Retrospective, Observational Analysis on the Impact of SARS-CoV-2 Variant Omicron in Hospitalized Immunocompromised Patients in a German Hospital Network—The VISAGE Study
Abstract
1. Introduction
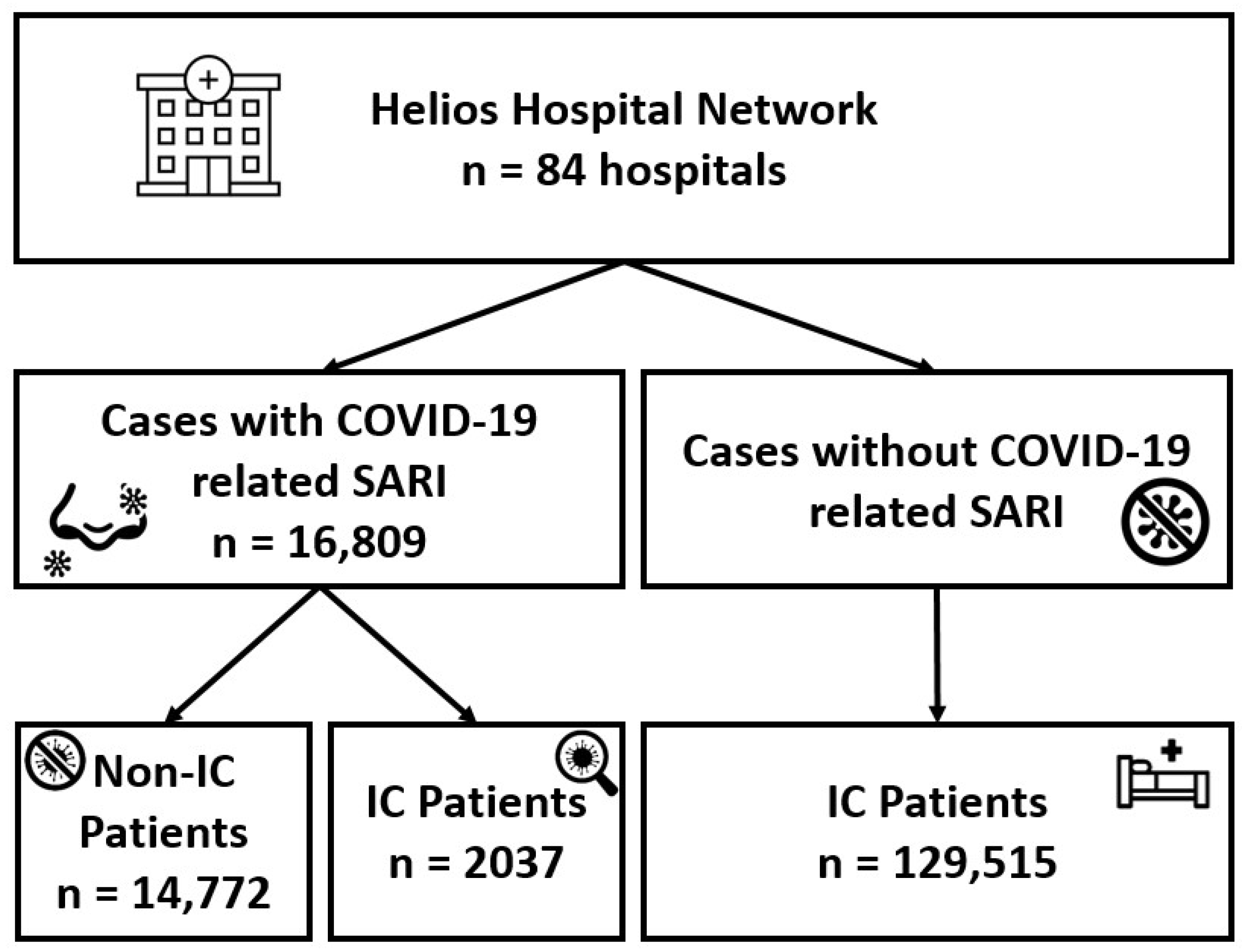
2. Methods
3. Results
3.1. Baseline Characteristics of Patients with COVID-19-Related SARI, with and without IC
3.2. In-Hospital Outcome for Patients with COVID-19-Related SARI with and without IC
3.3. Baseline Characteristics of Hospitalized IC Patients, with and without COVID-19-Related SARI
3.4. In-Hospital Outcome for IC Patients with and without COVID-19-Related SARI
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence intervals |
| COVID-19 | coronavirus disease 2019 |
| GLMM | generalized linear mixed models |
| IC | immunocompromised |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems |
| ICU | intensive care unit |
| LMM | linear mixed models |
| Non IC | non-immunocompromised |
| OPS | operations and procedures (German adaption) |
| OR | odds ratio |
| SARI | severe acute respiratory infection |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus type 2 |
References
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: Insights from the observational population-based INFORM study. Lancet Reg. Health Eur. 2023, 35, 100747. [Google Scholar] [CrossRef] [PubMed]
- Flahault, A.; Calmy, A.; Costagliola, D.; Drapkina, O.; Eckerle, I.; Larson, H.J.; Legido-Quigley, H.; Noakes, C.; Kazatchkine, M.; Kluge, H. No time for complacency on COVID-19 in Europe. Lancet 2023, 401, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Jank, M.; Oechsle, A.-L.; Armann, J.; Behrends, U.; Berner, R.; Chao, C.-M.; Diffloth, N.; Doenhardt, M.; Hansen, G.; Hufnagel, M.; et al. Comparing SARS-CoV-2 variants among children and adolescents in Germany: Relative risk of COVID-19-related hospitalization, ICU admission and mortality. Infection 2023, 51, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.; Hohenstein, S.; Kodde, C.; Leiner, J.; Schwegmann, K.; Bollmann, A.; Möller, R.; Kuhlen, R.; Nachtigall, I. Burden of hospital-acquired SARS-CoV-2 infections in Germany: Occurrence and outcomes of different variants. J. Hosp. Infect. 2022, 129, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sakuramoto, K.; Wada, D.; Maruyama, S.; Muroya, T.; Saito, F.; Nakamori, Y.; Kuwagata, Y. Evaluation of characteristics and prognosis of COVID-19 patients requiring invasive mechanical ventilation during dominance of nonvariant, alpha, delta, and omicron variants in tertiary hospitals of Japan. BMC Infect. Dis. 2024, 24, 223. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Digitales Impfquoten-Monitoring COVID-19. Available online: http://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquotenmonitoring.xlsx?__blob=publicationFile (accessed on 4 January 2024).
- Bahremand, T.; Yao, J.A.; Mill, C.; Piszczek, J.; Grant, J.M.; Smolina, K. COVID-19 hospitalisations in immunocompromised individuals in the Omicron era: A population-based observational study using surveillance data in British Columbia, Canada. Lancet Reg. Health Am. 2023, 20, 100461. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, S.; Laracy, J.C.; Perales, M.-A.; Kamboj, M.; van den Brink, M.R.M.; Vardhana, S. SARS-CoV-2 in immunocompromised individuals. Immunity 2022, 55, 1779–1798. [Google Scholar] [CrossRef]
- Shoham, S.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Hassanain, M.; Hotez, P.; Kang, G.; Kim, J.H.; Lall, B.; Larson, H.J.; et al. Vaccines and therapeutics for immunocompromised patients with COVID-19. EClinicalMedicine 2023, 59, 101965. [Google Scholar] [CrossRef]
- Ketkar, A.; Willey, V.; Pollack, M.; Glasser, L.; Dobie, C.; Wenziger, C.; Teng, C.-C.; Dube, C.; Cunningham, D.; Verduzco-Gutierrez, M. Assessing the risk and costs of COVID-19 in immunocompromised populations in a large United States commercial insurance health plan: The EPOCH-US Study. Curr. Med. Res. Opin. 2023, 39, 1103–1118. [Google Scholar] [CrossRef]
- Lübbert, C.; Dykukha, I.; Pelz, J.-P.; Yearley, H.; Junker, W.; Gruber, N.; Escher, S.; Biereth, K.; Melnik, S.; Puschmann, J. Individuals at risk for severe COVID-19 in whom ritonavir-containing therapies are contraindicated or may lead to interactions with concomitant medications: A retrospective analysis of German health insurance claims data. Drugs Context 2023, 12. [Google Scholar] [CrossRef]
- Obeid, M.; Suffiotti, M.; Pellaton, C.; Bouchaab, H.; Cairoli, A.; Salvadé, V.; Stevenel, C.; Hottinger, R.; Pythoud, C.; Coutechier, L.; et al. Humoral Responses Against Variants of Concern by COVID-19 mRNA Vaccines in Immunocompromised Patients. JAMA Oncol. 2022, 8, e220446. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Morath, C.; Bartenschlager, M.; Kim, H.; Reineke, M.; Beimler, J.; Buylaert, M.; Nusshag, C.; Kälble, F.; Reichel, P.; et al. Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am. J. Transplant 2022, 22, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.S.; Ogun, O.A.; Simmons, S.R.; Zamparo, J.M.; et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: A retrospective cohort study. Lancet Reg. Health Am. 2022, 9, 100198. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, E.; Chandak, A.; Gottlieb, R.L.; Chima-Melton, C.; Read, S.H.; Jiang, H.; Chiang, M.; Lee, E.; Gupta, R.; Berry, M.; et al. Remdesivir Reduced Mortality in Immunocompromised Patients Hospitalized for COVID-19 Across Variant Waves: Findings from Routine Clinical Practice. Clin. Infect. Dis. 2023, 77, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Bausch-Jurken, M. The Burden of COVID-19 in the Immunocompromised Patient: Implications for Vaccination and Needs for the Future. J. Infect. Dis. 2023, 228, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut. Epidemiologisches Bulletin: Aktuelle Daten und Informationen zu Infektionserkrankungen und Public Health. Available online: https://www.openagrar.de/receive/zimport_mods_00003614 (accessed on 14 February 2024).
- Häckl, D.; Pignot, M.; Dang, P.L.; Lauenroth, V.; Jah, F.; Wendtner, C.-M. Klinische Verläufe und Kosten für Hospitalisierungen von COVID-19-Patienten mit potenziell eingeschränktem Immunsystem in Deutschland. Dtsch. Med. Wochenschr. 2024, 149, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Federal Institute for Drugs and Medical Devices. ICD-10-GM: International Statistical Classification of Diseases, German Modification. Available online: https://www.bfarm.de/EN/Code-systems/Classifications/ICD/ICD-10-GM/_node.html (accessed on 5 February 2024).
- Moore, B.J.; White, S.; Washington, R.; Coenen, N.; Elixhauser, A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med. Care 2017, 55, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, A. Comorbidity: An R package for computing comorbidity scores. JOSS 2018, 3, 648. [Google Scholar] [CrossRef]
- Buda, S.; Tolksdorf, K.; Schuler, E.; Kuhlen, R.; Haas, W. Establishing an ICD-10 code based SARI-surveillance in Germany—Description of the system and first results from five recent influenza seasons. BMC Public Health 2017, 17, 612. [Google Scholar] [CrossRef]
- Leiner, J.; Hohenstein, S.; Pellissier, V.; König, S.; Winklmair, C.; Nachtigall, I.; Bollmann, A.; Kuhlen, R. COVID-19 and Severe Acute Respiratory Infections: Monitoring Trends in 421 German Hospitals During the First Four Pandemic Waves. Infect. Drug Resist. 2023, 16, 2775–2781. [Google Scholar] [CrossRef]
- UK Department of Health & Social Care. Defining the Highest-Risk Clinical Subgroups upon Community Infection with SARS-CoV-2 When Considering the Use of Neutralising Monoclonal Antibodies (nMABs) and Antiviral Drugs: Independent Advisory Group Report. Available online: https://www.gov.uk/government/publications/higher-risk-patients-eligible-for-covid-19-treatments-independent-advisory-group-report/defining-the-highest-risk-clinical-subgroups-upon-community-infection-with-sars-cov-2-when-considering-the-use-of-neutralising-monoclonal-antibodies (accessed on 15 December 2023).
- Federal Institute for Drugs and Medical Devices. German Adaptation of the International Classification of the Procedures in Medicine of the World Health Organization, Version. Available online: https://www.bfarm.de/SharedDocs/Downloads/DE/Kodiersysteme/klassifikationen/ops/vorgaenger/ops2022_zip.html?nn=841246&cms_dlConfirm=true&cms_calledFromDoc=841246 (accessed on 5 February 2022).
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ketkar, A.; Willey, V.; Glasser, L.; Dobie, C.; Wenziger, C.; Teng, C.-C.; Dube, C.; Hirpara, S.; Cunningham, D.; Verduzco-Gutierrez, M. Assessing the Burden and Cost of COVID-19 across Variants in Commercially Insured Immunocompromised Populations in the United States: Updated Results and Trends from the Ongoing EPOCH-US Study. Adv. Ther. 2024, 41, 1075–1102. [Google Scholar] [CrossRef] [PubMed]
- Nevejan, L.; Ombelet, S.; Laenen, L.; Keyaerts, E.; Demuyser, T.; Seyler, L.; Soetens, O.; van Nedervelde, E.; Naesens, R.; Geysels, D.; et al. Severity of COVID-19 among Hospitalized Patients: Omicron Remains a Severe Threat for Immunocompromised Hosts. Viruses 2022, 14, 2736. [Google Scholar] [CrossRef] [PubMed]
- Singson, J.R.C.; Kirley, P.D.; Pham, H.; Rothrock, G.; Armistead, I.; Meek, J.; Anderson, E.J.; Reeg, L.; Lynfield, R.; Ropp, S.; et al. Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19-COVID-NET, 10 States, March 2020-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.; Nevitt, S.; Madsen, W.R.; Turtle, L.; Davies, G.; Palmieri, C. Differences in Outcomes and Factors Associated with Mortality Among Patients with SARS-CoV-2 Infection and Cancer Compared with Those Without Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2210880. [Google Scholar] [CrossRef] [PubMed]
- Vujčić, I. Outcomes of COVID-19 among patients with liver disease. World J. Gastroenterol. 2023, 29, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Aleshina, O.A.; Zakurdaeva, K.; Vasileva, A.N.; Dubov, S.K.; Dubov, V.S.; Vorobyev, V.I.; Butaev, L.S.; Sukhareva, A.M.; Gavrilova, L.V.; Toropova, I.Y.; et al. Clinical Outcomes in Patients With COVID-19 and Hematologic Disease. Clin. Lymphoma Myeloma Leuk. 2023, 23, 589–598. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Y.; Liu, Y.; Fan, C.; Jiang, Y. Baseline immune status and the effectiveness of response to enteral nutrition among ICU patients with COVID-19: An observational, retrospective study. Nutrition 2024, 122, 112387. [Google Scholar] [CrossRef]
- Turtle, L.; Thorpe, M.; Drake, T.M.; Swets, M.; Palmieri, C.; Russell, C.D.; Ho, A.; Aston, S.; Wootton, D.G.; Richter, A.; et al. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023, 20, e1004086. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.; Hohenstein, S.; Kodde, C.; Leiner, J.; Schwegmann, K.; Bollmann, A.; Möller, R.; Kuhlen, R.; Nachtigall, I. The Disease Course of Hospitalized COVID-19 Patients During the Delta and Omicron Periods in Consideration of Vaccination Status. Dtsch. Arztebl. Int. 2022, 119, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Scott, J.; Richterman, A.; Male, V.; Cevik, M. Clinical course and management of COVID-19 in the era of widespread population immunity. Nat. Rev. Microbiol. 2023, 22, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Embi, P.J.; Levy, M.E.; Naleway, A.L.; Patel, P.; Gaglani, M.; Natarajan, K.; Dascomb, K.; Ong, T.C.; Klein, N.P.; Liao, I.-C.; et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults—Nine States, January-September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Soeroto, A.Y.; Yanto, T.A.; Kurniawan, A.; Hariyanto, T.I. Efficacy and safety of tixagevimab-cilgavimab as pre-exposure prophylaxis for COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2420. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, Q.; Ren, J.; Guo, W.; Feng, K.; Li, Z.; Huang, T.; Cai, Y.-D. Immune responses of different COVID-19 vaccination strategies by analyzing single-cell RNA sequencing data from multiple tissues using machine learning methods. Front. Genet. 2023, 14, 1157305. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-X.; Gao, Q.; Zhou, X.-C.; Chen, L.; Guo, W.; Feng, K.-Y.; Lu, L.; Huang, T.; Cai, Y.-D. Identification of Gene Markers Associated with COVID-19 Severity and Recovery in Different Immune Cell Subtypes. Biology 2023, 12, 947. [Google Scholar] [CrossRef]
- Haidar, G.; Hodges, J.C.; Bilderback, A.; Lukanski, A.; Linstrum, K.; Postol, B.; Troyan, R.; Wisniewski, M.K.; Coughenour, L.; Heaps, A.; et al. Prospective Assessment of Humoral and Cellular Immune Responses to a Third COVID-19 mRNA Vaccine Dose Among Immunocompromised Individuals. J. Infect. Dis. 2024, 229, 1328–1340. [Google Scholar] [CrossRef]
- Wang, X.; Haeussler, K.; Spellman, A.; Phillips, L.E.; Ramiller, A.; Bausch-Jurken, M.T.; Sharma, P.; Krivelyova, A.; Vats, S.; van de Velde, N. Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines in immunocompromised individuals: A systematic review and meta-analysis using the GRADE framework. Front. Immunol. 2023, 14, 1204831. [Google Scholar] [CrossRef]
- Kluge, S. S3-Leitlinie—Empfehlungen zur Therapie von Patienten mit COVID-19. 2024. Available online: https://register.awmf.org/assets/guidelines/113-001l_S3_Empfehlungen-zur-Therapie-von-Patienten-mit-COVID-19_2024-01.pdf (accessed on 14 April 2024).
- Robert-Koch-Institut. Deutscher Elektronischer Sequenzdaten-Hub (DESH). Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/DESH/Schaubild-Phasen-1-und-2.pdf?__blob=publicationFile (accessed on 14 April 2022).

| Characteristic 1 | Non-IC COVID-19-Related SARICOVID-19-Related SARI | IC COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | Solid Tumors with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | Hematological Diseases with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | Solid Organ Transplants with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | End-Stage Renal Disease with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | End-Stage Chronic Liver Diseases with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | HIV with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | Autoimmune Diseases with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 | Congenital Immunodeficiency with COVID-19-Related SARICOVID-19-Related SARI | p-Value 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case numbers | N = 14,772 | N = 2037 | N = 1000 | N = 133 | N = 138 | N = 27 | N = 243 | N = 7 | N = 494 | N = 347 | |||||||||
| Age (years) | 73.1 (19.7) | 71.2 (13.1) | <0.001 | 73.6 (11.1) | 0.182 | 70.0 (13.6) | 0.010 | 61.6 (13.1) | <0.001 | 74.1 (11.5) | 0.665 | 67.4 (12.3) | <0.001 | 46.6 (6.3) | <0.001 | 71.3 (14.5) | 0.007 | 66.9 (15.1) | <0.001 |
| Age group | <0.001 | <0.001 | <0.001 | <0.001 | 0.695 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| ≤59 years | 2310 (16%) | 360 (18%) | 106 (11%) | 25 (19%) | 52 (38%) | 4 (15%) | 66 (27%) | 7 (100%) | 99 (20%) | 98 (28%) | |||||||||
| 60−69 years | 1797 (12%) | 470 (23%) | 231 (23%) | 34 (26%) | 51 (37%) | 5 (19%) | 64 (26%) | 0 (0%) | 96 (19%) | 83 (24%) | |||||||||
| 70−79 years | 3356 (23%) | 574 (28%) | 309 (31%) | 39 (29%) | 26 (19%) | 7 (26%) | 68 (28%) | 0 (0%) | 124 (25%) | 92 (27%) | |||||||||
| ≥80 years | 7309 (49%) | 633 (31%) | 354 (35%) | 35 (26%) | 9 (6.5%) | 11 (41%) | 45 (19%) | 0 (0%) | 175 (35%) | 74 (21%) | |||||||||
| Sex | 0.015 | <0.001 | 0.004 | 0.193 | 1.000 | <0.001 | 0.238 | <0.001 | 0.643 | ||||||||||
| Male | 8337 (56%) | 1208 (59%) | 644 (64%) | 92 (69%) | 86 (62%) | 15 (56%) | 169 (70%) | 6 (86%) | 208 (42%) | 191 (55%) | |||||||||
| Female | 6435 (44%) | 829 (41%) | 356 (36%) | 41 (31%) | 52 (38%) | 12 (44%) | 74 (30%) | 1 (14%) | 286 (58%) | 156 (45%) | |||||||||
| Elixhauser comorbidity score | 12.1 (10.4) | 21.4 (13.5) | <0.001 | 27.2 (13.0) | <0.001 | 18.5 (13.2) | <0.001 | 15.1 (11.1) | 0.002 | 17.6 (10.0) | 0.009 | 24.5 (12.3) | <0.001 | 14.4 (12.1) | 0.632 | 13.6 (10.8) | 0.002 | 15.8 (12.8) | <0.001 |
| Non-IC with COVID-19-Related SARI, N = 14,772 | IC with COVID-19-Related SARI, N = 2037 | Odds Ratio [95% CI] | p-Value | Solid Tumors with COVID-19-Related SARI, N = 1000 | Odds Ratio [95% CI] | p-Value | Hematological Diseases with COVID-19-Related SARI, N = 133 | Odds Ratio [95% CI] | p-Value | Solid Organ Transplants with COVID-19-Related SARI, N = 138 | Odds Ratio [95% CI] | p-Value | End-Stage Renal Disease with COVID-19-Related SARI, N = 27 | Odds Ratio [95% CI] | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
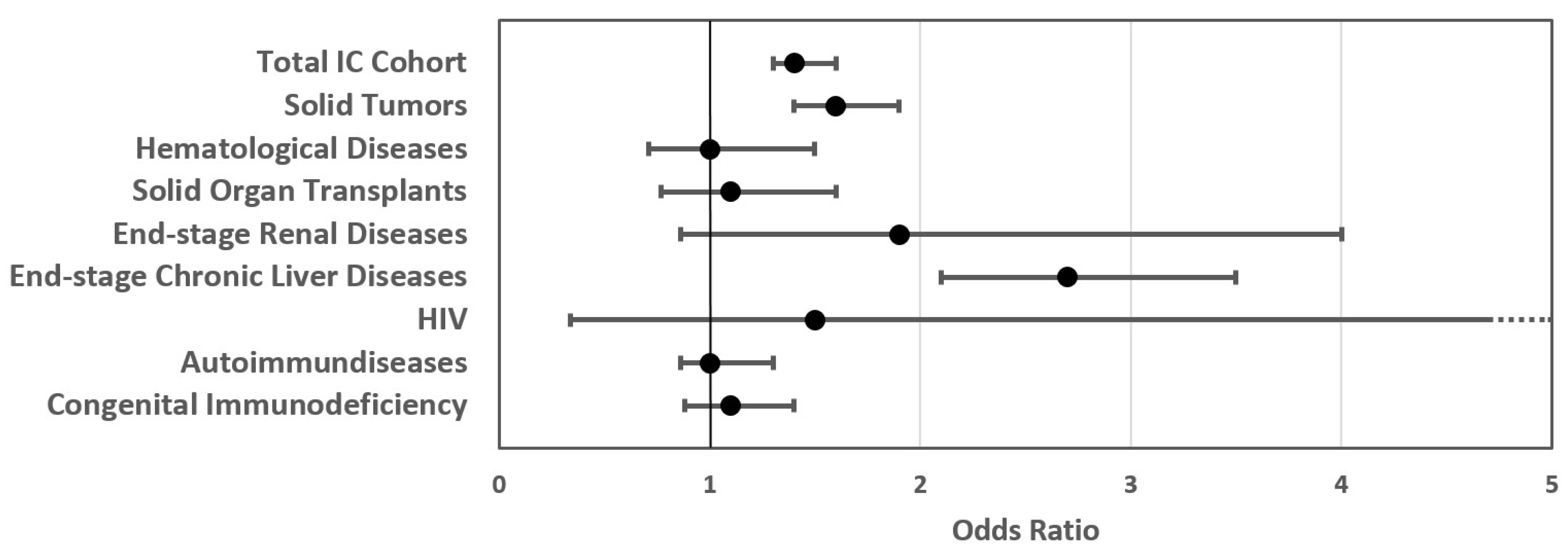
| Intensive care (n (%)) | 3273 (22%) | 577 (28%) | 1.3 [1.2–1.4] | <0.001 | 249 (25%) | 1.1 [0.9–1.3] | 0.272 | 35 (26%) | 1.0 [0.7–1.5] | 0.910 | 44 (32%) | 1.5 [1.0–2.2] | 0.035 | 9 (33%) | 1.5 [0.7–3.5] | 0.321 |
| Mechanical ventilation (n (%)) | 1984 (13%) | 336 (16%) | 1.2 [1.0–1.3] | 0.020 | 145 (15%) | 1.0 [0.8–1.2] | 0.979 | 24 (18%) | 1.1 [0.7–1.7] | 0.721 | 26 (19%) | 1.4 [0.9–2.1] | 0.178 | 7 (26%) | 1.7 [0.7–4.1] | 0.243 |
| Severe course (n (%)) | 4693 (32%) | 840 (42%) | 1.4 [1.3–1.6] | <0.001 | 439 (45%) | 1.6 [1.4–1.9] | <0.001 | 50 (38%) | 1.0 [0.7–1.5] | 0.933 | 47 (37%) | 1.1 [0.8–1.6] | 0.582 | 14 (52%) | 1.9 [0.9–4.0] | 0.114 |
| N/A | 312 | 50 | 27 | 1 | 10 | 0 | ||||||||||
| In-hospital mortality (n (%)) | 2331 (17%) | 458 (24%) | 1.5 [1.4–1.7] | <0.001 | 275 (29%) | 2.0 [1.7–2.3] | <0.001 | 33 (25%) | 1.5 [1.0–2.2] | 0.059 | 17 (15%) | 0.8 [0.5–1.4] | 0.446 | 6 (24%) | 1.3 [0.5–3.4] | 0.536 |
| N/A | 693 | 120 | 52 | 3 | 21 | 2 | ||||||||||
| Length of stay (d) | 11.2 (12.6) | 15.8 (15.3) | <0.001 | 17.0 (16.0) | <0.001 | 19.8 (17.1) | <0.001 | 11.8 (12.2) | 0.632 | 18.3 (17.1) | 0.028 | |||||
| Costs (€) | 8318.8 (14,958.9) | 12,377.7 (18,772.4) | <0.001 | 12,611.9 (19,733.9) | <0.001 | 17,857.8 (20,548.9) | <0.001 | 11,232.6 (13,757.8) | <0.001 | 16,688.0 (30,440.2) | 0.007 | |||||
| End-Stage Chronic Liver Disease with COVID-19-related SARI, N = 243 | Odds Ratio [95% CI] | p-Value | HIV with COVID-19-related SARI, N = 7 | Odds Ratio [95% CI] | p-Value | Autoimmune Disease with COVID-19-related SARI, N = 494 | Odds Ratio [95% CI] | p-Value | Congenital Immunodeficiency with COVID-19-related SARI, N = 347 | Odds Ratio [95% CI] | p-Value | |||||
| 103 (42%) | 2.5 [1.9–3.3] | <0.001 | 3 (43%) | 2.8 [0.6–13.0] | 0.193 | 132 (27%) | 1.3 [1.0–1.5] | 0.031 | 94 (27%) | 1.2 [1.0–1.6] | 0.085 | |||||
| 53 (22%) | 1.7 [1.2–2.3] | 0.002 | 2 (29%) | 2.7 [0.5–15.0] | 0.253 | 79 (16%) | 1.2 [0.9–1.5] | 0.179 | 59 (17%) | 1.3 [0.9–1.7] | 0.129 | |||||
| 137 (57%) | 2.7 [2.1–3.5] | <0.001 | 3 (43%) | 1.5 [0.3–7] | 0.575 | 164 (34%) | 1.0 [0.9–1.3] | 0.695 | 120 (36%) | 1.1 [0.9–1.4] | 0.389 | |||||
| 4 | 0 | 7 | 9 | |||||||||||||
| 76 (33%) | 2.5 [1.9–3.3] | <0.001 | 1 (14%) | 0.8 [0.1–6.5] | 0.820 | 71 (15%) | 0.9 [0.7–1.1] | 0.327 | 58 (18%) | 1.1 [0.8–1.4] | 0.639 | |||||
| 16 | 0 | 24 | 20 | |||||||||||||
| 19.5 (18.2) | <0.001 | 19.3 (18.0) | 0.072 | 12.7 (12.9) | <0.001 | 13.3 (12.8) | <0.001 | |||||||||
| 15,673.2 (20,015.1) | <0.001 | 19,703.3 (22,122.9) | 0.014 | 9835.0 (16,659.7) | <0.001 | 11,488.5 (18,718.4) | <0.001 | |||||||||
| Characteristic 1 | IC, N = 129,515 | IC with COVID-19-Related SARI, N = 2037 | p-Value 2 | Solid Tumors, N = 92,376 | Solid Tumors with COVID-19-Related SARI, N = 10,001 | p-Value 2 | Hematological Diseases, N = 5217 | Hematological Diseases with COVID-19-Related SARI, N = 133 | p-Value2 | Solid Organ Transplants, N = 1842 | Solid Organ Transplants with COVID-19-Related SARI, N = 138 | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 66.3 (15.1) | 71.2 (13.1) | <0.001 | 67.9 (13.7) | 73.6 (11.1) | <0.001 | 56.3 (24.9) | 70.0 (13.6) | <0.001 | 59.5 (14.9) | 61.6 (13.1) | 0.076 | ||||
| Age group | <0.001 | <0.001 | <0.001 | 0.768 | ||||||||||||
| ≤59 years | 35,067 (27%) | 360 (18%) | 21,316 (23%) | 106 (11%) | 2155 (41%) | 25 (19%) | 771 (42%) | 52 (38%) | ||||||||
| 60−69 years | 35,529 (27%) | 470 (23%) | 26,017 (28%) | 231 (23%) | 1166 (22%) | 34 (26%) | 630 (34%) | 51 (37%) | ||||||||
| 70−79 years | 33,218 (26%) | 574 (28%) | 25,419 (28%) | 309 (31%) | 1091 (21%) | 39 (29%) | 342 (19%) | 26 (19%) | ||||||||
| ≥80 years | 25,701 (20%) | 633 (31%) | 19,624 (21%) | 354 (35%) | 805 (15%) | 35 (26%) | 99 (5.4%) | 9 (6.5%) | ||||||||
| Sex | <0.001 | <0.001 | 0.012 | 1.000 | ||||||||||||
| Male | 70,686 (55%) | 1208 (59%) | 52,865 (57%) | 644 (64%) | 3021 (58%) | 92 (69%) | 1151 (62%) | 86 (62%) | ||||||||
| Female | 58,827 (45%) | 829 (41%) | 39,510 (43%) | 356 (36%) | 2196 (42%) | 41 (31%) | 691 (38%) | 52 (38%) | ||||||||
| N/A | 2 | 0 | 1 | 0 | ||||||||||||
| Elixhauser comorbidity score | 15.3 (11.8) | 21.4 (13.5) | <0.001 | 18.1 (11.4) | 27.2 (13.0) | <0.001 | 11.5 (10.4) | 18.5 (13.2) | <0.001 | 10.6 (9.5) | 15.1 (11.1) | <0.001 | ||||
| Elixhauser comorbidity index | <0.001 | <0.001 | 0.014 | <0.001 | ||||||||||||
| <0 | 5933 (4.6%) | 48 (2.4%) | 252 (0.3%) | 2 (0.2%) | 107 (2.1%) | 2 (1.5%) | 110 (6.0%) | 4 (2.9%) | ||||||||
| 0 | 5157 (4.0%) | 45 (2.2%) | 40 (<0.1%) | 0 (0%) | 742 (14%) | 7 (5.3%) | 186 (10%) | 2 (1.4%) | ||||||||
| 1–4 | 8587 (6.6%) | 69 (3.4%) | 3701 (4.0%) | 5 (0.5%) | 298 (5.7%) | 5 (3.8%) | 149 (8.1%) | 7 (5.1%) | ||||||||
| ≥5 | 109,838 (85%) | 1875 (92%) | 88,383 (96%) | 993 (99%) | 4070 (78%) | 119 (89%) | 1397 (76%) | 125 (91%) | ||||||||
| End-stage renal disease | End-stage chronic liver diseases | HIV | Autoimmune diseases | Congenital immunodeficiency | ||||||||||||
| End-stage renal disease, N = 709 | End-stage renal disease with COVID-19-related SARI, N = 27 | p-value 2 | End-stage chronic liver diseases, N = 11,040 | End-stage chronic liver diseases with COVID-19-related SARI, N = 243 | p-value 2 | HIV, N = 176 | HIV with COVID-19-related SARI, N = 7 | p-value 2 | Autoimmune diseases, N = 20,976 | Autoimmune diseases with COVID-19-related SARI, N = 494 | p-value 2 | Congenital immunodeficiency, N = 6508 | Congenital immunodeficiency with COVID-19-related SARI, N = 347 | p-value 2 | ||
| 69.0 (13.7) | 74.1 (11.5) | 0.034 | 65.6 (12.2) | 67.4 (12.3) | 0.022 | 50.9 (13.8) | 46.6 (6.3) | 0.138 | 63.8 (16.0) | 71.3 (14.5) | <0.001 | 52.2 (25.7) | 66.9 (15.1) | <0.001 | ||
| 0.443 | 0.085 | 0.473 | <0.001 | <0.001 | ||||||||||||
| 161 (23%) | 4 (15%) | 3316 (30%) | 66 (27%) | 129 (73%) | 7 (100%) | 7571 (36%) | 99 (20%) | 3296 (51%) | 98 (28%) | |||||||
| 158 (22%) | 5 (19%) | 3445 (31%) | 64 (26%) | 24 (14%) | 0 (0%) | 5109 (24%) | 96 (19%) | 1346 (21%) | 83 (24%) | |||||||
| 198 (28%) | 7 (26%) | 2701 (24%) | 68 (28%) | 18 (10%) | 0 (0%) | 4422 (21%) | 124 (25%) | 1042 (16%) | 92 (27%) | |||||||
| 192 (27%) | 11 (41%) | 1578 (14%) | 45 (19%) | 5 (2.8%) | 0 (0%) | 3874 (18%) | 175 (35%) | 824 (13%) | 74 (21%) | |||||||
| 0.809 | 0.176 | 1.000 | 0.013 | 0.205 | ||||||||||||
| 424 (60%) | 15 (56%) | 7194 (65%) | 169 (70%) | 143 (81%) | 6 (86%) | 7665 (37%) | 208 (42%) | 3345 (51%) | 191 (55%) | |||||||
| 285 (40%) | 12 (44%) | 3846 (35%) | 74 (30%) | 33 (19%) | 1 (14%) | 13,310 (63%) | 286 (58%) | 3163 (49%) | 156 (45%) | |||||||
| 1 | 0 | |||||||||||||||
| 13.5 (9.8) | 17.6 (10.0) | 0.047 | 15.6 (11.8) | 24.5 (12.3) | <0.001 | 6.4 (9.6) | 14.4 (12.1) | 0.131 | 6.1 (9.4) | 13.6 (10.8) | <0.001 | 8.7 (11.4) | 15.8 (12.8) | <0.001 | ||
| 0.245 | <0.001 | 0.193 | <0.001 | <0.001 | ||||||||||||
| 10 (1.4%) | 0 (0%) | 453 (4.1%) | 2 (0.8%) | 34 (19%) | 0 (0%) | 4747 (23%) | 31 (6.3%) | 842 (13%) | 16 (4.6%) | |||||||
| 26 (3.7%) | 0 (0%) | 109 (1.0%) | 0 (0%) | 38 (22%) | 0 (0%) | 3672 (18%) | 28 (5.7%) | 1453 (22%) | 26 (7.5%) | |||||||
| 59 (8.3%) | 0 (0%) | 2048 (19%) | 11 (4.5%) | 14 (8.0%) | 1 (14%) | 2323 (11%) | 34 (6.9%) | 592 (9.1%) | 22 (6.3%) | |||||||
| 614 (87%) | 27 (100%) | 8430 (76%) | 230 (95%) | 90 (51%) | 6 (86%) | 10,234 (49%) | 401 (81%) | 3621 (56%) | 283 (82%) | |||||||
| Characteristic | (Total) | ||||
|---|---|---|---|---|---|
| IC without COVID-19-Related SARI, N = 129,515 | IC with COVID-19-Related SARI, N = 2037 | Odds Ratio | 95% CI | p-Value | |
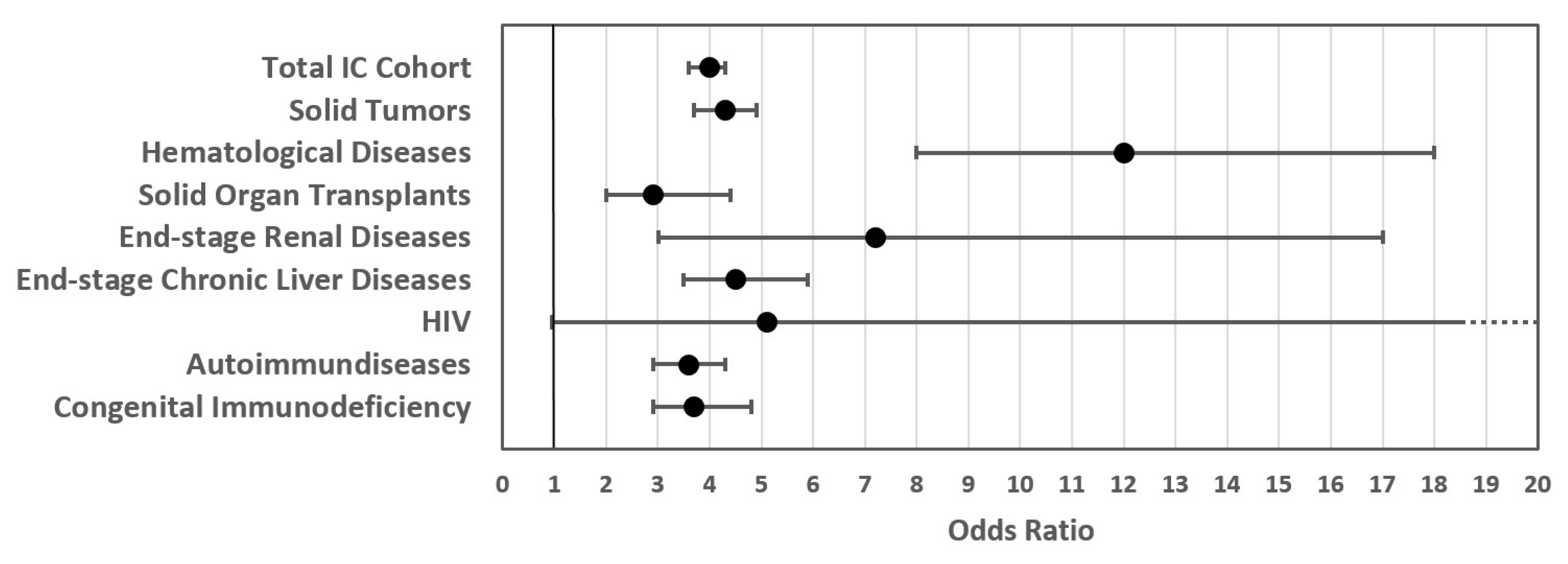
| Intensive care | 13,310 (10%) | 577 (28%) | 3.1 | 2.8, 3.4 | <0.001 |
| Mechanical ventilation | 2372 (1.8%) | 336 (16%) | 10 | 8.9, 11 | <0.001 |
| Severe course | 18,090 (14%) | 840 (42%) | 4.0 | 3.6, 4.3 | <0.001 |
| N/A | 3175 | 50 | |||
| In-hospital mortality | 5836 (4.7%) | 458 (24%) | 6.0 | 5.3, 6.6 | <0.001 |
| N/A | 4352 | 120 | |||
| Length of stay (d) | 6.1 (7.2) | 15.8 (15.3) | <0.001 | ||
| Costs (€) | 5645.3 (6877.4) | 12,377.7 (18,772.4) | <0.001 | ||
| ICU Treatment | Mechanical Ventilation | In-Hospital Mortality | Severe Courses | Length of Stay | Costs of Hospitalization | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | Coefficient (95% CI) | p Value | Coefficient (95% CI) | p Value |
| Male sex | 1.10 (1.06–1.14) | <0.001 | 1.14 (1.05–1.23) | 0.001 | 1.04 (0.98–1.10) | 0.167 | 1.07 (1.04–1.11) | <0.001 | −0.07 (−0.08–0.06) | <0.001 | −0.03 (−0.04–0.03) | <0.001 |
| Age | 0.98 (0.96–1.00) | 0.029 | 0.87 (0.84–0.91) | <0.001 | 1.39 (1.34–1.44) | <0.001 | 1.07 (1.05–1.09) | <0.001 | 0.05 (0.04–0.05) | <0.001 | 0.00 (0.00–0.01) | 0.403 |
| Elixhauser comorbidity score | 2.14 (2.08–2.20) | <0.001 | 2.91 (2.75–3.09) | <0.001 | 4.14 (3.97–4.31) | <0.001 | 2.67 (2.60–2.74) | <0.001 | 0.39 (0.38–0.39) | <0.001 | 0.27 (0.26–0.28) | <0.001 |
| COVID-19-related SARI | 2.59 (2.34–2.87) | <0.001 | 8.07 (7.07–9.22) | <0.001 | 4.44 (3.93–5.00) | <0.001 | 3.23 (2.94–3.56) | <0.001 | 0.81 (0.78–0.85) | <0.001 | 0.51 (0.48–0.54) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachtigall, I.; Kwast, S.; Hohenstein, S.; König, S.; Dang, P.L.; Leiner, J.; Giesen, N.; Schleenvoigt, B.T.; Bonsignore, M.; Bollmann, A.; et al. Retrospective, Observational Analysis on the Impact of SARS-CoV-2 Variant Omicron in Hospitalized Immunocompromised Patients in a German Hospital Network—The VISAGE Study. Vaccines 2024, 12, 634. https://doi.org/10.3390/vaccines12060634
Nachtigall I, Kwast S, Hohenstein S, König S, Dang PL, Leiner J, Giesen N, Schleenvoigt BT, Bonsignore M, Bollmann A, et al. Retrospective, Observational Analysis on the Impact of SARS-CoV-2 Variant Omicron in Hospitalized Immunocompromised Patients in a German Hospital Network—The VISAGE Study. Vaccines. 2024; 12(6):634. https://doi.org/10.3390/vaccines12060634
Chicago/Turabian StyleNachtigall, Irit, Stefan Kwast, Sven Hohenstein, Sebastian König, Phi Long Dang, Johannes Leiner, Nicola Giesen, Benjamin Thomas Schleenvoigt, Marzia Bonsignore, Andreas Bollmann, and et al. 2024. "Retrospective, Observational Analysis on the Impact of SARS-CoV-2 Variant Omicron in Hospitalized Immunocompromised Patients in a German Hospital Network—The VISAGE Study" Vaccines 12, no. 6: 634. https://doi.org/10.3390/vaccines12060634
APA StyleNachtigall, I., Kwast, S., Hohenstein, S., König, S., Dang, P. L., Leiner, J., Giesen, N., Schleenvoigt, B. T., Bonsignore, M., Bollmann, A., Kuhlen, R., & Jah, F. (2024). Retrospective, Observational Analysis on the Impact of SARS-CoV-2 Variant Omicron in Hospitalized Immunocompromised Patients in a German Hospital Network—The VISAGE Study. Vaccines, 12(6), 634. https://doi.org/10.3390/vaccines12060634







