Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update
Abstract
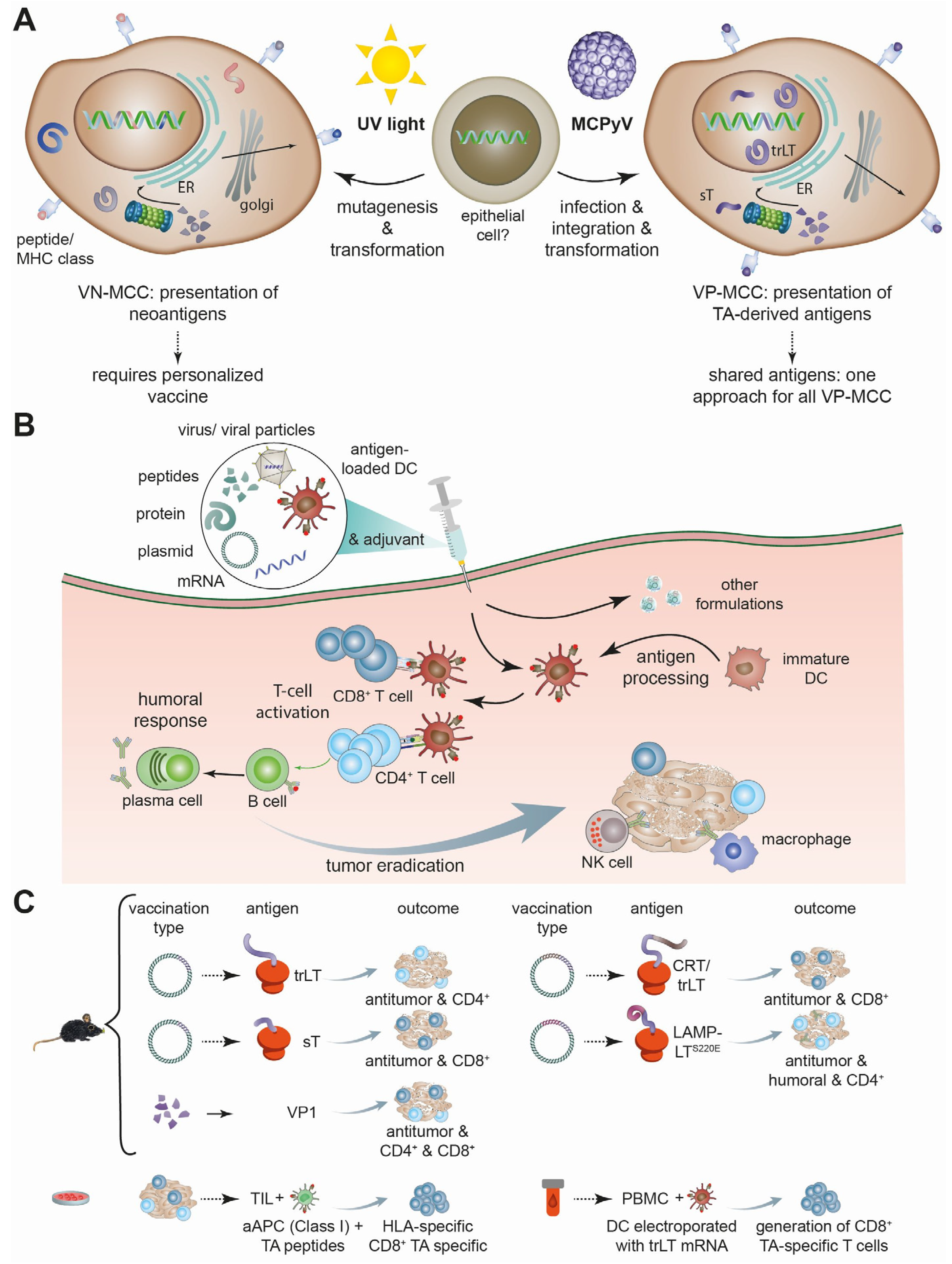
1. Introduction
1.1. Vaccines in General
1.1.1. Vaccine Principles in Oncology
1.1.2. Peptides
1.1.3. mRNA
1.1.4. Oncolytic-Virus-Based Vaccines
1.1.5. Oncolytic Plasmids/Oncotoxic Proteins
1.1.6. Exosome-Based Cancer Vaccine
1.2. Merkel Cell Carcinoma
2. Therapeutic Vaccines for MCC
2.1. Published Preclinical and Clinical Studies
| Reference/Year | Phase of Study | Vaccine Principle | Target/Effect |
|---|---|---|---|
| Zeng et al., 2012 [50] | 0 | DNA vaccine encoding MCPyV-LT aal-258 | Syngeneic C57BL/6 mice model with B16 tumors (melanoma) expressing LT/vaccinations was efficient in prophylactic and therapeutic setting; antitumor effect in vaccinated animals was largely dependent on CD4+ T cells |
| Xu et al., 2021 [51] | 0 | VP1 peptide-based vaccine | Syngeneic BALB/c mice model with CMS-5 tumors (sarcoma) expressing VP1/ Antitumor effect of the vaccine mainly mediated by VP1-specific CD4+ and CD8+ T cell responses |
| Bhatia et al., 2020 [49] | II | IL12-encoding plasmid-DNA | Intra-tumoral administration of plasmid and in vivo electroporation/ IL12 promotes adaptive type I immunity and has antitumor activity (15 patients); vaccine proved to be a safe therapeutic option |
| Jing et al., 2020 [44] | 0 | In vitro stimulation of TIL | MCC TILs/TA specificity of TILs could be detected in 9 of 12 patients |
| Gomez et al., 2012 [45] | 0 | DNA vaccine encoding LT-calreticulin fusion protein | Syngeneic C57BL/6 mice model with B16 tumors expressing LT/vaccinated mice with LT-calreticulin produced more LT-specific CD8+ cells and lived longer |
| Gomez et al., 2013 [46] | 0 | DNA vaccine encoding sT | Syngeneic C57BL/6 mice model with B16 tumors expressing sT/ st-targeting vaccine resulted in prolonged survival, decreased tumor size, and increased sT-specific CD8+ cells |
| Gerer et al., 2017 [47] | 0 | truncLT-mRNA-transfected dendritic cells (DC) | PBMCs of control and MCC patients/optimized DC are able to induce MCPyV-antigen-specific immune responses in vitro in both cohort (3 of 5 MCC patients) |
| Almansour 2022 [8] | 0 | In silico multiepitope vaccine design | Chimeric multi-epitopes-based vaccine to capsid VP1 and VP2 generated in silico strong immune responses with production of interferons and cytokines |
| Imon et al., 2023 [48] | 0 | In silico multiepitope vaccine design | Vaccine candidate consisted of peptides derived from LT, sT, VP1, VP2, and VP3 antigens, and demonstrated real-life-like immune response by computer-aided immune simulation |
| Buchta Rosean et al., 2023 [7] | 0 | DNA vaccine encoding LTS220A-LAMP1 fusion protein | Syngeneic C57BL/6 mice model with B16 tumors expressing LTS220A/antigen-specific CD4+ cells and humoral response |
| Brohl et al., 2023 [55] | I | DNA plasmid encoding immunogenic bacterial protein Emm5 | Treatment of ICI-resistant MCC by up to three intralesional injections of IFx-Hu/well tolerated at the applied doses; best response to therapy was SD, but 4 of 5 MCC patients experienced objective response to ICI re-challenge |
2.2. Currently Registered Trials on Vaccines against Merkel Cell Carcinoma
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montero, D.A.; Vidal, R.M.; Velasco, J.; Carreño, L.J.; Torres, J.P.; Benachi O, M.A.; Tovar-Rosero, Y.-Y.; Oñate, A.A.; O’Ryan, M. Two centuries of vaccination: Historical and conceptual approach and future perspectives. Front. Public Health 2023, 11, 1326154. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Sherpa, N.; Manyanga, J.; Johanns, T.M. Considerations for personalized neoantigen vaccination in Malignant glioma. Adv. Drug Deliv. Rev. 2022, 186, 114312. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Stroffolini, G. Vaccination Campaign against Hepatitis B Virus in Italy: A History of Successful Achievements. Vaccines 2023, 11, 1531. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Wu, S.-E.; Chen, Y.-H.; Hung, C.-T.; Yang, B.-H. Therapeutic Cancer Vaccines for Nonmelanoma Skin Cancer. Curr. Treat. Options Oncol. 2023, 24, 496–514. [Google Scholar] [CrossRef]
- Buchta Rosean, C.; Leyder, E.C.; Hamilton, J.; Carter, J.J.; Galloway, D.A.; Koelle, D.M.; Nghiem, P.; Heiland, T. LAMP1 targeting of the large T antigen of Merkel cell polyomavirus results in potent CD4 T cell responses and tumor inhibition. Front. Immunol. 2023, 14, 1253568. [Google Scholar] [CrossRef]
- Almansour, N.M. Immunoinformatics- and Bioinformatics-Assisted Computational Designing of a Novel Multiepitopes Vaccine Against Cancer-Causing Merkel Cell Polyomavirus. Front. Microbiol. 2022, 13, 929669. [Google Scholar] [CrossRef]
- Joshi, T.P.; Farr, M.A.; Hsiou, D.A.; Nugent, S.; Fathy, R.A.; Lewis, D.J. Therapeutic targets for vaccination in polyomavirus-driven Merkel cell carcinoma. Dermatol. Ther. 2022, 35, e15580. [Google Scholar] [CrossRef]
- Houben, R.; Celikdemir, B.; Kervarrec, T.; Schrama, D. Merkel Cell Polyomavirus: Infection, Genome, Transcripts and Its Role in Development of Merkel Cell Carcinoma. Cancers 2023, 15, 444. [Google Scholar] [CrossRef]
- Celikdemir, B.; Houben, R.; Kervarrec, T.; Samimi, M.; Schrama, D. Current and preclinical treatment options for Merkel cell carcinoma. Expert Opin. Biol. Ther. 2023, 23, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Koff, W.C.; Rappuoli, R.; Plotkin, S.A. Historical Advances in Structural and Molecular Biology and How They Impacted Vaccine Development. J. Mol. Biol. 2023, 435, 168113. [Google Scholar] [CrossRef] [PubMed]
- Lista, F.; Peragallo, M.S.; Biselli, R.; de Santis, R.; Mariotti, S.; Nisini, R.; D’Amelio, R. Have Diagnostics, Therapies, and Vaccines Made the Difference in the Pandemic Evolution of COVID-19 in Comparison with “Spanish Flu”? Pathogens 2023, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Coventry, B.J. Therapeutic vaccination immunomodulation: Forming the basis of all cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135519862234. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick-Cherny, S.; Pulliam, T.; Church, C.; Koelle, D.M.; Nghiem, P. Polyomavirus-driven Merkel cell carcinoma: Prospects for therapeutic vaccine development. Mol. Carcinog. 2020, 59, 807–821. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, P.J.; Bilusic, M. Cancer Vaccines. Hematol. Oncol. Clin. N. Am. 2019, 33, 199–214. [Google Scholar] [CrossRef]
- Zahavi, D.; AlDeghaither, D.; O’Connell, A.; Weiner, L.M. Enhancing antibody-dependent cell-mediated cytotoxicity: A strategy for improving antibody-based immunotherapy. Antib. Ther. 2018, 1, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, E.J.M.; Griffioen, A.W. The revival of cancer vaccines—The eminent need to activate humoral immunity. Hum. Vaccines Immunother. 2017, 13, 1112–1114. [Google Scholar] [CrossRef][Green Version]
- Souza-Fonseca-Guimaraes, F.; Cursons, J.; Huntington, N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019, 40, 142–158. [Google Scholar] [CrossRef]
- Mahmood, F.; Xu, R.; Awan, M.U.N.; Song, Y.; Han, Q.; Xia, X.; Wei, J.; Xu, J.; Peng, J.; Zhang, J. HBV Vaccines: Advances and Development. Vaccines 2023, 11, 1862. [Google Scholar] [CrossRef]
- Barra, F.; Leone Roberti Maggiore, U.; Bogani, G.; Ditto, A.; Signorelli, M.; Martinelli, F.; Chiappa, V.; Lorusso, D.; Raspagliesi, F.; Ferrero, S. New prophylactics human papilloma virus (HPV) vaccines against cervical cancer. J. Obstet. Gynaecol. 2019, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, A.; Mustafa, A.S.; Hanif, A.; Tunio, J.H.; Hanif, S.N.M. Recent Advances in Cancer Immunotherapy with a Focus on FDA-Approved Vaccines and Neoantigen-Based Vaccines. Vaccines 2023, 11, 1633. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, S.; Persano, F. Lipid-Based Vectors for Therapeutic mRNA-Based Anti-Cancer Vaccines. Curr. Pharm. Des. 2019, 25, 1443–1454. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.-Y. Therapeutic cancer vaccines: Past, present, and future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Cao, W.; Song, Y.; Jiang, Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp. Hematol. Oncol. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Butterfield, L.H. Dendritic Cell-Based Cancer Vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Noh, Y.-W.; Kang, T.H.; Kim, J.-E.; Kim, S.; Um, S.H.; Oh, D.-B.; Park, Y.-M.; Lim, Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 2017, 130, 56–66. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Zaggana, E.; Konstantinou, M.P.; Krasagakis, G.H.; de Bree, E.; Kalpakis, K.; Mavroudis, D.; Krasagakis, K. Merkel Cell Carcinoma-Update on Diagnosis, Management and Future Perspectives. Cancers 2022, 15, 103. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Mondal, M.; Guo, J.; He, P.; Zhou, D. Recent advances of oncolytic virus in cancer therapy. Hum. Vaccin. Immunother. 2020, 16, 2389–2402. [Google Scholar] [CrossRef]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef]
- Diep, Y.N.; Kim, T.J.; Cho, H.; Lee, L.P. Nanomedicine for advanced cancer immunotherapy. J. Control. Release 2022, 351, 1017–1037. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. Exosomes/microvesicles: Mediators of cancer-associated immunosuppressive microenvironments. Semin. Immunopathol. 2011, 33, 441–454. [Google Scholar] [CrossRef]
- Silling, S.; Kreuter, A.; Gambichler, T.; Meyer, T.; Stockfleth, E.; Wieland, U. Epidemiology of Merkel Cell Polyomavirus Infection and Merkel Cell Carcinoma. Cancers 2022, 14, 6176. [Google Scholar] [CrossRef]
- Becker, J.C.; Beer, A.J.; DeTemple, V.K.; Eigentler, T.; Flaig, M.; Gambichler, T.; Grabbe, S.; Höller, U.; Klumpp, B.; Lang, S.; et al. S2k Guideline—Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)—Update 2022. J. Dtsch. Dermatol. Ges. 2023, 21, 305–320. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; Schrama, D.; Ugurel, S. Merkel Cell Carcinoma: Integrating Epidemiology, Immunology, and Therapeutic Updates. Am. J. Clin. Dermatol. 2024. [Google Scholar] [CrossRef]
- Jing, L.; Ott, M.; Church, C.D.; Kulikauskas, R.M.; Ibrani, D.; Iyer, J.G.; Afanasiev, O.K.; Colunga, A.; Cook, M.M.; Xie, H.; et al. Prevalent and Diverse Intratumoral Oncoprotein-Specific CD8+ T Cells within Polyomavirus-Driven Merkel Cell Carcinomas. Cancer Immunol. Res. 2020, 8, 648–659. [Google Scholar] [CrossRef]
- Gomez, B.P.; Wang, C.; Viscidi, R.P.; Peng, S.; He, L.; Wu, T.-C.; Hung, C.-F. Strategy for eliciting antigen-specific CD8+ T cell-mediated immune response against a cryptic CTL epitope of merkel cell polyomavirus large T antigen. Cell Biosci. 2012, 2, 36. [Google Scholar] [CrossRef]
- Gomez, B.; He, L.; Tsai, Y.C.; Wu, T.-C.; Viscidi, R.P.; Hung, C.-F. Creation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigen. Cell Biosci. 2013, 3, 29. [Google Scholar] [CrossRef]
- Gerer, K.F.; Erdmann, M.; Hadrup, S.R.; Lyngaa, R.; Martin, L.-M.; Voll, R.E.; Schuler-Thurner, B.; Schuler, G.; Schaft, N.; Hoyer, S.; et al. Preclinical evaluation of NF-κB-triggered dendritic cells expressing the viral oncogenic driver of Merkel cell carcinoma for therapeutic vaccination. Ther. Adv. Med. Oncol. 2017, 9, 451–464. [Google Scholar] [CrossRef]
- Imon, R.R.; Samad, A.; Alam, R.; Alsaiari, A.A.; Talukder, M.E.K.; Almehmadi, M.; Ahammad, F.; Mohammad, F. Computational formulation of a multiepitope vaccine unveils an exceptional prophylactic candidate against Merkel cell polyomavirus. Front. Immunol. 2023, 14, 1160260. [Google Scholar] [CrossRef]
- Bhatia, S.; Longino, N.V.; Miller, N.J.; Kulikauskas, R.; Iyer, J.G.; Ibrani, D.; Blom, A.; Byrd, D.R.; Parvathaneni, U.; Twitty, C.G.; et al. Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 598–607. [Google Scholar] [CrossRef]
- Zeng, Q.; Gomez, B.P.; Viscidi, R.P.; Peng, S.; He, L.; Ma, B.; Wu, T.-C.; Hung, C.-F. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine 2012, 30, 1322–1329. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, S.; He, Y.; Jin, X.; Zhao, G.; Wang, B. Development of a therapeutic vaccine targeting Merkel cell polyomavirus capsid protein VP1 against Merkel cell carcinoma. NPJ Vaccines 2021, 6, 119. [Google Scholar] [CrossRef]
- Verhaegen, M.E.; Harms, P.W.; van Goor, J.J.; Arche, J.; Patrick, M.T.; Wilbert, D.; Zabawa, H.; Grachtchouk, M.; Liu, C.-J.; Hu, K.; et al. Direct cellular reprogramming enables development of viral T antigen-driven Merkel cell carcinoma in mice. J. Clin. Investig. 2022, 132, e152069. [Google Scholar] [CrossRef]
- Schrama, D.; Hesbacher, S.; Angermeyer, S.; Schlosser, A.; Haferkamp, S.; Aue, A.; Adam, C.; Weber, A.; Schmidt, M.; Houben, R. Serine 220 phosphorylation of the Merkel cell polyomavirus large T antigen crucially supports growth of Merkel cell carcinoma cells. Int. J. Cancer 2016, 138, 1153–1162. [Google Scholar] [CrossRef]
- Hansen, U.K.; Lyngaa, R.; Ibrani, D.; Church, C.; Verhaegen, M.; Dlugosz, A.A.; Becker, J.C.; Straten, P.T.; Nghiem, P.; Hadrup, S.R. Extended T-Cell Epitope Landscape in Merkel Cell Polyomavirus Large T and Small T Oncoproteins Identified Uniquely in Patients with Cancer. J. Investig. Dermatol. 2022, 142, 239–243. [Google Scholar] [CrossRef]
- Brohl, A.S.; Markowitz, J.; Eroglu, Z.; Silk, A.W.; Hyngstrom, J.R.; Khushalani, N.I.; Tarhini, A.A.; De-Aquino, D.B.; Abraham, E.; Tsai, K.Y.; et al. Phase 1b trial of IFx-Hu2.0, a novel personalized cancer vaccine, in checkpoint inhibitor resistant merkel cell carcinoma and cutaneous squamous cell carcinoma. J. Clin. Oncol. 2023, 41, 9534. [Google Scholar] [CrossRef]
- Boitano, T.K.L.; Kako, T.; Leath, C.A., 3rd. New Paradigms in the Treatment of Cervical Cancer. Obstet. Gynecol. 2023, 142, 1322–1332. [Google Scholar] [CrossRef]
- Yurchenko, K.A.; Laikova, K.V.; Golovkin, I.O.; Novikov, I.A.; Yurchenko, A.A.; Makalish, T.P.; Oberemok, V.V. Inhibitory Effect of Phosphorothioate Oligonucleotide Complementary to G6PD mRNA on Murine Melanoma. Curr. Issues Mol. Biol. 2023, 45, 3180–3192. [Google Scholar] [CrossRef]
- Gambichler, T.; Majchrzak-Stiller, B.; Peters, I.; Becker, J.C.; Strotmann, J.; Abu Rached, N.; Müller, T.; Uhl, W.; Buchholz, M.; Braumann, C. The effect of GP-2250 on cultured virus-negative Merkel cell carcinoma cells: Preliminary results. J. Cancer Res. Clin. Oncol. 2023, 149, 10831–10840. [Google Scholar] [CrossRef]
- Geletneky, K.; Nüesch, J.P.; Angelova, A.; Kiprianova, I.; Rommelaere, J. Double-faceted mechanism of parvoviral oncosuppression. Curr. Opin. Virol. 2015, 13, 17–24. [Google Scholar] [CrossRef]
| NCT/Status | Design of Study | Vaccine Category | Intervention/Treatment |
|---|---|---|---|
| NCT04160065 currently recruiting | Phase I, non-randomized, multicenter | Plasmid DNA encoding immunogenic bacterial protein Emm55 | Intralesional IFx-Hu2.0 monotherapy in MCC resistant to ICI; |
| NCT05269381 currently recruiting | Phase I, open-label |
Personalized neoantigen peptide-based | PNeoVCA: cyclophosphamide, personalized s.c. neoantigen vaccine with sargramostim, given as monotherapy or with pembrolizumab |
| NCT05076760 currently recruiting | Phase I, open-label |
Conditionally replication-competent oncolytic adenovirus type 5
encoding transgenes for IFNβ and a recombinant chimeric form of CD40-ligand | Intratumoral injection of MEM-288 (selectively replicative in cancer cells) with and without nivolumab |
| NCT04349436 currently recruiting | Phase IB/II, multicenter, open-label | Oncolytic, modified herpes simplex type 1 virus (RP1) | Intra-tumoral injection of RP1 |
| NCT04725331 currently recruiting | Phase I/II multicenter, open-label | Oncolytic vaccinia-based virus encoding CTLA4 and GM-CSF (BT-001) | Intra-tumoral BT-001 (TG6030) administered alone or in combination with pembrolizumab |
| NCT06014086 currently recruiting | Phase I, open Label | RNAi targeting PD-1 (PH-762) | Neoadjuvant monotherapy using PH-762 |
| NCT05859074 currently recruiting | Phase I, open Label | Non-replicative recombinant modified vaccinia virus Ankara encoding FLT3L and Ox40L (MQ710) | Intra-tumoral MQ710 alone or in combination with pembrolizumab |
| NCT02819843 active, not recruiting | Phase II open Label | Modified oncolytic herpes virus type 1 encoding GM-CSF (talimogene laherparepvec; T-VEC) | Intralesional T-VEC with or without hypofractionated radiotherapy |
| NCT02978625 active, not recruiting | Phase II, open Label | Modified oncolytic herpes virus type 1 encoding GM-CSF (talimogene laherparepvec; T-VEC) | Intratumoral T-VEC plus anti-PD-1 antibody (Nivolumab) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Schrama, D.; Käpynen, R.; Weyer-Fahlbusch, S.S.; Becker, J.C.; Susok, L.; Kreppel, F.; Abu Rached, N. Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update. Vaccines 2024, 12, 533. https://doi.org/10.3390/vaccines12050533
Gambichler T, Schrama D, Käpynen R, Weyer-Fahlbusch SS, Becker JC, Susok L, Kreppel F, Abu Rached N. Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update. Vaccines. 2024; 12(5):533. https://doi.org/10.3390/vaccines12050533
Chicago/Turabian StyleGambichler, Thilo, David Schrama, Riina Käpynen, Sera S. Weyer-Fahlbusch, Jürgen C. Becker, Laura Susok, Florian Kreppel, and Nessr Abu Rached. 2024. "Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update" Vaccines 12, no. 5: 533. https://doi.org/10.3390/vaccines12050533
APA StyleGambichler, T., Schrama, D., Käpynen, R., Weyer-Fahlbusch, S. S., Becker, J. C., Susok, L., Kreppel, F., & Abu Rached, N. (2024). Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update. Vaccines, 12(5), 533. https://doi.org/10.3390/vaccines12050533








