How to Accelerate Early Stage of Malaria Vaccine Development by Optimizing Functional Assays
Abstract
:1. Introduction
2. Correlate of Protection (CoP) vs. Surrogate of Protection (SoP)
3. Reported CoP for RTS,S, R21, and Some BSV
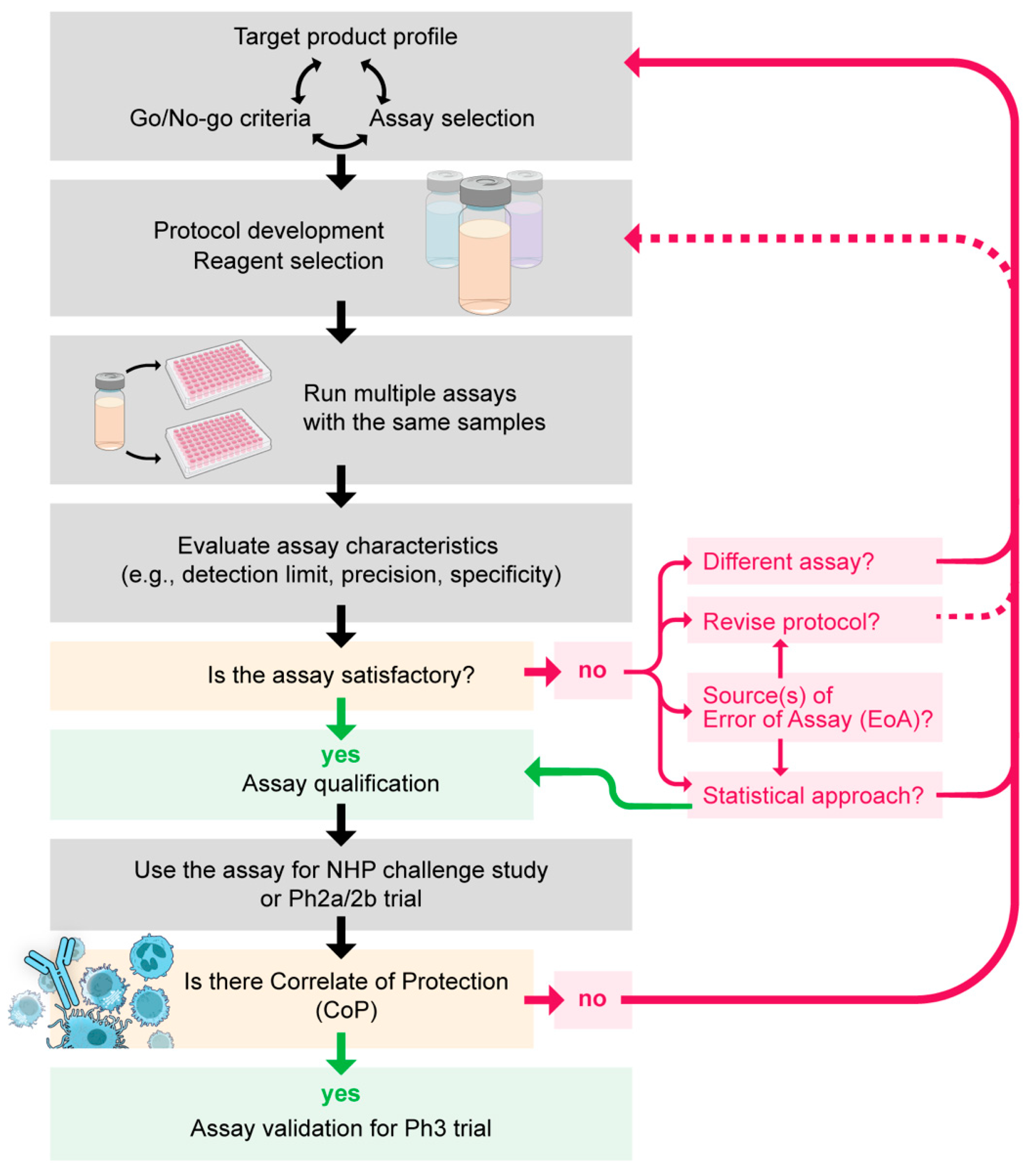
4. Assay Standardization, Qualification, and Validation
5. Species Selection for Preclinical Immunization Studies
6. How to Select an Assay for Vaccine Development?
7. How to Standardize, Qualify, and Validate an Assay?
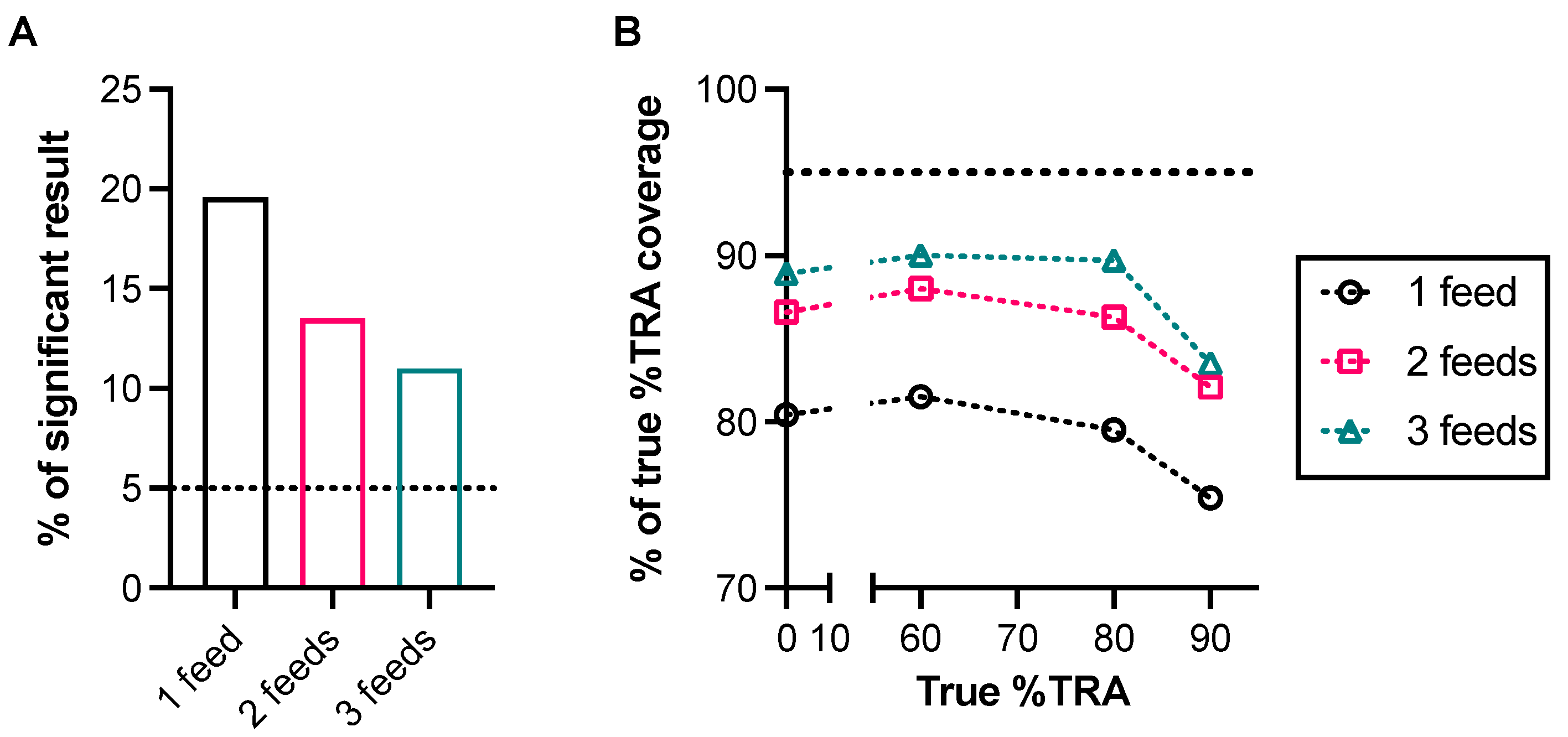
8. How to Interpret and Report the Results of an Assay?
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 22 April 2024).
- Topazian, H.M.; Schmit, N.; Gerard-Ursin, I.; Charles, G.D.; Thompson, H.; Ghani, A.C.; Winskill, P. Modelling the relative cost-effectiveness of the RTS,S/AS01 malaria vaccine compared to investment in vector control or chemoprophylaxis. Vaccine 2023, 41, 3215–3223. [Google Scholar] [CrossRef]
- Schmit, N.; Topazian, H.M.; Natama, H.M.; Bellamy, D.; Traore, O.; Some, M.A.; Rouamba, T.; Tahita, M.C.; Bonko, M.D.A.; Sourabie, A.; et al. The public health impact and cost-effectiveness of the R21/Matrix-M malaria vaccine: A mathematical modelling study. Lancet Infect. Dis. 2024, 24, 465–475. [Google Scholar] [CrossRef]
- Miura, K.; Flores-Garcia, Y.; Long, C.; Zavala, F. Vaccines and monoclonal antibodies: New tools for malaria control. Clin. Microbiol. Rev. 2024, e0007123. [Google Scholar] [CrossRef]
- Takashima, E.; Otsuki, H.; Morita, M.; Ito, D.; Nagaoka, H.; Yuguchi, T.; Hassan, I.; Tsuboi, T. The need for novel asexual blood-stage malaria vaccine candidates for Plasmodium falciparum. Biomolecules 2024, 14, 100. [Google Scholar] [CrossRef]
- Jakhar, R.; Sehrawat, N.; Gakhar, S.K. An analytical review of vector- and pathogen-based transmission-blocking vaccine for malaria control. J. Vector Borne Dis. 2022, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Renia, L.; Tadepalli, G.; Bayry, J.; Kumar, H.M.S. Plasmodium falciparum malaria vaccines and vaccine adjuvants. Vaccines 2021, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E.; Patrick Gorres, J. Malaria vaccines since 2000: Progress, priorities, products. NPJ Vaccines 2020, 5, 48. [Google Scholar] [CrossRef]
- Miura, K.; Tachibana, M.; Takashima, E.; Morita, M.; Kanoi, B.N.; Nagaoka, H.; Baba, M.; Torii, M.; Ishino, T.; Tsuboi, T. Malaria transmission-blocking vaccines: Wheat germ cell-free technology can accelerate vaccine development. Expert. Rev. Vaccines 2019, 18, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; McCall, M.B.B. Correlates of malaria vaccine efficacy. Expert Rev. Vaccines 2021, 20, 143–161. [Google Scholar] [CrossRef]
- Miura, K. Progress and prospects for blood-stage malaria vaccines. Expert Rev. Vaccines 2016, 15, 765–781. [Google Scholar] [CrossRef]
- Teo, A.; Feng, G.; Brown, G.V.; Beeson, J.G.; Rogerson, S.J. Functional antibodies and protection against blood-stage malaria. Trends Parasitol. 2016, 32, 887–898. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Correlates of Vaccine-Induced Protection: Methods and Implications. Available online: https://www.who.int/publications/i/item/WHO-IVB-13.01 (accessed on 22 April 2024).
- Thakur, A.; Pedersen, L.E.; Jungersen, G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine 2012, 30, 4907–4920. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2022, 13, 1081107. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Natama, H.M.; Some, A.; Bellamy, D.; Traore, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: A phase 1/2b randomised controlled trial. Lancet Infect. Dis. 2022, 22, 1728–1736. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouedraogo, J.B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Seaton, K.E.; Spreng, R.L.; Abraha, M.; Reichartz, M.; Rojas, M.; Feely, F., 2nd; Huntwork, R.H.C.; Dutta, S.; Mudrak, S.V.; Alam, S.M.; et al. Subclass and avidity of circumsporozoite protein specific antibodies associate with protection status against malaria infection. NPJ Vaccines 2021, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.A.; Hogan, A.B.; Walker, P.G.T.; White, M.T.; Cunnington, A.J.; Ockenhouse, C.F.; Ghani, A.C. Modelling the roles of antibody titre and avidity in protection from Plasmodium falciparum malaria infection following RTS,S/AS01 vaccination. Vaccine 2020, 38, 7498–7507. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; MacGill, R.S.; Early, A.M.; Bolton, J.S.; King, C.R.; Locke, E.; Pierson, T.; Wirth, D.F.; Neafsey, D.E.; Bergmann-Leitner, E.S. Breadth of humoral immune responses to the C-terminus of the circumsporozoite protein is associated with protective efficacy induced by the RTS,S malaria vaccine. Vaccine 2021, 39, 968–975. [Google Scholar] [CrossRef]
- Suscovich, T.J.; Fallon, J.K.; Das, J.; Demas, A.R.; Crain, J.; Linde, C.H.; Michell, A.; Natarajan, H.; Arevalo, C.; Broge, T.; et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci. Transl. Med. 2020, 12, eabb4757. [Google Scholar] [CrossRef]
- Moncunill, G.; Carnes, J.; Chad Young, W.; Carpp, L.; De Rosa, S.; Campo, J.J.; Nhabomba, A.; Mpina, M.; Jairoce, C.; Finak, G.; et al. Transcriptional correlates of malaria in RTS,S/AS01-vaccinated African children: A matched case-control study. eLife 2022, 11, e70393. [Google Scholar] [CrossRef] [PubMed]
- Macia, D.; Campo, J.J.; Moncunill, G.; Jairoce, C.; Nhabomba, A.J.; Mpina, M.; Sorgho, H.; Dosoo, D.; Traore, O.; Kusi, K.A.; et al. Strong off-target antibody reactivity to malarial antigens induced by RTS,S/AS01E vaccination is associated with protection. JCI Insight 2022, 7, e158030. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Idris, A.H.; Berkowitz, N.M.; Happe, M.; Gaudinski, M.R.; Buettner, C.; Strom, L.; Awan, S.F.; Holman, L.A.; Mendoza, F.; et al. Low-dose subcutaneous or intravenous monoclonal antibody to prevent malaria. N. Engl. J. Med. 2022, 387, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kayentao, K.; Ongoiba, A.; Preston, A.C.; Healy, S.A.; Doumbo, S.; Doumtabe, D.; Traore, A.; Traore, H.; Djiguiba, A.; Li, S.; et al. Safety and efficacy of a monoclonal antibody against malaria in Mali. N. Engl. J. Med. 2022, 387, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Locke, E.; Flores-Garcia, Y.; Mayer, B.T.; MacGill, R.S.; Borate, B.; Salgado-Jimenez, B.; Gerber, M.W.; Mathis-Torres, S.; Shapiro, S.; King, C.R.; et al. Establishing RTS,S/AS01 as a benchmark for comparison to next-generation malaria vaccines in a mouse model. NPJ Vaccines 2024, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Genton, B.; Betuela, I.; Felger, I.; Al-Yaman, F.; Anders, R.F.; Saul, A.; Rare, L.; Baisor, M.; Lorry, K.; Brown, G.V.; et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 2002, 185, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Thera, M.A.; Doumbo, O.K.; Coulibaly, D.; Laurens, M.B.; Ouattara, A.; Kone, A.K.; Guindo, A.B.; Traore, K.; Traore, I.; Kouriba, B.; et al. A field trial to assess a blood-stage malaria vaccine. N. Engl. J. Med. 2011, 365, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Cousens, S.; Druilhe, P. Protection against malaria by MSP3 candidate vaccine. N. Engl. J. Med. 2011, 365, 1062–1064. [Google Scholar] [CrossRef]
- Palacpac, N.M.; Ntege, E.; Yeka, A.; Balikagala, B.; Suzuki, N.; Shirai, H.; Yagi, M.; Ito, K.; Fukushima, W.; Hirota, Y.; et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS ONE 2013, 8, e64073. [Google Scholar] [CrossRef]
- Sirima, S.B.; Mordmuller, B.; Milligan, P.; Ngoa, U.A.; Kironde, F.; Atuguba, F.; Tiono, A.B.; Issifou, S.; Kaddumukasa, M.; Bangre, O.; et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine 2016, 34, 4536–4542. [Google Scholar] [CrossRef]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T.; et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med 2021, 2, 701–719.e719. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, M.P.; Jogdand, P.S.; Singh, S.K.; Esen, M.; Christiansen, M.; Issifou, S.; Hounkpatin, A.B.; Ateba-Ngoa, U.; Kremsner, P.G.; Dziegiel, M.H.; et al. The malaria vaccine candidate GMZ2 elicits functional antibodies in individuals from malaria endemic and non-endemic areas. J. Infect. Dis. 2013, 208, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Dassah, S.; Adu, B.; Tiendrebeogo, R.W.; Singh, S.K.; Arthur, F.K.N.; Sirima, S.B.; Theisen, M. GMZ2 vaccine-induced antibody responses, naturally acquired immunity and the incidence of malaria in Burkinabe children. Front. Immunol. 2022, 13, 899223. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.O.; Milne, K.H.; Elias, S.C.; Edwards, N.J.; Douglas, A.D.; Brown, R.E.; Silk, S.E.; Biswas, S.; Miura, K.; Roberts, R.; et al. Demonstration of the blood-stage Plasmodium falciparum controlled human malaria infection model to assess efficacy of the P. falciparum Apical Membrane Antigen 1 vaccine, FMP2.1/AS01. J. Infect. Dis. 2016, 213, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.D.; Baldeviano, G.C.; Jin, J.; Miura, K.; Diouf, A.; Zenonos, Z.A.; Ventocilla, J.A.; Silk, S.E.; Marshall, J.M.; Alanine, D.G.W.; et al. A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non-human primates. Nat. Commun. 2019, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Barrett, J.R.; Themistocleous, Y.; Rawlinson, T.A.; Diouf, A.; Martinez, F.J.; Nielsen, C.M.; Lias, A.M.; King, L.D.W.; Edwards, N.J.; et al. Vaccination with Plasmodium vivax Duffy-binding protein inhibits parasite growth during controlled human malaria infection. Sci. Transl. Med. 2023, 15, eadf1782. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R.; Silk, S.E.; Mkindi, C.G.; Kwiatkowska, K.M.; Hou, M.M.; Lias, A.M.; Kalinga, W.F.; Mtaka, I.M.; McHugh, K.; Bardelli, M.; et al. Analyses of human vaccine-specific circulating and bone marrow-resident B cell populations reveal benefit of delayed vaccine booster dosing with blood-stage malaria antigens. Front. Immunol. 2023, 14, 1193079. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.; Amaravadi, L.; Eck, S.; Stevenson, L.; Wang, Y.M.C.; Devanarayan, V.; Allinson, J.; Lundsten, K.; Gunsior, M.; Ni, Y.G.; et al. Best practices for the development and fit- for-purpose validation of biomarker methods: A conference report. AAPS Open 2022, 8, 2. [Google Scholar] [CrossRef]
- Clement, F.; Dewar, V.; Van Braeckel, E.; Desombere, I.; Dewerchin, M.; Swysen, C.; Demoitie, M.A.; Jongert, E.; Cohen, J.; Leroux-Roels, G.; et al. Validation of an enzyme-linked immunosorbent assay for the quantification of human IgG directed against the repeat region of the circumsporozoite protein of the parasite Plasmodium falciparum. Malar. J. 2012, 11, 384. [Google Scholar] [CrossRef]
- Raghunandan, R.; Mayer, B.T.; Flores-Garcia, Y.; Gerber, M.W.; Gottardo, R.; Jhun, H.; Herrera, S.M.; Perez-Ramos, D.W.; Locke, E.; King, C.R.; et al. Characterization of two in vivo challenge models to measure functional activity of monoclonal antibodies to Plasmodium falciparum circumsporozoite protein. Malar. J. 2020, 19, 113. [Google Scholar] [CrossRef]
- Miura, K.; Diouf, A.; Fay, M.P.; Barrett, J.R.; Payne, R.O.; Olotu, A.I.; Minassian, A.M.; Silk, S.E.; Draper, S.J.; Long, C.A. Assessment of precision in growth inhibition assay (GIA) using human anti-PfRH5 antibodies. Malar. J. 2023, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Deng, B.; Tullo, G.; Diouf, A.; Moretz, S.E.; Locke, E.; Morin, M.; Fay, M.P.; Long, C.A. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLoS ONE 2013, 8, e57909. [Google Scholar] [CrossRef] [PubMed]
- Churcher, T.S.; Blagborough, A.M.; Delves, M.; Ramakrishnan, C.; Kapulu, M.C.; Williams, A.R.; Biswas, S.; Da, D.F.; Cohuet, A.; Sinden, R.E. Measuring the blockade of malaria transmission—An analysis of the standard membrane feeding assay. Int. J. Parasitol. 2012, 42, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.P.; Udhayakumar, V.; Oloo, A.J.; Nahlen, B.L.; Lal, A.A. Differential effect and interaction of monocytes, hyperimmune sera, and immunoglobulin G on the growth of asexual stage Plasmodium falciparum parasites. Am. J. Trop. Med. Hyg. 1999, 60, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.; Miura, K.; Fay, M.P.; Williams, A.R.; Murungi, L.M.; Shi, J.; Hodgson, S.H.; Douglas, A.D.; Osier, F.H.; Fairhurst, R.M.; et al. Standardization of the antibody-dependent respiratory burst assay with human neutrophils and Plasmodium falciparum malaria. Sci. Rep. 2015, 5, 14081. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, C.; Heno, K.K.; Adams, Y.; Resende, M.; Mathiesen, L.; Soegaard, M.; de Jongh, W.A.; Theander, T.G.; Salanti, A.; Nielsen, M.A. Comparison of functional assays used in the clinical development of a placental malaria vaccine. Vaccine 2017, 35, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Wilson, H.L.; Meurens, F.; van Drunen Littel-van den Hurk, S.; Wilson, D.; Walker, S.; Wheler, C.; Townsend, H.; Potter, A.A. Large animal models for vaccine development and testing. ILAR J. 2015, 56, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Herati, R.S.; Wherry, E.J. What is the predictive value of animal models for vaccine efficacy in humans? Consideration of strategies to improve the value of animal models. Cold Spring Harb. Perspect. Biol. 2018, 10, a031583. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, S.J.; Knight, G.M.; Kirschner, D.E.; White, R.G.; Evans, T.G. Dose finding for new vaccines: The role for immunostimulation/immunodynamic modelling. J. Theor. Biol. 2019, 465, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Zhou, H.; Diouf, A.; Moretz, S.E.; Fay, M.P.; Miller, L.H.; Martin, L.B.; Pierce, M.A.; Ellis, R.D.; Mullen, G.E.; et al. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin. Vaccine Immunol. 2009, 16, 963–968. [Google Scholar] [CrossRef]
- Cheru, L.; Wu, Y.; Diouf, A.; Moretz, S.E.; Muratova, O.V.; Song, G.; Fay, M.P.; Miller, L.H.; Long, C.A.; Miura, K. The IC(50) of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine 2010, 28, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Zaric, M.; Marini, A.; Nielsen, C.M.; Gupta, G.; Mekhaiel, D.; Pham, T.P.; Elias, S.C.; Taylor, I.J.; de Graaf, H.; Payne, R.O.; et al. Poor CD4+ T cell immunogenicity limits humoral immunity to P. falciparum transmission-blocking candidate Pfs25 in humans. Front. Immunol. 2021, 12, 732667. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.; Binter, S.; Kosmac, M.; Ghraichy, M.; von Niederhausern, V.; Kovaltsuk, A.; Galson, J.D.; Truck, J.; Kelly, D.F.; Deane, C.M.; et al. Characterisation of the immune repertoire of a humanised transgenic mouse through immunophenotyping and high-throughput sequencing. eLife 2023, 12, e81629. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.A.; Anderson, C.F.; Swihart, B.J.; Mwakingwe-Omari, A.; Gabriel, E.E.; Decederfelt, H.; Hobbs, C.V.; Rausch, K.M.; Zhu, D.; Muratova, O.; et al. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Investig. 2021, 131, e146221. [Google Scholar] [CrossRef] [PubMed]
- Taghavian, O.; Jain, A.; Joyner, C.J.; Ketchum, S.; Nakajima, R.; Jasinskas, A.; Liang, L.; Fong, R.; King, C.; Greenhouse, B.; et al. Antibody profiling by proteome microarray with multiplex isotype detection reveals overlap between human and Aotus nancymaae controlled malaria infections. Proteomics 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Noe, A.; Datoo, M.S.; Flaxman, A.; Husainy, M.A.; Jenkin, D.; Bellamy, D.; Makinson, R.A.; Morter, R.; Ramos Lopez, F.; Sheridan, J.; et al. Deep immune phenotyping and single-cell transcriptomics allow identification of circulating TRM-like cells which correlate with liver-stage immunity and vaccine-induced protection from malaria. Front. Immunol. 2022, 13, 795463. [Google Scholar] [CrossRef]
- Raj, D.K.; Nixon, C.P.; Nixon, C.E.; Dvorin, J.D.; DiPetrillo, C.G.; Pond-Tor, S.; Wu, H.W.; Jolly, G.; Pischel, L.; Lu, A.; et al. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 2014, 344, 871–877. [Google Scholar] [CrossRef]
- Raj, D.K.; Das Mohapatra, A.; Jnawali, A.; Zuromski, J.; Jha, A.; Cham-Kpu, G.; Sherman, B.; Rudlaff, R.M.; Nixon, C.E.; Hilton, N.; et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature 2020, 582, 104–108. [Google Scholar] [CrossRef]
- Gamain, B.; Chene, A.; Viebig, N.K.; Tuikue Ndam, N.; Nielsen, M.A. Progress and insights toward an effective placental malaria vaccine. Front. Immunol. 2021, 12, 634508. [Google Scholar] [CrossRef]
- Tiendrebeogo, R.W.; Adu, B.; Singh, S.K.; Dziegiel, M.H.; Nebie, I.; Sirima, S.B.; Christiansen, M.; Dodoo, D.; Theisen, M. Antibody-dependent cellular inhibition Is associated with reduced risk against febrile malaria in a longitudinal cohort study involving Ghanaian children. Open Forum Infect. Dis. 2015, 2, ofv044. [Google Scholar] [CrossRef]
- Kyei-Baafour, E.; Kusi, K.A.; Arthur, F.K.N.; Tiendrebeogo, R.W.; Owusu-Yeboa, E.; Singh, S.K.; Friedrich, S.; Gerds, T.A.; Dodoo, D.; Theisen, M.; et al. High opsonic phagocytosis activity and growth inhibition of merozoites are associated with RON4 antibody levels and protect against febrile malaria in Ghanaian children. Front. Immunol. 2023, 14, 1161301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Senosiain, A.; Kana, I.H.; Singh, S.; Das, M.K.; Dziegiel, M.H.; Hertegonne, S.; Adu, B.; Theisen, M. Neutrophils dominate in opsonic phagocytosis of P. falciparum blood-stage merozoites and protect against febrile malaria. Commun. Biol. 2021, 4, 984. [Google Scholar] [CrossRef] [PubMed]
- Kana, I.H.; Adu, B.; Tiendrebeogo, R.W.; Singh, S.K.; Dodoo, D.; Theisen, M. Naturally acquired antibodies target the glutamate-rich protein on intact merozoites and predict protection against febrile malaria. J. Infect. Dis. 2017, 215, 623–630. [Google Scholar] [CrossRef]
- Osier, F.H.; Feng, G.; Boyle, M.J.; Langer, C.; Zhou, J.; Richards, J.S.; McCallum, F.J.; Reiling, L.; Jaworowski, A.; Anders, R.F.; et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: Mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Mansourou, A.; Joos, C.; Niass, O.; Diouf, B.; Tall, A.; Perraut, R.; Niang, M.; Toure-Balde, A. Improvement of the antibody-dependent respiratory burst assay for assessing protective immune responses to malaria. Open Biol. 2022, 12, 210288. [Google Scholar] [CrossRef] [PubMed]
- Joos, C.; Marrama, L.; Polson, H.E.; Corre, S.; Diatta, A.M.; Diouf, B.; Trape, J.F.; Tall, A.; Longacre, S.; Perraut, R. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS ONE 2010, 5, e9871. [Google Scholar] [CrossRef] [PubMed]
- Greve, B.; Lehman, L.G.; Lell, B.; Luckner, D.; Schmidt-Ott, R.; Kremsner, P.G. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J. Infect. Dis. 1999, 179, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.E.; Lugaajju, A.; Nylander, M.; Danielsson, L.; Tijani, M.K.; Beeson, J.G.; Persson, K.E.M. Acquisition of complement fixing antibodies targeting Plasmodium falciparum merozoites in infants and their mothers in Uganda. Front. Immunol. 2023, 14, 1295543. [Google Scholar] [CrossRef] [PubMed]
- Reiling, L.; Boyle, M.J.; White, M.T.; Wilson, D.W.; Feng, G.; Weaver, R.; Opi, D.H.; Persson, K.E.M.; Richards, J.S.; Siba, P.M.; et al. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat. Commun. 2019, 10, 610. [Google Scholar] [CrossRef]
- Boyle, M.J.; Reiling, L.; Feng, G.; Langer, C.; Osier, F.H.; Aspeling-Jones, H.; Cheng, Y.S.; Stubbs, J.; Tetteh, K.K.; Conway, D.J.; et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42, 580–590. [Google Scholar] [CrossRef]
- Hassan, I.; Kanoi, B.N.; Nagaoka, H.; Sattabongkot, J.; Udomsangpetch, R.; Tsuboi, T.; Takashima, E. High-throughput antibody profiling identifies targets of protective immunity against P. falciparum malaria in Thailand. Biomolecules 2023, 13, 1267. [Google Scholar] [CrossRef]
- Kanoi, B.N.; Nagaoka, H.; White, M.T.; Morita, M.; Palacpac, N.M.Q.; Ntege, E.H.; Balikagala, B.; Yeka, A.; Egwang, T.G.; Horii, T.; et al. Global repertoire of human antibodies against Plasmodium falciparum RIFINs, SURFINs, and STEVORs in a malaria exposed population. Front. Immunol. 2020, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Kusi, K.A.; Aguiar, J.; Kumordjie, S.; Aggor, F.; Bolton, J.; Renner, A.; Kyei-Baafour, E.; Puplampu, N.; Belmonte, M.; Dodoo, D.; et al. Antigenicity and immune correlate assessment of seven Plasmodium falciparum antigens in a longitudinal infant cohort from northern Ghana. Sci. Rep. 2019, 9, 8621. [Google Scholar] [CrossRef]
- Kana, I.H.; Singh, S.K.; Garcia-Senosiain, A.; Dodoo, D.; Singh, S.; Adu, B.; Theisen, M. Breadth of functional antibodies is associated with Plasmodium falciparum merozoite phagocytosis and protection sgainst febrile malaria. J. Infect. Dis. 2019, 220, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Valmaseda, A.; Macete, E.; Nhabomba, A.; Guinovart, C.; Aide, P.; Bardaji, A.; Bassat, Q.; Nhampossa, T.; Maculuve, S.; Casellas, A.; et al. Identifying immune correlates of protection against Plasmodium falciparum through a novel approach to account for heterogeneity in malaria exposure. Clin. Infect. Dis. 2018, 66, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Partey, F.D.; Castberg, F.C.; Sarbah, E.W.; Silk, S.E.; Awandare, G.A.; Draper, S.J.; Opoku, N.; Kweku, M.; Ofori, M.F.; Hviid, L.; et al. Kinetics of antibody responses to PfRH5-complex antigens in Ghanaian children with Plasmodium falciparum malaria. PLoS ONE 2018, 13, e0198371. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Arumugam, T.U.; Reiling, L.; Healer, J.; Hodder, A.N.; Fowkes, F.J.; Cross, N.; Langer, C.; Takeo, S.; Uboldi, A.D.; et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J. Immunol. 2013, 191, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Cutts, J.C.; Agius, P.A.; Zaw, L.; Powell, R.; Moore, K.; Draper, B.; Simpson, J.A.; Fowkes, F.J.I. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: A systematic review. BMC Med. 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.J.; Richards, J.S.; Simpson, J.A.; Beeson, J.G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010, 7, e1000218. [Google Scholar] [CrossRef]
- Duncan, C.J.; Hill, A.V. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum. Vaccines Immunother. 2012, 8, 706–714. [Google Scholar] [CrossRef]
- Guevara Patino, J.A.; Holder, A.A.; McBride, J.S.; Blackman, M.J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 1997, 186, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.S.; Marjason, J.; Elliott, S.; Fahey, P.; Bang, G.; Malkin, E.; Tierney, E.; Aked-Hurditch, H.; Adda, C.; Cross, N.; et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide(R) ISA 720. PLoS ONE 2011, 6, e24413. [Google Scholar] [CrossRef]
- Druilhe, P.; Spertini, F.; Soesoe, D.; Corradin, G.; Mejia, P.; Singh, S.; Audran, R.; Bouzidi, A.; Oeuvray, C.; Roussilhon, C. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2005, 2, e344. [Google Scholar] [CrossRef]
- Yagi, M.; Bang, G.; Tougan, T.; Palacpac, N.M.; Arisue, N.; Aoshi, T.; Matsumoto, Y.; Ishii, K.J.; Egwang, T.G.; Druilhe, P.; et al. Protective epitopes of the Plasmodium falciparum SERA5 malaria vaccine reside in intrinsically unstructured N-terminal repetitive sequences. PLoS ONE 2014, 9, e98460. [Google Scholar] [CrossRef]
- Steiner-Monard, V.; Kamaka, K.; Karoui, O.; Roethlisberger, S.; Audran, R.; Daubenberger, C.; Fayet-Mello, A.; Erdmann-Voisin, A.; Felger, I.; Geiger, K.; et al. The candidate blood stage malaria vaccine P27A induces a robust humoral response in a fast track to the field phase I trial in exposed and non exposed volunteers. Clin. Infect. Dis. 2018, 68, 466–474. [Google Scholar] [CrossRef]
- Blank, A.; Furle, K.; Jaschke, A.; Mikus, G.; Lehmann, M.; Husing, J.; Heiss, K.; Giese, T.; Carter, D.; Bohnlein, E.; et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Richert, L.; Chene, A.; Konate, A.T.; Campion, C.; Dechavanne, S.; Semblat, J.P.; Benhamouda, N.; Bahuaud, M.; Loulergue, P.; et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: A first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. 2020, 20, 585–597. [Google Scholar] [CrossRef]
- Mordmuller, B.; Sulyok, M.; Egger-Adam, D.; Resende, M.; de Jongh, W.A.; Jensen, M.H.; Smedegaard, H.H.; Ditlev, S.B.; Soegaard, M.; Poulsen, L.; et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 2019, 69, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Delves, M.J.; Miguel-Blanco, C.; Matthews, H.; Molina, I.; Ruecker, A.; Yahiya, S.; Straschil, U.; Abraham, M.; Leon, M.L.; Fischer, O.J.; et al. A high throughput screen for next-generation leads targeting malaria parasite transmission. Nat. Commun. 2018, 9, 3805. [Google Scholar] [CrossRef]
- Delves, M.; Lafuente-Monasterio, M.J.; Upton, L.; Ruecker, A.; Leroy, D.; Gamo, F.J.; Sinden, R. Fueling open innovation for malaria transmission-blocking drugs: Hundreds of molecules targeting early parasite mosquito stages. Front. Microbiol. 2019, 10, 2134. [Google Scholar] [CrossRef]
- Hill, D.L.; Eriksson, E.M.; Li Wai Suen, C.S.; Chiu, C.Y.; Ryg-Cornejo, V.; Robinson, L.J.; Siba, P.M.; Mueller, I.; Hansen, D.S.; Schofield, L. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS ONE 2013, 8, e74627. [Google Scholar] [CrossRef] [PubMed]
- Ghumra, A.; Khunrae, P.; Ataide, R.; Raza, A.; Rogerson, S.J.; Higgins, M.K.; Rowe, J.A. Immunisation with recombinant PfEMP1 domains elicits functional rosette-inhibiting and phagocytosis-inducing antibodies to Plasmodium falciparum. PLoS ONE 2011, 6, e16414. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Howell, K.B.; Reiling, L.; Ataide, R.; Mackintosh, C.L.; Fowkes, F.J.; Petter, M.; Chesson, J.M.; Langer, C.; Warimwe, G.M.; et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. Clin. Infect. Dis. 2012, 122, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Fleit, H.B.; Kobasiuk, C.D. The human monocyte-like cell line THP-1 expresses Fc gamma RI and Fc gamma RII. J. Leukoc. Biol. 1991, 49, 556–565. [Google Scholar] [CrossRef]
- Miura, K.; Stone, W.J.; Koolen, K.M.; Deng, B.; Zhou, L.; van Gemert, G.J.; Locke, E.; Morin, M.; Bousema, T.; Sauerwein, R.W.; et al. An inter-laboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar. J. 2016, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Eldering, M.; Bompard, A.; Miura, K.; Stone, W.; Morlais, I.; Cohuet, A.; van Gemert, G.J.; Brock, P.M.; Rijpma, S.R.; van de Vegte-Bolmer, M.; et al. Comparative assessment of An. gambiae and An. stephensi mosquitoes to determine transmission-reducing activity of antibodies against P. falciparum sexual stage antigens. Parasit. Vectors 2017, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Premawansa, S.; Gamage-Mendis, A.; Perera, L.; Begarnie, S.; Mendis, K.; Carter, R. Plasmodium falciparum malaria transmission-blocking immunity under conditions of low endemicity as in Sri Lanka. Parasite Immunol. 1994, 16, 35–42. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, M.; de Vlas, S.J.; Sauerwein, R.W. Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. Int. J. Parasitol. 2006, 36, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.; Bousema, T.; Sauerwein, R.; Drakeley, C. Two-faced immunity? The evidence for antibody enhancement of malaria transmission. Trends Parasitol. 2018, 35, 140–153. [Google Scholar] [CrossRef]
- Bansal, G.P.; Araujo Md, S.; Cao, Y.; Shaffer, E.; Araujo, J.E.; Medeiros, J.F.; Hayashi, C.; Vinetz, J.; Kumar, N. Transmission-reducing and -enhancing monoclonal antibodies against Plasmodium vivax gamete surface protein Pvs48/45. Infect. Immun. 2024, 92, e0037423. [Google Scholar] [CrossRef]
- Medley, G.F.; Sinden, R.E.; Fleck, S.; Billingsley, P.F.; Tirawanchai, N.; Rodriguez, M.H. Heterogeneity in patterns of malarial oocyst infections in the mosquito vector. Parasitology 1993, 106 Pt 5, 441–449. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, M.; De Vlas, S.J.; Saul, A.; van de Vegte-Bolmer, M.; Eling, W.M.; Sauerwein, R.W. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology 2005, 130, 13–22. [Google Scholar] [CrossRef]
- Miura, K.; Swihart, B.J.; Fay, M.P.; Kumpitak, C.; Kiattibutr, K.; Sattabongkot, J.; Long, C.A. Evaluation and modeling of direct membrane-feeding assay with Plasmodium vivax to support development of transmission blocking vaccines. Sci. Rep. 2020, 10, 12569. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Knezevic, I.; Mattiuzzo, G.; Page, M.; Minor, P.; Griffiths, E.; Nuebling, M.; Moorthy, V. WHO International Standard for evaluation of the antibody response to COVID-19 vaccines: Call for urgent action by the scientific community. Lancet Microbe 2021, 3, e235–e240. [Google Scholar] [CrossRef]
| Characteristics | Example of Interpretation 1 |
|---|---|
| Specificity | Whether we can detect a signal of test antibody in the presence of an unrelated substance that may be expected to be present in a test sample |
| Selectivity | Whether we do not detect any signal when an analyte contains no specific antibody |
| Sensitivity | How low (or high) we can detect a signal of test antibody |
| Detection Limit | The lowest (or the highest) signal can be detected (but not necessarily quantitated) |
| Quantitation Limit | The lowest (or the highest) signal can be quantitatively detected |
| Range | The interval between the upper and lower levels of signals in which the analytical procedure has a suitable level of the other parameters |
| Precision | How much variation in a signal is observed when the same antibody is tested repeatedly |
| Repeatability | Intra-assay variability |
| Intermediated Precision | Inter-assay variability |
| Reproducibility | Inter-laboratory variability |
| Stability | Whether we can detect the same signal when a test antibody is kept at a storage temperature and/or after freeze–thaw |
| Dilution effects | Whether we can obtain the same result (a signal multiple by a dilution factor) when a test sample is diluted (but still within a “range” of assays) |
| Linearity | Whether (a transformation of) a signal of a test antibody is directly proportional to (a transformation of) the concentration of test antibody |
| Accuracy | Agreement between a conventional true value and an observed value |
| Robustness | How much variation in the analytical procedure (e.g., incubation time and temperature) is allowed to detect the same signal for the same sample |
| Assay | Principle | Characteristics |
|---|---|---|
| Growth inhibition assay (GIA) or invasion inhibition assay (IIA) | A test antibody is incubated with cultured parasites to determine the direct killing or invasion blocking effect of the antibody. |
|
| Antibody-dependent cellular inhibition (ADCI) assay | A test antibody is incubated with human monocytes and cultured parasites to determine the parasite-killing effect by the monocytes. |
|
| Opsonic phagocytosis assay | A test antibody is incubated with parasites first; then, the opsonized parasites are further incubated with cells. A proportion of cells that internalize the parasites is measured. |
|
| Antibody dependent respiratory burst (ADRB) assay | A test antibody is incubated with purified merozoites first; then, the opsonized merozoites are further incubated with neutrophils. ROS released from the neutrophils are measured. |
|
| Complement fixation assay | A test serum/plasma is incubated with purified merozoites; then, the amount of C1q that binds to the merozoite-specific antibodies is measured. |
|
| Infected RBC binding inhibition assay | A test antibody is incubated with infected RBC and CSA to determine how much binding between infected RBC and CSA is interfered with by the antibody. |
|
| Assay | Principle | Characteristics |
|---|---|---|
| Standard-membrane-feeding assay (SMFA) | A mixture of a test antibody and in vitro cultured gametocytes is fed to mosquitoes; then, a reduction in oocysts (intensity or prevalence) in the mosquitoes is determined. |
|
| Direct-membrane-feeding assay (DMFA) | Similar to SMFA but uses gametocytes in blood from an infected human (or monkey), instead of cultured gametocytes in SMFA. |
|
| Direct skin feed assays (DSF) | Mosquitoes feed directly on the skin of a vaccinated human. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, K. How to Accelerate Early Stage of Malaria Vaccine Development by Optimizing Functional Assays. Vaccines 2024, 12, 586. https://doi.org/10.3390/vaccines12060586
Miura K. How to Accelerate Early Stage of Malaria Vaccine Development by Optimizing Functional Assays. Vaccines. 2024; 12(6):586. https://doi.org/10.3390/vaccines12060586
Chicago/Turabian StyleMiura, Kazutoyo. 2024. "How to Accelerate Early Stage of Malaria Vaccine Development by Optimizing Functional Assays" Vaccines 12, no. 6: 586. https://doi.org/10.3390/vaccines12060586





