A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations
Abstract
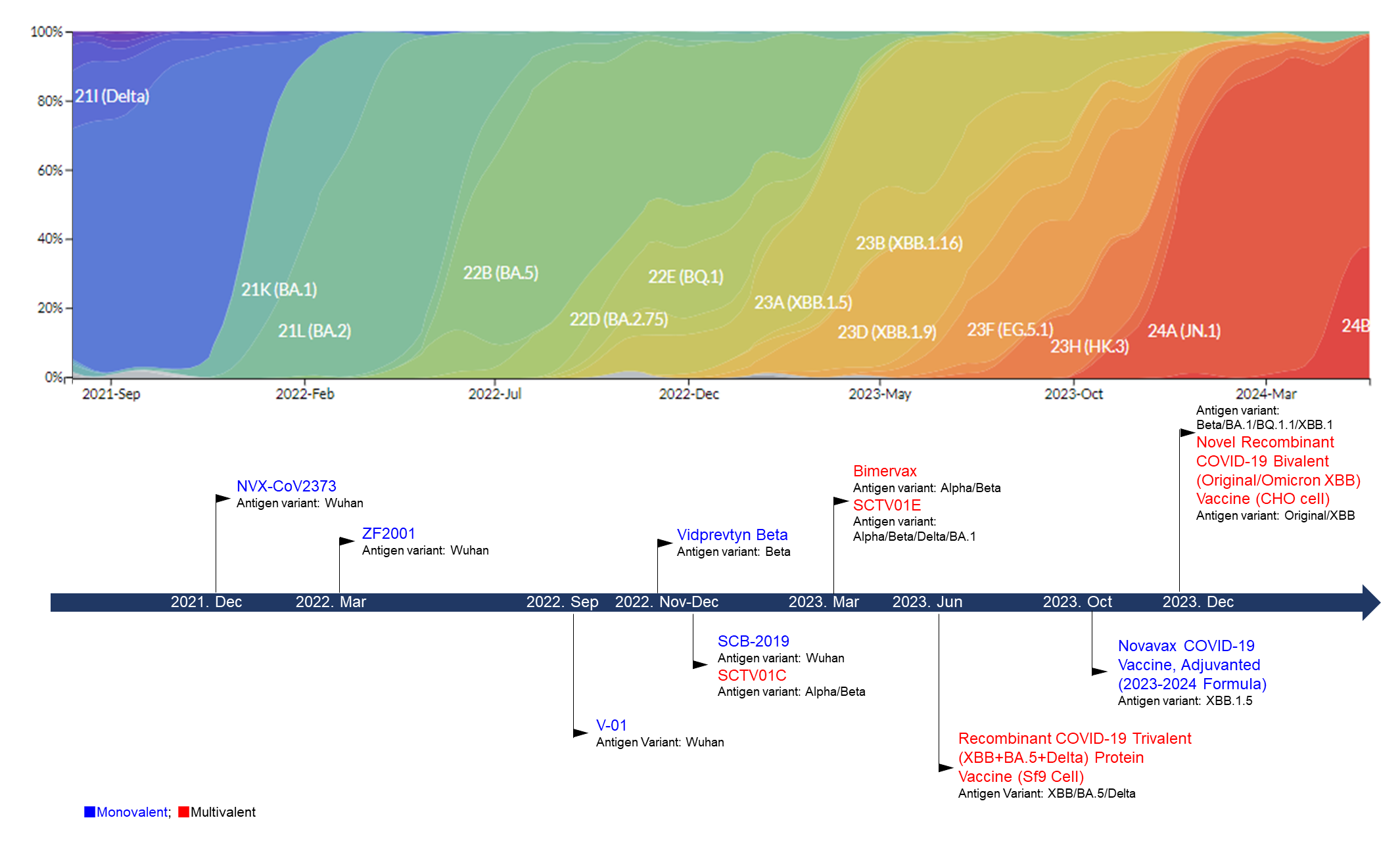
1. Introduction
2. Protein-Based COVID-19 Vaccines
2.1. Monovalent COVID-19 Vaccines
2.1.1. Nuvaxovid (NVX-CoV2373)/Novavax XBB.1.5 Vaccine 2023–2024 Formulation
2.1.2. ZF2001
2.1.3. V-01
2.1.4. Vidprevtyn Beta
2.1.5. SCB-2019
2.2. Multivalent COVID-19 Vaccines
2.2.1. SCTV01C/SCTV01E/SCTV01E-2
2.2.2. Bimervax
2.2.3. Recombinant COVID-19 Trivalent (XBB + BA.5 + Delta) Protein Vaccine (Sf9 Cell)
2.2.4. Novel Recombinant COVID-19 Bivalent (Original/Omicron XBB) Vaccine (CHO Cell)
3. Future Strategies Dealing with Constantly Emerging SARS-CoV-2 Variants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adil, M.T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 18 February 2024).
- Zenk, L.; Steiner, G.; Pina, E.C.M.; Laubichler, M.D.; Bertau, M.; Kainz, M.J.; Jager, C.; Schernhammer, E.S. Fast Response to Superspreading: Uncertainty and Complexity in the Context of COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 7884. [Google Scholar] [CrossRef]
- Farsalinos, K.; Poulas, K.; Kouretas, D.; Vantarakis, A.; Leotsinidis, M.; Kouvelas, D.; Docea, A.O.; Kostoff, R.; Gerotziafas, G.T.; Antoniou, M.N.; et al. Improved strategies to counter the COVID-19 pandemic: Lockdowns vs. primary and community healthcare. Toxicol. Rep. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Pandey, K.R.; Subedee, A.; Khanal, B.; Koirala, B. COVID-19 control strategies and intervention effects in resource limited settings: A modeling study. PLoS ONE 2021, 16, e0252570. [Google Scholar] [CrossRef]
- Chen, H.Z.; Tang, L.L.; Yu, X.L.; Zhou, J.; Chang, Y.F.; Wu, X. Bioinformatics analysis of epitope-based vaccine design against the novel SARS-CoV-2. Infect. Dis. Poverty 2020, 9, 88. [Google Scholar] [CrossRef]
- Boehm, E.; Kronig, I.; Neher, R.A.; Eckerle, I.; Vetter, P.; Kaiser, L.; The Geneva Centre for Emerging Viral Diseases. Novel SARS-CoV-2 variants: The pandemics within the pandemic. Clin. Microbiol. Infect. 2021, 27, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- McMenamin, M.E.; Nealon, J.; Lin, Y.; Wong, J.Y.; Cheung, J.K.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; Cowling, B.J. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: A population-based observational study. Lancet Infect. Dis. 2022, 22, 1435–1443. [Google Scholar] [CrossRef]
- Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally since Pandemic Start. Available online: https://nextstrain.org/ncov/gisaid/global/all-time (accessed on 11 March 2024).
- Vaccines and Related Biological Products Advisory Committee June 15, 2023 Meeting Summary Minutes. Available online: https://www.fda.gov/media/173590/download (accessed on 18 May 2023).
- Statement on the Antigen Composition of COVID-19 Vaccines. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 2 December 2022).
- Pfizer/BioNTech: Comirnaty Bivalent Original/Omicron BA.1. Available online: https://covid19.trackvaccines.org/vaccines/223/ (accessed on 2 December 2022).
- Pfizer/BioNTech: Comirnaty Bivalent Original/Omicron BA.4/BA.5. Available online: https://covid19.trackvaccines.org/vaccines/225/ (accessed on 2 December 2022).
- Moderna: Spikevax Bivalent Original/Omicron BA.1. Available online: https://covid19.trackvaccines.org/vaccines/210/ (accessed on 2 December 2022).
- Moderna: Spikevax Bivalent Original/Omicron BA.4/BA.5. Available online: https://covid19.trackvaccines.org/vaccines/224/ (accessed on 2 December 2022).
- United Arab Emirates. Available online: https://covid19.trackvaccines.org/country/united-arab-emirates/ (accessed on 2 December 2022).
- Xu, K.; Lei, W.; Kang, B.; Yang, H.; Wang, Y.; Lu, Y.; Lv, L.; Sun, Y.; Zhang, J.; Wang, X.; et al. A novel mRNA vaccine, SYS6006, against SARS-CoV-2. Front. Immunol. 2022, 13, 1051576. [Google Scholar] [CrossRef] [PubMed]
- The COVID-19 mRNA Vaccine Produced by CSPC Pharmaceutical Group Ltd. Was Granted for Emergency Use Authorization. Available online: http://www.e-cspc.com/details/details_92_4894.html (accessed on 21 March 2023).
- A Circular on the Issuance of the Implementation Plan for the Second Dose of the Novel Coronavirus Vaccine. Available online: http://www.gov.cn/xinwen/2022-12/14/content_5731899.htm (accessed on 14 December 2022).
- COVID-19 Vaccine SCTV01E Was Granted for Emergency Use Authorization. Available online: https://cs.com.cn/ssgs/gsxw/202303/t20230322_6331383.html (accessed on 14 December 2022).
- Wang, R.; Huang, H.; Yu, C.; Sun, C.; Ma, J.; Kong, D.; Lin, Y.; Zhao, D.; Zhou, S.; Lu, J.; et al. A spike-trimer protein-based tetravalent COVID-19 vaccine elicits enhanced breadth of neutralization against SARS-CoV-2 Omicron subvariants and other variants. Sci. China Life Sci. 2022, 66, 1818–1830. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; Lopez-Cortes, A.; Gonzalez, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Cid, R.; Bolivar, J. Platforms for Production of Protein-Based Vaccines: From Classical to Next-Generation Strategies. Biomolecules 2021, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Vaccines for COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html (accessed on 12 January 2024).
- COVID-19 Medicines. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 7 May 2024).
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Anez, G.; Adelglass, J.M.; Barrat Hernandez, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.A.; Galloway, J.; et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin. Infect. Dis. 2023, 76, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Anez, G.; Dunkle, L.M.; Gay, C.L.; Kotloff, K.L.; Adelglass, J.M.; Essink, B.; Campbell, J.D.; Cloney-Clark, S.; Zhu, M.; Plested, J.S.; et al. Safety, Immunogenicity and Efficacy of NVX-CoV2373 in Adolescents in PREVENT-19: A Randomized, Phase 3 Trial. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Áñez, G.; Dunkle, L.M.; Gay, C.L.; Kotloff, K.L.; Patel, N.; Plested, J.S.; McGarry, A.; Woo, W.; Cai, R.; Cho, I. Safety and Immunogenicity of a Booster Dose of Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) in Adults from the PREVENT-19 Trial in the United States. Open Forum Infect. Dis. 2022, 9, ofac492.1869. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Patel, N.; Trost, J.F.; Guebre-Xabier, M.; Zhou, H.; Norton, J.; Jiang, D.; Cai, Z.; Zhu, M.; Marchese, A.M.; Greene, A.M.; et al. XBB.1.5 spike protein COVID-19 vaccine induces broadly neutralizing and cellular immune responses against EG.5.1 and emerging XBB variants. Sci. Rep. 2023, 13, 19176. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer-Based Covid-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, Y.; He, P.; Chen, Z.; Yang, H.; Li, F.; Zhang, S.; Wang, D.; Wang, G.; Yang, S.; et al. Safety and immunogenicity of a protein subunit COVID-19 vaccine (ZF2001) in healthy children and adolescents aged 3–17 years in China: A randomised, double-blind, placebo-controlled, phase 1 trial and an open-label, non-randomised, non-inferiority, phase 2 trial. Lancet Child. Adolesc. Health 2023, 7, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Mahmood, S.F.; Jin, F.; Cheah, W.K.; Ahmad, M.; Sohail, M.A.; Ahmad, W.; Suppan, V.K.; Sayeed, M.A.; Luxmi, S.; et al. Efficacy of heterologous boosting against SARS-CoV-2 using a recombinant interferon-armed fusion protein vaccine (V-01): A randomized, double-blind and placebo-controlled phase III trial. Emerg. Microbes Infect. 2022, 11, 1910–1919. [Google Scholar] [CrossRef]
- Launay, O.; Gupta, R.; Machabert, T.; Konate, E.; Rousseau, A.; Claire, V.; Beckers, F.; Chicz, R.; Botelho-Nevers, E.; Cachanado, M.; et al. Beta-variant recombinant SARS-CoV-2 vaccine induces durable cross-reactive antibodies against Omicron variants. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Bravo, L.; Smolenov, I.; Han, H.H.; Li, P.; Hosain, R.; Rockhold, F.; Clemens, S.A.C.; Roa, C., Jr.; Borja-Tabora, C.; Quinsaat, A.; et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: A phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2022, 399, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Bravo, L.; Buntinx, E.; Borja-Tabora, C.; Velasquez, H.; Rodriquez, E.J.; Rodriguez, C.A.; Carlos, J.; Montellano, M.E.B.; Alberto, E.R.; et al. Safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit COVID-19 vaccine in healthy 12–17 year-old adolescents. Hum. Vaccin. Immunother. 2023, 19, 2206359. [Google Scholar] [CrossRef] [PubMed]
- Roa, C.C., Jr.; de Los Reyes, M.R.A.; Plennevaux, E.; Smolenov, I.; Hu, B.; Gao, F.; Ilagan, H.; Ambrosino, D.; Siber, G.; Clemens, R.; et al. SCB-2019 protein vaccine as heterologous booster of neutralizing activity against SARS-CoV-2 Omicron variants after immunization with other COVID-19 vaccines. Hum. Vaccin. Immunother. 2024, 20, 2301632. [Google Scholar] [CrossRef]
- Hannawi, S.; Saf Eldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Li, J.; Chen, Y.; Xie, L. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C in adults previously vaccinated with inactivated vaccine: A randomized, double-blind, placebo-controlled phase 1/2 clinical trial. J. Infect. 2023, 86, 154–225. [Google Scholar] [CrossRef]
- Hannawi, S.; Saifeldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Liu, D.; Yan, L.; Xie, L. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C, in adults previously vaccinated with mRNA vaccine: A randomized, double-blind, placebo-controlled phase 1/2 clinical trial. eBioMedicine 2023, 87, 104386. [Google Scholar] [CrossRef]
- Hannawi, S.; Yan, L.; Saifeldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Zhang, M.; Gao, C.; Chen, Y.; et al. Safety and immunogenicity of multivalent SARS-CoV-2 protein vaccines: A randomized phase 3 trial. eClinicalMedicine 2023, 64, 102195. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, S.; Saf Eldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Xu, S.; Li, J.; Liu, D.; Baidoo, A.A.H.; et al. Safety and immunogenicity of a tetravalent and bivalent SARS-CoV-2 protein booster vaccine in men. Nat. Commun. 2023, 14, 4043. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, S.; Ma, W.; Li, X.; Wei, K.; Xie, F.; Zhao, C.; Zhao, X.; Wang, S.; Li, C.; et al. Enhanced neutralization of SARS-CoV-2 variant BA.2.86 and XBB sub-lineages by a tetravalent COVID-19 vaccine booster. Cell Host Microbe 2024, 32, 25–34.e5. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, Q.; Zhu, C.; Xuan, K.; Li, T.; Li, Q.; Pang, X.; Zha, Z.; Li, J.; Qiao, L.; et al. Immunogenicity of Tetravalent Protein Vaccine SCTV01E-2 against SARS-CoV-2 EG.5 Subvaraint: A Phase 2 Trial. Vaccines 2024, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Corominas, J.; Garriga, C.; Prenafeta, A.; Moros, A.; Canete, M.; Barreiro, A.; Gonzalez-Gonzalez, L.; Madrenas, L.; Guell, I.; Clotet, B.; et al. Safety and immunogenicity of the protein-based PHH-1V compared to BNT162b2 as a heterologous SARS-CoV-2 booster vaccine in adults vaccinated against COVID-19: A multicentre, randomised, double-blind, non-inferiority phase IIb trial. Lancet Reg. Health Eur. 2023, 28, 100613. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shang, J.; Jiang, S.; Du, L. Subunit Vaccines against Emerging Pathogenic Human Coronaviruses. Front. Microbiol. 2020, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Hadj Hassine, I. COVID-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022, 32, e2313. [Google Scholar] [CrossRef] [PubMed]
- Regulatory Approval of COVID-19 Vaccine Nuvaxovid. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-nuvaxovid (accessed on 3 February 2022).
- EMA Recommends Nuvaxovid for Authorization in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu (accessed on 3 October 2023).
- Novavax Letter of Authorization 10192022. Available online: https://www.fda.gov/media/159902/download (accessed on 3 October 2023).
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Last Update 11/2022-Product Information Leaflet (PIL) for Nuvaxovid Dispersion for Injection. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-nuvaxovid/product-information-leaflet-pil-for-nuvaxovid-dispersion-for-injection (accessed on 3 February 2022).
- Public Health England. Investigation of Novel SARS-CoV-2 Variant: Variant of Concern 202012/01. Technical Briefing 5. 14 January 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959426/Variant_of_Concern_VOC_202012_01_Technical_Briefing_5.pdf (accessed on 20 December 2021).
- Label of Recombinant COVID-19 Trivalent (XBB+BA.5+Delta) Protein Vaccine (Sf9 Cell). Available online: http://www.westvacpharma.com/detail/122 (accessed on 20 December 2021).
- Regan, J.J.; Moulia, D.L.; Link-Gelles, R.; Godfrey, M.; Mak, J.; Najdowski, M.; Rosenblum, H.G.; Shah, M.M.; Twentyman, E.; Meyer, S.; et al. Use of Updated COVID-19 Vaccines 2023–2024 Formula for Persons Aged >/=6 Months: Recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb. Mortal Wkly. Rep. 2023, 72, 1140–1146. [Google Scholar] [CrossRef]
- Approved Vaccines. Available online: https://covid19.trackvaccines.org/vaccines/approved (accessed on 20 December 2021).
- Instructions for Recombinant Novel Coronavirus Protein Vaccine (CHO Cells). Available online: http://www.zhifeishengwu.com/d/file/product/ybcp/2022-04-01/049f602bcb54c0e00c336d44264301d8.pdf (accessed on 2 December 2022).
- Cao, Y.; Yisimayi, A.; Bai, Y.; Huang, W.; Li, X.; Zhang, Z.; Yuan, T.; An, R.; Wang, J.; Xiao, T.; et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Res. 2021, 31, 732–741. [Google Scholar] [CrossRef] [PubMed]
- VidPrevtyn Beta; COVID-19 Vaccine (Recombinant, Adjuvanted). Available online: https://www.ema.europa.eu/en/documents/product-information/vidprevtyn-beta-epar-product-information_en.pdf (accessed on 1 March 2022).
- Summaru of Product Charicteristics. Available online: https://www.ema.europa.eu/system/files/documents/product-information/ema-combined-h-6058_en_1.pdf (accessed on 10 November 2022).
- Sun, S.; Cai, Y.; Song, T.Z.; Pu, Y.; Cheng, L.; Xu, H.; Sun, J.; Meng, C.; Lin, Y.; Huang, H.; et al. Interferon-armed RBD dimer enhances the immunogenicity of RBD for sterilizing immunity against SARS-CoV-2. Cell Res. 2021, 31, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- EMA Recommends Approval of VidPrevtyn Beta as a COVID-19 Booster Vaccine. Available online: https://www.ema.europa.eu/en/news/ema-recommends-approval-vidprevtyn-beta-covid-19-booster-vaccine (accessed on 30 March 2023).
- Liang, J.G.; Su, D.; Song, T.Z.; Zeng, Y.; Huang, W.; Wu, J.; Xu, R.; Luo, P.; Yang, X.; Zhang, X.; et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat. Commun. 2021, 12, 1346. [Google Scholar] [CrossRef]
- Richmond, P.C.; Hatchuel, L.; Pacciarini, F.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J.; Clemens, R. Persistence of the Immune Responses and Cross-Neutralizing Activity with Variants of Concern Following 2 Doses of Adjuvanted SCB-2019 Coronavirus Disease 2019 Vaccine. J. Infect. Dis. 2021, 224, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Clover Biopharmaceuticals Completes Enrollment of Adult and Elderly Population in SPECTRA Global Phase 2/3 Clinical Trial for Its COVID-19 Vaccine Candidate. Available online: https://ir.cloverbiopharma.com/news-releases/news-release-details/clover-biopharmaceuticals-completes-enrollment-adult-and-elderly (accessed on 10 November 2022).
- Clover Announces Corporate Updates and Full Year 2022 Financial Results. 28 March 2023. Available online: https://ir.cloverbiopharma.com/node/7441/pdf (accessed on 6 July 2021).
- Wang, R.; Sun, C.; Ma, J.; Yu, C.; Kong, D.; Chen, M.; Liu, X.; Zhao, D.; Gao, S.; Kou, S.; et al. A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants. Vaccines 2022, 10, 702. [Google Scholar] [CrossRef]
- Authorised COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 7 May 2024).
- He, C.; Yang, J.; Hong, W.; Chen, Z.; Peng, D.; Lei, H.; Alu, A.; He, X.; Bi, Z.; Jiang, X.; et al. A self-assembled trimeric protein vaccine induces protective immunity against Omicron variant. Nat. Commun. 2022, 13, 5459. [Google Scholar] [CrossRef]
- Announcement on Inclusion of Novel Recombinant COVID-19 Bivalent (Original/Omicron XBB) Vaccine (CHO Cell) for Emergency Use. Available online: https://static.cninfo.com.cn/finalpage/2023-12-04/1218505191.PDF (accessed on 4 December 2023).
- About Wastewater Data. Available online: https://www.cdc.gov/nwss/about-data.html (accessed on 14 May 2024).
- Advisory Committee Calendar. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar (accessed on 15 June 2023).
- Kandeel, M.; Mohamed, M.E.M.; Abd El-Lateef, H.M.; Venugopala, K.N.; El-Beltagi, H.S. Omicron variant genome evolution and phylogenetics. J. Med. Virol. 2022, 94, 1627–1632. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
| Vaccine | Manufacture | Antigen Variant | Adjuvant | Approved Authorities | Approved Indication and Populations |
|---|---|---|---|---|---|
| NVX-CoV2373 | Novavax | Wuhan | Matrix-M | FDA and EMA | Primary series for individual aged ≥ 12 years Booster for individual ≥ 18 years |
| Novavax COVID-19 Vaccine (2023–2024 Formula) | Novavax | XBB.1.5 | Matrix-M | FDA and EMA | Primary series and booster for individuals aged ≥ 12 years |
| ZF2001 | Anhui Zhifei Longcom Biopharmaceutical | Wuhan-Hu-1 | Aluminum hydroxide | NMPA | Primary series and booster for individuals aged ≥ 3 years |
| V-01 | Livzon Mabpharm Inc | Wuhan | Aluminum hydroxide | NMPA | Booster for individuals ≥ 18 years |
| Vidprevtyn Beta | Sanofi & GSK | Beta | AS03 | EMA | Booster for individuals ≥ 18 years |
| SCB-2019 | Clover Biopharmaceuticals | Wuhan-Hu-1 | CpG 1018/Aluminum | NMPA | Booster for individuals ≥ 18 years |
| SCTV01C | Sinocelltech Ltd. | Alpha, Beta | SCT-VA02B | NMPA | Booster for individuals ≥ 18 years |
| SCTV01E | Sinocelltech Ltd. | Alpha, Beta, Delta, BA.1 | SCT-VA02B | NMPA | Booster for individuals ≥ 18 years |
| SCTV01E-2 | Sinocelltech Ltd. | Beta, BA.1, BQ.1.1, XBB.1 | SCT-VA02B | NMPA | Booster for individuals ≥ 18 years |
| Bimervax | Laboratorios Hipra, S.A. | Alpha, Beta | SQBA | EMA | Booster for individuals ≥ 16 years |
| Recombinant Trivalent Protein Vaccine (Sf9 Cell) | WestVac Biopharma | XBB, BA.5, Delta | MF59-like adjuvant | NMPA | Booster for individuals ≥ 18 years |
| Novel Recombinant Bivalent (Original/Omicron XBB) Vaccine (CHO cell) | Livzon Mabpharm Inc | Original, Omicron XBB | Aluminum hydroxide | NMPA | Booster for individuals ≥ 18 years |
| Vaccine Name | Antigen Variant | Year | Journal | Content | Reference |
|---|---|---|---|---|---|
| NVX-CoV2373 | Wuhan | 2021 | The New England Journal of Medicine | Efficacy and safety | [29] |
| 2022 | The New England Journal of Medicine | Efficacy and safety | [30] | ||
| 2023 | Clinical Infectious Diseases | Efficacy and safety | [31] | ||
| 2022 | medRxiv | Immunogenicity and efficacy | [32] | ||
| 2022 | Open Forum Infectious Diseases | Immunogenicity and safety | [33] | ||
| 2021 | Lancet | Immunogenicity and safety | [34] | ||
| Novavax COVID-19 Vaccine (2023–2024 Formula) | XBB.1.5 | 2023 | Scientific Reports | Immunogenicity (pre-clinical study) | [35] |
| ZF2001 | Wuhan-Hu-1 | 2021 | Lancet Infectious Disease | Safety and immunogenicity | [36] |
| 2022 | The New England Journal of Medicine | Efficacy and safety | [37] | ||
| 2023 | The Lancet Child & Adolescent Health | Immunogenicity and safety | [38] | ||
| V-01 | Wuhan | 2022 | Emerging Microbes & Infections | Efficacy and safety | [39] |
| Vidprevtyn Beta | Beta | 2023 | Research Square | Immunogenicity and safety | [40] |
| SCB-2019 | Wuhan-Hu-1 | 2022 | Lancet | Efficacy and safety | [41] |
| 2023 | Human Vaccines & Immunotherapeutics | Immunogenicity and safety | [42] | ||
| 2024 | Human Vaccines & Immunotherapeutics | Immunogenicity and safety | [43] | ||
| SCTV01C | Alpha, Beta | 2023 | Journal of Infection | Immunogenicity and safety | [44] |
| 2023 | EBioMedicine | Immunogenicity and safety | [45] | ||
| SCTV01E | Alpha, Beta, Delta, BA.1 | 2023 | EClinicalMedicine | Immunogenicity and safety | [46] |
| 2023 | Nat Communication | Immunogenicity and safety | [47] | ||
| 2024 | Cell Host Microbe | Immunogenicity and safety | [48] | ||
| SCTV01E-2 | Beta, BA.1, BQ.1.1, XBB.1 | 2024 | Vaccines (Basel) | Immunogenicity and safety | [49] |
| Bimervax | Alpha, Beta | 2023 | Lancet Regional Health Europe | Immunogenicity and safety | [50] |
| Vaccine | Identifier | Phase | Primary/Booster Dose | Age (Years) | n | Location | Status |
|---|---|---|---|---|---|---|---|
| NVX-CoV2373 | NCT04583995 | III | Primary | 18–84 | 14,039 | UK | Completed |
| NCT04611802 | III | Primary | ≥12 | 31,829 | US and Mexico (≥18 years); US (≥12 years) | Completed | |
| ISRCTN 73765130 | II | Booster | ≥30 | 2878 | UK | Completed | |
| ZF2001 | NCT04646590 | III | Primary | ≥18 | 28,873 | China, Ecuador, Indonesia, Pakistan, Uzbekistan | Completed |
| NCT05109598 | II | Primary | 3~17 | 400 | China | Completed | |
| V-01 | NCT05096832 | III | Booster | ≥18 | 10,241 | Pakistan, Malaysia | Completed |
| Vidprevtyn Beta | NCT04762680 | II/III | Booster | ≥18 | 543 | US, Honduras, Kenya, Mexico, New Zealand, Panama, Spain, UK | Completed |
| NCT05124171 | III | Booster | ≥18 | 162 | France | Completed | |
| SCB-2019 | NCT04672395 | II/III | Primary | ≥18 | 30,128 | Belgium, Brazil, Colombia, Philippines, and South Africa | Completed |
| Extended of NCT04672395 | Primary | 12–17 | 1280 | Belgium, Colombia, Philippines | Completed | ||
| NCT05188677 | III | Booster | 18–80 | 1330 | Philippines | Completed | |
| SCTV01C | NCT05043285 | I/II | Booster | ≥18 | 234 | United Arab Emirates | Completed |
| NCT05043311 | I/II | Booster | ≥18 | 234 | United Arab Emirates | Completed | |
| SCTV01E | NCT05323461 | III | Booster | ≥18 | 1351 | United Arab Emirates | Completed |
| Booster | ≥18 | 451 | United Arab Emirates | Completed | |||
| NCT05308576 | III | Booster | ≥18 | 9223 | China | ongoing | |
| SCTV01E-2 | NCT05933512 | II | Booster | ≥18 | 429 | China | ongoing |
| Bimervax | NCT05142553 | IIb | Booster | ≥18 | 887 | Spain | Completed |
| NCT05246137 | III | Booster | ≥18 | 2661 | Italy, Spain | Completed | |
| Recombinant Trivalent Protein Vaccine (Sf9 Cell) | NCT05911061 | III | Booster | ≥18 | 1905 | -- | Completed |
| Vaccines | Age (Years) | n | Dosage | Time for Efficacy | Median Time for Efficacy Follow-Up | Efficacy | Reference | |
|---|---|---|---|---|---|---|---|---|
| Overall Efficacy | Efficacy for Specific Variants | |||||||
| NVX-CoV2373 | 18–84 | 14,039 | 2 | 7 days after the second dose | 3 months | 89.7% | Alpha: 86.3% Non-Alpha: 96.4% | [29] |
| 7 days after the second dose | 4.5 months | 82.7% | [31] | |||||
| ≥18 | 29,582 | 2 | 7 days after the second dose | 3 months | 90.4% | Alpha: 93.6% Non-Alpha: 92.6% | [30] | |
| 12~17 | 2247 | 2 | 7 days after the second dose | 2 months | 79.5% | Delta: 82.0% | [32] | |
| ZF2001 | ≥18 | 28,873 | 3 | 7 days after the third dose | 50 days † | 81.4% | Delta: 81.4% Alpha: 92.7% Kappa+ B.1.617.3: 84.8% | [37] |
| 178 days † | 75.7% | Delta: 76.1% Alpha: 88.3% Kappa: 75.2% | ||||||
| V-01 | ≥18 | 10,241 | 1 | 14 days after vaccination | 60 days | 47.8% | Omicron: 47.0% Delta: 79.9% | [39] |
| SCB-2019 | ≥18 | 30,128 | 2 | 14 days after the second dose | 82 days | 67.2% | Delta: 78.7% Gamma: 91.8% Mu: 58.6% | [41] |
| Recombinant Trivalent Protein Vaccine (Sf9 Cell) | ≥18 years | 5855 | 1 | 14 days after the second dose | -- | 93.28% | -- | [59] |
| Vaccine | Age (Years) | n | Dosage | Pseudo/ Live-Virus | Variant | GMT | GMR (95% CI) | Seroconversion Rate | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ZF2001 | 3–17 | 75 | 3 | Live | Prototype | 176.5 (118.6, 262.8) | 93% | [38] | |
| 3–17 | 400 | 3 | Live | Prototype BA.2 | 245.4 (220.0, 273.7) 42.9 (37.9, 48.5) | 3–17 years/18–59 years 8.6 (7.0, 10.4) 10.6 (9.1, 12.5) | 99% 95% | [38] | |
| Vidprevtyn Beta | ≥18 | 162 | 1 | Pseudo | BA.1 BA.4/5 D614G | Vidprevtyn Beta/BNT162b2 2.53 (1.80, 3.57) 2.50 (1.70, 3.67) 1.43 (1.06, 1.94) | 100.0% 96.2% | [40,64] | |
| ≥18 | 543 | 1 | Pseudo | D614G Beta D614G Beta | mRNA vaccine primed 10,814 (9793, 11,941) 7501 (6754, 8330) Ad-vector vaccine primed 6565 (5397, 7986) 5077 (4168, 6185) | [64] | |||
| SCB-2019 | 12–17 | 1280 | 2 | Live | Prototype | 12–17 years/18–25 years 1.9 (1.3–3.0) | 86% | [42] | |
| 18–80 | 1330 | 1 | Live | Prototype | SCB-2019/Comirnaty 0.36 (0.31, 0.41); SCB-2019/CoronaVac 4.63 (3.96, 5.41); SCB-2019/Vaxevria 1.68 (1.46, 1.93) | [43] | |||
| SCTV01C | ≥18 | 234 | 1 | Live | Delta Omicron | 3891 (3432, 4412) 870 (752, 1007) | 13.1 (10.3, 16.9) 14.7 (11.0, 19.7) | [44] | |
| ≥18 | 234 | 1 | Live | Delta Omicron | 3816 (3382, 4305) 833 (713, 973) | 3.1 (2.5, 3.8) 4.0 (3.1, 5.1) | [45] | ||
| SCTV01E | ≥18 | 1351 | 1 | Live | Delta BA.1 BA.5 | BBIBP-CorV: 667 (541, 823) SCTV01C: 4171 (3545, 4906) SCTV01E: 4760 (3939, 5752) BBIBP-CorV: 219 (167, 286) SCTV01C: 1262 (1056, 1509) SCTV01E: 1926 (1557, 2382) BBIBP-CorV: 324 (251, 419) SCTV01C: 2203 (1872, 2593) SCTV01E: 2636 (2227, 3120) | SCTV01C/BBIBP-CorV: 6.26 SCTV01E/BBIBP-CorV: 7.26 SCTV01E/SCTV01C: 1.15 SCTV01C/BBIBP-CorV: 6.49 SCTV01E/BBIBP-CorV: 9.56 SCTV01E/SCTV01C: 1.50 SCTV01C/BBIBP-CorV: 7.11 SCTV01E/BBIBP-CorV: 8.61 SCTV01E/SCTV01C: 1.20 | [46] | |
| ≥18 | 451 | 1 | Live | BA.1 BA.5 | BNT162b2: 1049 (923, 1193) SCTV01C: 1189 (1027, 1376) SCTV01E: 1659 (1445, 1904) BNT162b2: 1687 (1471, 1936) SCTV01C: 1736 (1517, 1987) SCTV01E: 2281 (1993, 2610) | SCTV01E/BNT162b2: 1.55 (1.30, 1.85) SCTV01E/SCTV01C: 1.44 (1.19, 1.74) SCTV01E/BNT162b2: 1.28 (1.07, 1.54) SCTV01E/SCTV01C: 1.33 (1.10, 1.61) | [47] | ||
| SCTV01E-2 | ≥18 | 429 | 1 | Live | EG.5 XBB.1 | SCTV01E-2: 924 (823, 1037) SCTV01E: 510 (454, 573) SCTV01E-2: 1887 (1686, 2112) SCTV01E: 1435 (1267, 1626) | 1.8 (1.5, 2.1) 1.3 (1.1, 1.5) | SCTV01E-2: 78.9% SCTV01E: 61.6% SCTV01E-2: 68.5% SCTV01E: 62.4% | [49] |
| Bimervax | ≥18 | 765 | 1 | Pseudo | D614G Beta Delta BA.1 | 1.71 (1.45, 2.02) 0.62 (0.52, 0.75) 1.02 (0.86, 1.21) 0.60 (0.50, 0.72 | [50,65] | ||
| ≥16 | 2661 | 1 | Pseudo | D614G/Beta/Delta/BA.1 D614G/Beta/Delta/BA.1 D614G/Beta/Delta/BA.1 | Comirnaty primed 4753.65/8820.74/7564.79/5757.43 Ad26.COV2-S primed 2298.81/5009.47/2600.31/1847.41 Spikevax primed 4437.27/6857.95/5811.47/4379.81 | [65] | |||
| Recombinant Trivalent Protein Vaccine (Sf9 Cell) | ≥18 | 2905 | 1 | Live | XBB.1.5/XBB.1.16 XBB.1.9.1/XBB.2.3/BQ.1 BF.7/BA.4/5/BA.2.75 | 1728.6/1093.67 616.03/1112.53/1329.77 2052.44/3235.68/3681.23 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, G.; Gao, C.; Zhang, M.; Chen, Y.; Xie, L. A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations. Vaccines 2024, 12, 579. https://doi.org/10.3390/vaccines12060579
Qian G, Gao C, Zhang M, Chen Y, Xie L. A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations. Vaccines. 2024; 12(6):579. https://doi.org/10.3390/vaccines12060579
Chicago/Turabian StyleQian, Gui, Cuige Gao, Miaomiao Zhang, Yuanxin Chen, and Liangzhi Xie. 2024. "A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations" Vaccines 12, no. 6: 579. https://doi.org/10.3390/vaccines12060579
APA StyleQian, G., Gao, C., Zhang, M., Chen, Y., & Xie, L. (2024). A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations. Vaccines, 12(6), 579. https://doi.org/10.3390/vaccines12060579





