Exploring the Potentiality of a Plant Platform for Monoclonal Antibody Production in Veterinary Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Molecular Design and cDNA Assemblies
2.3. Preparation of Plasmids
2.4. Plant Cultivation and Transient Expression
2.5. Extraction and Purification of Recombinant Canine IL-31 in Plants (rcIL-31 (Plant))
2.6. Extraction and Purification of a Recombinant Monoclonal Antibody Specific for cIL-31 (M1)
2.7. SDS-PAGE and Western Immunoblot Analyses
2.8. Dynamic Light Scattering
2.9. Size Exclusion Chromatography
2.10. N-Linked Glycan Analysis
2.11. Antibodies Binding to cIL-31 According to Biolayer Interferometry
2.12. Interaction between Neonatal Fc Receptor and Antibodies Using Bioluminescent Immunoassay
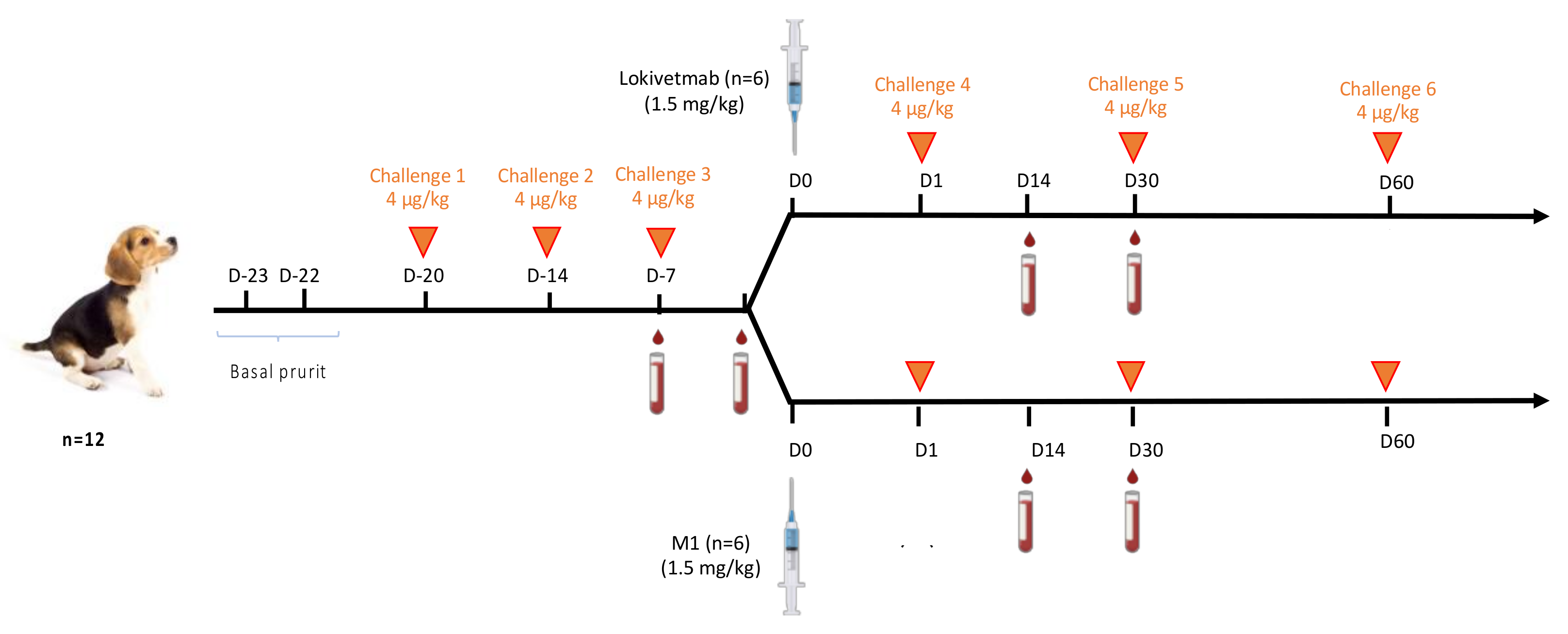
2.13. Challenges and Injection Regimen
2.14. Quantification of cIL-31-Specific IgG
3. Results
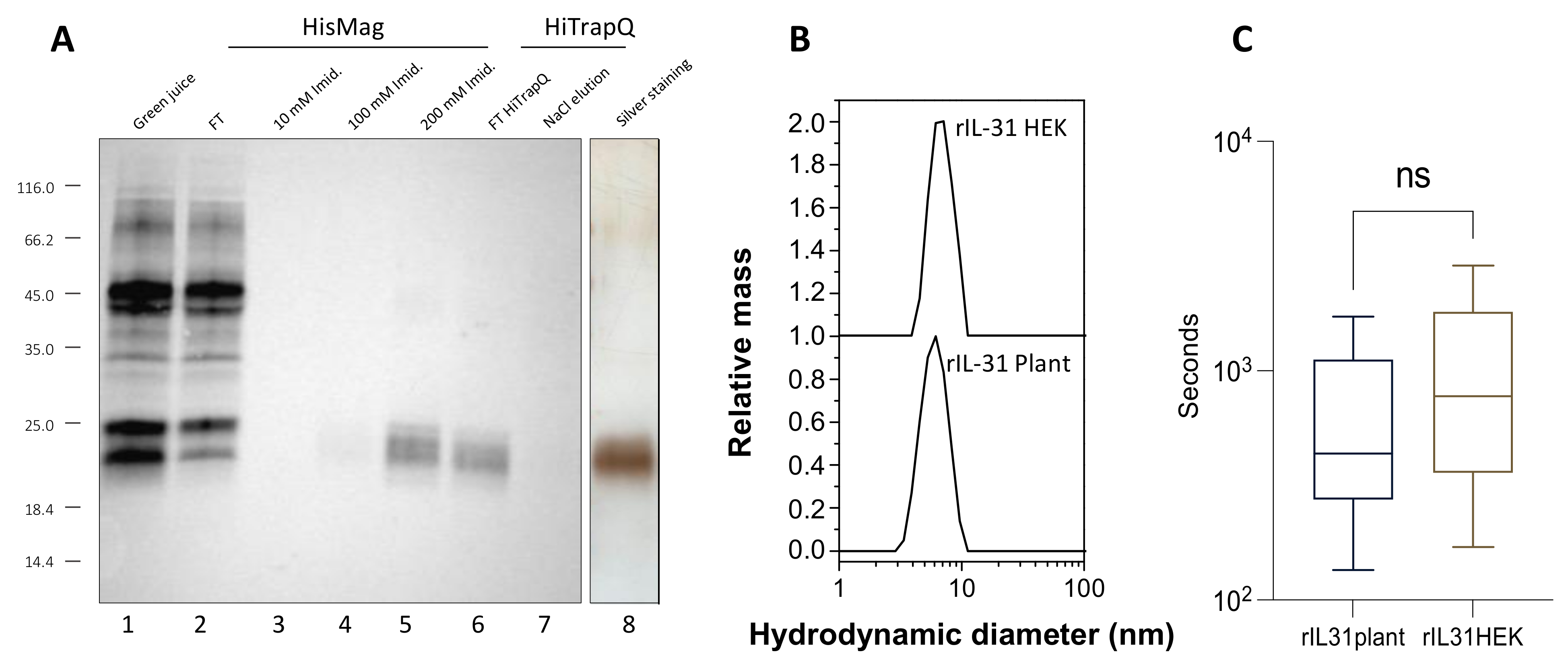
3.1. Production, Purification, and Biological Activity of Plant-Made rcIL-31 (Plant)
3.2. Production, Purification, and Characterization of the Plant-Made Anti-cIL-31 Monoclonal Antibody
3.3. N-Linked Glycosylation Patterns of M1 and Lokivetmab
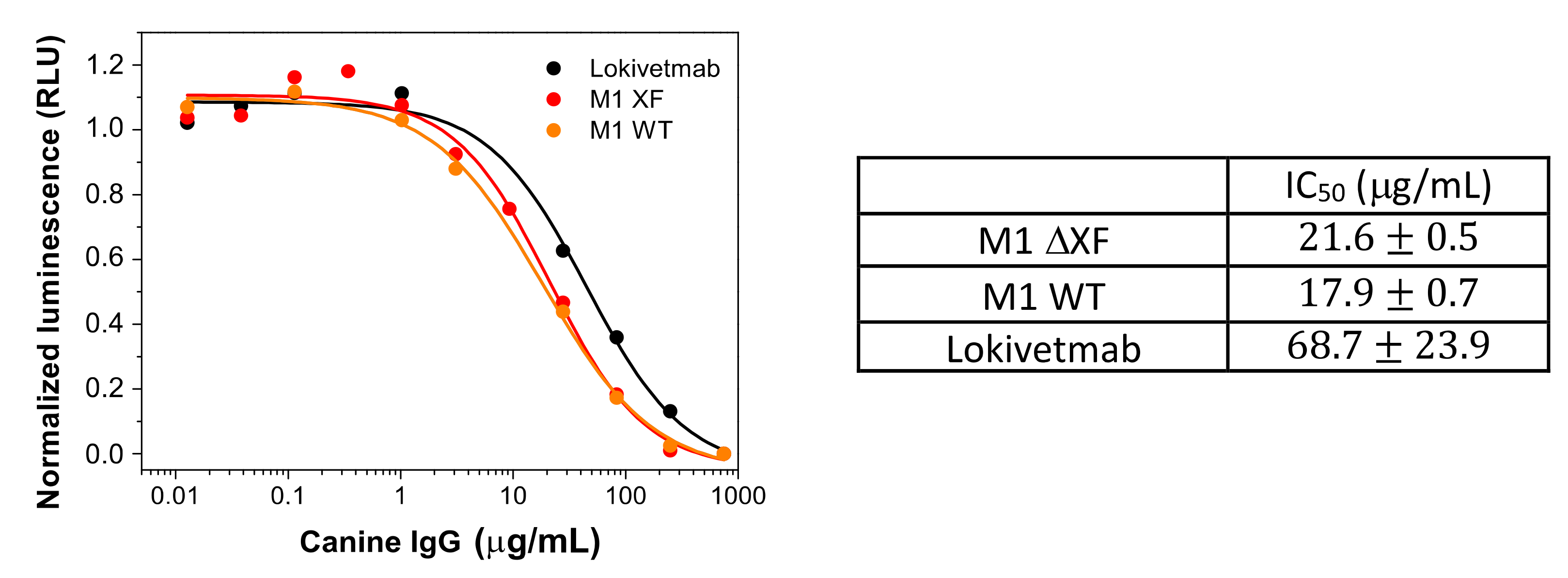
3.4. In Vitro Functional Characterization of Plant-Made Antibody
3.5. Binding to FcRn
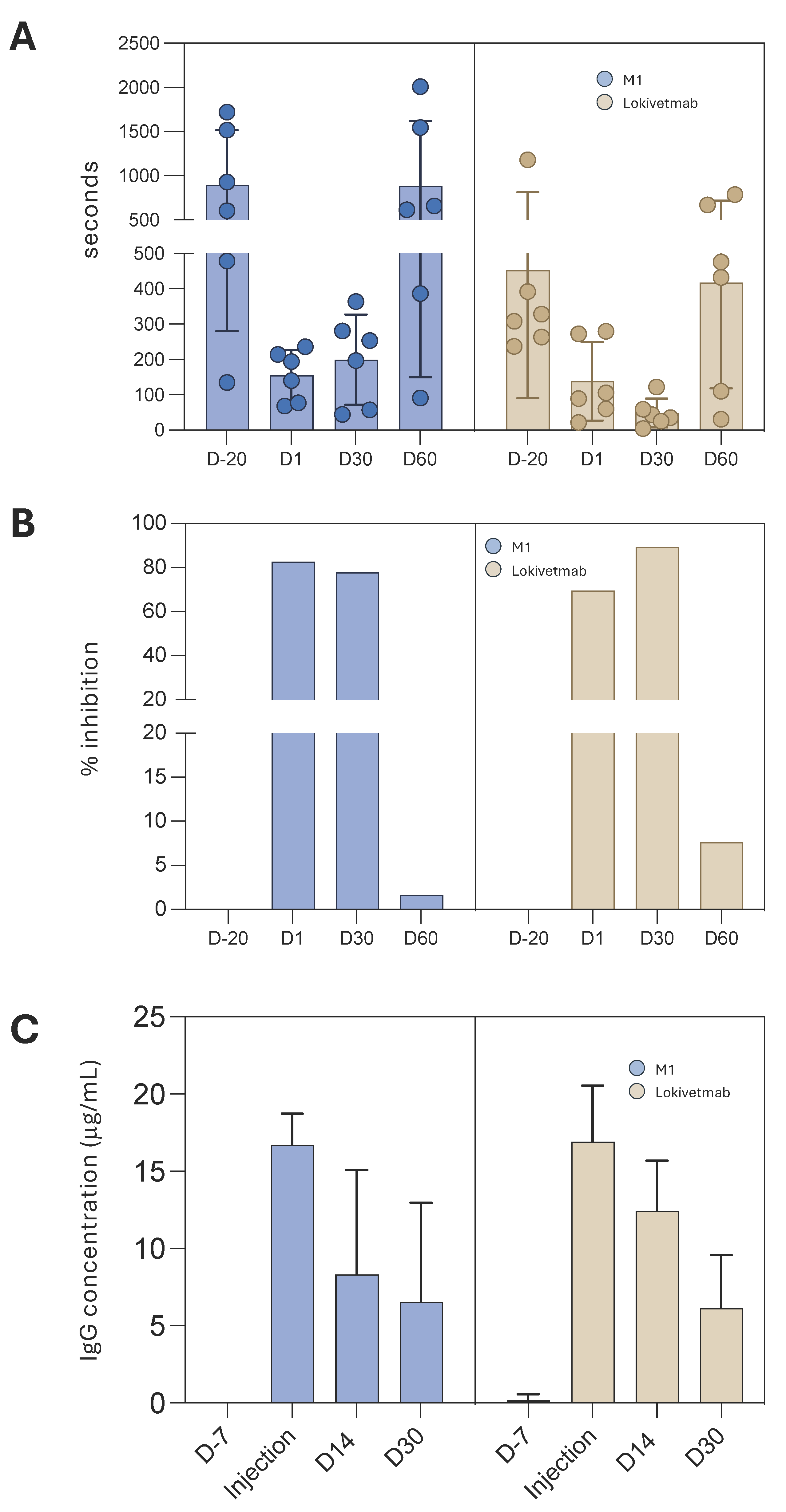
3.6. M1 Efficacy in Controlling Dog Pruritus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffin, C.E.; Hillier, A. The ACVD task force on canine atopic dermatitis (XXIV): Allergen-specific immunotherapy. Vet. Immunol. Immunopathol. 2001, 81, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.B.; Hillier, A.; Olivry, T. The ACVD task force on canine atopic dermatitis (VI): IgE-induced immediate and late-phase reactions, two inflammatory sequences at sites of intradermal allergen injections. Vet. Immunol. Immunopathol. 2001, 81, 199–204. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, D.J. The future of immunotherapy for canine atopic dermatitis: A review. Vet. Dermatol. 2017, 28, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fennis, E.E.M.; van Damme, C.M.M.; Schlotter, Y.M.; Sinke, J.D.; Leistra, M.H.G.; Bartels, R.T.; Broere, F. Efficacy of subcutaneous allergen immunotherapy in atopic dogs: A retrospective study of 664 cases. Vet. Dermatol. 2022, 33, 321-e75. [Google Scholar] [CrossRef]
- Mueller, R.S. Update on Allergen Immunotherapy. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Mantyh, P.W.; Murrell, J.; Innes, J.F.; Lascelles, B.D.X. Anti-nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet. Rec. 2019, 184, 23. [Google Scholar] [CrossRef]
- Gober, M.; Hillier, A.; Vasquez-Hidalgo, M.A.; Amodie, D.; Mellencamp, M.A. Use of Cytopoint in the Allergic Dog. Front. Vet. Sci. 2022, 9, 909776. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Knazovicky, D.; Case, B.; Freire, M.; Innes, J.F.; Drew, A.C.; Gearing, D.P. A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet. Res. 2015, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Kato, Y.; Kaneko, M.K.; Sakai, Y.; Shiga, T.; Kato, M.; Tsukui, T.; Takemoto, H.; Tokimasa, A.; Baba, K.; et al. Generation of a canine anti-canine CD20 antibody for canine lymphoma treatment. Sci. Rep. 2020, 10, 11476. [Google Scholar] [CrossRef]
- Dias, J.N.R.; Almeida, A.; André, A.S.; Aguiar, S.I.; Bule, P.; Nogueira, S.; Oliveira, S.S.; Carrapiço, B.; Gil, S.; Tavares, L.; et al. Characterization of the canine CD20 as a therapeutic target for comparative passive immunotherapy. Sci. Rep. 2022, 12, 2678. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Olivry, T. An update on the treatment of canine atopic dermatitis. Vet. J. 2016, 207, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bagci, S.; Ruzicka, T. IL-31: A new key player in dermatology and beyond. J. Allergy Clin. Immunol. 2018, 141, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.J.; Humphrey, W.R.; Messamore, J.E.; Fleck, T.J.; Fici, G.J.; Shelly, J.A.; Teel, J.F.; Bammert, G.F.; Dunham, S.A.; Fuller, T.E.; et al. Interleukin-31: Its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet. Dermatol. 2013, 24, 48-e12. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.M.; Ramsey, D.S.; Walsh, K.F.; Martinon, O.M.; Mahabir, S.P.; Hoevers, J.D.; Walters, R.R.; Dunham, S.A. A blinded, randomized, placebo-controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet. Dermatol. 2016, 27, 478-e129. [Google Scholar] [CrossRef] [PubMed]
- Fleck, T.J.; Norris, L.R.; Mahabir, S.; Walters, R.R.; Martinon, O.; Dunham, S.A.; Gonzales, A.J. Onset and duration of action of lokivetmab in a canine model of IL-31 induced pruritus. Vet. Dermatol. 2021, 32, 681-e182. [Google Scholar] [CrossRef]
- Krautmann, M.; Walters, R.R.; King, V.L.; Esch, K.; Mahabir, S.P.; Gonzales, A.; Dominowski, P.J.; Sly, L.; Mwangi, D.; Foss, D.L.; et al. Laboratory safety evaluation of lokivetmab, a canine anti-interleukin-31 monoclonal antibody, in dogs. Vet. Immunol. Immunopathol. 2023, 258, 110574. [Google Scholar] [CrossRef] [PubMed]
- Bammert, G.F.; Dunham, S.A. Interleukin-31 Monoclonal Antibody. US20140315251A1, 25 September 2014. [Google Scholar]
- Bensignor, E.; Videmont, E. Weekly topical therapy based on plant extracts combined with lokivetmab in canine atopic dermatitis. Vet. Dermatol. 2022, 33, 68-e22. [Google Scholar] [PubMed]
- Eidenberger, L.; Kogelmann, B.; Steinkellner, H. Plant-based biopharmaceutical engineering. Nat. Rev. Bioeng. 2023, 1, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; Andreani, G.; Dubé, C.; Berubé, M.; Bussière, D.; Couture, M.M.J.; Dargis, M.; Hendin, H.E.; Landry, N.; Lavoie, P.-O.; et al. Development and characterization of a plant-derived norovirus-like particle vaccine. Vaccine 2023, 41, 6008–6016. [Google Scholar] [CrossRef]
- Shin, M.; Kang, H.; Shin, K.R.; Lee, R.; Kim, K.; Min, K.; Cho, K.-N.; Sohn, E.-J.; Kim, K.S.; Kim, S.-H.; et al. Plant-expressed Zika virus envelope protein elicited protective immunity against the Zika virus in immunocompetent mice. Sci. Rep. 2023, 13, 22955. [Google Scholar] [CrossRef]
- Su, H.; van Eerde, A.; Rimstad, E.; Bock, R.; Branza-Nichita, N.; Yakovlev, I.A.; Clarke, J.L. Plant-made vaccines against viral diseases in humans and farm animals. Front. Plant Sci. 2023, 14, 1170815. [Google Scholar] [CrossRef] [PubMed]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.M.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.-Y.I.; Ly, L.H.; Marcel, S. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; Caffferkey, R.; Bowdish, K. Production of antibodies in transgenic plants. Nature 1989, 342, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kwong, A.T.; Holtz, B.R.; Erwin, R.L.; Marcel, S.; McDonald, K.A. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs 2016, 8, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Gomord, V.; Stordeur, V.; Fitchette, A.C.; Fixman, E.D.; Tropper, G.; Garnier, L.; Desgagnes, R.; Viel, S.; Couillard, J.; Beauverger, G.; et al. Design, production and immunomodulatory potency of a novel allergen bioparticle. PLoS ONE 2020, 15, e0242867. [Google Scholar] [CrossRef] [PubMed]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking b-1,2-xylose and core a-1,3-fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.; Grünwald-Gruber, C.; Vezina, L.-P.; Gomord, V.; Morel, B. A fast and easy one-step purification strategy for plant-made antibodies using Protein A magnetic beads. Front. Plant Sci. 2024, 14, 1276148. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Neto, M.; Ferreira, J.R., Jr.; Colau, D.; Fischer, H.; Nascimento, A.S.; Craievich, A.F.; Dumoutier, L.; Renauld, J.C.; Polikarpov, I. Interleukin-22 forms dimers that are recognized by two interleukin-22R1 receptor chains. Biophys. J. 2008, 94, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010, 10, 301–316. [Google Scholar] [CrossRef]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Chartrain, M.; Chu, L. Development and Production of Commercial Therapeutic Monoclonal Antibodies in Mammalian Cell Expression Systems: An Overview of the Current Upstream Technologies. Curr. Pharm. Biotechnol. 2008, 9, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Moussavou, G.; Ko, K.; Lee, J.H.; Choo, Y.K. Production of monoclonal antibodies in plants for cancer immunotherapy. BioMed Res. Int. 2015, 2015, 306164. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2022, 7, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Vézina, L.-P.; Faye, L.; Lerouge, P.; D’Aoust, M.-A.; Marquet-Blouin, E.; Burel, C.; Lavoie, P.-O.; Bardor, M.; Gomord, V. Transient co-expression for fast and high-yield production of antibodies with human-like N-glycans in plants. Plant Biotechnol. J. 2009, 7, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, C.; Nagy, N.A.; Kroon, P.Z.; Auger, L.; Desgagnés, R.; Martel, C.; Mirande, L.; Morel, B.; Roberge, J.; Stordeur, V.; et al. A novel peanut allergy immunotherapy: Plant-based enveloped Ara h 2 Bioparticles activate dendritic cells and polarize T cell responses to Th1. World Allergy Organ. J. 2023, 16, 100839. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, C.; Stigler, M.; Kirpas, M.E.; Versteeg, S.; Akkerdaas, J.H.; Pena-Castellanos, G.; Blokhuis, B.R.; Dreskin, S.C.; Auger, L.; Desgagnés, R.; et al. Plant-based enveloped Ara h 2 bioparticles display exceptional hypo-allergenicity. Clin. Exp. Allergy 2023, 53, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Busold, S.; Aglas, L.; Menage, C.; Auger, L.; Desgagnés, R.; Faye, L.; Fitchette, A.-C.; de Jong, E.C.; Martel, C.; Stigler, M.; et al. Fel d 1 surface expression on plant-made eBioparticles combines potent immune activation and hypoallergenicity. Allergy 2022, 77, 3124–3126. [Google Scholar] [CrossRef] [PubMed]
- Pogue, G.P.; Vojdani, F.; Palmer, K.E.; Hiatt, E.; Hume, S.; Phelps, J.; Long, L.; Bohorova, N.; Kim, D.; Pauly, M.; et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol. J. 2010, 8, 638–654. [Google Scholar] [CrossRef]
- Xu, J.; Ge, X.; Dolan, M.C. Towards high yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol. Adv. 2011, 29, 278–299. [Google Scholar] [CrossRef]
- Ridgley, L.A.; Falci Finardi, N.; Gengenbach, B.B.; Opdensteinen, P.; Croxford, Z.; Ma, J.K.-C.; Bodman-Smith, M.; Buyel, J.F.; Teh, A.Y.-H. Killer to cure: Expression and production costs calculation of tobacco plant-made cancer-immune checkpoint inhibitors. Plant Biotechnol. J. 2023, 21, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Walwyn, D.R.; Huddy, S.M.; Rybicki, E.P. Techno-Economic Analysis of Horseradish Peroxidase Production Using a Transient Expression System in Nicotiana benthamiana. Appl. Biochem. Biotechnol. 2015, 175, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.G. Economic aspects of commercial manufacture of biopharmaceuticals. J. Biotechnol. 2004, 113, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Gomord, V.; Fitchette, A.-C.; Menu-Bouaouiche, L.; Saint-Jore-Dupas, C.; Plasson, C.; Michaud, D.; Faye, L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 2010, 8, 564–587. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Castilho, A.; Strasser, R.; Grünwald-Gruber, C.; Altmann, F.; Strubl, S.; Galuska, C.E.; Zlatina, K.; Galuska, S.P.; Werner, S.; et al. Engineering of complex protein sialylation in plants. Proc. Natl. Acad. Sci. USA 2016, 113, 9498–9503. [Google Scholar] [CrossRef]
- Mercx, S.; Smargiasso, N.; Chaumont, F.; De Pauw, E.; Boutry, M.; Navarre, C. Inactivation of the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes in Nicotiana tabacum BY-2 Cells by a Multiplex CRISPR/Cas9 Strategy Results in Glycoproteins without Plant-Specific Glycans. Front. Plant Sci. 2017, 8, 253887. [Google Scholar] [CrossRef]
- Montero-Morales, L.; Steinkellner, H. Advanced Plant-Based Glycan Engineering. Front. Bioeng. Biotechnol. 2018, 6, 81. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morel, B.; Favrot, C.; Mirande, L.; Grünwald-Gruber, C.; Stordeur, V.; Vezina, L.P.; Faye, L.; Gomord, V. Exploring the Potentiality of a Plant Platform for Monoclonal Antibody Production in Veterinary Medicine. Vaccines 2024, 12, 620. https://doi.org/10.3390/vaccines12060620
Morel B, Favrot C, Mirande L, Grünwald-Gruber C, Stordeur V, Vezina LP, Faye L, Gomord V. Exploring the Potentiality of a Plant Platform for Monoclonal Antibody Production in Veterinary Medicine. Vaccines. 2024; 12(6):620. https://doi.org/10.3390/vaccines12060620
Chicago/Turabian StyleMorel, Bertrand, Claude Favrot, Lucie Mirande, Clemens Grünwald-Gruber, Virginie Stordeur, Louis Philippe Vezina, Loïc Faye, and Véronique Gomord. 2024. "Exploring the Potentiality of a Plant Platform for Monoclonal Antibody Production in Veterinary Medicine" Vaccines 12, no. 6: 620. https://doi.org/10.3390/vaccines12060620
APA StyleMorel, B., Favrot, C., Mirande, L., Grünwald-Gruber, C., Stordeur, V., Vezina, L. P., Faye, L., & Gomord, V. (2024). Exploring the Potentiality of a Plant Platform for Monoclonal Antibody Production in Veterinary Medicine. Vaccines, 12(6), 620. https://doi.org/10.3390/vaccines12060620




