Exploring the Impact of mRNA Modifications on Translation Efficiency and Immune Tolerance to Self-Antigens
Abstract
1. Introduction
2. Materials and Methods
2.1. Nucleotides, Antibodies, and Enzymes
2.2. Cell Culture
2.3. Plasmids
2.4. In Vitro Transcription
2.5. Agarose Gel Electrophoresis
2.6. Transfection
2.7. Cell Viability
2.8. Western Blot Analysis
2.9. Induction of Cytokines by the In Vitro Transcribed RNAs
2.10. Purification of His-Tagged WT from HEK293T Cells
2.11. Animals
2.12. Immunization
2.13. Analysis of WT1-Antibody Response by ELISA
2.14. Statistics
3. Results
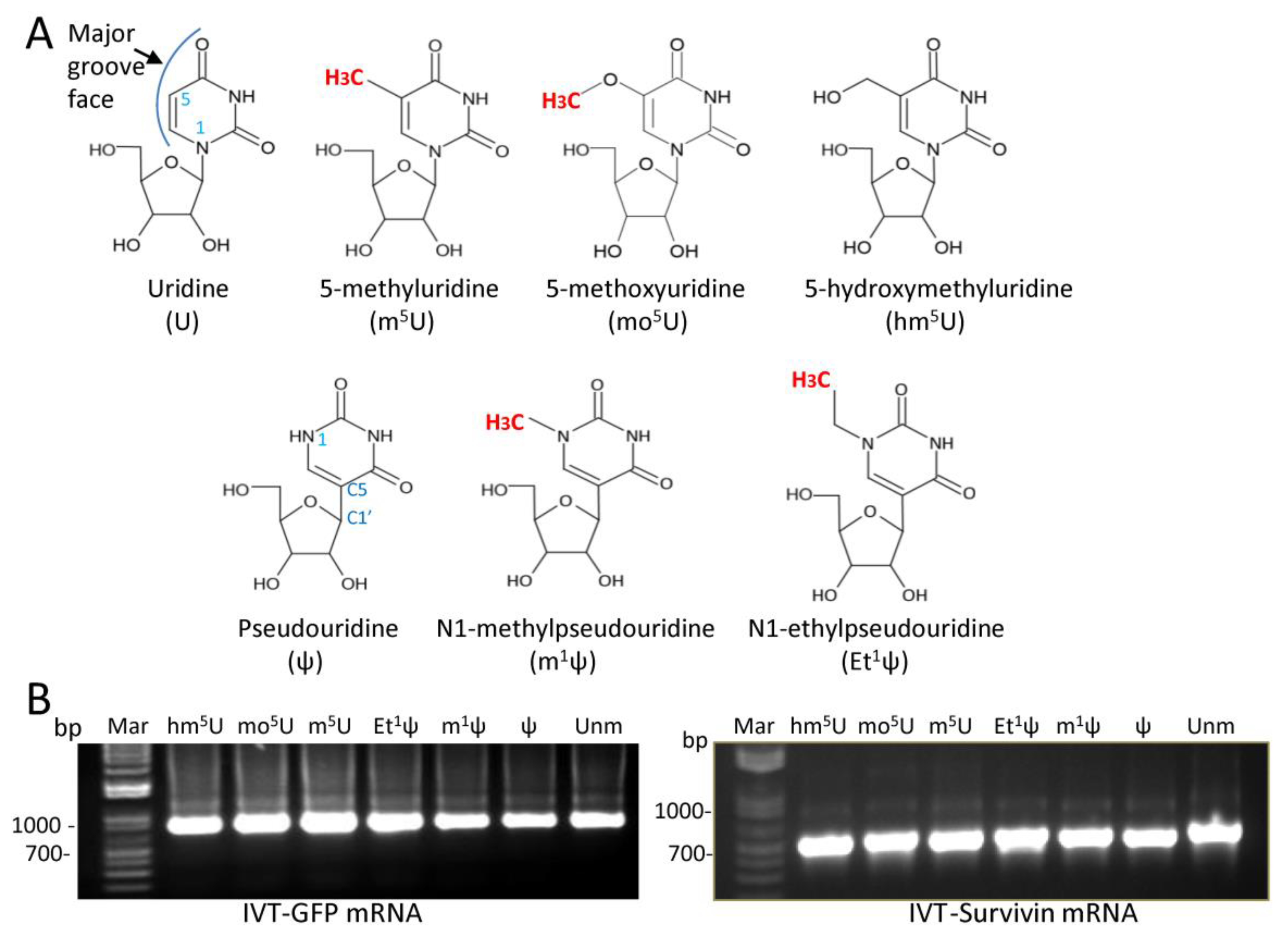
3.1. T7 RNA Polymerase Tolerates Modifications at the C5 Position of Uridine or the N1 Position of ψ
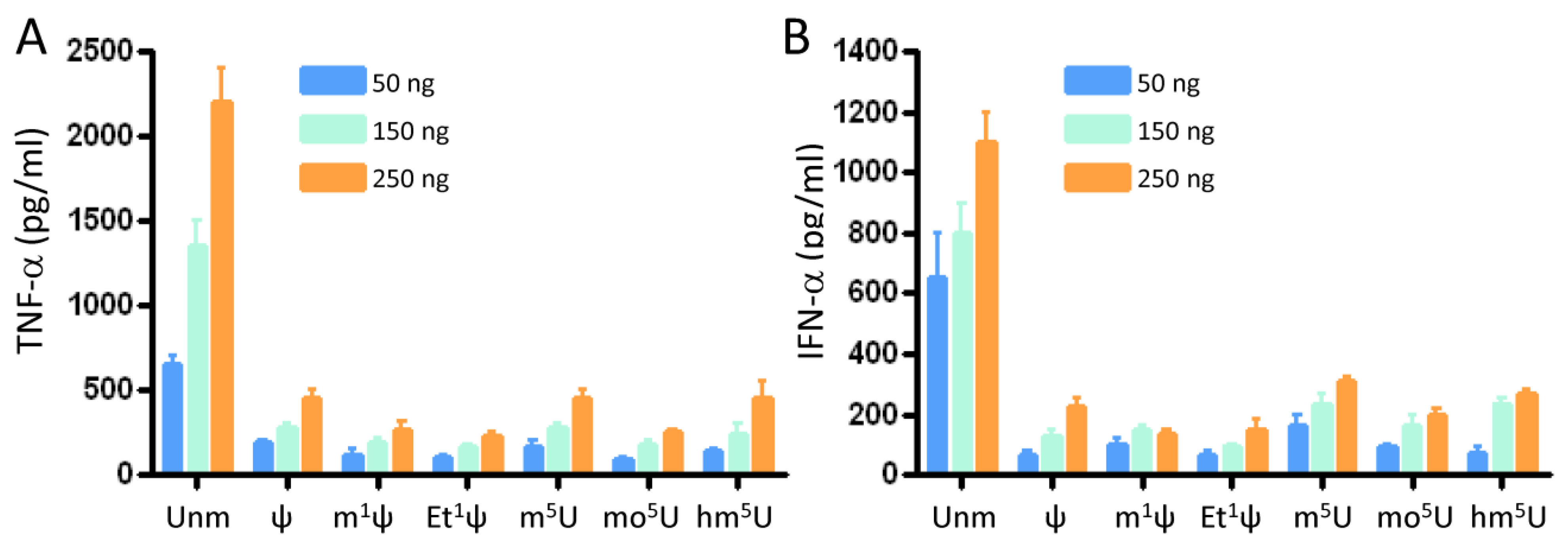
3.2. All Tested Modifications at the C5 Position of Uridine and the N1 Position of ψ Inhibited Innate Immune Activation
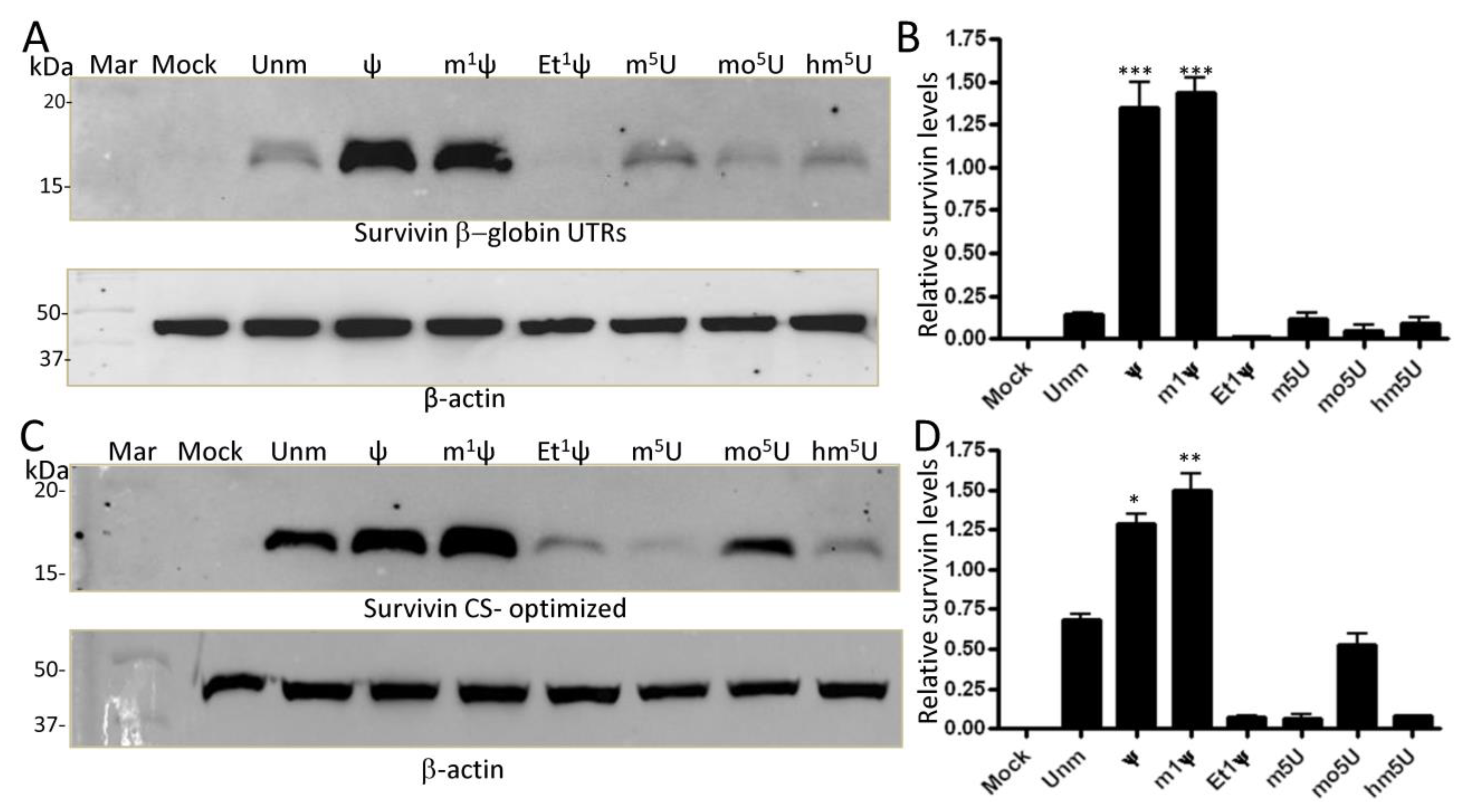
3.3. Ethylation at the N1 Position of ψ Abrogates Translation in Human Cells
3.4. Sequence Optimization Does Not Rescue the Inhibitory Effect of N1 Ethylation of ψ on Translation
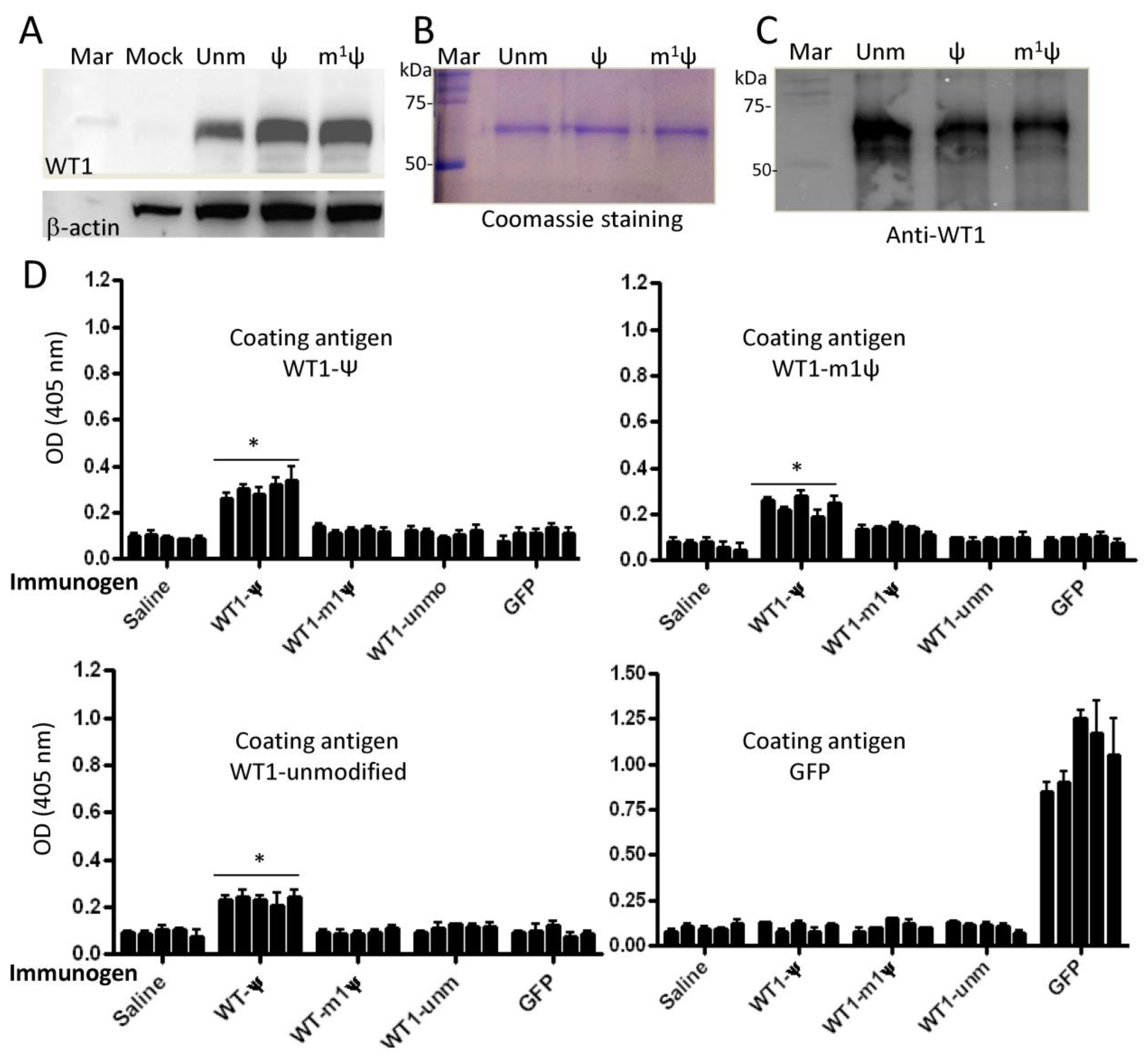
3.5. Ψ-Modified mRNA Vaccine Induces Antibody Responses to Syngeneic Wilms’ Tumor Antigen 1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| GFP | Green Fluorescent protein |
| hm5U | 5-hydroxymethyluridine |
| IVT | In vitro transcription |
| IFN-α | Interferon alpha |
| mo5U | 5-methoxyuridine |
| m5U | 5-methyluridine |
| Et1ψ | N1-ethylpseudouridine |
| m1ψ | N1-methylpseudouridine |
| ψ | Pseudouridine |
| siRNA | Small interfering RNA |
| TAAs | Tumor-associated antigens |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| U | Uridine |
| WT1 | Wilms’ Tumor Antigen-1 |
References
- Sun, H.; Zhang, Y.; Wang, G.; Yang, W.; Xu, Y. mRNA-based therapeutics in cancer treatment. Pharmaceutics 2023, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Breaker, R.R. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2‘-Hydroxyl Group. J. Am. Chem. 1999, 23, 5364–5372. [Google Scholar] [CrossRef]
- Lennox, K.A.; Behlke, M.A. Chemical Modifications in RNA Interference and CRISPR/Cas Genome Editing Reagents. Methods Mol. Biol. 2020, 2115, 23–55. [Google Scholar] [PubMed]
- Thiel, K.W.; Giangrande, P.H. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides 2009, 19, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Sørensen, D.R. A nuclease-resistant protein kinase C alpha ribozyme blocks glioma cell growth. Nat. Biotech. 1998, 16, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Leirdal, M.; Sioud, M. High cleavage activity and stability of hammerhead r ibozymes with a uniform 2’-amino pyrimidine modification. Biochem. Biophys. Res. Commun. 1998, 250, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 8, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Releasing the immune system brakes using siRNAs enhances cancer immunotherapy. Cancers 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Sørensen, D.R. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem. Biophys. Res. Commun. 2003, 312, 1220–1225. [Google Scholar] [CrossRef]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotech. 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Hornung, V.; Guenthner-Biller, M.; Bourquin, C.; Ablasser, A.; Schlee, M.; Uematsu, S.; Noronha, A.; Manoharan, M.; Akira, S.; de Fougerolles, A.; et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 2005, 11, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence dependent and requires endosomal localization. J. Mol. Biol. 2005, 348, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Longo, M.; Han, Y.; Lundberg, P.; Cantin, E.; Rossi, J.J. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat. Biotech. 2004, 22, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.K.; Schlee, M.; et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Sioud, M. On the delivery of small interfering RNAs into mammalian cells. Expert Opin. Drug Deliv. 2005, 2, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechn. 2005, 23, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: A central role for 2’-hydroxyl uridines in immune responses. Eur. J. Immunol. 2006, 36, 1222–1230. [Google Scholar] [CrossRef]
- Höfler, S.; Carlomagno, T. Structural and functional roles of 2′-O-ribose methylations and their enzymatic machinery across multiple classes of RNAs. Curr. Opin. Struc. Biol. 2020, 65, 42–50. [Google Scholar] [CrossRef]
- Meier, U.T. RNA modification in Cajal bodies. RNA Biol. 2017, 14, 693–700. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechn. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Saebøe-Larssen, S.; Fossberg, E.; Gaudernack, G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). J. Immunol. Methods 2002, 259, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Saebøe-Larssen, S.; Hetland, T.E.; Kaern, J.; Mobergslien, A.; Kvalheim, G. Silencing of indoleamine 2,3-dioxygenase enhances dendritic cell immunogenicity and antitumour immunity in cancer patients. Int. J. Oncol. 2013, 43, 280–288. [Google Scholar] [CrossRef]
- Piao, X.; Yadav, V.; Wang, E.; Chang, W.; Tau, L.; Lindenmuth, B.E.; Wang, S.X. Double-stranded RNA reduction by chaotropic agents during in vitro transcription of messenger RNA. Mol. Ther. Nucleic Acids. 2022, 29, 618–624. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef] [PubMed]
- Charette, M.; Gray, M.W. Pseudouridine in RNA: What, where, how, and why. IUBMB Life 2000, 49, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl Acad. Sci. USA 2019, 116, 24075–24240. [Google Scholar] [CrossRef]
- Mulroney, T.E.; Pöyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024, 625, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Moradian, H.; Roch, T.; Anthofer, L.; Lendlein, A.; Gossen, M. Chemical modification of uridine modulates mRNA-mediated proinflammatory and antiviral response in primary human macrophages. Mol. Ther. Nucleic Acids 2022, 27, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Thor, S.P. Survivin—A universal tumor antigen. Histol. Histopathol. 2002, 17, 669–675. [Google Scholar] [PubMed]
- Strong, T.V.; Hampton, T.A.; Louro, I.; Bilbao, G.; Conry, R.M.; Curiel, D.T. Incorporation of beta-globin untranslated regions into a Sindbis virus vector for augmentation of heterologous mRNA expression. Gene Ther. 1997, 4, 624–627. [Google Scholar] [CrossRef]
- Kirshina, A.; Vasileva, O.; Kunyk, D.; Seregina, K.; Muslimov, A.; Ivanov, R.; Reshetnikov, V. Effects of Combinations of Untranslated-Region Sequences on Translation of mRNA. Biomolecules 2023, 13, 1677. [Google Scholar] [CrossRef]
- Kim, S.C.; Sekhon, S.S.; Shin, W.R.; Ahn, G.; Cho, B.K.; Ahn, J.Y.; Kim, Y.H. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell. Toxicol. 2022, 18, 1–8. [Google Scholar] [CrossRef]
- Agris, P.F.; Narendran, A.; Sarachan, K.; Väre, V.Y.P.; Eruysal, E. The importance of being modified: The role of RNA modifications in translational fidelity. Enzymes 2017, 41, 1–50. [Google Scholar] [PubMed]
- Motorin, Y.; Helm, M. tRNA stabilization by modified nucleotides. Biochemistry 2010, 49, 4934–4944. [Google Scholar] [CrossRef] [PubMed]
- Väre, V.Y.; Eruysal, E.R.; Narendran, A.; Sarachan, K.L.; Agris, P.F. Chemical and conformational diversity of modified nucleosides Affects tRNA structure and function. Biomolecules 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Ueda, T.; Watanabe, K. The presence of pseudouridine in the anticodon alters the genetic code: A possible mechanism for assignment of the AAA lysine codon as asparagine in echinoderm mitochondria. Nucleic Acids Res. 1999, 27, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Karijolich, J.; Yu, Y.T. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 2011, 474, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Eyler, D.E.; Franco, M.K.; Batool, Z.; Wu, M.Z.; Dubuke, M.L.; Dobosz-Bartoszek, M.; Jones, J.D.; Polikanov, Y.S.; Roy, B.; Koutmou, K.S. Pseudouridinylation of mRNA coding sequences alters translation. Proc. Natl Acad. Sci. USA 2019, 116, 23068–23074. [Google Scholar] [CrossRef] [PubMed]
- Lhuillier, C.; Rudqvist, N.P.; Elemento, O.; Formenti, S.C.; Demaria, S. Radiation therapy and anti-tumor immunity: Exposing immunogenic mutations to the immune system. Genome Med. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H. WT1 (Wilms’ tumor gene 1): Biology and cancer immunotherapy. Jpn. J. Clin. Oncol. 2010, 40, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sæbøe-Larssen, S.; Sioud, M. Improving dendritic cell cancer vaccine potency using RNA interference. Methods Mol. Biol. 2020, 2115, 249–258. [Google Scholar]
- Melamed, J.R.; Hajj, K.A.; Chaudhary, N.; Strelkova, D.; Arral, M.L.; Pardi, N.; Alameh, M.G.; Miller, J.B.; Farbiak, L.; Siegwart, D.J.; et al. Lipid nanoparticle chemistry determines how nucleoside base modifications alter mRNA delivery. J. Control. Release 2022, 341, 206–214. [Google Scholar] [CrossRef]
- Hall, K.B.; McLaughlin, L.W. Properties of pseudouridine N1 imino protons located in the major groove of an A-form RNA duplex. Nucleic Acids Res. 1992, 20, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.R.; Veltri, C.A.; Nielsen, L. An RNA model system for investigation of pseudouridine stabilization of the codon-anticodon interaction in tRNALys, tRNAHis and tRNATyr. J. Biomol. Struct. Dyn. 1998, 15, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Kierzek, E.; Malgowska, M.; Lisowiec, J.; Turner, D.H.; Gdaniec, Z.; Kierzek, R. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res. 2014, 42, 3492–3501. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol. Med. 2006, 12, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kagra, D.; Mahmi, A.S.; Kumar, N.V.S.; Prabhakar, P.S.; Sharma, P. Influence of the number, nature and position of methyl posttranscriptional modifications on nucleobase stacking in RNA. ChemPhysChemistry 2021, 22, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Yarian, C.S.; Basti, M.M.; Cain, R.J.; Ansari, G.; Guenther, R.H.; Sochacka, E.; Czerwinska, G.; Malkiewicz, A.; Agris, P.F. Structural and functional roles of the N1- and N3-protons of psi at tRNA’s position 39. Nucleic Acids Res. 1999, 27, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Sowers, L.C.; Shaw, B.R.; Sedwick, W.D. Base stacking and molecular polarizability: Effect of a methyl group in the 5-position of pyrimidines. Biochem. Biophys. Res. Commun. 1987, 148, 790–794. [Google Scholar] [CrossRef]
- Dutta, N.; Deb, I.; Sarzynska, J.; Lahiri, A. Structural and thermodynamic consequences of base pairs containing pseudouridine and N1-methylpseudouridine in RNA duplexes. BioRxiv 2023. [Google Scholar] [CrossRef]
- Svitkin, Y.V.; Gingras, A.C.; Sonenberg, N. Membrane-dependent relief of translation elongation arrest on pseudouridine- and N1-methyl-pseudouridine-modified mRNAs. Nucleic Acids Res. 2022, 50, 7202–7215. [Google Scholar] [CrossRef]
- Agris, P.F.; Eruysal, E.R.; Narendran, A.; Väre, V.Y.P.; Vangaveti, S.; Ranganathan, S.V. Celebrating wobble decoding: Half a century and still much is new. RNA Biol. 2018, 15, 537–553. [Google Scholar] [CrossRef]
- Kim, K.Q.; Burgute, B.D.; Tzeng, S.C.; Jing, C.; Jungers, C.; Zhang, J.; Yan, L.L.; Vierstra, R.D.; Djuranovic, S.; Evans, B.S.; et al. N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Rep. 2022, 40, 111300. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Potapov, V.; Dai, N.; Ong, J.L.; Roy, B. N1-methyl-pseudouridine is incorporated with higher fidelity than pseudouridine in synthetic RNAs. Sci. Rep. 2022, 12, 13017. [Google Scholar] [CrossRef] [PubMed]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Boon, T.; Kellermann, O. Rejection by syngeneic mice of cell variants obtained by mutagenesis of a malignant teratocarcinoma cell line. Proc. Natl. Acad. Sci. USA 1977, 74, 272–275. [Google Scholar] [CrossRef]
- Boon, T.; Van Pel, A. Teratocarcinoma cell variants rejected by syngeneic mice: Protection of mice immunized with these variants against other variants and against the original malignant cell line. Proc. Natl. Acad. Sci USA 1978, 75, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Engelhorn, M.E.; Guevara-Patiño, J.A.; Noffz, G.; Hooper, A.T.; Lou, O.; Gold, J.S.; Kappel, B.J.; Houghton, A.N. Autoimmunity and tumor immunity induced by immune responses to mutations in self. Nat. Med. 2006, 12, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Patiño, J.A.; Turk, M.J.; Wolchok, J.D.; Houghton, A.N. Immunity to cancer through immune recognition of altered self: Studies with melanoma. Adv Cancer Res. 2003, 90, 157–177. [Google Scholar] [PubMed]
- Guevara-Patiño, J.A.; Engelhorn, M.E.; Turk, M.J.; Liu, C.; Duan, F.; Rizzuto, G.; Cohen, A.D.; Merghoub, T.; Wolchok, J.D.; Houghton, A.N. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J. Clin. Investig. 2006, 116, 1382–1390. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.P. OAS proteins and cGAS: Unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 2014, 14, 521–528. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Jha, B.K.; Silverman, R.H.; Weissman, D.; Karikó, K. Nucleoside modifications in RNA limit activation of 2’-5’-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011, 39, 9329–9338. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sioud, M.; Juzeniene, A.; Sæbøe-Larssen, S. Exploring the Impact of mRNA Modifications on Translation Efficiency and Immune Tolerance to Self-Antigens. Vaccines 2024, 12, 624. https://doi.org/10.3390/vaccines12060624
Sioud M, Juzeniene A, Sæbøe-Larssen S. Exploring the Impact of mRNA Modifications on Translation Efficiency and Immune Tolerance to Self-Antigens. Vaccines. 2024; 12(6):624. https://doi.org/10.3390/vaccines12060624
Chicago/Turabian StyleSioud, Mouldy, Asta Juzeniene, and Stein Sæbøe-Larssen. 2024. "Exploring the Impact of mRNA Modifications on Translation Efficiency and Immune Tolerance to Self-Antigens" Vaccines 12, no. 6: 624. https://doi.org/10.3390/vaccines12060624
APA StyleSioud, M., Juzeniene, A., & Sæbøe-Larssen, S. (2024). Exploring the Impact of mRNA Modifications on Translation Efficiency and Immune Tolerance to Self-Antigens. Vaccines, 12(6), 624. https://doi.org/10.3390/vaccines12060624







