Long Prime–Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patient Inclusion Criteria
2.2. Study Design
2.3. Blood Collection, Serum Anti-Vaccine Antibody by ELISA, and Dectin-1 SNP Genotyping
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
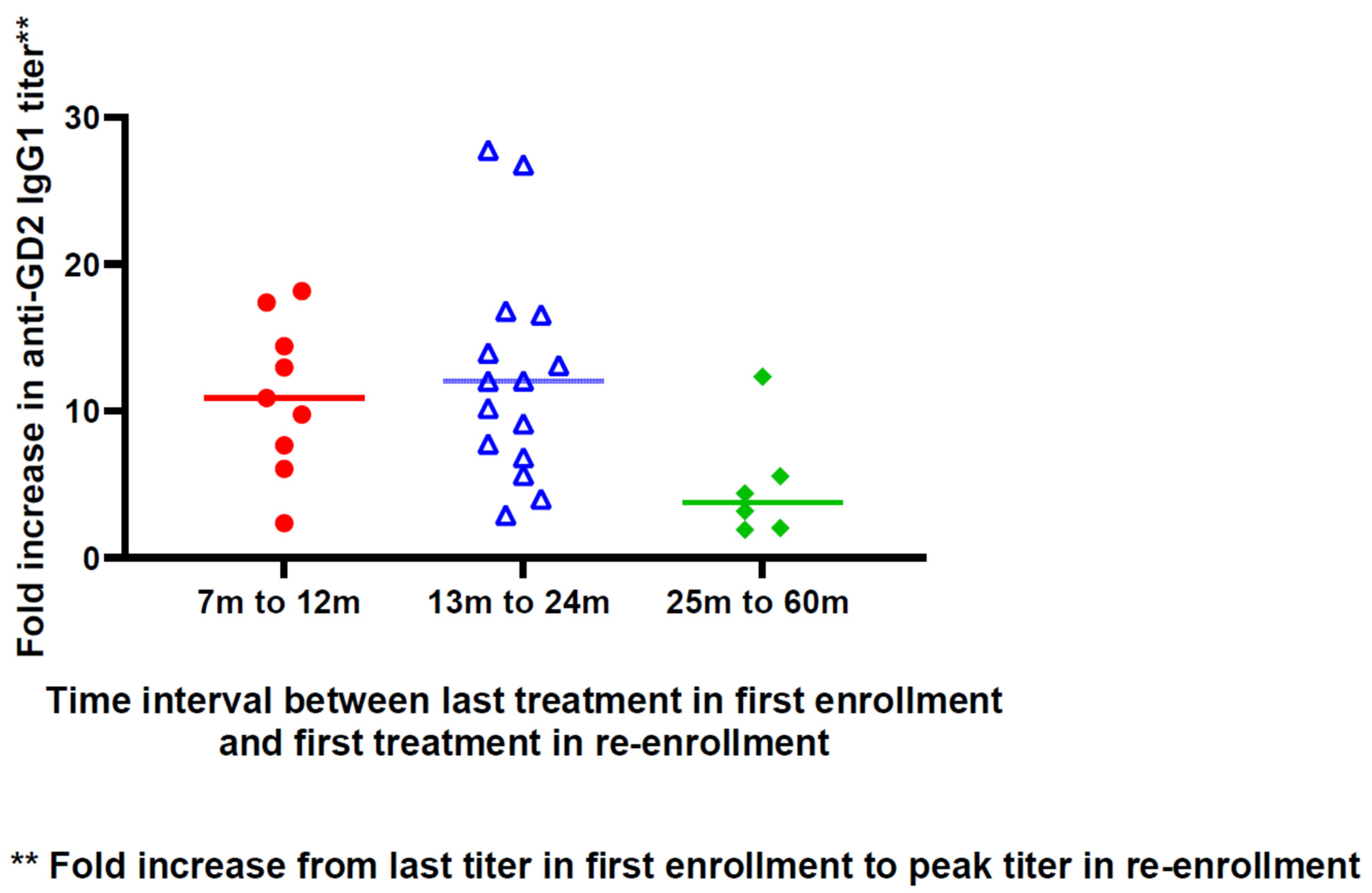
3.2. Anti-Vaccine Antibody Response
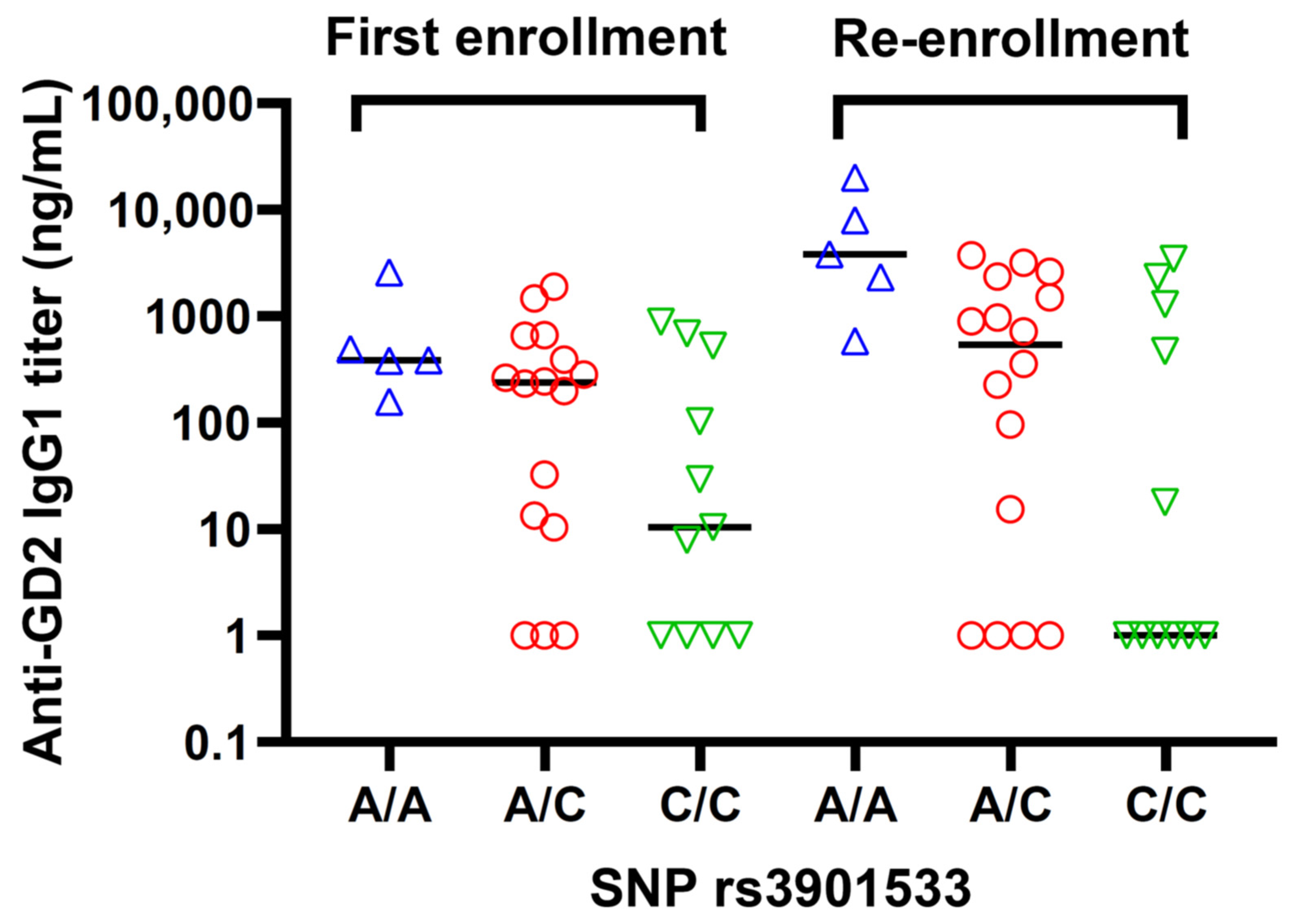
3.3. Antibody Response and Dectin-1 SNP rs3901533
3.4. Patient Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.M.C.; Plotkin, S.A. The influence of interval between doses on response to vaccines. Vaccine 2021, 39, 7123–7127. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Grunau, B.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Golding, L.; Kirkham, T.L.; Demers, P.A.; Lavoie, P.M. Immunogenicity of Extended mRNA SARS-CoV-2 Vaccine Dosing Intervals. JAMA 2022, 327, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Chung, H.; Jung, J.K.; Buchan, S.A.; Campigotto, A.; Campitelli, M.A.; Crowcroft, N.S.; Gubbay, J.B.; Karnauchow, T.; Katz, K.; et al. The impact of repeated vaccination using 10-year vaccination history on protection against influenza in older adults: A test-negative design study across the 2010/11 to 2015/16 influenza seasons in Ontario, Canada. Eurosurveillance 2020, 25, 1900245. [Google Scholar] [CrossRef] [PubMed]
- Valenciano, M.; Kissling, E.; Larrauri, A.; Nunes, B.; Pitigoi, D.; O’Donnell, J.; Reuss, A.; Horváth, J.K.; Paradowska-Stankiewicz, I.; Rizzo, C.; et al. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: Results of the European I-MOVE multicentre test-negative case-control study, 2011/2012–2016/2017. Influenza Other Respir. Viruses 2018, 12, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Skowronski, D.M.; McLean, H.Q.; Chambers, C.; Sundaram, M.E.; De Serres, G. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Rev. Vaccines 2017, 16, 723–736. [Google Scholar] [CrossRef]
- Thompson, M.G.; Naleway, A.; Fry, A.M.; Ball, S.; Spencer, S.M.; Reynolds, S.; Bozeman, S.; Levine, M.; Katz, J.M.; Gaglani, M. Effects of Repeated Annual Inactivated Influenza Vaccination among Healthcare Personnel on Serum Hemagglutinin Inhibition Antibody Response to A/Perth/16/2009 (H3N2)-like virus during 2010-11. Vaccine 2016, 34, 981–988. [Google Scholar] [CrossRef]
- Sanyal, M.; Holmes, T.H.; Maecker, H.T.; Albrecht, R.A.; Dekker, C.L.; He, X.-S.; Greenberg, H.B. Diminished B-Cell Response After Repeat In-fluenza Vaccination. J. Infect. Dis. 2019, 219, 1586–1595. [Google Scholar] [CrossRef]
- Khurana, S.; Hahn, M.; Coyle, E.M.; King, L.R.; Lin, T.-L.; Treanor, J.; Sant, A.; Golding, H. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat. Commun. 2019, 10, 3338. [Google Scholar] [CrossRef]
- Music, N.; Tzeng, W.P.; Liaini Gross, F.; Levine, M.Z.; Xu, X.; Shieh, W.J.; Tumpey, T.M.; Katz, J.M.; York, I.A. Repeated vaccination against matched H3N2 influenza virus gives less protection than single vaccination in ferrets. NPJ Vaccines 2019, 4, 28. [Google Scholar] [CrossRef]
- Goldblatt, D. Conjugate vaccines. Clin. Exp. Immunol. 2000, 119, 1–3. [Google Scholar] [CrossRef]
- Adamo, R. Advancing homogeneous antimicrobial glycoconjugate vaccines. Acc. Chem. Res. 2017, 50, 1270–1279. [Google Scholar] [CrossRef]
- Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate vaccines: Current approaches towards faster vaccine design. Expert Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Sorieul, C.; Papi, F.; Carboni, F.; Pecetta, S.; Phogat, S.; Adamo, R. Recent advances and future perspectives on carbohydrate-based cancer vaccines and therapeutics. Pharmacol. Ther. 2022, 235, 108158. [Google Scholar] [CrossRef]
- Wilson, R.M.; Danishefsky, S.J. A Vision for Vaccines Built from Fully Synthetic Tumor-Associated Antigens: From the Laboratory to the Clinic. J. Am. Chem. Soc. 2013, 135, 14462–14472. [Google Scholar] [CrossRef] [PubMed]
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–8826. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing with Granulocyte-Macrophage Colony-Stimulating Factor in Patients with Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018, 4, 1729–1735. [Google Scholar] [CrossRef]
- Livingston, P.O.; Adluri, S.; Helling, F.; Yao, T.-J.; Kensilt, C.R.; Newman, M.J.; Marciani, D. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine 1994, 12, 1275–1280. [Google Scholar] [CrossRef]
- Helling, F.; Zhang, S.; Shang, A.; Adluri, S.; Calves, M.; Koganty, R.; Longenecker, B.M.; Yao, T.J.; Oettgen, H.F.; O Livingston, P. GM2-KLH conjugate vaccine: Increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res. 1995, 55, 2783–2788. [Google Scholar]
- Ragupathi, G.; Livingston, P.O.; Hood, C.; Gathuru, J.; Krown, S.E.; Chapman, P.B.; Wolchok, J.D.; Williams, L.J.; Oldfield, R.C.; Hwu, W.J. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin. Cancer Res. 2003, 9, 5214–5220. [Google Scholar]
- Ragupathi, G.; Meyers, M.; Adluri, S.; Howard, L.; Musselli, C.; Livingston, P.O. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. Int. J. Cancer 2000, 85, 659–666. [Google Scholar] [CrossRef]
- Chapman, P.B.; Williams, L.; Salibi, N.; Hwu, W.-J.; Krown, S.E.; Livingston, P.O. A phase II trial comparing five dose levels of BEC2 anti-idiotypic monoclonal antibody vaccine that mimics GD3 ganglioside. Vaccine 2004, 22, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Gilewski, T.; Ragupathi, G.; Bhuta, S.; Williams, L.J.; Musselli, C.; Zhang, X.F.; Bencsath, K.P.; Panageas, K.S.; Chin, J.; Hudis, C.A.; et al. Immunization of metastatic breast cancer pa-tients with a fully synthetic globo H conjugate: A phase I trial. Proc. Natl. Acad. Sci. USA 2001, 98, 3270–3275. [Google Scholar] [CrossRef]
- Huang, C.S.; Yu, A.L.; Tseng, L.M.; Chow, L.W.; Hou, M.F.; Hurvitz, S.A.; Schwab, R.B.; Wong, C.H.; Murray, J.L.; Chang, H.K.; et al. Randomized phase II/III trial of active immuno-therapy with OPT-822/OPT-821 in patients with metastatic breast cancer. J. Clin. Oncol. 2016, 34 (Suppl. 15), 1003. [Google Scholar] [CrossRef]
- O’cearbhaill, R.E.; Ragupathi, G.; Zhu, J.; Wan, Q.; Mironov, S.; Yang, G.; Spassova, M.K.; Iasonos, A.; Kravetz, S.; Ouerfelli, O.; et al. A Phase I Study of Unimolecular Pentavalent (Globo-H-GM2-sTn-TF-Tn) Immunization of Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer in First Remission. Cancers 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.Y.; Cheung, N.K.V.; Modak, S.; Mauguen, A.; Feng, Y.; Basu, E.; Roberts, S.S.; Ragupathi, G.; Kushner, B.H. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients with High-Risk Neuroblastoma With Prior Disease Progression. J. Clin. Oncol. 2021, 39, 215–226. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Mauguen, A.; Modak, S.; Ragupathi, G.; Basu, E.M.; Roberts, S.S.; Kushner, B.H.; Cheung, N.-K. Effect of Oral β-Glucan on Antibody Response to Ganglioside Vaccine in Patients with High-Risk Neuroblastoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement from the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Palgen, J.-L.; Feraoun, Y.; Dzangué-Tchoupou, G.; Joly, C.; Martinon, F.; Le Grand, R.; Beignon, A.-S. Optimize Prime/Boost Vaccine Strategies: Trained Immunity as a New Player in the Game. Front. Immunol. 2021, 12, 612747. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef]
- Saz-Leal, P.; del Fresno, C.; Brandi, P.; Martínez-Cano, S.; Dungan, O.M.; Chisholm, J.D.; Kerr, W.G.; Sancho, D. Targeting SHIP-1 in Myeloid Cells Enhances Trained Immunity and Boosts Response to Infection. Cell Rep. 2018, 25, 1118–1126. [Google Scholar] [CrossRef]
- Dagan, R.; Poolman, J.; Siegrist, C.-A. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010, 28, 5513–5523. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Tontini, M.; Faenzi, E.; Cioncada, R.; Proietti, D.; Seubert, A.; Nuti, S.; Berti, F.; Romano, M.R. Carrier priming effect of CRM197 is related to an enhanced B and T cell activation in meningococcal serogroup A conjugate vaccination. Immunological comparison between CRM197 and diphtheria toxoid. Vaccine 2016, 34, 2334–2341. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Chu, C.; Schönbrunn, A.; Fischer, D.; Liu, Y.; Hocher, J.-G.; Weinerth, J.; Klemm, K.; von Baehr, V.; Krämer, B.K.; Elitok, S.; et al. Immune response of heterologous versus homologous prime-boost regimens with adenoviral vectored and mRNA COVID-19 vaccines in immunocompromised patients. Front. Immunol. 2023, 14, 1187880. [Google Scholar] [CrossRef]
- Ramirez-Valdez, R.A.; Baharom, F.; Khalilnezhad, A.; Fussell, S.C.; Hermans, D.J.; Schrager, A.M.; Tobin, K.K.; Lynn, G.M.; Khalilnezhad, S.; Ginhoux, F.; et al. Intravenous heterologous prime-boost vaccination activates innate and adaptive immunity to promote tumor regression. Cell Rep. 2023, 42, 112599. [Google Scholar] [CrossRef]
- Niavarani, S.R.; St-Cyr, G.; Daniel, L.; Lawson, C.; Giguère, H.; Alkayyal, A.A.; Tai, L.H. Heterologous prime-boost cellular vaccination induces potent antitumor immunity against triple negative breast cancer. Front. Immunol. 2023, 14, 1098344. [Google Scholar] [CrossRef]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef]
- Shinefield, H.R. Overview of the development and current use of CRM197 conjugate vaccines for pediatric use. Vaccine 2010, 28, 4335–4339. [Google Scholar] [CrossRef] [PubMed]
- Bröker, M.; Costantino, P.; DeTora, L.; McIntosh, E.D.; Rappuoli, R. Biochemical and biological characteristics of cross-reacting material 197 (CRM197), a non-toxic mutant of diphtheria toxin: Use as a conjugation protein in vaccines and other potential clinical applications. Biol. J. Int. Assoc. Biol. Stand. 2011, 39, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.-W.; Fang, J.-M.; Ren, S.-X.; Li, W.; Wang, J.; Zhao, Y.; Chen, G.-Y.; Xu, Q.; Zhou, C.-C. A Novel Combined Conjugate Therapeutic Cancer Vaccine, Recombinant EGF-CRM197, in Patients with Advanced Solid Tumors: A Phase I Clinical Study. Front. Oncol. 2021, 11, 745699. [Google Scholar] [CrossRef] [PubMed]
- Ede, N.J.; Good, A.J.; Tobias, J.; Garner-Spitzer, E.; Zielinski, C.C.; Wiedermann, U. Development of the B cell cancer vaccine HER-vaxx for the treatment of her-2 expressing cancers. Front. Oncol. 2022, 12, 939356. [Google Scholar] [CrossRef]
- Hickey, J.M.; Toprani, V.M.; Kaur, K.; Mishra, R.P.N.; Goel, A.; Oganesyan, N.; Lees, A.; Sitrin, R.; Joshi, S.B.; Volkin, D.B. Analytical Comparability Assessments of 5 Re-combinant CRM(197) Proteins From Different Manufacturers and Expression Systems. J. Pharm. Sci. 2018, 107, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Pettini, E.; Pastore, G.; Fiorino, F.; Medaglini, D.; Ciabattini, A. Short or Long Interval between Priming and Boosting: Does It Impact on the Vaccine Immunogenicity? Vaccines 2021, 9, 289. [Google Scholar] [CrossRef]
- Nicolas, A.; Sannier, G.; Dubé, M.; Nayrac, M.; Tauzin, A.; Painter, M.M.; Goel, R.R.; Laporte, M.; Gendron-Lepage, G.; Medjahed, H.; et al. An extended SARS-CoV-2 mRNA vaccine prime-boost interval enhances B cell immunity with limited impact on T cells. iScience 2023, 26, 105904. [Google Scholar] [CrossRef]
- Simondon, F.; Preziosi, M.P.; Pinchinat, S.; Yam, A.; Chabirand, L.; Wassilak, S.; Pines, E.; Trape, J.F.; Salomon, H.; Hoffenbach, A. Randomised Study of the Possible Adjuvant Effect of BCG Vaccine on the Immunogenicity of Diphtheria-Tetanus-Acellular Pertussis Vaccine in Senegalese Infants. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 23–29. [Google Scholar] [CrossRef]
- Booy, R.; Taylor, S.; Tudor-Williams, G.; Moxon, E.; Aitken, S.; Griffiths, H.; Chapel, H.; Ashworth, L.; Macfarlane, J.; Mayon-White, R. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet 1992, 339, 507–510. [Google Scholar] [CrossRef]
- Rümke, H.C.; Loch, H.P.; Hoppenbrouwers, K.; Vandermeulen, C.; Malfroot, A.; Helm, K.; Douha, M.; Willems, P. Immunogenicity and safety of a measles–mumps–rubella–varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine 2011, 29, 3842–3849. [Google Scholar] [CrossRef]
- Dobrenkov, K.; Cheung, N.-K.V. GD2-Targeted Immunotherapy and Radioimmunotherapy. Semin. Oncol. 2014, 41, 589–612. [Google Scholar] [CrossRef]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.V. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr. Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Bosslet, K.; Mennel, H.; Rodden, F.; Bauer, B.; Wagner, F.; Altmannsberger, A.; Sedlacek, H.; Wiegandt, H. Monoclonal antibodies against epitopes on ganglioside GD2 and the lactones. Markers for gliomas and neuroblastomas. Cancer Immunol. Immunother. 1989, 29, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ritter, G.; Boosfeld, E.; Adluri, R.; Calves, M.; Oettgen, H.F.; Old, L.J.; Livingston, P. Antibody response to immunization with ganglioside GD3 and GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int. J. Cancer 1991, 48, 379–385. [Google Scholar] [CrossRef]
- Cardenas, F.I.; Mauguen, A.; Cheung, I.Y.; Kramer, K.; Kushner, B.H.; Ragupathi, G.; Cheung, N.-K.V.; Modak, S. Phase I Trial of Oral Yeast-Derived β-Glucan to Enhance Anti-GD2 Immunotherapy of Resistant High-Risk Neuroblastoma. Cancers 2021, 13, 6265. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Kramer, K.; Ragupathi, G.; Cheung, N.K. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission. Clin. Cancer Res. 2014, 20, 1375–1382. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.-K.V.; Ross, G.D. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of immune responses to vaccination by the microbiota: Implica-tions and potential mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef]
- Hong, S.-H. Influence of Microbiota on Vaccine Effectiveness: “Is the Microbiota the Key to Vaccine-induced Responses?”. J. Microbiol. 2023, 61, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed]
- Marriott, M.; Post, B.; Chablani, L. A comparison of cancer vaccine adjuvants in clinical trials. Cancer Treat. Res. Commun. 2023, 34, 100667. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.M.; Martinez, E.A.; Bogaert, L.; Kast, W.M. Investigation of the Optimal Prime Boost Spacing Regimen for a Cancer Therapeutic Vaccine Targeting Human Papillomavirus. Cancers 2022, 14, 4339. [Google Scholar] [CrossRef]
- Castiglione, F.; Mantile, F.; De Berardinis, P.; Prisco, A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput. Math. Methods Med. 2012, 2012, 842329. [Google Scholar] [CrossRef]
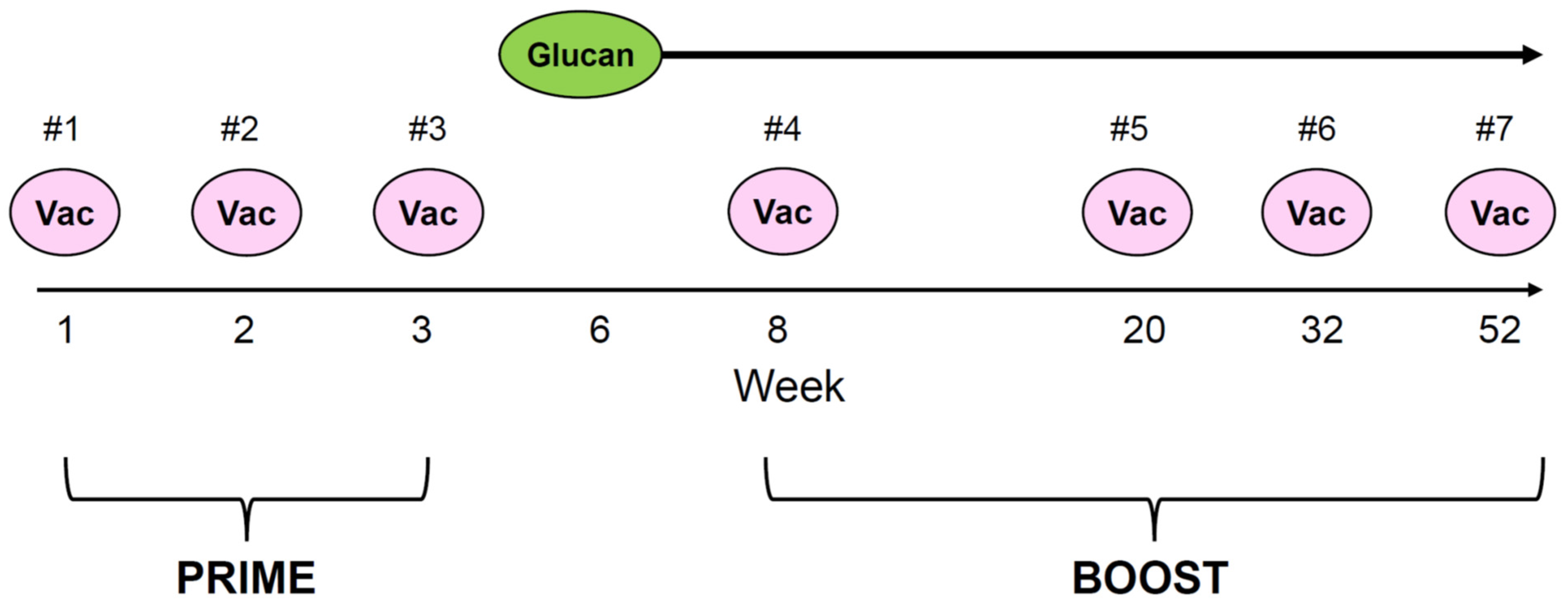
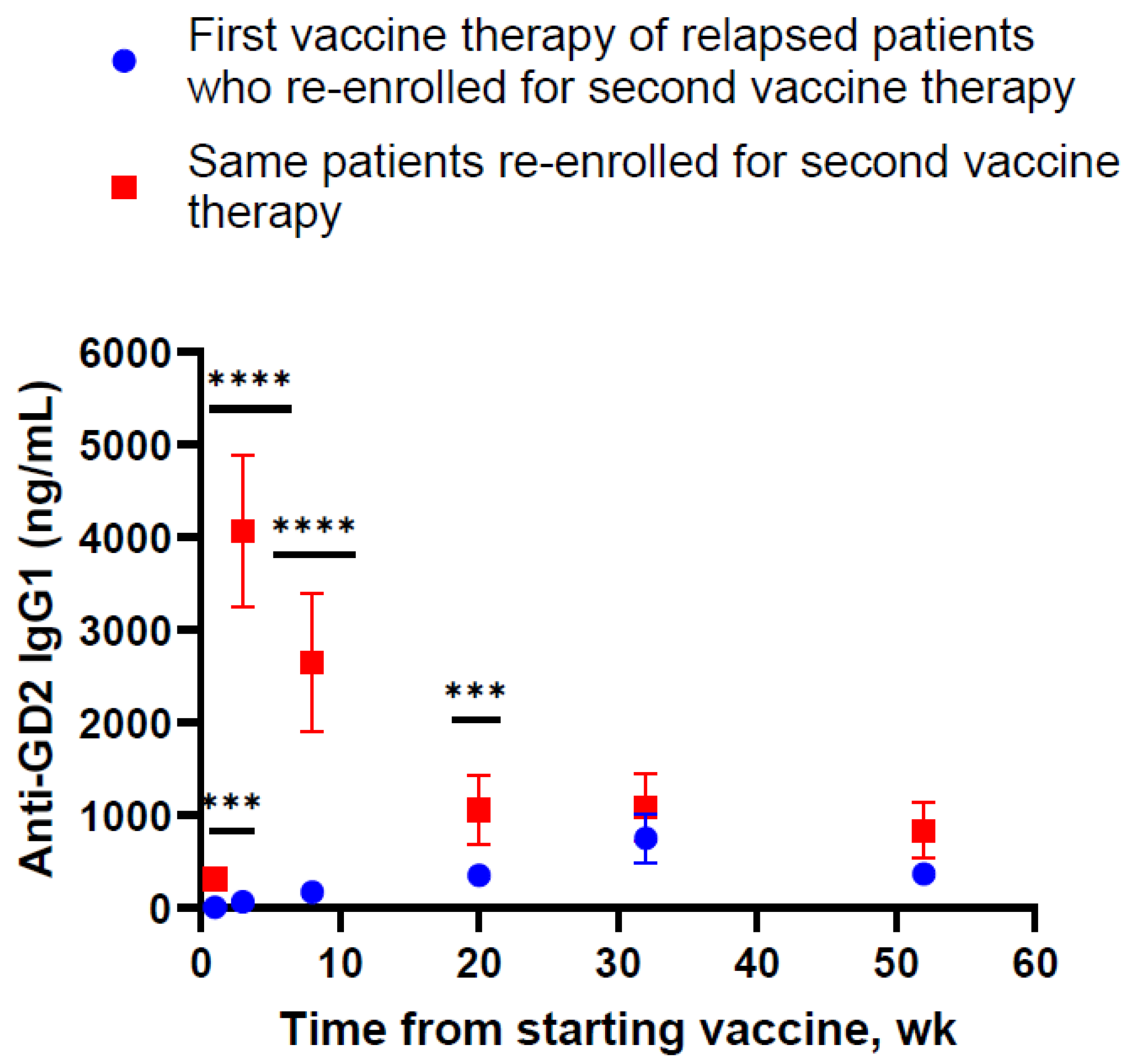
 ) to the last injection at re-enrollment (
) to the last injection at re-enrollment ( ). The timing of prime and boost was indicated. Months from the last upfront vaccine to the first injection during re-enrollment were as follows: 16.6 m for patient #1, 13.4 m for patient #2, and 12.0 m for patient #3.
). The timing of prime and boost was indicated. Months from the last upfront vaccine to the first injection during re-enrollment were as follows: 16.6 m for patient #1, 13.4 m for patient #2, and 12.0 m for patient #3.
 ) to the last injection at re-enrollment (
) to the last injection at re-enrollment ( ). The timing of prime and boost was indicated. Months from the last upfront vaccine to the first injection during re-enrollment were as follows: 16.6 m for patient #1, 13.4 m for patient #2, and 12.0 m for patient #3.
). The timing of prime and boost was indicated. Months from the last upfront vaccine to the first injection during re-enrollment were as follows: 16.6 m for patient #1, 13.4 m for patient #2, and 12.0 m for patient #3.


| Characteristics | N = 32 1 |
|---|---|
| Sex | |
| Female | 13 (41%) |
| Male | 19 (59%) |
| Diagnosis | 2010-09-24 to 2018-05-02 |
| Age at diagnosis (y) | 3.3 [0.1–10.4] |
| <18 months | 4 (12%) |
| ≥18 months | 28 (88%) |
| Neuroblastoma stage | |
| 3 | 1 |
| 4 | 31 |
| Disease status at the first vaccine trial | |
| First CR | 16 (50%) |
| Second CR with prior PD | 16 (50%) |
| Time to PD on/after the first vaccine trial (m) | 10.7 [2.3–59.1] |
| <12 | 19 (60%) |
| 12–30 | 10 (31%) |
| >30 | 3 (9%) |
| Dectin-1 SNP rs3901533 | |
| A/A | 5 (16%) |
| A/C | 16 (50%) |
| C/C | 11(34%) |
| First vaccine date after the first enrollment | 2014-06-04 to 2019-09-17 |
| First vaccine relapse date | 2015-10-05 to 2020-05-11 |
| First vaccine date after re-enrollment | 2017-01-12 to 2021-07-13 |
| Interval between the last vaccine dose of the first enrollment and the first vaccine dose of re-enrollment (m) | 16.1 [7.4–59.5] |
| 1 N (%); Median [Range] | |
| CR, clinical remission; PD, progressive disease | |
| SNP, single nucleotide polymorphism | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, I.Y.; Mauguen, A.; Modak, S.; Basu, E.M.; Feng, Y.; Kushner, B.H.; Cheung, N.K. Long Prime–Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine. Vaccines 2024, 12, 587. https://doi.org/10.3390/vaccines12060587
Cheung IY, Mauguen A, Modak S, Basu EM, Feng Y, Kushner BH, Cheung NK. Long Prime–Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine. Vaccines. 2024; 12(6):587. https://doi.org/10.3390/vaccines12060587
Chicago/Turabian StyleCheung, Irene Y., Audrey Mauguen, Shakeel Modak, Ellen M. Basu, Yi Feng, Brian H. Kushner, and Nai Kong Cheung. 2024. "Long Prime–Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine" Vaccines 12, no. 6: 587. https://doi.org/10.3390/vaccines12060587






