |
| Female (percent) | 32 (56%) | 28 (54%) |
| Male (percent) | 25 (44%) | 24 (46%) |
| Participants | (A) Primary Series | |||||
|---|---|---|---|---|---|---|
| BNT162b2 | mRNA-1273 | |||||
| Number (n) | Time to Collection (Days ± SD) | Number (n) | Time to Collection (Days ± SD) | |||
| Month 1 | 57 | 25 ± 11 | 52 | 20 ± 8 | ||
| Month 4 | 53 | 116 ± 12 | 49 | 114 ± 11 | ||
| Month 6 | 53 | 183 ± 18 | 46 | 192 ± 18 | ||
| Total receiving primary vaccine | 57 | 52 | ||||
| Participants | (B) Primary Series Plus Boost (third Dose) * | |||||
| BNT162b2 | mRNA-1273 | |||||
| Number (n) | Time to Collection (Days ± SD) | Number (n) | Time to Collection (Days ± SD) | |||
| Month 1 | 36 | 37 ± 16 | 22 | 36 ± 18 | ||
| Month 3 | 35 | 95 ± 8 | 30 | 93 ± 7 | ||
| Month 6 | 28 | 183 ± 6 | 27 | 184 ± 6 | ||
| Month 9 | 26 | 278 ± 7 | 18 | 275 ± 5 | ||
| Total receiving primary vaccine | 36 | 32 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullock, J.L., Jr.; Hickey, T.E.; Kemp, T.J.; Metz, J.; Loftus, S.; Haynesworth, K.; Castro, N.; Luke, B.T.; Lowy, D.R.; Pinto, L.A. Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination. Vaccines 2024, 12, 516. https://doi.org/10.3390/vaccines12050516
Bullock JL Jr., Hickey TE, Kemp TJ, Metz J, Loftus S, Haynesworth K, Castro N, Luke BT, Lowy DR, Pinto LA. Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination. Vaccines. 2024; 12(5):516. https://doi.org/10.3390/vaccines12050516
Chicago/Turabian StyleBullock, Jimmie L., Jr., Thomas E. Hickey, Troy J. Kemp, Jordan Metz, Sarah Loftus, Katarzyna Haynesworth, Nicholas Castro, Brian T. Luke, Douglas R. Lowy, and Ligia A. Pinto. 2024. "Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination" Vaccines 12, no. 5: 516. https://doi.org/10.3390/vaccines12050516
APA StyleBullock, J. L., Jr., Hickey, T. E., Kemp, T. J., Metz, J., Loftus, S., Haynesworth, K., Castro, N., Luke, B. T., Lowy, D. R., & Pinto, L. A. (2024). Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination. Vaccines, 12(5), 516. https://doi.org/10.3390/vaccines12050516





