Abstract
The yellow fever (YF) vaccine is one of the safest and most effective vaccines currently available. Still, its administration in people living with HIV (PLWH) is limited due to safety concerns and a lack of consensus regarding decreased immunogenicity and long-lasting protection for this population. The mechanisms associated with impaired YF vaccine immunogenicity in PLWH are not fully understood, but the general immune deregulation during HIV infection may play an important role. To assess if HIV infection impacts YF vaccine immunogenicity and if markers of immune deregulation could predict lower immunogenicity, we evaluated the association of YF neutralization antibody (NAb) titers with the pre-vaccination frequency of activated and exhausted T cells, levels of pro-inflammatory cytokines, and frequency of T cells, B cells, and monocyte subsets in PLWH and HIV-negative controls. We observed impaired YF vaccine immunogenicity in PLWH with lower titers of YF-NAbs 30 days after vaccination, mainly in individuals with CD4 count <350 cells/mm3. At the baseline, those individuals were characterized by having a higher frequency of activated and exhausted T cells and tissue-like memory B cells. Elevated levels of those markers were also observed in individuals with CD4 count between 500 and 350 cells/mm3. We observed a negative correlation between the pre-vaccination level of CD8+ T cell exhaustion and CD4+ T cell activation with YF-NAb titers at D365 and the pre-vaccination level of IP-10 with YF-NAb titers at D30 and D365. Our results emphasize the impact of immune activation, exhaustion, and inflammation in YF vaccine immunogenicity in PLWH.
1. Introduction
Yellow fever (YF) is a sylvatic arboviral disease endemic to the tropical and subtropical regions of Latin America, the Caribbean, and Africa. The disease is a major concern for public health due to the high fatality rate [1,2] and the increasing risk of urban outbreaks associated with the increased circulation of Aedes aegypti and the narrowing of the sylvatic borders [3]. In Brazil, urban YF was eradicated in the late 1960s through vector control and vaccination campaigns, but the disease remains restricted to the Amazon region [4], which extends through nine states that house approximately 15% of the country’s total population living with HIV, estimated at around 1 million [5].
YF antiviral-specific treatment is not available, but live attenuated vaccines are available as a primary prevention strategy and widely prescribed to travelers/residents of endemic areas and the general population during outbreaks [3]. During the last decade, wide vaccination campaigns were called in response to outbreaks in 2014–2018, which reached the borders of greatly populated states in the southeast region [6,7]. Together, these states accounted for 162 thousand notified HIV cases in the last decade, representing approximately 40% of HIV infections in Brazil during the historical series [5].
Although safe and highly effective for the general population, precautions are taken while recommending the YF vaccine to immunocompromised individuals due to concerns regarding serious adverse events for live vaccines in those individuals, including people living with HIV (PLWH) [8]. For PLWH, safety and immunogenicity reports are mostly limited to small observational studies and case reports [9,10,11,12,13,14,15], except for a few cohort studies [16,17,18,19,20,21], and indicate that markers of HIV disease progression are associated with a lower level and shorter persistence of YF neutralization antibody (NAb) titers [11,17,18,20]. To address this, a longitudinal prospective non-randomized interventional trial with 280 PLWH was conducted and confirmed the safety of the YF vaccine in PLWH with CD4+ cell count ≥200 cells/mm3 [22]. However, immunogenicity was impaired in PLWH, particularly in those with a high viral load, low CD4+ T cell count, and low CD4/CD8 ratio at vaccination.
Several mechanisms could impact vaccination efficacy in PLWH. Beyond a severe immunodeficiency in advanced stages, HIV infection leads to chronic and generalized immune deregulation that triggers immune activation, affecting several components of the immune response [23,24]. The consequences of this activation state include a shift in T cells towards most differentiated/effector phenotypes [23,25,26,27] and decrease in central memory cells [28,29,30,31]; altered dynamics of monocytes, with an increase in CD16++ subsets [32,33,34]; increased frequency of T cells expressing exhaustion markers (e.g., PD-1, TIM-3, TIGIT, and LAG-3) [35,36,37,38,39,40,41,42,43]; and premature aging and immune senescence profiles in cytotoxic T cells [44,45,46,47,48]. In addition, B cell subsets in PLWH present an activation profile similar to T cells, with decreased frequencies of resting memory and increased tissue-like and activated memory subsets [49,50,51,52,53]. Those alterations are associated with disease progression and can be attenuated by antiretroviral treatment, although levels of several markers of immune activation and exhaustion do not fully normalize even after long-term viral suppression [36,54,55,56,57,58]. Furthermore, a study evaluating the response to the YF vaccine in HIV-negative individuals associated the lower level of immune response with an increased level of immune activation and exhaustion in individuals from Uganda [59], highlighting the impact of immune activation in YF vaccine immunogenicity.
To further investigate the mechanisms that could impair YF vaccine immunogenicity in PLWH, our study evaluated the pre-vaccination frequencies of several cellular subsets and inflammation markers that are affected by HIV infection in a longitudinal interventional trial that evaluated the immunogenicity and reactogenicity of the YF vaccine in PLWH and HIV-negative controls [22].
2. Materials and Methods
2.1. Study Population
This sub-study was conducted within a longitudinal prospective non-randomized interventional trial with PLWH and HIV-negative controls at the Instituto Nacional de Infectologia Evandro Chagas from Fundação Oswaldo Cruz (INI-FIOCRUZ), Rio de Janeiro, Brazil, to evaluate the immunogenicity and safety of a standard single dose of 17DD YFV in PLWH (NCT03132311). The trial design and results have been reported previously [22]. Between May 2017 and May 2018, adults (18 to 59 years old) with no history of prior YF vaccination or disease and no contraindications to the vaccine were eligible for this study [22]. PLWH were required to have a CD4+ T cell count >200 cell/mm3 at least 6 months before enrolment in this study. The HIV-negative participants were required to have a non-reactive anti-HIV rapid test at enrolment.
At enrolment (D0 visit), all participants received a single standard dose (0.5 mL, subcutaneous) of the 17DD YF vaccine (Bio-Manguinhos - Fiocruz, Rio de Janeiro, Brazil) (21), containing approximately 105 viral particles. Clinical data, including time since HIV diagnosis, time in ART, and nadir CD4 were collected from PLWH. After vaccination, participants were followed on Day 5 and Day 30 and 1 year after enrolment. Blood samples collected at each visit were used for the assessment of CD4+ and CD8+ T cell counts, quantification of HIV viral load in PLWH, and isolation of peripheral blood mononuclear cells (PBMCs).
2.2. Sample Preparation
PBMCs were isolated from whole blood using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) by density gradient centrifugation, cryopreserved in fetal bovine serum supplemented with 10% DMSO at a concentration of 7–10 × 106 cells/cryovial and stored in liquid nitrogen until use.
2.3. CD4+ and CD8+ T Cell Counts and Plasma Viral Load Quantification
Absolute CD4+ and CD8+ T cell counts were obtained from whole blood using the MultiTest TruCount-kit and the MultiSet software v3.1x on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Plasma HIV-1 viral loads were measured using the Abbott RealTime HIV-1 assay (Abbott Laboratories, Wiesbaden, Germany), with a lower detection limit of 40 copies/mL.
2.4. Flow Cytometry
Cryopreserved PBMCs were thawed and rested overnight in RPMI 1640 (Sigma-Aldrich, USA) supplemented with 10% of fetal bovine serum (Gibco, Waltham, MA, USA) at 37 °C, with 5% of CO2 and under controlled humidity.
After resting, cells were counted and aliquoted for staining with antibodies for the evaluation of the following cellular populations: naïve, effector, memory, exhausted T cell (Panel 1), senescent T cell (Panel 2), activated T cell, monocyte (Panel 3), peripheral T follicular helper cell (Panel 4), and B cell (Panel 5) subsets. A complete description of the panels and antibodies used in each panel is available in Table S1.
All the cell samples were stained with FVS450 (BD Biosciences, USA) for dead cell exclusion prior to specific panel staining. After staining, the samples were fixed using PBS-PFA 1% solution and acquired using a BD FACSAria™ IIu flow cytometer (BD Biosciences, USA). Analyses were performed with FlowJo v.10.0.7 (TreeStar, Woodburn, OR, USA).
2.5. Plasmatic Markers
The plasmatic levels of IL-4, IL-6, IL-18, IL-21, CXCL10/IP-10, CCL4, and sCD163 were assessed using a custom ProcartaPlex multiplex immunoassay (Invitrogen, Waltham, MA, USA), according to the manufacturer’s instructions, and a MAGPIX reader (Luminex Corp, Austin, TX, USA).
2.6. Neutralization Assay
The YF-NAb titers at D30 and D365 were quantified using the micro plaque-reduction neutralization-Horseradish Peroxidase test (μPRN-HRP), carried out at Laboratório de Tecnologia Virológica, Bio-Manguinhos (LATEV, FIOCRUZ-RJ, Brazil), as previously described [60]. The assay values indicate the highest serum dilution tested capable of neutralizing the challenge virus by 50%, with 1:1458 being a higher limit. Titers ≥1:100 (3.15 log10 mIU/mL) were considered reactive and protective, and titers <1:70 were considered non-reactive and non-protective. Samples with results >1:70 and <1:100 were considered inconclusive and reevaluated.
2.7. Statistical Analysis
In addition to a group of HIV-negative individuals (HIVneg; n = 26), PLWH participants were divided into three groups based on pre-vaccination CD4 counts: CD4 count >500 cells/ mm3 (CD4high; n = 30), CD4 count between 350 and 499 cells/ mm3 (CD4med; n = 20), and CD4 count between 200 and 349 cells/ mm3 (CD4low; n = 13).
For the descriptive analysis, differences between the groups were verified through the Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables. For the inferential analyses, linear regression fixed-effects models adjusted by age and sex were calculated for each variable evaluated in this study to investigate associations between the main exploratory variable and participant groups. The HIVneg group was considered as a reference category for all models, and p-values < 0.05 were considered significant for covariate coefficient estimates. The complete spreadsheet with the models’ statistics is available in File S1.
Further, correlation analysis between variables included in this study was evaluated using the Spearman method. Correlations were considered relevant only if p < 0.05 and classified as weak (rho between 0.3 and 0.5 or between −0.3 and −0.5), mild (rho between 0.5 and 0.7 or between −0.5 and −0.7), and strong (rho > 0.8 or <−0.8). All statistical analyses were performed in R 4.2.2.
3. Results
3.1. Clinical and Demographic Characteristics of the Study Groups
A total of 64 PLWH (CD4high [n = 31], CD4med [n = 20], and CD4low [n = 13]) and 27 HIV-negative individuals were included in this study. Table 1 shows the clinical and demographic characteristics of the groups. No significant differences in age and sex were observed between the groups. In PLWH, all individuals were under ART and had an undetectable HIV viral load (<40 copies/mL). There were no significant differences in the time since HIV-1 diagnosis and time since ART initiation, although PLWH with CD4low had lower medians for both variables. In addition, nadir CD4+ counts were lower in PLWH with CD4low than in PLWH with CD4high (p < 0.001).

Table 1.
Clinical and sociodemographic characteristics of the studied groups.
3.2. Immunophenotyping of T Cell Subsets Commonly Affected by HIV Infection
HIV affects T cell homeostasis, leading to the proliferation of effector, exhausted, and senescent populations, which present limited functionality and may contribute to impaired vaccinal responses. Thus, we sought to evaluate alterations in the subsets in our cohort that could explain differences in YF vaccine immunogenicity.
Based on the data resulting from multivariable linear regression models adjusted by age and sex, we observed increased T cell exhaustion mainly in the CD4med and CD4low groups (Figure 1). Relative to HIV-negative controls, the frequencies of PD-1+ cells among total CD4+ T cells were higher in the CD4med and CD4low groups (p < 0.0001 for both, Figure 1a). The frequency of PD-1+ cells among total CD8+ T cells was also higher in the CD4med group than in HIV-negative controls (p = 0.0028). When evaluating PD-1 expression in T cell subsets, we observed that the population that contributed more to the elevated PD-1 expression among CD4+ T cells was TEM cells. Among CD8+ T cells, most PD-1+ cells were TEM cells.
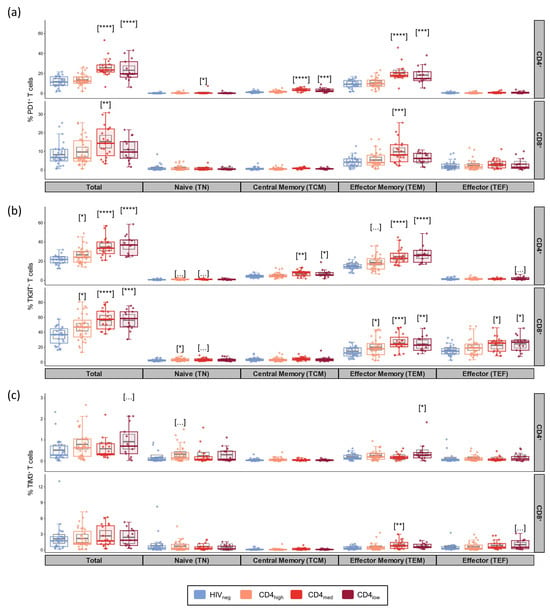
Figure 1.
Exhaustion markers in total T cells and their subsets in PLWH and control groups. Frequencies of PD-1+ (a), TIGIT+ (b), and TIM-3+ (c) cells among CD4+ and CD8+ total, naïve (TN; CD45RA+CCR7+CD27+), central memory (TCM; CD45RA-CCR7+CD27+), effector memory (TEM; CD45RA-CCR7-CD27-), and effector (TEFF; CD45RA+CCR7-CD27-) subsets. The colored dots and boxplots are colored for each group evaluated according to the legend. For the colored boxplots, the horizontal bars represent the IQR and sample median, and the whiskers extend until the lower and upper fences. Gray boxplots represent marginal mean estimates and confidence intervals calculated for multivariate linear models fitted by ordinary least square regressions. p-values represent coefficient significance for the group in the calculated model using HIVneg as a reference and are represented between brackets as: … p < 0.1; * p < 0.05; ** p < 0.01; *** p < 0.001; and **** p < 0.0001.
When evaluating TIGIT as a marker of exhaustion (Figure 1b), adjusted models indicated higher frequencies of TIGIT+ cells among all the PLWH groups compared to HIV-negative controls for both total CD4+ (p = 0.01 for CD4high and p < 0.0001 for CD4med and CD4low) and total CD8+ T cells (p = 0.033 for CD4high; p < 0.0001 for CD4med; and p = 0.0002 for CD4low). As observed for PD-1, TEM also accounted for most of the CD4+ T cells expressing TIGIT in the CD4med and CD4low groups. For CD8+ T cells, the majority of TIGIT+ cells were TEM and TEF.
For TIM-3, no differences were observed between the groups, and only low frequencies of TIM-3+ cells were observed in all the evaluated subsets (Figure 1c). Boolean analysis for the evaluation of the co-expression of exhaustion markers indicated higher frequencies of CD4+ T cells co-expressing TIGIT and PD-1 in all the PLWH groups (p = 0.039 for CD4high and p < 0.0001 for CD4med and CD4low) (Figure S2). Among CD8+ T cells, significantly higher frequencies of PD-1+TIGIT+ T cells were observed for CD4med (p = 0.014), while a tendency of higher frequency was observed for CD4low (p = 0.094). Significantly higher frequencies were also observed for all the PLWH groups when evaluating CD4+ T cells co-expressing three exhaustion markers (p = 0.011 for CD4high; p = 0.086 for CD4med; and p = 0.0069 for CD4low).
We also evaluated the frequency of general T cell subsets. As expected, the CD4med and CD4low groups presented a tendency of lower median frequencies of CD8+ TN cells, and the CD4med group presented a tendency of higher median frequencies of TEM in comparison to HIVneg (Figure S1).
In addition to an increase in exhaustion, we also observed elevated levels of CD4+ activation in the CD4med (p = 0.021) and CD4low groups (p = 0.0072) compared to HIVneg (Figure 2). For CD8+ T cell activation, a significantly higher frequency was observed for CD4low (p = 0.038), while CD4med presented a tendency of higher frequencies of CD38+HLA-DR+ cells (p = 0.056).
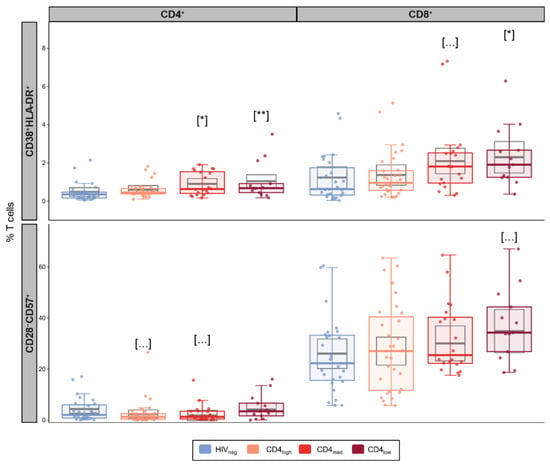
Figure 2.
Senescence and activation in T cells in PLWH and control groups. Frequencies of activated (CD38+HLA-DR+) and senescent (CD28-CD57+) cells among CD4+ and CD8+ T cells. The colored dots and boxplots are colored for each group evaluated according to the legend. For the colored boxplots, the horizontal bars represent the IQR and sample median, and the whiskers extend until the lower and upper fences. Gray boxplots represent marginal mean estimates and confidence intervals calculated for multivariate linear models fitted by ordinary least square regressions. p-values represent coefficient significance for the group in the calculated model using HIVneg as a reference and are represented between brackets as: … p < 0.1; * p < 0.05; and ** p < 0.01.
Finally, we did not observe significant effects for any of the PLWH groups in the adjusted linear models used to evaluate senescent T cells (Figure 2), although the CD4low group presented a significantly higher median frequency of CD8+CD28−CD57+ cells compared to HIVneg.
3.3. Immunophenotyping of Monocytes and B Cell Subsets
In addition to T cells, we aimed to investigate the subset profiles of monocytes as the balance between classical, intermediate, and non-classical monocytes is affected in HIV infection and correlates with an inflammatory setting in PLWH [32,33,34]. We observed a similar frequency of classical, intermediate, and non-classical monocytes between the PLWH and HIVneg groups (Figure S3).
As for T cells, we also evaluated the frequency of naïve, activated memory (AM), resting memory (RM), and tissue-like memory (TLM) B cell subsets since the balance between those cells is impacted by HIV infection and because they drive humoral response. In addition, we also investigated the frequency of peripheral T follicular helper cells (pTfh, CD4+CD45RA−CXCR5+) as this subset has an essential role in the generation of high-affinity antibodies, memory B cells, and long-lived plasma cells [61]. The frequency of pTfh cells exhibited no significant differences between the groups, regardless of HIV status or CD4 count (Figure 3a). According to the multivariable analyses, all the PLWH groups presented significantly lower frequencies of RM B cells compared to HIVneg (p = 0.0026 for CD4high; p = 0.0004 for CD4med; and p < 0.0001 for CD4low), while increased frequencies were observed in the TLM subset for CD4med (p = 0.028) and CD4low (p < 0.0001) and in the AM subset for the CD4low group (p = 0.03).
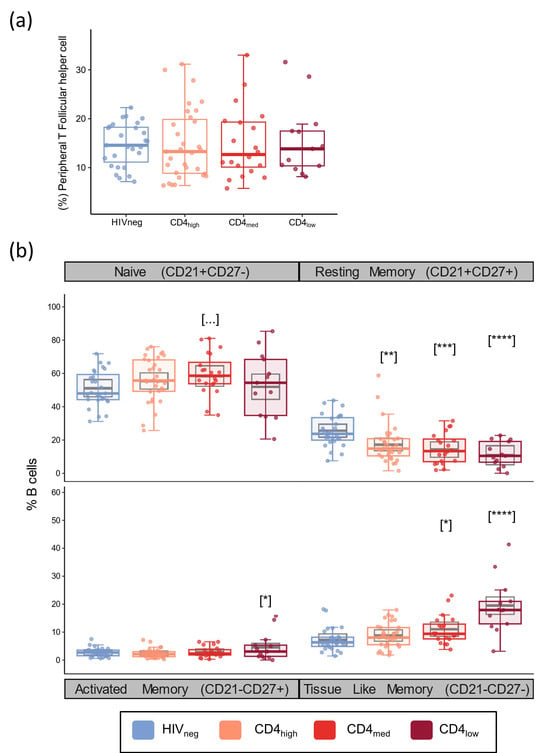
Figure 3.
Peripheral T Follicular helper and B cell subsets in PLWH and control groups. Frequencies of peripheral T follicular helper cells (CD4+CD45RA-CXCR5+) (a), and naïve (CD21+CD27-), resting memory (CD21+CD27+), activated memory (CD21-CD27+), and tissue-like memory (CD21-CD27-) B cell subsets (b). The colored dots and boxplots are colored for each group evaluated according to the legend. For the colored boxplots, the horizontal bars represent the IQR and sample median, and the whiskers extend until the lower and upper fences. Gray boxplots represent marginal mean estimates and confidence intervals calculated for multivariate linear models fitted by ordinary least square regressions. p-values represent coefficient significance for the group in the calculated model using HIVneg as a reference and are represented between brackets as: … p < 0.1; * p < 0.05; ** p < 0.01; *** p < 0.001; and **** p < 0.0001.
In addition to naïve and memory subsets, we also evaluated the frequency of total B cells, immature B cells, and plasmablasts (Figure S4). From these populations, a significant effect of a higher coefficient was observed for HIVmed.
3.4. Higher Level of CXCL10/IP-10 in PLWH with Lower CD4 Counts
In addition to the frequency of cellular subsets, we also evaluated the plasmatic concentration of sCD163 and six cytokines that are consistently associated with inflammation or the regulation of the humoral response upon the activation/inhibition of B cells. Adjusted models using HIVneg as a reference group indicated higher concentrations of CXCL10/IP-10 in the CD4low group (p = 0.013) and sCD163 in the CD4med group (p = 0.023) (Figure 4). IL-18 values were similar among the groups, and most samples presented levels of IL-4, CCL4, IL-6, and IL-21 below the limit of detection.
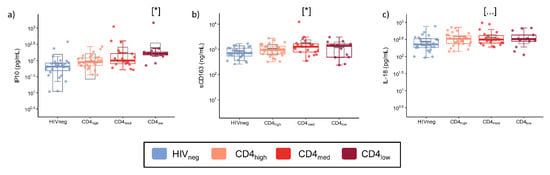
Figure 4.
Plasma levels of inflammatory cytokines, cytokines involved in humoral response, and sCD163 in PLWH and control groups. The graphs represent the concentrations measured by a multiplex immunofluorescence assay of (a) IP10; (b) sCD163; and (c) IL-18. The colored dots and boxplots are colored for each group evaluated according to the legend. For the colored boxplots, the horizontal bars represent the IQR and sample median, and the whiskers extend until the lower and upper fences. Gray boxplots represent marginal mean estimates and confidence intervals calculated for multivariate linear models fitted by ordinary least square regressions. p-values represent coefficient significance for the group in the calculated model using HIVneg as a reference and are represented between brackets as: … p < 0.1; and * p < 0.05.
3.5. YF-NAbs Are Lower in PLWH with Lower CD4 Counts and Correlated Negatively with T Cell Activation and Exhaustion
At D30, the HIVneg, CD4high, and CD4med groups presented median titers of 1458, the upper limit of the neutralization test. For these groups, more than 70% of the individuals presented titers above 1:1000 (14/27 from HIVneg, 19/31 from CD4high, and 13/20 from CD4med). For the CD4low group, we observed lower immunogenicity for the YF vaccine as the median YF-NAb titer in this group was 812, and linear model analysis showed a significantly lower linear coefficient for the group (p = 0.005, Figure 5).
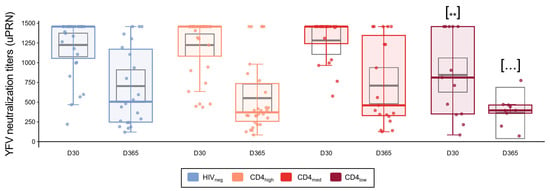
Figure 5.
YFV neutralization titers in PLWH and control groups. YF neutralization antibodies titers at D30 and D365 as measured by the micro plaque-reduction neutralization-Horseradish Peroxidase test (YFV μPRN-HRP). For the colored boxplots, the horizontal bars represent the IQR and sample median, and the whiskers extend until the lower and upper fences. Gray boxplots represent marginal mean estimates and confidence intervals calculated for multivariate linear models fitted by ordinary least square regressions. p-values represent coefficient significance for the group in the calculated model using HIVneg as a reference and are represented as … p < 0.1; ** p < 0.01.
Correlations between the D30 and D365 YF-NAb titers and the pre-vaccination levels of exhaustion, activation, and inflammation markers evaluated here were calculated (Figure 6). We observed a negative correlation between the frequency of exhausted CD8+ T cells (PD-1+, p < 0.028) and activated CD4+ T cells (CD38+HLA-DR+, p < 0.038) with the μPRN titers at D365.
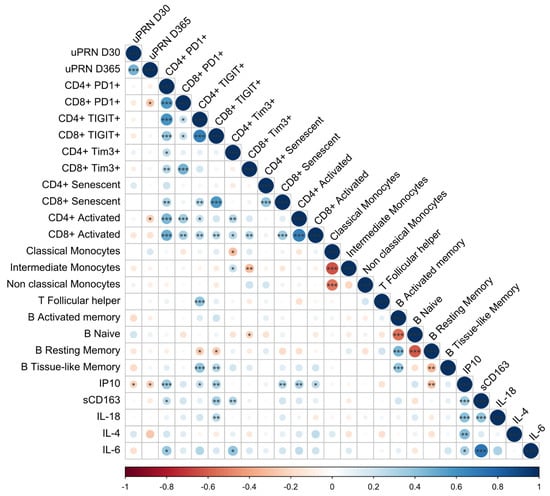
Figure 6.
Spearman correlations between the markers evaluated in this study. Spearman correlation data are shown in the matrix and represent the correlation between the evaluated markers. Circles’ sizes and colors are equivalent to Spearman rho values obtained for each comparison. Significant p-values are represented inside the circles as * p < 0.05; ** p < 0.01; and *** p < 0.001.
4. Discussion
YF vaccine is one of the most effective vaccines currently available. Safety and efficacy have been extensively studied for the general population [21,62,63,64]. However, controversial data suggest that vaccine efficacy is impaired in PLWH [11,13,17,18,19,20], and the associated mechanisms are not clear. In a previous study [18], our group demonstrated lower YF vaccine immunogenicity in PLWH with low CD4 counts. However, the HIV-driven immunodysregulation associated with this reduced response was not evaluated. In the present study, we conducted an extensive immunological analysis to identify factors associated with lower YF vaccine immunogenicity in PLWH with low CD4 counts. We observed higher T and B cell activation and exhaustion in the PLWH groups with lower CD4 counts compared to the HIVneg group. Additionally, the PLWH group with lower CD4 counts also showed higher concentrations of IP-10, sCD163, and IL-18 compared to the HIVneg group [18]. We also observed a negative correlation between the pre-vaccination level of CD8+ T cell exhaustion and CD4+ T cell activation with YF-NAb titers at D365 and the pre-vaccination level of IP-10 with YF-NAb titers at D30 and D365.
The immune deregulation in HIV infection is driven by multiple factors, with generalized and chronic immune activation being considered key factors [23]. Here, we observed higher frequencies of activated T cells in the groups with lower CD4 ranges (CD4med/CD4low). Additionally, this suggests that, although more subtle than observed in other studies, our categorization of PLWH based on CD4 ranges allowed us to observe that these participants present different stages of immunological impairment, which could impact the vaccine response.
In a previous study by Muyianja et al. in 2014 [59], a negative effect on the magnitude of humoral/cellular response against the YF vaccine was observed in HIV-negative individuals with higher baseline activation and exhaustion markers. This study underscored the impact of immune activation on YF vaccine immunogenicity. In agreement with Muyianja’s findings, we observed a negative correlation between the frequency of activated CD4+ T cells with YF-NAb one year after vaccination.
A clear effect of immune impairment in PLWH observed in our study was an increased frequency of exhausted T cells compared to HIV-negative individuals, particularly in participants with lower CD4 counts. T cell exhaustion is characterized as a dysfunctional state driven by persistent antigenic stimulation, resulting in decreased proliferative capacity for general T cells, lower cytotoxic potential for CD8+ T cells, and lower polyfunctionality for CD4+ T cells [35]. In our study, we observed a profile of exhausted T cells in the CD4med and CD4low groups as participants from these groups presented higher frequencies of cells expressing PD-1 and TIGIT.
PD-1 is an inhibitory receptor that regulates T cell effector functions during various events, including acute and chronic infection, cancer, and immune homeostasis. In our study, we observed higher exhaustion in CD4+ T cells, with increased frequencies of PD-1+ T cells in total and TCM subsets for CD4med and CD4low compared to HIVneg. For the CD8+ T cells, increased medians were observed for the CD4low group, but significance in the multivariable models was achieved only for the CD4med group. This result confirms that, while CD8+ T cell cytotoxicity is crucial for the control of viral infections, exhaustion profiles also significantly impact CD4+ T cells and should widely impact the humoral immune response as these cells present an important role in antibody affinity maturation and the development of B cell memory.
While PD-1 plays a central role in the regulation of T cell responses and is considered a main exhaustion marker, several other co-inhibitory molecules are overexpressed in the context of T cell exhaustion. Among them, TIGIT and TIM-3 are widely co-expressed with PD-1, and their combination blockade leads to an increase in cytotoxic and antitumoral activity [40,65,66]. In our study, the frequencies of TIM-3+ cells were very low in all T cell subsets observed for all the groups, although elevated levels were described for T and NK cells in PLWH [41,67]. However, elevated TIGIT expression was observed even for the CD4high group. These results agree with previous data that show that the exhaustion profile is not fully reversible in response to ART [27,68,69].
A negative effect of the increased expression of PD-1 was also observed by Muyianja et al. in 2014, with negative correlations between YF-NAb titers and the frequency of PD-1+ CD8+ memory T cells. Here, we also found a negative correlation between the frequency of PD-1+ CD8+ T cells before vaccination and the YF-NAb titers one year after vaccination, highlighting the impact of T cell exhaustion in antibody response.
In addition to T cell exhaustion, chronic immune activation accelerates T cell immune senescence, a profile associated with aging that affects the renewal capability of those cells and increases the secretion of pro-inflammatory cytokines [70]. In our study, we observed a significantly higher frequency of CD28−CD57+ T cells in the CD4low group compared to HIVneg. However, we did not observe a correlation between senescence and NAb titers.
The CD4+ T cell is the primary cell subset impacted by HIV infection, but HIV-associated immune deregulation impacts other cell populations that are important for immune response. Monocytes, for example, are impacted by immune activation, and HIV infection favors differentiation towards CD16+ populations as intermediate and non-classical subsets [32,33,34]. In our study, however, the frequencies of the three monocyte subsets evaluated were similar in the PLWH and HIVneg groups and were not correlated with YF-NAb titers.
On the other hand, in our cohort, we observed the effects of immune activation in B cell subsets. As for T cells, B lymphocytes in PLWH present a shift towards more differentiated phenotypes in response to chronic stimulation of the immune system [49,50,51,52,53]. This shift was reflected in significantly higher frequencies of tissue-like memory B cells in the CD4med and CD4low groups. These cells are present at higher frequencies in PLWH [49,51,53,71,72] and are of particular importance as their profile resembles the one observed for exhausted T cells [51]. In this context, the increased frequency of these cells in individuals with lower CD4 T cell counts is another indicator of humoral response impairment in comparison to HIV-negative individuals or treated individuals with higher CD4 counts. Associations between frequencies of these cells and lower YF-NAb titers were also observed in the study by Muyianja et al. in 2014, but here, we did not observe a negative correlation between the frequency of TLM B cells with YF vaccine immunogenicity.
In addition to those observed in TLM B cells, we also observed lower frequencies of RM B cells in all the groups of PLWH, despite no significant alterations in the frequency of naïve or AM B cells. RM B cells are also named classic memory B cells as they represent the main circulating B cell subset [49], and several studies highlight that those cells are essential for the humoral response as they are long-lived memory cells with increased responsiveness compared to naïve B cells [73,74] and do not need continuous stimuli for long-term survival [73,75]. In HIV infection, however, these cells do not only occur in lower levels [52,53,71,76] but also present signals of dysfunctionality associated with impaired humoral response in viremic individuals [76,77,78]. Although B cell dynamics restoration was observed to some extent as a response to ART [52,78,79], we did not observe this effect in our cohort as participants from the CD4high group presented RM B cell frequencies similar to the other PLWH groups. This should be reflected in an impaired humoral response as the preservation of this subset is associated with better immunological responses against HIV [80,81], similar to those observed for T central memory cells [30,31].
Although we evaluated an extensive panel of cells and molecules, our study presented some limitations. One of these limitations is the lower number of individuals in the CD4low group and the absence of individuals without ART and a detectable viral load. The absence of individuals with detectable viremia in our cohort precluded the evaluation of the direct influence of HIV replication in vaccine response. Also, our study evaluated the YF-NAb titer only until one year after vaccination. Our cohort is still being followed, and the results from five and ten years after vaccination will allow us to evaluate if PLWH will maintain protective YF-NAb titers for a long time or if they will need a second dose of vaccine. Moreover, the limit of quantification of our YF-NAb assay probably impacted our correlation analyses when other studies evaluated higher YF-NAb titers.
5. Conclusions
We highlighted that immune activation, exhaustion, and inflammation persist in a group of PLWH with low CD4 counts, even with a suppressed viral load. These alterations could contribute to the diminished vaccine response observed in this group, as evidenced by the negative correlation between markers of activation, exhaustion, and inflammation with the YF-Nab titers after 30 days and one year after vaccination. Therefore, implementing strategies to decrease immune activation, exhaustion, and inflammation before vaccination could enhance vaccine immunogenicity in this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12060578/s1, Figure S1: Frequencies of T cell subsets in PLWH and control groups; Figure S2: Boolean combination of exhaustion markers in total T cells in PLWH and control groups; Figure S3: Monocyte subsets in PLWH and control groups; Figure S4: Total B cells, plasmablasts, and immature B cells in PLWH and control groups; Figure S5: Plasma levels of soluble markers in PLWH and control groups; Table S1: Antibodies and screened populations for each flow cytometry stain; File S1: Data of linear models; File S2: Data of Spearman correlations.
Author Contributions
Conceptualization, L.E.C. and F.H.C.; Data curation, D.G.C., M.M., B.G., and S.W.C.; Formal analysis, D.G.C., S.M.B.d.L., A.d.S.A., M.D.d.C., and L.G.P.B.; Funding acquisition, C.B.W.G.-G., D.V.d.A., B.G., D.S.-A., L.E.C., and F.H.C.; Investigation, D.G.C., T.S.T., A.C.S.d.L., C.B.W.G.-G., D.V.d.A., A.d.S.A., M.D.d.C., and L.G.P.B.; Methodology, S.M.B.d.L., A.d.S.A., M.D.d.C., L.G.P.B., A.M.B.d.F., L.E.C., and F.H.C.; Project administration, L.E.C.; Resources, S.M.B.d.L., A.d.S.A., M.M., B.G., S.W.C., M.D.d.C., L.G.P.B., A.M.B.d.F., D.S.-A., and L.E.C.; Supervision, C.B.W.G.-G., D.V.d.A., L.E.C., and F.H.C.; Validation, S.M.B.d.L., A.d.S.A., L.G.P.B., A.M.B.d.F., L.E.C., and F.H.C.; Visualization, D.G.C.; Writing—original draft, D.G.C. and F.H.C.; Writing—review and editing, D.G.C., T.S.T., A.C.S.d.L., C.B.W.G.-G., D.V.d.A., S.M.B.d.L., A.d.S.A., M.M., B.G., S.W.C., M.D.d.C., L.G.P.B., A.M.B.d.F., D.S.-A., L.E.C., and F.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Council for Scientific and Technological Development–CNPq and by Presidência da Fundação Oswaldo Cruz/Vice Presidência de Pesquisa e Coleções Biológicas VPPCB/Fiocruz-Chamada CNPq/Fiocruz N° 16/2017-PROEP/PEC (# 420674/2017–9), and ANRS Emerging infectious diseases (ANRS-12403).
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the INI/Fiocruz Ethics Committee (CAAE: #67136517.9.0000.5262 at 01/05/2017), and the clinical trial was registered at Clinicaltrials.gov (NCT03132311 at 24 April 2017).
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
Data supporting this manuscript may be available upon reasonable request to the corresponding author.
Acknowledgments
We thank the patients, nurses, and clinicians who participated in this study and all the INI staff from the blood collection sector. Finally, for the provision of CD4+ T cell counts and HIV-1 viral load clinical services, we thank the Brazilian Ministry of Health National Network located in the Multiuser Laboratorial Platform from INI and the FIOCRUZ PDTIS Flow Cytometry Platform (RPT08A).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Burke, D.; De La Hoz, F.; et al. Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow Fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Global Strategy to Eliminate Yellow Fever Epidemics 2017–2026; WHO: Geneva, Switzerland, 2018; ISBN 9789241513661. [Google Scholar]
- Chippaux, J.-P.; Chippaux, A. Yellow Fever in Africa and the Americas: A Historical and Epidemiological Perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Brasil Boletim Epidemiológico HIV/Aids|2023; Ministério da Saúde: Brasília, Brasil, 2023.
- Rosser, J.I.; Nielsen-Saines, K.; Saad, E.; Fuller, T. Reemergence of Yellow Fever Virus in Southeastern Brazil, 2017–2018: What Sparked the Spread? PLoS Neglected Trop. Dis. 2022, 16, e0010133. [Google Scholar] [CrossRef] [PubMed]
- Mir, D.; Delatorre, E.; Bonaldo, M.; Lourenço-De-Oliveira, R.; Vicente, A.C.; Bello, G. Phylodynamics of Yellow Fever Virus in the Americas: New Insights into the Origin of the 2017 Brazilian Outbreak. Sci. Rep. 2017, 7, 7385. [Google Scholar] [CrossRef]
- Eibl, M.M.; Wolf, H.M. Vaccination in Patients with Primary Immune Deficiency, Secondary Immune Deficiency and Autoimmunity with Immune Regulatory Abnormalities. Immunotherapy 2015, 7, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Kengsakul, K.; Sathirapongsasuti, K.; Punyagupta, S. Fatal Myeloencephalitis Following Yellow Fever Vaccination in a Case with HIV Infection. J. Med. Assoc. Thai. 2002, 85, 131–134. [Google Scholar] [PubMed]
- Pistone, T.; Verdiere, C.-H.; Receveur, M.-C.; Ezzedine, K.; Lafon, M.-E.; Malvy, D. Immunogenicity and Tolerability of Yellow Fever Vaccination in 23 French HIV-Infected Patients. Curr. HIV Res. 2010, 8, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Veit, O.; Domingo, C.; Niedrig, M.; Staehelin, C.; Sonderegger, B.; Héquet, D.; Stoeckle, M.; Calmy, A.; Schiffer, V.; Bernasconi, E.; et al. Long-Term Immune Response to Yellow Fever Vaccination in Human Immunodeficiency Virus (HIV)–Infected Individuals Depends on HIV RNA Suppression Status: Implications for Vaccination Schedule. Clin. Infect. Dis. 2018, 66, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Depatureaux, A.G.; Chapplain, J.M.; Dupont, M.; Souala, F.; Arvieux, C.; Poveda, J.D.; Michelet, C. Yellow Fever Vaccine Is Safe and Effective in HIV-Infected Patients. AIDS 2004, 18, 825–827. [Google Scholar] [CrossRef]
- Sibailly, T.S.; Wiktor, S.Z.; Tsai, T.F.; Cropp, B.C.; Ekpini, E.R.; Adjorlolo-Johnson, G.; Gnaore, E.; DeCock, K.M.; Greenberg, A.E. Poor Antibody Response to Yellow Fever Vaccination in Children Infected with Human Immunodeficiency Virus Type 1. Pediatr. Infect. Dis. J. 1997, 16, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Receveur, M.C.; Thiébaut, R.; Vedy, S.; Malvy, D.; Mercié, P.; Bras, M.L. Yellow Fever Vaccination of Human Immunodeficiency Virus-Infected Patients: Report of 2 Cases. Clin. Infect. Dis. 2000, 31, E7–E8. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.L.; Enohata, T.; Lopes, M.H.; Dos Santos, S.D.S. Vaccination in Brazilian HIV-Infected Adults: A Cross-Sectional Study. AIDS Patient Care STDS 2008, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Veit, O.; Niedrig, M.; Chapuis-Taillard, C.; Cavassini, M.; Mossdorf, E.; Schmid, P.; Bae, H.G.; Litzba, N.; Staub, T.; Hatz, C.; et al. Immunogenicity and Safety of Yellow Fever Vaccination for 102 HIV-Infected Patients. Clin. Infect. Dis. 2009, 48, 659–666. [Google Scholar] [CrossRef]
- Pacanowski, J.; Lacombe, K.; Campa, P.; Dabrowska, M.; Poveda, J.D.; Meynard, J.L.; Poirot, J.L.; Fonquernie, L.; Girard, P.M. Plasma HIV-RNA Is the Key Determinant of Long-Term Antibody Persistence after Yellow Fever Immunization in a Cohort of 364 HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2012, 59, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Avelino-Silva, V.I.; Miyaji, K.T.; Mathias, A.; Costa, D.A.; De Carvalho Dias, J.Z.; Lima, S.B.; Simoes, M.; Freire, M.S.; Caiaffa-Filho, H.H.; Hong, M.A.; et al. CD4/CD8 Ratio Predicts Yellow Fever Vaccine-Induced Antibody Titers in Virologically Suppressed HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2016, 71, 189–195. [Google Scholar] [CrossRef] [PubMed]
- De Verdiere, N.C.; Durier, C.; Samri, A.; Meiffredy, V.; Launay, O.; Matheron, S.; Mercier-Delarue, S.; Even, S.; Aboulker, J.P.; Molina, J.M.; et al. Immunogenicity and Safety of Yellow Fever Vaccine in HIV-1-Infected Patients. AIDS 2018, 32, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Florence, E.; Domingo, C.; Delforge, M.; De Wit, S.; Dauby, N. Seroconversion and Antibody Persistence after Yellow Fever Vaccination in People Living with HIV: Impact of Baseline HIV Viral Load and Yellow Fever Seropositivity. J. Travel Med. 2022, 29, taac024. [Google Scholar] [CrossRef] [PubMed]
- Kimathi, D.; Juan, A.; Bejon, P.; Grais, R.F.; Warimwe, G.M. Randomized, Double-Blinded, Controlled Non-Inferiority Trials Evaluating the Immunogenicity and Safety of Fractional Doses of Yellow Fever Vaccines in Kenya and Uganda. Wellcome Open Res. 2019, 4, 182. [Google Scholar] [CrossRef]
- Motta, E.; Camacho, L.A.B.; Cunha, M.; de Filippis, A.B.; Lima, S.M.B.; Costa, M.; Pedro, L.; Cardoso, S.W.; Cortes, F.; Gripp, C.; et al. Immunogenicity and Reactogenicity of Yellow Fever Vaccine in People Living with HIV. AIDS 2023, 37, 2319–2329. [Google Scholar] [CrossRef]
- Moir, S.; Chun, T.-W.; Fauci, A.S. Pathogenic Mechanisms of HIV Disease. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 223–248. [Google Scholar] [CrossRef] [PubMed]
- Sokoya, T.; Steel, H.C.; Nieuwoudt, M.; Rossouw, T.M. HIV as a Cause of Immune Activation and Immunosenescence. Mediat. Inflamm. 2017, 2017, 6825493. [Google Scholar] [CrossRef] [PubMed]
- Ghiglione, Y.; Falivene, J.; Ruiz, M.J.; Laufer, N.; Socías, M.E.; Cahn, P.; Giavedoni, L.; Sued, O.; Gherardi, M.M.; Salomón, H.; et al. Early Skewed Distribution of Total and HIV-Specific CD8+ T-Cell Memory Phenotypes during Primary HIV Infection Is Related to Reduced Antiviral Activity and Faster Disease Progression. PLoS ONE 2014, 9, e104235. [Google Scholar] [CrossRef]
- Douek, D.C.; Picker, L.J.; Koup, R.A. T Cell Dynamics In HIV-1 Infection. Annu. Rev. Immunol. 2003, 21, 265–304. [Google Scholar] [CrossRef] [PubMed]
- Breton, G.; Chomont, N.; Takata, H.; Fromentin, R.; Ahlers, J.; Filali-Mouhim, A.; Riou, C.; Boulassel, M.-R.; Routy, J.-P.; Yassine-Diab, B.; et al. Programmed Death-1 Is a Marker for Abnormal Distribution of Naive/Memory T Cell Subsets in HIV-1 Infection. J. Immunol. 2013, 191, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.P.; Milush, J.M.; Cunha-Neto, E.; Kallas, E.G.; Kalil, J.; Somsouk, M.; Hunt, P.W.; Deeks, S.G.; Nixon, D.F.; SenGupta, D. The CD8+ Memory Stem T Cell (TSCM) Subset Is Associated with Improved Prognosis in Chronic HIV-1 Infection. J. Virol. 2014, 88, 13836–13844. [Google Scholar] [CrossRef] [PubMed]
- Elrefaei, M.; Martin, J.; Deeks, S.; Hoh, R.; McElroy, M.D.; Preas, C.P.; Cao, H. Central Memory CD4+ T Cell Responses in Chronic HIV Infection Are Not Restored by Antiretroviral Therapy. J. Immunol. 2014, 173, 2184–2189. [Google Scholar] [CrossRef]
- Potter, S.J.; Lacabaratz, C.; Lambotte, O.; Perez-Patrigeon, S.; Vingert, B.; Sinet, M.; Colle, J.-H.; Urrutia, A.; Scott-Algara, D.; Boufassa, F.; et al. Preserved Central Memory and Activated Effector Memory CD4+ T-Cell Subsets in Human Immunodeficiency Virus Controllers: An ANRS EP36 Study. J. Virol. 2007, 81, 13904–13915. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Bosinger, S.E.; Peck, M.; Richert-Spuhler, L.E.; Heigele, A.; Gile, J.P.; Patel, N.; Taaffe, J.; Julg, B.; Camerini, D.; et al. Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals. PLoS Pathog. 2014, 10, e1004345. [Google Scholar] [CrossRef]
- Funderburg, N.T.; Zidar, D.A.; Shive, C.; Lioi, A.; Mudd, J.; Musselwhite, L.W.; Simon, D.I.; Costa, M.A.; Rodriguez, B.; Sieg, S.F.; et al. Shared Monocyte Subset Phenotypes in HIV-1 Infection and in Uninfected Subjects with Acute Coronary Syndrome. Blood 2012, 120, 4599–4608. [Google Scholar] [CrossRef]
- Chen, P.; Su, B.; Zhang, T.; Zhu, X.; Xia, W.; Fu, Y.; Zhao, G.; Xia, H.; Dai, L.; Sun, L.; et al. Perturbations of Monocyte Subsets and Their Association with T Helper Cell Differentiation in Acute and Chronic HIV-1-Infected Patients. Front. Immunol. 2017, 8, 272. [Google Scholar] [CrossRef]
- Han, J.; Wang, B.; Han, N.; Zhao, Y.; Song, C.; Feng, X.; Mao, Y.; Zhang, F.; Zhao, H.; Zeng, H. CD14highCD16+ Rather than CD14lowCD16+ Monocytes Correlate with Disease Progression in Chronic HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2009, 52, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell Exhaustion in HIV Infection. Immunol. Rev. 2019, 292, 149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Segal, F.P.; Bosch, R.J.; Lalama, C.M.; Roberts-Toler, C.; Delagreverie, H.; Getz, R.; Garcia-Broncano, P.; Kinslow, J.; Tressler, R.; et al. ART Reduces T Cell Activation and Immune Exhaustion Markers in HIV Controllers. Clin. Infect. Dis. 2019, 70, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 Expression on HIV-Specific T Cells Is Associated with T-Cell Exhaustion and Disease Progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 Expression on HIV-Specific CD8+ T Cells Leads to Reversible Immune Dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pantazis, N.; Martin, G.E.; Hickling, S.; Hurst, J.; Meyerowitz, J.; Willberg, C.B.; Robinson, N.; Brown, H.; Fisher, M.; et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016, 12, e1005661. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-Cell Exhaustion during Chronic Viral Infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Ndhlovu, L.C.; Barbour, J.D.; Sheth, P.M.; Jha, A.R.; Long, B.R.; Wong, J.C.; Satkunarajah, M.; Schweneker, M.; Chapman, J.M.; et al. Tim-3 Expression Defines a Novel Population of Dysfunctional T Cells with Highly Elevated Frequencies in Progressive HIV-1 Infection. J. Exp. Med. 2008, 205, 2763. [Google Scholar] [CrossRef]
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016, 12, e1005349. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T Cell Exhaustion during Chronic Viral Infection by Multiple Inhibitory Receptors. Nat. Immunol. 2009, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Duffau, P.; Wittkop, L.; Lazaro, E.; Le Marec, F.; Cognet, C.; Blanco, P.; Moreau, J.F.; Dauchy, F.A.; Cazanave, C.; Vandenhende, M.A.; et al. Association of Immune-Activation and Senescence Markers with Non-AIDS-Defining Comorbidities in HIV-Suppressed Patients. AIDS 2015, 29, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Papagno, L.; Spina, C.A.; Marchant, A.; Salio, M.; Rufer, N.; Little, S.; Dong, T.; Chesney, G.; Waters, A.; Easterbrook, P.; et al. Immune Activation and CD8+ T-Cell Differentiation towards Senescence in HIV-1 Infection. PLoS Biol. 2004, 2, e20. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.; Sauce, D. Mechanisms of Immune Aging in HIV. Clin. Sci. 2022, 136, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Karandikar, N.J.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Crotty, L.E.; Casazza, J.P.; Kuruppu, J.; Migueles, S.A.; Connors, M.; et al. Expression of CD57 Defines Replicative Senescence and Antigen-Induced Apoptotic Death of CD8+ T Cells. Blood 2003, 101, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jamieson, B.D.; Hultin, L.E.; Hultin, P.M.; Effros, R.B.; Detels, R. Premature Aging of T Cells Is Associated with Faster HIV-1 Disease Progression. JAIDS J. Acquir. Immune Defic. Syndr. 2009, 50, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Fauci, A.S. B-Cell Responses to HIV Infection. Immunol. Rev. 2017, 275, 33–48. [Google Scholar] [CrossRef]
- Hart, M.; Steel, A.; Clark, S.A.; Moyle, G.; Nelson, M.; Henderson, D.C.; Wilson, R.; Gotch, F.; Gazzard, B.; Kelleher, P. Loss of Discrete Memory B Cell Subsets Is Associated with Impaired Immunization Responses in HIV-1 Infection and May Be a Risk Factor for Invasive Pneumococcal Disease 1. J. Immunol. 2007, 178, 8212–8220. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Ho, J.; Malaspina, A.; Wang, W.; DiPoto, A.C.; O’Shea, M.A.; Roby, G.; Kottilil, S.; Arthos, J.; Proschan, M.A.; et al. Evidence for HIV-Associated B Cell Exhaustion in a Dysfunctional Memory B Cell Compartment in HIV-Infected Viremic Individuals. J. Exp. Med. 2008, 205, 1797–1805. [Google Scholar] [CrossRef]
- Longwe, H.; Gordon, S.; Malamba, R.; French, N. Characterising B Cell Numbers and Memory B Cells in HIV Infected and Uninfected Malawian Adults. BMC Infect. Dis. 2010, 10, 280. [Google Scholar] [CrossRef]
- Pensieroso, S.; Galli, L.; Nozza, S.; Ruffin, N.; Castagna, A.; Tambussi, G.; Hejdeman, B.; Misciagna, D.; Riva, A.; Malnati, M.; et al. B-Cell Subset Alterations and Correlated Factors in HIV-1 Infection. AIDS 2013, 27, 1209–1217. [Google Scholar] [CrossRef]
- Wada, N.I.; Jacobson, L.P.; Margolick, J.B.; Breen, E.C.; Macatangay, B.; Penugonda, S.; Martínez-Maza, O.; Bream, J.H. The Effect of HAART-Induced HIV Suppression on Circulating Markers of Inflammation and Immune Activation. AIDS 2015, 29, 463–471. [Google Scholar] [CrossRef]
- Caetano, D.G.; de Paula, H.H.S.; Bello, G.; Hoagland, B.; Villela, L.M.; Grinsztejn, B.; Veloso, V.G.; Morgado, M.G.; Guimarães, M.L.; Côrtes, F.H. HIV-1 Elite Controllers Present a High Frequency of Activated Regulatory T and Th17 Cells. PLoS ONE 2020, 15, e0228745. [Google Scholar] [CrossRef] [PubMed]
- Caetano, D.G.; Ribeiro-Alves, M.; Hottz, E.D.; Vilela, L.M.; Cardoso, S.W.; Hoagland, B.; Grinsztejn, B.; Veloso, V.G.; Morgado, M.G.; Bozza, P.T.; et al. Increased Biomarkers of Cardiovascular Risk in HIV-1 Viremic Controllers and Low Persistent Inflammation in Elite Controllers and Art-Suppressed Individuals. Sci. Rep. 2022, 12, 6569. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.A.; King, M.S.S.; Tschampa, J.M.M.; da Silva, B.A.A.; Landay, A.L.L. Serum Immune Activation Markers Are Persistently Increased in Patients with HIV Infection after 6 Years of Antiretroviral Therapy despite Suppression of Viral Replication and Reconstitution of CD4+ T Cells. J. Infect. Dis. 2009, 200, 1212–1215. [Google Scholar] [CrossRef]
- Lederman, M.M.; Calabrese, L.; Funderburg, N.T.; Clagett, B.; Medvik, K.; Bonilla, H.; Gripshover, B.; Salata, R.A.; Taege, A.; Lisgaris, M.; et al. Immunologic Failure despite Suppressive Antiretroviral Therapy Is Related to Activation and Turnover of Memory CD4 Cells. J. Infect. Dis. 2011, 204, 1217–1226. [Google Scholar] [CrossRef]
- Muyanja, E.; Ssemaganda, A.; Ngauv, P.; Cubas, R.; Perrin, H.; Srinivasan, D.; Canderan, G.; Lawson, B.; Kopycinski, J.; Graham, A.S.; et al. Immune Activation Alters Cellular and Humoral Responses to Yellow Fever 17D Vaccine. J. Clin. Investig. 2014, 124, 3147–3158. [Google Scholar] [CrossRef]
- Simões, M.; Camacho, L.A.B.; Yamamura, A.M.Y.; Miranda, E.H.; Cajaraville, A.C.R.A.; da Silva Freire, M. Evaluation of Accuracy and Reliability of the Plaque Reduction Neutralization Test (Micro-PRNT) in Detection of Yellow Fever Virus Antibodies. Biologicals 2012, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Antonio Bastos Camacho, L.; da Silva Freire, M.; da Luz Fernandes Leal, M.; Gomes de Aguiar, S.; Pereira do Nascimento, J.; Iguchi, T.; de Azevedo Lozana, J.; Henrique Guedes Farias, R.; Vaccines, F.; Antonio Bastos Camacho Fiocruz, L. Immunogenicity of WHO-17D and Brazilian 17DD Yellow Fever Vaccines: A Randomized Trial Imunogenicidade Das Vacinas Contra Febre Amarela WHO-17D e 17DD: Ensaio Randomizado. Rev. Saude Publica 2004, 38, 671–679. [Google Scholar] [CrossRef]
- Desai, S.; Anil, K.; Potey, A.V.; Sindhu, Y.; Grappi, S.; Lapini, G.; Manney, S.; Tyagi, P.; Montomoli, E.; Poonawalla, C.S.; et al. A Phase I Clinical Study to Assess Safety and Immunogenicity of Yellow Fever Vaccine. NPJ Vaccines 2022, 7, 170. [Google Scholar] [CrossRef]
- Casey, R.M.; Harris, J.B.; Ahuka-Mundeke, S.; Dixon, M.G.; Kizito, G.M.; Nsele, P.M.; Umutesi, G.; Laven, J.; Kosoy, O.; Paluku, G.; et al. Immunogenicity of Fractional-Dose Vaccine during a Yellow Fever Outbreak—Final Report. N. Engl. J. Med. 2019, 381, 444. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 Comes of Age as an Inhibitory Receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Finney, C.A.M.; Ayi, K.; Wasmuth, J.D.; Sheth, P.M.; Kaul, R.; Loutfy, M.; Kain, K.C.; Serghides, L. HIV Infection Deregulates Tim-3 Expression on Innate Cells. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 63, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.E.; Sen, D.R.; Pace, M.; Robinson, N.; Meyerowitz, J.; Adland, E.; Thornhill, J.P.; Jones, M.; Ogbe, A.; Parolini, L.; et al. Epigenetic Features of HIV-Induced T-Cell Exhaustion Persist Despite Early Antiretroviral Therapy. Front. Immunol. 2021, 12, 647688. [Google Scholar] [CrossRef] [PubMed]
- Cockerham, L.R.; Jain, V.; Sinclair, E.; Glidden, D.V.; Hartogenesis, W.; Hatano, H.; Hunt, P.W.; Martin, J.N.; Pilcher, C.D.; Sekaly, R.; et al. Programmed Death-1 Expression on CD4+ and CD8+ T Cells in Treated and Untreated HIV Disease. AIDS 2014, 28, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Landay, A. Early Immune Senescence in HIV Disease. Curr. HIV/AIDS Rep. 2010, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Amu, S.; Lavy-Shahaf, G.; Cagigi, A.; Hejdeman, B.; Nozza, S.; Lopalco, L.; Mehr, R.; Chiodi, F. Frequency and Phenotype of B Cell Subpopulations in Young and Aged HIV-1 Infected Patients Receiving ART. Retrovirology 2014, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Kardava, L.; Moir, S.; Wang, W.; Ho, J.; Buckner, C.M.; Posada, J.G.; O’Shea, M.A.; Roby, G.; Chen, J.; Sohn, H.W.; et al. Attenuation of HIV-Associated Human B Cell Exhaustion by SiRNA Downregulation of Inhibitory Receptors. J. Clin. Investig. 2011, 121, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Avery, D.T.; Deenick, E.K.; Hodgkin, P.D. Intrinsic Differences in the Proliferation of Naive and Memory Human B cells as a Mechanism for Enhanced Secondary Immune Responses 1. J. Immunol. 2003, 170, 686–694. [Google Scholar] [CrossRef]
- Good, K.L.; Avery, D.T.; Tangye, S.G. Resting Human Memory B cells Are Intrinsically Programmed for Enhanced Survival and Responsiveness to Diverse Stimuli Compared to Naive B cells. J. Immunol. 2009, 182, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Lam, K.P.; Rajewsky, K. Memory B-Cell Persistence Is Independent of Persisting Immunizing Antigen. Nature 2000, 407, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Titanji, K.; Chiodi, F.; Bellocco, R.; Schepis, D.; Osorio, L.; Tassandin, C.; Tambussi, G.; Grutzmeier, S.; Lopalco, L.; De Milito, A. Primary HIV-1 Infection Sets the Stage for Important B Lymphocyte Dysfunctions. AIDS 2005, 19, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, A.; Moir, S.; DiPoto, A.C.; Ho, J.; Wang, W.; Roby, G.; O’Shea, M.A.; Fauci, A.S. CpG Oligonucleotides Enhance Proliferative and Effector Responses of B cells in HIV-Infected Individuals. J. Immunol. 2008, 181, 1199. [Google Scholar] [CrossRef] [PubMed]
- Kardava, L.; Moir, S.; Shah, N.; Wang, W.; Wilson, R.; Buckner, C.M.; Santich, B.H.; Kim, L.J.Y.; Spurlin, E.E.; Nelson, A.K.; et al. Abnormal B Cell Memory Subsets Dominate HIV-Specific Responses in Infected Individuals. J. Clin. Investig. 2014, 124, 3252–3262. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Buckner, C.M.; Ho, J.; Wang, W.; Chen, J.; Waldner, A.J.; Posada, J.G.; Kardava, L.; O’Shea, M.A.; Kottilil, S.; et al. B cells in Early and Chronic HIV Infection: Evidence for Preservation of Immune Function Associated with Early Initiation of Antiretroviral Therapy. Blood 2010, 116, 5571–5579. [Google Scholar] [CrossRef]
- Buckner, C.M.; Kardava, L.; Zhang, X.; Gittens, K.; Justement, J.S.; Kovacs, C.; McDermott, A.B.; Li, Y.; Sajadi, M.M.; Chun, T.-W.; et al. Maintenance of HIV-Specific Memory B-Cell Responses in Elite Controllers Despite Low Viral Burden. J. Infect. Dis. 2016, 214, 390–398. [Google Scholar] [CrossRef]
- Bussmann, B.M.; Reiche, S.; Bieniek, B.; Krznaric, I.; Ackermann, F.; Jassoy, C. Loss of HIV-Specific Memory B-Cells as a Potential Mechanism for the Dysfunction of the Humoral Immune Response against HIV. Virology 2010, 397, 7–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).