Association between Reactogenicity and Immunogenicity in a Vaccinated Cohort with Two mRNA SARS-CoV-2 Vaccines at a High-Complexity Reference Hospital: A Post Hoc Analysis on Immunology Aspects of a Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Collected Data
2.2. Statistical Analysis
2.2.1. Sample Size Calculation and Planned Analyses
2.2.2. Study Endpoints
2.2.3. Statistical Analysis
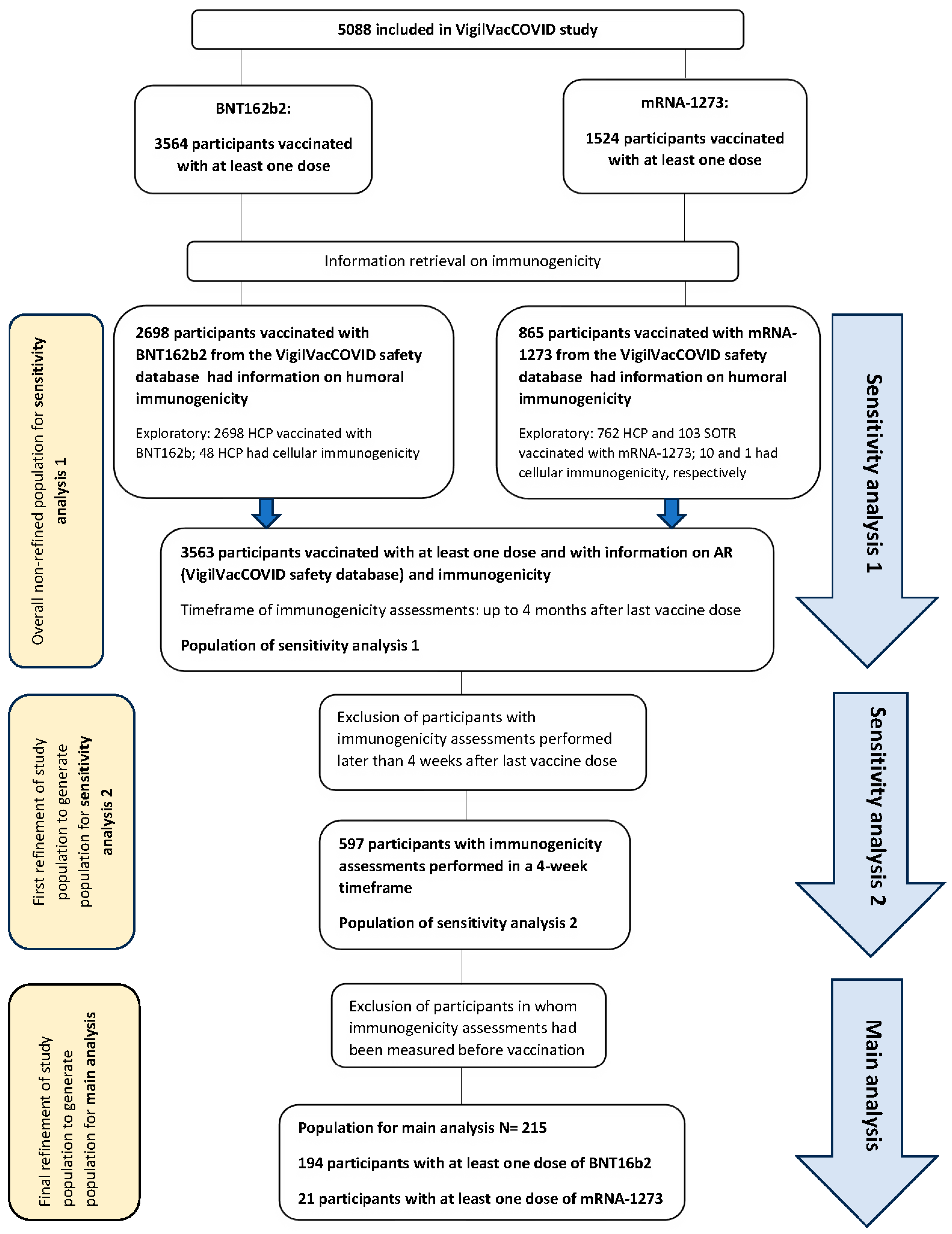
3. Results
3.1. Immunogenicity and Reactogenicity Results
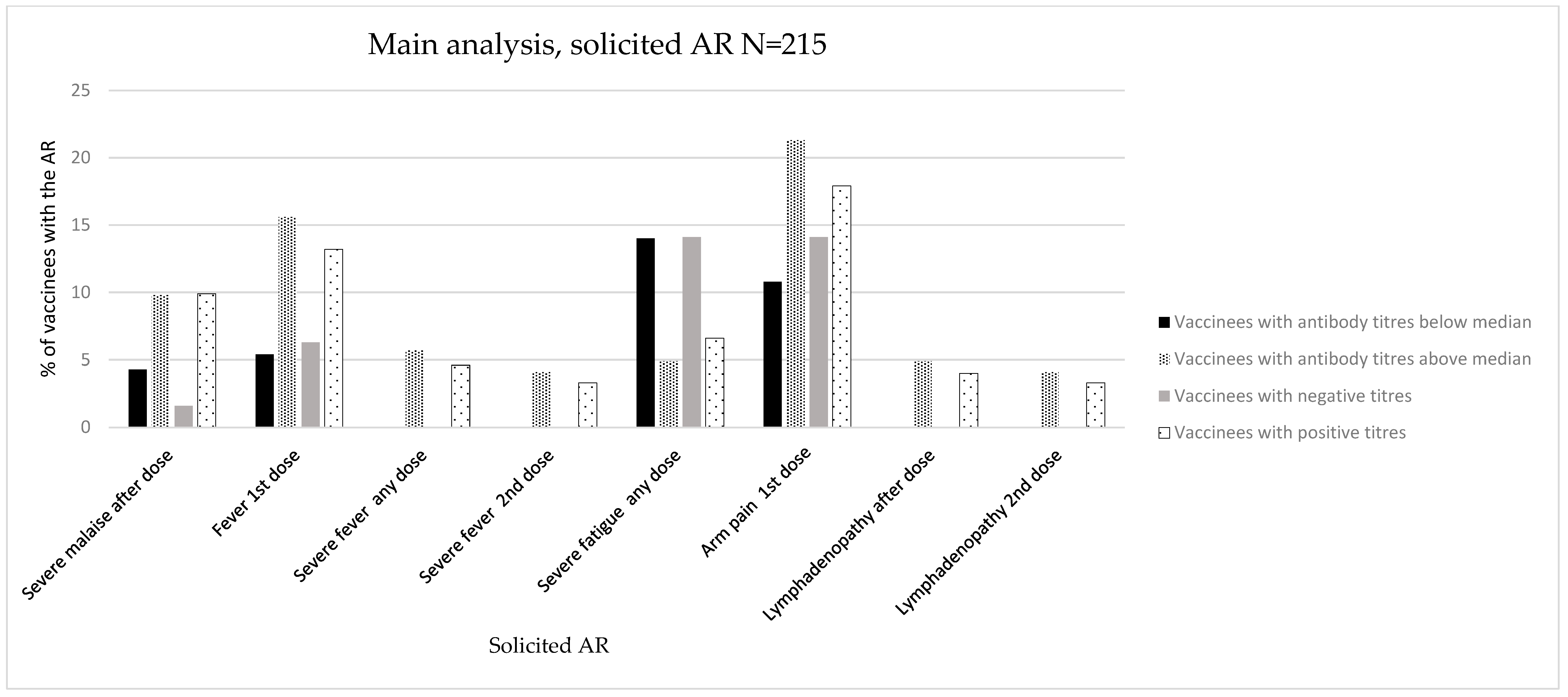
3.1.1. Main Analysis (Main Population, N = 215)
Immunogenicity Assessed as Positive/Negative Antibody Titres
Immunogenicity Assessed as Antibody Titres above/below Median
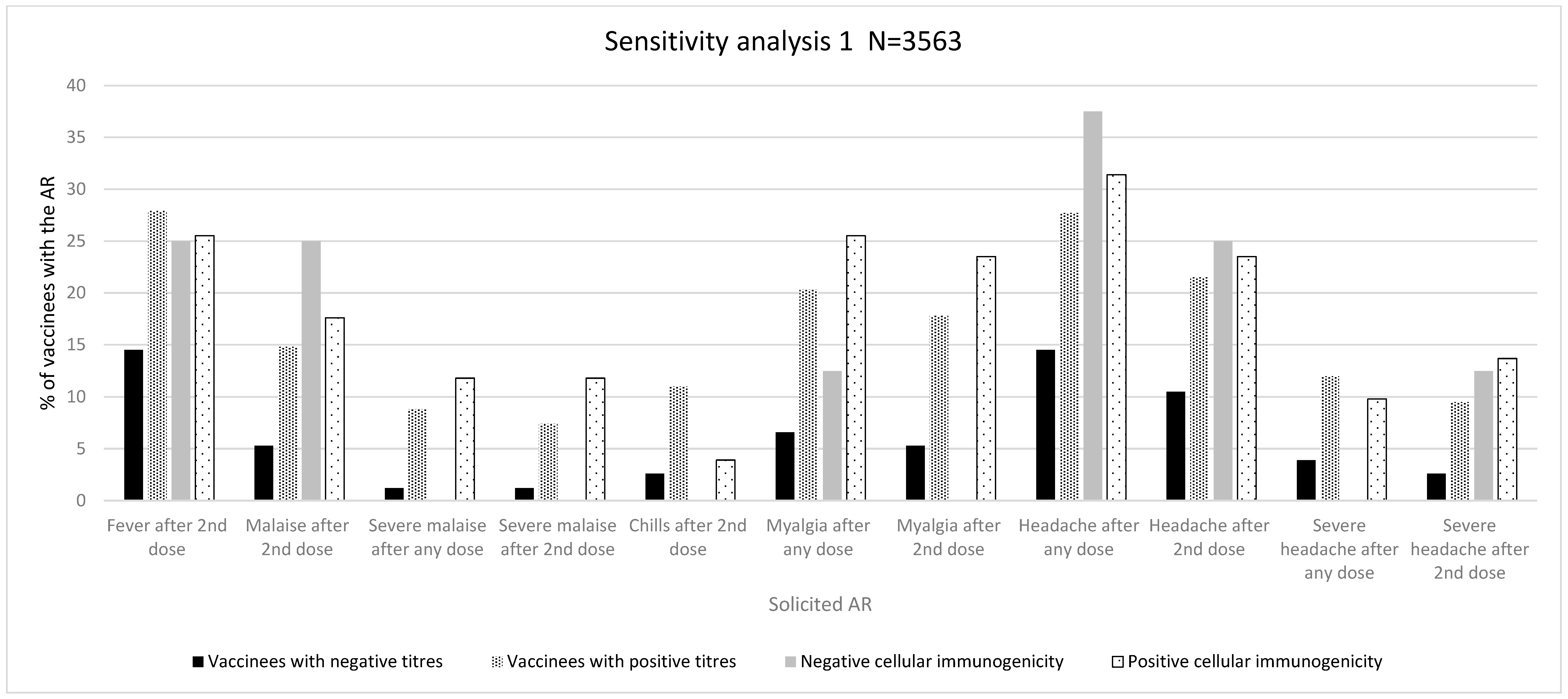
3.1.2. Sensitivity Analysis 1 (N = 3563)
Immunogenicity Assessed as Positive/Negative Antibody Titres
Immunogenicity Assessed as Antibody Titres above/below Median
Cellular Immunogenicity
Descriptive Analysis According to Type of Vaccine and Population
3.1.3. Sensitivity Analysis 2 (N = 597)
Immunogenicity Assessed as Positive/Negative Antibody Titres
Immunogenicity Assessed as Antibody Titres above/below Median
3.1.4. Overall Reactogenicity and Immunogenicity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef]
- CDC Morbidity and Mortality Weekly Report (MMWR) [Internet]. 2021. Available online: https://www.cdc.gov/mmwr/index.html (accessed on 7 June 2024).
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.-H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Casella, C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017, 47, 17–25. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P.; et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Ciccimarra, F.; Luxi, N.; Bellitto, C.; L’Abbate, L.; Raethke, M.; van Hunsel, F.; Lieber, T.; Mulder, E.; Riefolo, F.; Dureau-Pournin, C.; et al. Safety Monitoring of COVID-19 Vaccines in Persons with Prior SARS-CoV-2 Infection: A European Multi-Country Study. Vaccines 2024, 12, 241. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Debes, A.K.; Xiao, S.; Colantuoni, E.; Egbert, E.R.; Caturegli, P.; Gadala, A.; Milstone, A.M. Association of Vaccine Type and Prior SARS-CoV-2 Infection with Symptoms and Antibody Measurements Following Vaccination among Health CareWorkers. JAMA Intern. Med. 2021, 181, 1660–1662. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.H.; Chung, J.W.; Hwang, M.-H.; Kim, M.-C. Systemic Adverse Events and Use of Antipyretics Predict the Neutralizing Antibody Positivity Early after the First Dose of ChAdOx1 Coronavirus Disease Vaccine. J. Clin. Med. 2021, 10, 2844. [Google Scholar] [CrossRef]
- Lee, S.W.; Moon, J.Y.; Lee, S.K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.-H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.M.; Lim, S.; Shin, K.-H.; Kim, T.; Kim, W.-J.; Yun, M.; Oh, S.-H. Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines 2021, 9, 1473. [Google Scholar] [CrossRef]
- Son, S.; Lee, K. Development of mRNA Vaccines/Therapeutics and Their Delivery System. Mol. Cells 2023, 46, 41–47. [Google Scholar] [CrossRef]
- Sáez-Peñataro, J.; Torres, F.; Bartra, J.; Bascuas, J.; Vilella, A.; Tortajada, M.; Quesada, S.; González, E.; López-Suñé, E.; Castells, C.; et al. Tolerability and Reactogenicity Profile of mRNA SARS-Cov-2 Vaccines from a Mass Vaccination Campaign in a Tertiary Hospital: Between-Vaccine and Between-Population Prospective Observational Study (VigilVacCOVID Study). BioDrugs 2022, 36, 509–520. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Akashi, Y.; Horie, M.; Kiyotaki, J.; Takeuchi, Y.; Togashi, K.; Adachi, Y.; Ueda, A.; Notake, S.; Nakamura, K.; Terada, N.; et al. Clinical Performance of the cobas Liat SARS-CoV-2 & Influenza A/B Assay in Nasal Samples. Mol. Diagn. Ther. 2022, 26, 323–331. [Google Scholar]
- Florin, L.; Maelegheer, K.; Vandewal, W.; Bernard, D.; Robbrecht, J. Performance Evaluation of the Siemens SARS-CoV-2 Total Antibody and IgG Antibody Test. Lab. Med. 2021, 52, e147–e153. [Google Scholar] [CrossRef]
- Egri, N.; Olivé, V.; Hernández-Rodríguez, J.; Castro, P.; De Guzman, C.; Heredia, L.; Segura, A.C.; Fernandez, M.D.; de Moner, N.; Torradeflot, M.; et al. CoVITEST: A Fast and Reliable Method to Monitor Anti-SARS-CoV-2 Specific T Cells From Whole Blood. Front. Immunol. 2022, 13, 848586. [Google Scholar] [CrossRef]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukunaga, A.; Tanaka, A.; Takeuchi, J.S.; Inoue, Y.; Kimura, M.; Maeda, K.; Ueda, G.; Mizoue, T.; Ujiie, M.; et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine 2022, 40, 1924–1927. [Google Scholar] [CrossRef]
- Rechavi, Y.; Shashar, M.; Lellouche, J.; Yana, M.; Yakubovich, D.; Sharon, N. Occurrence of BNT162b2 Vaccine Adverse Reactions Is Associated with Enhanced SARS-CoV-2 IgG Antibody Response. Vaccines 2021, 9, 977. [Google Scholar] [CrossRef]
- Oyebanji, O.A.; Wilson, B.; Keresztesy, D.; Carias, L.; Wilk, D.; Payne, M.; Aung, H.; St Denis, K.; Lam, E.C.; Rowley, C.F.; et al. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin. Exp. Res. 2021, 33, 3151–3160. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kadowaki, T.; Matsuo, R.; Sasaki, A.; Miyaji, C.; Higuchi, C.; Nakayama, M.; Sakurada, Y.; Hagiya, H.; Takao, S.; et al. Association Between Fever and Antibody Titer Trends After a Third Dose of the mRNA-1273 Vaccine. J. Epidemiol. 2022, 32, 567–569. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, J.Y.; Park, S.; Kwon, J.-S.; Park, J.Y.; Cha, H.H.; Suh, M.H.; Lee, H.J.; Lim, J.S.; Bae, S.; et al. Correlation between Reactogenicity and Immunogenicity after the ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccination. Immune Netw. 2021, 21, e41. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE 2021, 16, e0257668. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Song, K.H.; Choi, Y.; Go, S.; Choi, S.-J.; Jung, J.; Kang, C.K.; Choe, P.G.; Kim, N.-J.; Park, W.B.; et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021, 36, 1486–1491. [Google Scholar] [CrossRef]
- Held, J.; Esse, J.; Tascilar, K.; Steininger, P.; Schober, K.; Irrgang, P.; Alsalameh, R.; Tenbusch, M.; Seggewies, C.; Bogdan, C. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®). Vaccines 2021, 9, 1063. [Google Scholar] [CrossRef]
- Bauernfeind, S.; Salzberger, B.; Hitzenbichler, F.; Scigala, K.; Einhauser, S.; Wagner, R.; Gessner, A.; Koestler, J.; Peterhoff, D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines 2021, 9, 1089. [Google Scholar] [CrossRef]
- Egri, N.; Juan, M. Immunology in COVID-19; more than diagnosis of infection or the basis of vaccination. Med. Clin. (Barc.) 2022, 158, 324–326. [Google Scholar] [CrossRef]
- European Medicines Agency. Safety of COVID-19 Vaccines. 2023. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines/safety-covid-19-vaccines (accessed on 7 June 2024).
- Pharmacovigilance Risk Assessment Committee (PRAC) Meeting Highlights. 3–6 May 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-3-6-may-2021 (accessed on 7 June 2024).
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Pharmacovigilance Risk Assessment Committee (PRAC) Meeting Highlights, 29 November–2 Deacember 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-29-november-2-december-2021 (accessed on 7 June 2024).
- Elizalde, M.U.; Eguinoa, F.J.G.; de Las Huertas, A.G.L.; Jiménez-González, M.; Ramírez, E. Myocarditis and pericarditis risk with mRNA COVID-19 vaccination compared to unvaccinated individuals: A retrospective cohort study in a Spanish Tertiary Hospital. Biomed. Pharmacother. 2024, 171, 116181. [Google Scholar] [CrossRef]
- Bouchlarhem, A.; Boulouiz, S.; Bazid, Z.; Ismaili, N.; El ouafi, N. Is There a Causal Link Between Acute Myocarditis and COVID-19 Vaccination: An Umbrella Review of Published Systematic Reviews and Meta-Analyses. Clin. Med. Insights Cardiol. 2024, 18, 11795468231221406. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- Jeet Kaur, R.; Dutta, S.; Charan, J.; Bhardwaj, P.; Tandon, A.; Yadav, D.; Islam, S.; Haque, M. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int. J. Gen. Med. 2021, 14, 3909–3927. [Google Scholar] [CrossRef]
- Sun, C.L.F.; Jaffe, E.; Levi, R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci. Rep. 2022, 12, 6978, Erratum in Sci Rep. 2023, 13, 13276. [Google Scholar] [CrossRef]
- Chang, Y.; Lv, G.; Liu, C.; Huang, E.; Luo, B. Cardiovascular safety of COVID-19 vaccines in real-world studies: A systematic review and meta-analysis. Expert Rev. Vaccines 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Ho, J.S.Y.; Sia, C.H.; Ngiam, J.N.; Loh, P.H.; Chew, N.W.S.; Kong, W.K.-F.; Poh, K.-K. A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med. J. 2023, 64, 543–549. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Urdaneta, V.; Esposito, D.B.; Dharia, P.; Moraga, M.S.; Anteyi, K.; Oduyebo-Omotosho, T.; Rossi, M.; Burton, P.; Vega, J.M.; Dawson, R.; et al. Global Safety Assessment of Adverse Events of Special Interest Following 2 Years of Use and 772 Million Administered Doses of mRNA-1273. Open Forum Infect Dis. 2024, 11, ofae067. [Google Scholar] [CrossRef]
- Chen, C.Y.; Su, T.C. Benefits and Harms of COVID-19 Vaccines in Cardiovascular Disease: A Comprehensive Review. J. Lipid Atheroscler. 2023, 12, 119–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, W.; Wen, X.; Cong, X.; Jiang, W. COVID-19 mRNA vaccine, but not a viral vector-based vaccine, promotes neutralizing anti-type I interferon autoantibody production in a small group of healthy individuals. J. Med. Virol. 2023, 95, e29137. [Google Scholar] [CrossRef]
- Ning, W.; Xu, W.; Cong, X.; Fan, H.; Gilkeson, G.; Wu, X.; Hughes, H.; Jiang, W. COVID-19 mRNA vaccine BNT162b2 induces autoantibodies against type I interferons in a healthy woman. J. Autoimmun. 2022, 132, 102896. [Google Scholar] [CrossRef]
- Bastard, P.; Michailidis, E.; Hoffmann, H.H.; Chbihi, M.; Voyer, T.L.; Rosain, J.; Philippot, Q.; Seeleuthner, Y.; Gervais, A.; Materna, M.; et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021, 218, e20202486. [Google Scholar] [CrossRef]
- Wolff, A.S.B.; Hansen, L.; Grytaas, M.A.; Oftedal, B.E.; Breivik, L.; Zhou, F.; Hufthammer, K.O.; Sjøgren, T.; Olofsson, J.S.; Trieu, M.C.; et al. Vaccination prevents severe COVID-19 outcome in patients with neutralizing type 1 interferon autoantibodies. iScience 2023, 26, 107084. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Cavalli, M.; Cederholm, A.; Aranda-Guillén, M.; Behere, A.; Mildner, H.; Lakshmikanth, T.; Gonzalez, L.; Mugabo, C.H.; Johnsson, A.; et al. No link between type I interferon autoantibody positivity and adverse reactions to COVID-19 vaccines. NPJ Vaccines 2024, 9, 42. [Google Scholar] [CrossRef]

| Covariates | Total Population (N = 215) |
|---|---|
| Age | |
| N | 215 |
| Mean (SD) | 41.13 (12.71) |
| Gender | |
| Male (n, %) | 70 (32.6) |
| Female (n, %) | 145 (67.4) |
| Occupational SARS-CoV-2 contact | |
| In contact with SARS-CoV-2 patients | 105 (48.8) |
| Without contact with SARS-CoV-2 patients | 110 (51.2) |
| a Previous SARS-CoV-2 infection | |
| Yes | 78 (36.3) |
| No | 137 (63.7) |
| Comorbidities | |
| b Any comorbidity | 83 (38.6) |
| Arterial hypertension | 12 (5.6) |
| Diabetes mellitus | 3 (1.4) |
| Heart failure | 1 (0.5) |
| Asthma | 8 (3.7) |
| Rheumatic/immune-mediated disease | 1 (0.5) |
| Drug allergies | 17 (7.9) |
| Food allergies | 10 (4.7) |
| Type of administered vaccine | |
| BNT16b2 | |
| First dose: | 194 (90.2) |
| Second dose: | 138 (64.2) |
| mRNA-1273 | |
| First dose: | 21 (9.8) |
| Second dose: | 17 (7.9) |
| Humoral immunogenicity (antibody positiveness on semiquantitative antibody titres) | |
| Number of vaccinees with positive antibody titres (titres of >1.0) (n, %) | 151 (70) |
| Number of vaccinees with negative antibody titres (titres of <1.0) (n, %) | 64 (30) |
| Humoral immunogenicity (antibody semiquantitative titres above/below median) | |
| Median value of antibody titres (P25, P75) * | 7.69 (0.73,10.00) |
| Number of vaccinees with antibody titres above median (n, %) | 93 (43%) |
| Number of vaccinees with antibody titres below median (n, %) | 122 (57%) |
| Main Analysis | |||||||
|---|---|---|---|---|---|---|---|
| N = 215 | Antibody Positiveness | p-Value | Antibody Titres | p-Value | |||
| Negative Antibody Titres (n = 64) | Positive Antibody Titres (n = 151) | Antibody Titres below Median * (n = 93) | Antibody Titres above Median * (n = 122) | ||||
| Proportion of AR (% of vaccinees with an AR) | Any mild AR after 1st dose (%) | 19 (29.7) | 28 (18.5) | 0.071 n | 22 (23.7) | 25 (20.5) | 0.578 |
| Proportion of specific AR (% of vaccinees with an AR) | Malaise after any dose (%) | 4 (6.3) | 24 (15.9) | 0.055 n | 9 (9.7) | 19 (15.6) | 0.203 |
| Malaise after 2nd dose (%) | 3 (4.7) | 19 (12.6) | 0.081 n | 8 (8.6) | 14 (11.5) | 0.491 | |
| Severe malaise after any dose (%) | 1 (1.6) | 15 (9.9) | 0.033 | 4 (4.3) | 12 (9.8) | 0.126 | |
| Severe malaise after 2nd dose (%) | 1 (1.6) | 13 (8.6) | 0.056 n | 4 (4.3) | 10 (8.2) | 0.251 | |
| Fever after 1st dose (%) | 4 (6.3) | 20 (13.2) | 0.136 | 5 (5.4) | 19 (15.6) | 0.019 | |
| Severe fever after any dose (%) | 0 (0.0) | 7 (4.6) | 0.080 n | 0 (0.0) | 7 (5.7) | 0.019 | |
| Severe fever after 2nd dose (%) | 0 (0.0) | 5 (3.3) | 0.141 | 0 (0.0) | 5 (4.1) | 0.048 | |
| Severe fatigue after any dose (%) | 9 (14.1) | 10 (6.6) | 0.079 n | 13 (14.0) | 6 (4.9) | 0.020 | |
| Arm pain after any dose (%) | 15 (23.4) | 38 (25.2) | 0.788 | 17 (18.3) | 36 (29.5) | 0.058 n | |
| Arm pain after 1st dose (%) | 9 (14.1) | 27 (17.9) | 0.493 | 10 (10.8) | 26 (21.3) | 0.040 | |
| Lymphadenopathy after any dose (%) | 0 (0.0) | 6 (4.0) | 0.106 | 0 (0.0) | 6 (4.9) | 0.030 | |
| Lymphadenopathy after 2nd dose (%) | 0 (0.0) | 5 (3.3) | 0.141 | 0 (0.0) | 5 (4.1) | 0.048 | |
| Severe myalgia after 1st dose (%) | 2 (3.1) | 7 (4.6) | 0.613 | 0 (0.0) | 4 (3.3) | 0.078 n | |
| Severe injection site swelling after any dose (%) | 0 (0.0) | 4 (2.6) | 0.189 | 0 (0.0) | 4 (3.3) | 0.078 n | |
| Severe injection site swelling after 1st dose (%) | 0 (0.0) | 4 (2.6) | 0.189 | 0 (0.0) | 4 (3.3) | 0.078 n | |
| Sensitivity Analysis 1 | |||||||
|---|---|---|---|---|---|---|---|
| N = 3563 | Antibody Positiveness | p-Value | Antibody Titres | p-Value | |||
| Negative Antibody Titres (n = 76) | Positive Antibody Titres (n = 3487) | Antibody Titres below Median * (n = 440) | Antibody Titres above Median * (n = 3123) | ||||
| Intensity (maximum intensity grade; % vaccinees at each grade) | Grade 1 after any vaccine dose (%) | 15 (23.8) | 350 (12.2) | 0.020 | 62 (16.9) | 303 (11.8) | 0.017 |
| Grade 2 after any vaccine dose (%) | 23 (36.5) | 1132 (39.3) | 142 (38.7) | 1013 (39.3) | |||
| Grade 3 after any vaccine dose (%) | 25 (39.7) | 1397 (48.5) | 163 (44.4) | 1259 (48.9) | |||
| Grade 1 after 2nd vaccine dose (%) | 12 (27.3) | 261 (11.6) | 0.003 | 47 (17.0) | 226 (11.2) | 0.006 | |
| Grade 2 after 2nd vaccine dose (%) | 19 (43.2) | 949 (42.3) | 121 (43.8) | 847 (42.1) | |||
| Grade 3 after 2nd vaccine dose (%) | 13 (29.5) | 1036 (46.1) | 108 (39.1) | 941 (46.7) | |||
| Intensity (mean, SD Likert score) | After any dose (mean, SD) | 5.48 (2.47) | 6.15 (2.36) | 0.031 | 5.91 (2.17) | 6.16 (2.38) | 0.067 n |
| After 2nd dose (mean, SD) | 4.95 (2.37) | 6.10 (2.06) | 0.001 | 5.64 (2.14) | 6.14 (2.05) | 0.003 | |
| Proportion of ARs (% of vaccinees with an AR) | Any AR after 1st dose (%) | 56 (73.7) | 2222 (63.7) | 0.074 n | 295 (67.0) | 1983 (63.5) | 0.147 |
| Any AR after 2nd dose (%) | 45 (59.2) | 2335 (67.0) | 0.156 | 291 (66.1) | 2089 (66.9) | 0.753 | |
| Any mild AR after any dose (%) | 29 (38.2) | 1089 (31.2) | 0.198 | 168 (38.2) | 950 (30.4) | 0.001 | |
| Any mild AR after 1st dose (%) | 21 (27.6) | 667 (19.1) | 0.063 n | 102 (23.2) | 586 (18.8) | 0.028 | |
| Any mild AR after 2nd dose (%) | 17 (22.4) | 622 (17.8) | 0.308 | 99 (22.5) | 540 (17.3) | 0.008 | |
| Any severe AR after 2nd dose (%) | 13 (17.1) | 1036 (29.7) | 0.017 | 108 (24.5) | 941 (30.1) | 0.016 | |
| Proportion of specific ARs (% of vaccinees with an AR) | Fever after 2nd dose (%) | 11 (14.5) | 974 (27.9) | 0.010 | 114 (25.9) | 871 (27.9) | 0.380 |
| Malaise after any dose (%) | 7 (9.2) | 622 (17.8) | 0.050 | 68 (15.5) | 561 (18.0) | 0.200 | |
| Malaise after 2nd dose (%) | 4 (5.3) | 517 (14.8) | 0.020 | 56 (12.7) | 465 (14.9) | 0.230 | |
| Severe malaise after any dose (%) | 1 (1.2) | 305 (8.8) | 0.010 | 32 (7.3) | 274 (8.8) | 0.290 | |
| Severe malaise after 2nd dose (%) | 1 (1.2) | 258 (7.4) | 0.030 | 25 (5.7) | 234 (7.5) | 0.170 | |
| Severe arm pain after 2nd dose (%) | 1 (1.3) | 187 (5.4) | 0.120 | 15 (3.4) | 173 (5.5) | 0.060 n | |
| Chills after 2nd dose (%) | 2 (2.6) | 385 (11.0) | 0.020 | 35 (8.0) | 352 (11.3) | 0.040 | |
| Myalgia after any dose (%) | 5 (6.6) | 709 (20.3) | 0.003 | 94 (21.4) | 620 (19.9) | 0.460 | |
| Myalgia after 2nd dose (%) | 4 (5.3) | 621 (17.8) | 0.004 | 79 (18.0) | 546 (17.5) | 0.810 | |
| Severe myalgia after 2nd dose (%) | 2 (2.6) | 296 (8.5) | 0.070 n | 29 (6.6) | 269 (8.6) | 0.150 | |
| Headache after any dose (%) | 11 (14.5) | 967 (27.7) | 0.010 | 118 (26.8) | 860 (27.5) | 0.750 | |
| Headache after 2nd dose (%) | 8 (10.5) | 750 (21.5) | 0.020 | 89 (20.2) | 669 (21.4) | 0.570 | |
| Severe headache after any dose (%) | 3 (3.9) | 419 (12.0) | 0.030 | 39 (8.9) | 383 (12.3) | 0.040 | |
| Severe headache after 2nd dose (%) | 2 (2.6) | 331 (9.5) | 0.040 | 28 (6.4) | 305 (9.8) | 0.020 | |
| Sensitivity Analysis 2 | |||||||
|---|---|---|---|---|---|---|---|
| N = 597 | Antibody Positiveness | p-Value | Antibody Titres | p-Value | |||
| Negative Antibody Titres (n = 273) | Positive Antibody Titres (n = 324) | Antibody Titres below Median * (n = 288) | Antibody Titres above Median * (n = 309) | ||||
| Intensity (maximum intensity grade; % vaccinees at each grade) | Grade 1 after 1st vaccine dose (%) | 48 (17.6) | 39 (12.0) | 0.050 n | 50 (26.2) | 37 (17.5) | 0.072 n |
| Grade 2 after 1st vaccine dose (%) | 79 (28.9) | 94 (29.0) | 81 (42.4) | 92 (43.4) | |||
| Grade 3 after 1st vaccine dose (%) | 53 (19.4) | 90 (27.8) | 60 (31.4) | 83 (39.2) | |||
| Intensity (mean, SD Likert score) | After 1st dose (mean, SD) | 3.37 (2.99) | 3.83 (3.21) | 0.083 n | 5.28 (2.18) | 5.65 (2.09) | 0.083 n |
| After 2nd dose (mean, SD) | 3.63 (3.31) | 3.01 (3.23) | 0.001 | 5.87 (2.13) | 5.77 (2.02) | 0.570 | |
| Proportion of ARs (% of vaccinees with) | Any AR after 2nd dose (%) | 187 (68.5) | 188 (58.0) | 0.008 | 193 (67.0) | 182 (58.9) | 0.040 |
| Any moderate AR after 1st dose (%) | 99 (36.3) | 139 (42.9) | 0.099 | 103 (35.8) | 135 (43.7) | 0.048 | |
| Any moderate AR after 2nd dose (%) | 126 (46.2) | 124 (38.3) | 0.052 n | 129 (44.8) | 121 (39.2) | 0.163 | |
| Any severe AR after 1st dose (%) | 53 (19.4) | 90 (27.8) | 0.017 | 60 (20.8) | 83 (26.9) | 0.085 n | |
| Proportion of specific ARs (% of vaccinees with) | Fatigue after 1st dose (%) | 21 (7.7) | 20 (6.2) | 0.465 | 24 (8.3) | 43 (13.9) | 0.031 |
| Fever after 1st dose (%) | 16 (5.9) | 73 (22.5) | <0.001 | 19 (6.6) | 70 (22.7) | <0.001 | |
| Severe fever after 1st dose (%) | 0 (0.0) | 11 (3.4) | 0.002 | 1 (0.3) | 10 (3.2) | 0.009 | |
| Malaise after any dose (%) | 37 (13.6) | 76 (23.5) | 0.002 | 41 (14.2) | 72 (23.3) | 0.004 | |
| Malaise after 1st dose (%) | 5 (1.8) | 36 (11.1) | <0.001 | 7 (2.4) | 34 (11.0) | <0.001 | |
| Severe malaise after any dose (%) | 17 (6.2) | 42 (13.0) | 0.006 | 19 (6.6) | 40 (12.9) | 0.009 | |
| Severe malaise after 1st dose (%) | 3 (1.1) | 18 (5.6) | 0.003 | 5 (1.7) | 16 (5.2) | 0.023 | |
| Chills after 1st dose (%) | 2 (0.7) | 25 (7.7) | <0.001 | 4 (1.4) | 23 (7.4) | 0.004 | |
| Myalgia after 1st dose (%) | 9 (3.3) | 36 (11.1) | 0.003 | 9 (3.1) | 36 (11.7) | <0.001 | |
| Severe myalgia after 1st dose (%) | 3 (1.0) | 18 (5.8) | 0.003 | 3 (1.0) | 18 (5.8) | 0.002 | |
| Other musculoskeletal disorders after 1st dose (%) | 2 (0.7) | 9 (2.8) | 0.064 n | 3 (1.0) | 8 (2.6) | 0.160 | |
| Arthralgia after 1st dose (%) | 0 (0.0) | 10 (3.1) | 0.034 | 1 (0.3) | 9 (2.9) | 0.015 | |
| Severe arthralgia after 1st dose (%) | 0 (0.0) | 6 (1.9) | 0.024 | 1 (0.3) | 5 (1.6) | 0.120 | |
| Headache after 1st dose (%) | 24 (8.8) | 48 (14.8) | 0.024 | 26 (9.0) | 46 (14.9) | 0.028 | |
| Severe headache after 1st dose (%) | 6 (2.2) | 20 (6.2) | 0.018 | 6 (2.1) | 20 (6.5) | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Peñataro, J.; Calvo, G.; Bascuas, J.; Mosquera, M.M.; Marcos, M.Á.; Egri, N.; Torres, F. Association between Reactogenicity and Immunogenicity in a Vaccinated Cohort with Two mRNA SARS-CoV-2 Vaccines at a High-Complexity Reference Hospital: A Post Hoc Analysis on Immunology Aspects of a Prospective Cohort Study. Vaccines 2024, 12, 665. https://doi.org/10.3390/vaccines12060665
Sáez-Peñataro J, Calvo G, Bascuas J, Mosquera MM, Marcos MÁ, Egri N, Torres F. Association between Reactogenicity and Immunogenicity in a Vaccinated Cohort with Two mRNA SARS-CoV-2 Vaccines at a High-Complexity Reference Hospital: A Post Hoc Analysis on Immunology Aspects of a Prospective Cohort Study. Vaccines. 2024; 12(6):665. https://doi.org/10.3390/vaccines12060665
Chicago/Turabian StyleSáez-Peñataro, Joaquín, Gonzalo Calvo, Juan Bascuas, Maria Mar Mosquera, Maria Ángeles Marcos, Natalia Egri, and Ferran Torres. 2024. "Association between Reactogenicity and Immunogenicity in a Vaccinated Cohort with Two mRNA SARS-CoV-2 Vaccines at a High-Complexity Reference Hospital: A Post Hoc Analysis on Immunology Aspects of a Prospective Cohort Study" Vaccines 12, no. 6: 665. https://doi.org/10.3390/vaccines12060665
APA StyleSáez-Peñataro, J., Calvo, G., Bascuas, J., Mosquera, M. M., Marcos, M. Á., Egri, N., & Torres, F. (2024). Association between Reactogenicity and Immunogenicity in a Vaccinated Cohort with Two mRNA SARS-CoV-2 Vaccines at a High-Complexity Reference Hospital: A Post Hoc Analysis on Immunology Aspects of a Prospective Cohort Study. Vaccines, 12(6), 665. https://doi.org/10.3390/vaccines12060665






