A Computationally Optimized Broadly Reactive Hemagglutinin and Neuraminidase Vaccine Boosts Antibody-Secreting Cells and Induces a Robust Serological Response, Preventing Lung Damage in a Pre-Immune Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of COBRA HA and NA Proteins
2.2. Production of Virus-like Particle
2.3. Pre-Immunization, Vaccinations, and Infections
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Hemagglutination Inhibition (HAI) Assay
2.6. Lung Viral Titers
2.7. Lung Histopathology Assessment
2.8. Fluorospot Assay
2.9. Quantification and Statistical Analysis
3. Results
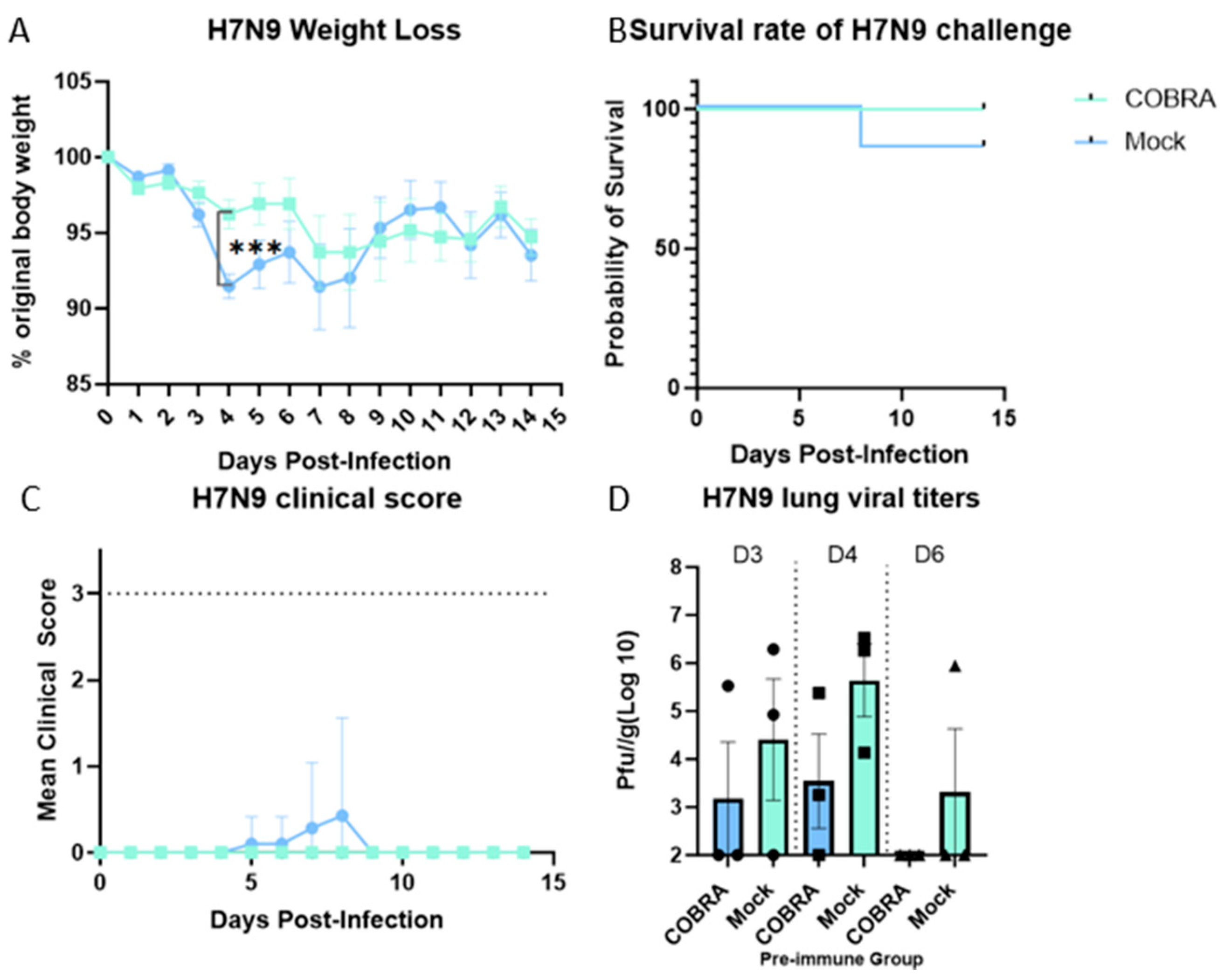
3.1. Vaccination and Infection of Mice
3.2. In Vivo Protection Efficacy Evaluation
3.3. Assessment of Lung Injury and Inflammation
3.4. Elicitation of HAI Antibodies by COBRA HA/NA Vaccines
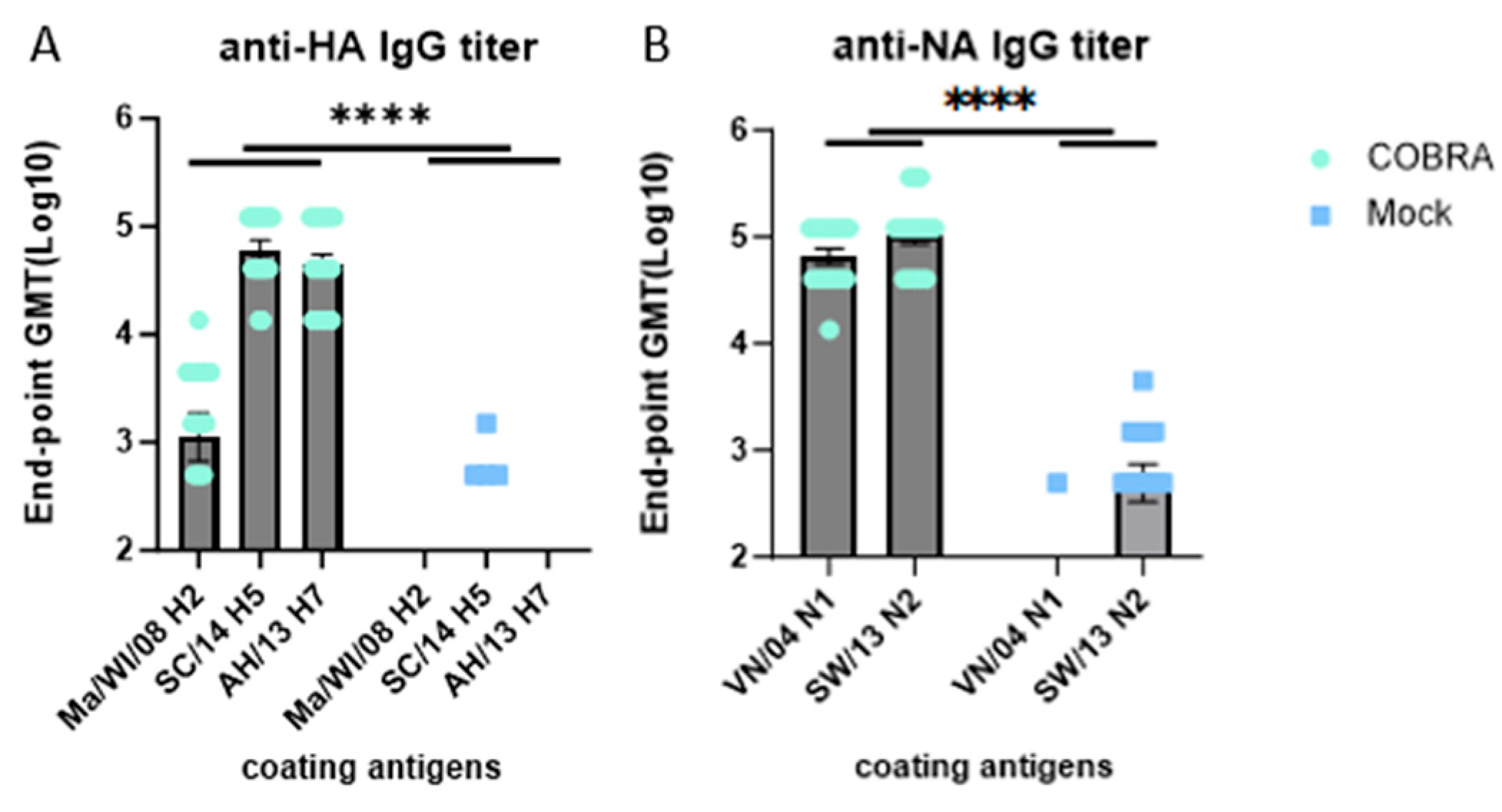
3.5. Elicitation of IgG Antibodies by COBRA HA/NA Vaccines
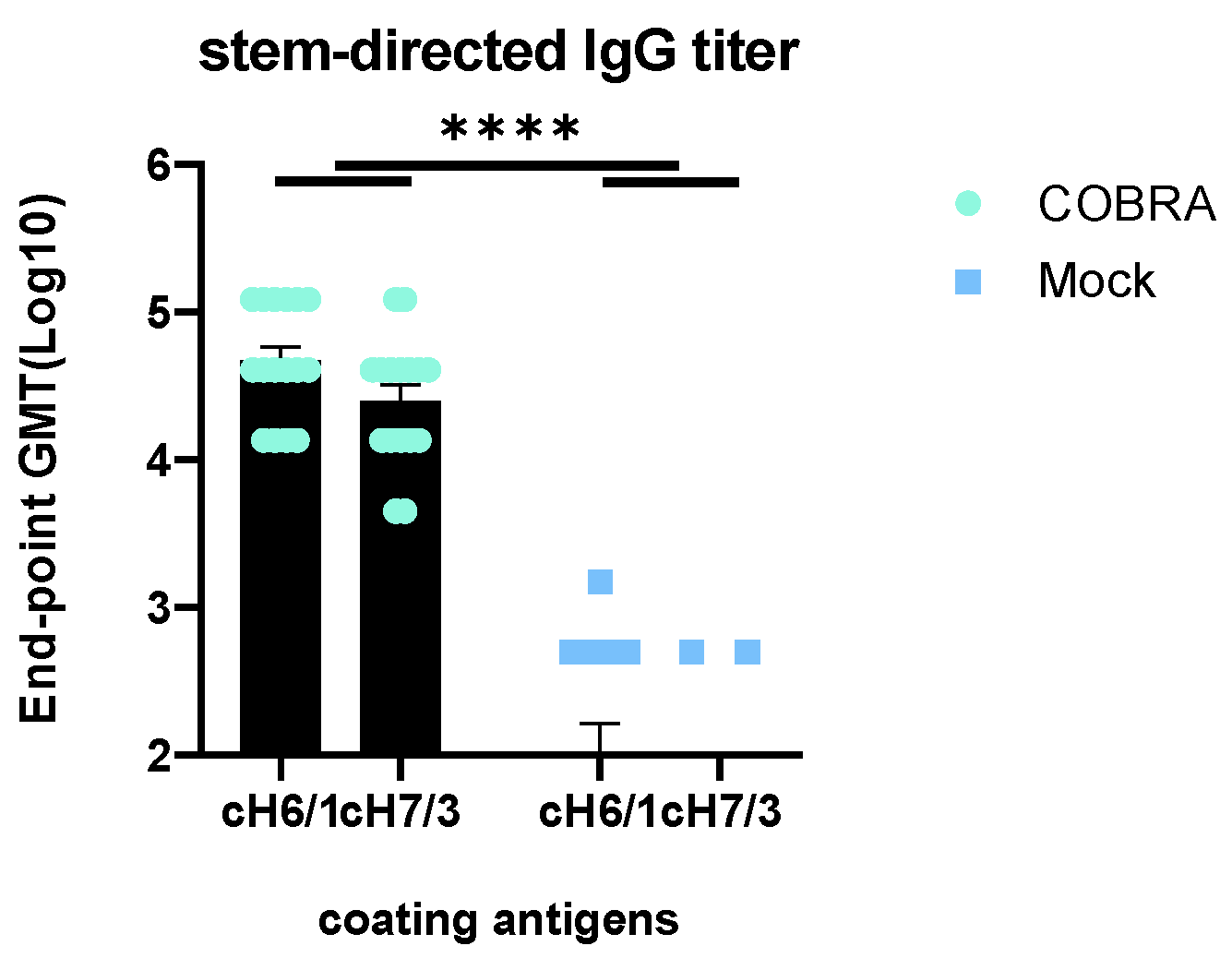
3.6. Elicitation of HA Stem-Directed Antibodies by COBRA HA/NA Vaccines
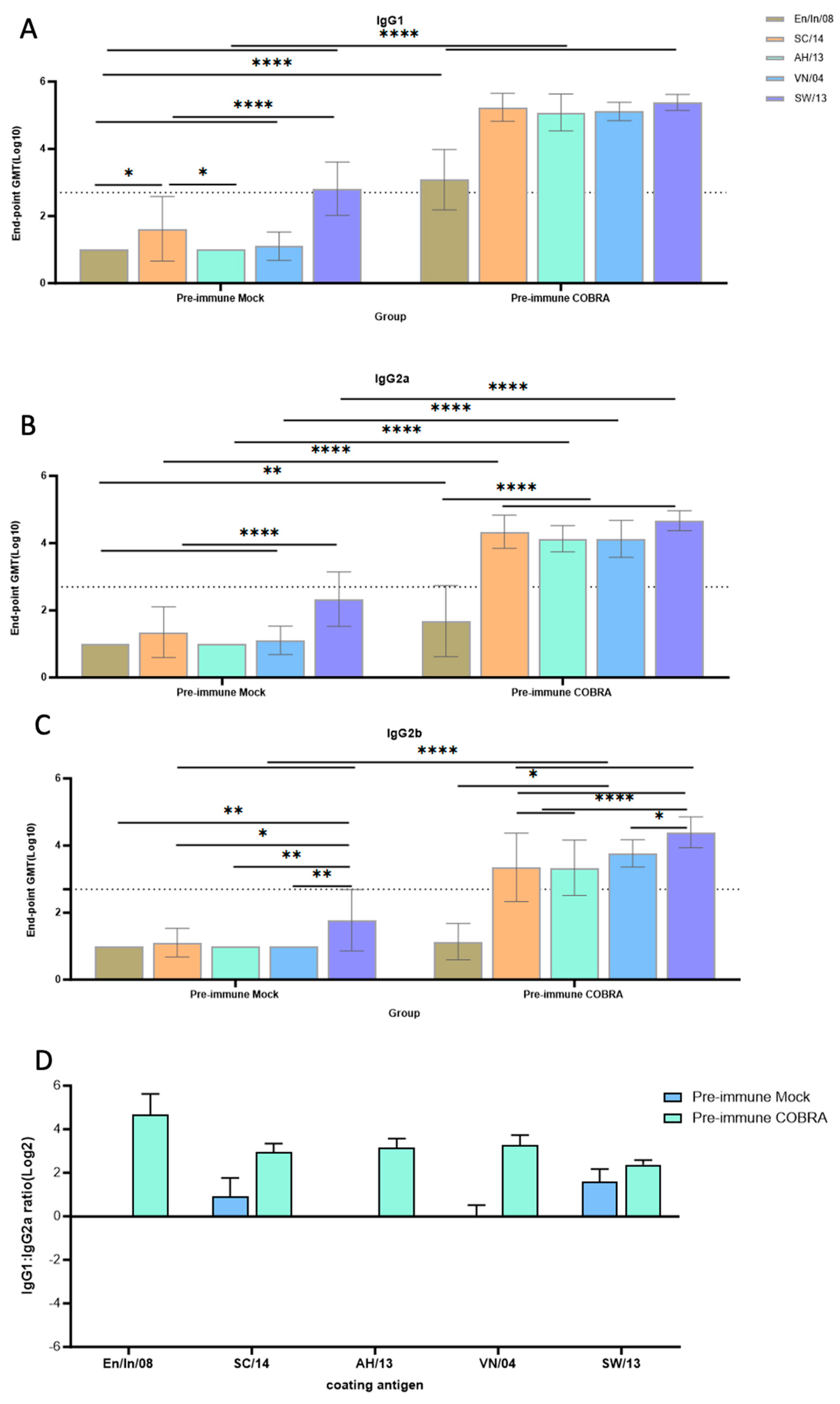
3.7. Th1/Th2 Antibodies Predominance and Trend
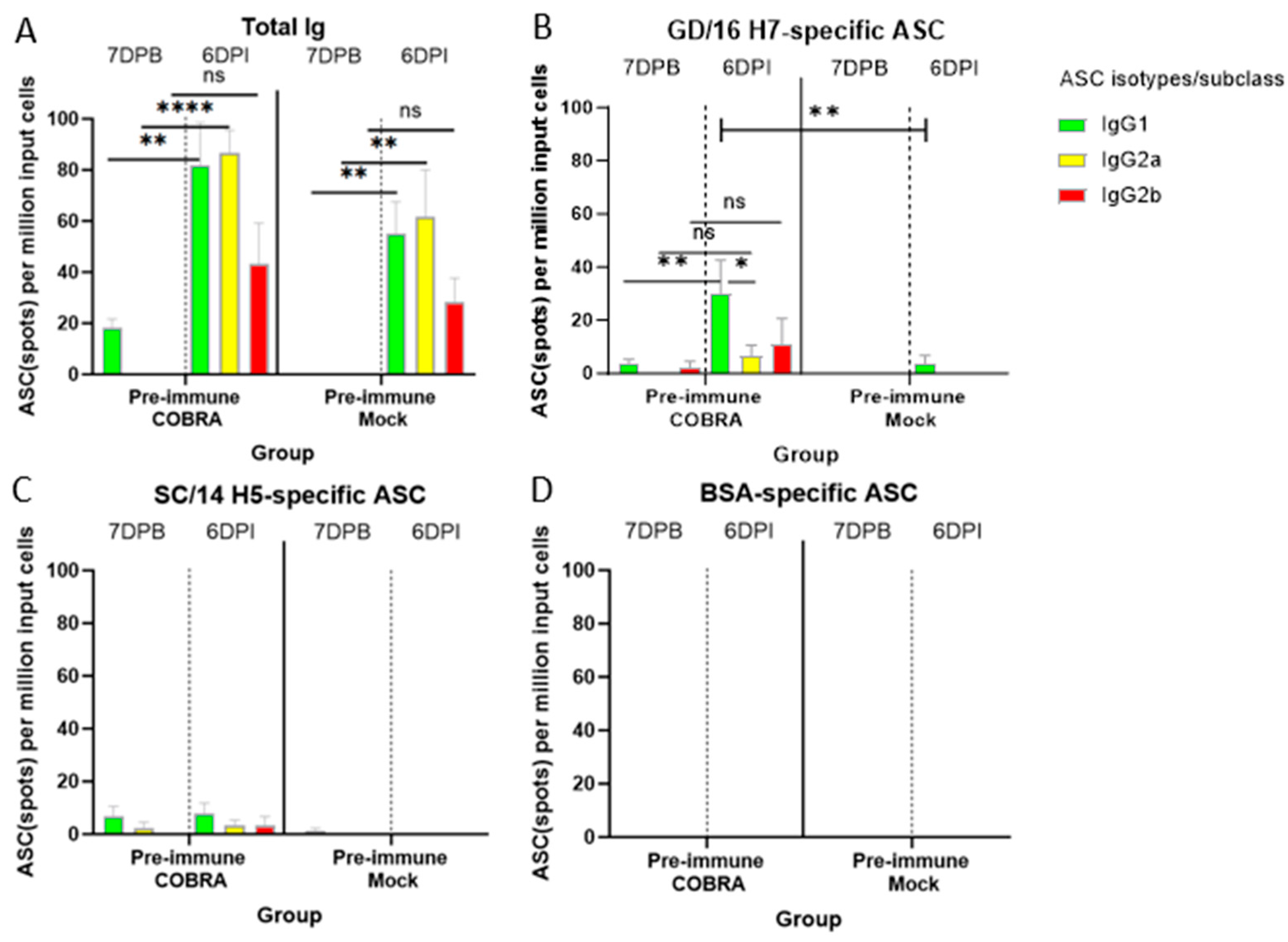
3.8. COBRA HA/NA Vaccines Elicit Robust Antibody-Secreting Cells (ASCs)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Uyeki, T.M. High-risk Groups for Influenza Complications. JAMA 2020, 324, 2334. [Google Scholar] [CrossRef]
- Kenmoe, S.; Takuissu, G.; Ebogo-Belobo, J.; Kengne-Ndé, C.; Mbaga, D.; Bowo-Ngandji, A.; Ndzie, J.O.; Kenfack-Momo, R.; Tchatchouang, S.; Fogang, R.L.; et al. A systematic review of influenza virus in water environments across human, poultry, and wild bird habitats. Water Res. X 2023, 22, 100210. [Google Scholar] [CrossRef]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V.; et al. Antigenic and Genetic Characteristics of Swine-Origin 2009 A(H1N1) Influenza Viruses Circulating in Humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef]
- Douglas, P.K.; Douglas, D.B.; Harrigan, D.C.; Douglas, K.M. Preparing for pandemic influenza and its aftermath: Mental health issues considered. Int. J. Emerg. Ment. Health 2009, 11, 137–144. [Google Scholar]
- Tapia, R.; Brito, B.; Saavedra, M.; Mena, J.; García-Salum, T.; Rathnasinghe, R.; Barriga, G.; Tapia, K.; García, V.; Bucarey, S.; et al. Novel influenza A viruses in pigs with zoonotic potential, Chile. Microbiol. Spectr. 2024, 12, e0218123. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Global Patterns of Influenza A Virus in Wild Birds. Science 2006, 312, 384. [Google Scholar] [CrossRef]
- Hill, N.J.; Bishop, M.A.; Trovão, N.S.; Ineson, K.M.; Schaefer, A.L.; Puryear, W.B.; Zhou, K.; Foss, A.D.; Clark, D.E.; MacKenzie, K.G.; et al. Ecological divergence of wild birds drives avian influenza spillover and global spread. PLoS Pathog. 2022, 18, e1010062. [Google Scholar] [CrossRef]
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Landreth, S.; Lu, Y.; Pandey, K.; Zhou, Y. A Replication-Defective Influenza Virus Harboring H5 and H7 Hemagglutinins Provides Protection against H5N1 and H7N9 Infection in Mice. J. Virol. 2021, 95, e02154-20. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Pang, X.; Yang, P.; Shu, Y.; Zhang, Y.; Wang, Q.; Chen, Z.; Liu, J.; Cheng, J.; Jiao, Y.; et al. Surveillance of the first case of human avian influenza A (H7N9) virus in Beijing, China. Infection 2014, 42, 127–133. [Google Scholar] [CrossRef]
- Chang, P.; Sadeyen, J.-R.; Bhat, S.; Daines, R.; Hussain, A.; Yilmaz, H.; Iqbal, M. Risk assessment of the newly emerged H7N9 avian influenza viruses. Emerg. Microbes Infect. 2023, 12, 2172965. [Google Scholar] [CrossRef]
- WattPoultry. Mozambique Records First Cases of H7 Avian Flu in Poultry. Available online: https://www.wattagnet.com/broilers-turkeys/diseases-health/article/15636846/mozambique-records-first-cases-of-h7-avian-flu-in-poultry (accessed on 16 June 2024).
- Monjane, I.V.A.; Djedje, H.; Tamele, E.; Nhabomba, V.; Tivane, A.R.; Massicame, Z.E.; Arone, D.M.; Pastori, A.; Bortolami, A.; Monne, I.; et al. H7N6 highly pathogenic avian influenza in Mozambique, 2023. Emerg. Microbes Infect. 2024, 13, 2321993. [Google Scholar] [CrossRef]
- Zhu, X.; Turner, H.L.; Lang, S.; McBride, R.; Bangaru, S.; Gilchuk, I.M.; Yu, W.; Paulson, J.C.; Crowe, J.E.; Ward, A.B.; et al. Structural basis of protection against H7N9 influenza virus by human anti-N9 neuraminidase antibodies. Cell Host Microbe 2019, 26, 729–738. [Google Scholar] [CrossRef]
- Vemula, S.V.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Vaccine approaches conferring cross-protection against influenza viruses. Expert. Rev. Vaccines 2017, 16, 1141–1154. [Google Scholar] [CrossRef]
- Huber, V.C.; Peltola, V.; Iverson, A.R.; McCullers, J.A. Contribution of vaccine-induced immunity toward either the HA or the NA component of influenza viruses limits secondary bacterial complications. J. Virol. 2010, 84, 4105–4108. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Stepanova, E.; Mezhenskaya, D.; Matyushenko, V.; Prokopenko, P.; Sychev, I.; Wong, P.-F.; Rudenko, L. Influenza vaccine: Progress in a vaccine that elicits a broad immune response. Expert. Rev. Vaccines 2021, 20, 1097–1112. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Palese, P. Is a Universal Influenza Virus Vaccine Possible? Annu. Rev. Med. 2020, 71, 315–327. [Google Scholar] [CrossRef]
- Mascola, J.R.; Fauci, A.S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Parra-Rojas, C.; Messling, V.V.; Hernandez-Vargas, E.A. Adjuvanted influenza vaccine dynamics. Sci. Rep. 2019, 9, 73. [Google Scholar] [CrossRef]
- Martin, J.T. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals 1997, 25, 209–213. [Google Scholar] [CrossRef]
- Reneer, Z.B.; Jamieson, P.J.; Skarlupka, A.L.; Huang, Y.; Ross, T.M. Computationally Optimized Broadly Reactive H2 HA Influenza Vaccines Elicited Broadly Cross-Reactive Antibodies and Protected Mice from Viral Challenges. J. Virol. 2020, 95, e01526-20. [Google Scholar] [CrossRef]
- Nuñez, I.A.; Huang, Y.; Ross, T.M. Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections. Pathogens 2021, 10, 1352. [Google Scholar] [CrossRef]
- Ge, P.; Ross, T.M. Evaluation of Pre-Pandemic Trivalent COBRA HA Vaccine in Mice Pre-Immune to Historical H1N1 and H3N2 Influenza Viruses. Viruses 2023, 15, 203. [Google Scholar] [CrossRef]
- Skarlupka, A.L.; Bebin-Blackwell, A.G.; Sumner, S.F.; Ross, T.M. Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains. J. Virol. 2021, 95, e0075921. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Kinzler, D.; Choi, A.; Hirsh, A.; Beaulieu, E.; Lecrenier, N.; Innis, B.L.; Palese, P.; Mallett, C.P.; Krammer, F. A chimeric haemagglutinin-based influenza split virion vaccine adjuvanted with AS03 induces protective stalk-reactive antibodies in mice. NPJ Vaccines 2016, 1, 16015. [Google Scholar] [CrossRef]
- Francis, M.E.; King, M.L.; Kelvin, A.A. Back to the Future for Influenza Preimmunity-Looking Back at Influenza Virus History to Infer the Outcome of Future Infections. Viruses 2019, 11, 122. [Google Scholar] [CrossRef]
- Sautto, G.A.; Kirchenbaum, G.A.; Abreu, R.B.; Ecker, J.W.; Pierce, S.R.; Kleanthous, H.; Ross, T.M. A Computationally Optimized Broadly Reactive Antigen Subtype-Specific Influenza Vaccine Strategy Elicits Unique Potent Broadly Neutralizing Antibodies against Hemagglutinin. J. Immunol. 2020, 204, 375–385. [Google Scholar] [CrossRef]
- Wagner, R.; Matrosovich, M.; Klenk, H.D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 2002, 12, 159–166. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antigenic and Genetic Characteristics of Zoonotic Influenza A Viruses and Development of Candidate Vaccine Viruses for Pandemic Preparedness. Available online: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-southern-hemisphere-recommendation-2020/201909-zoonotic-vaccinevirusupdate.pdf?sfvrsn=dea4da8b_7 (accessed on 6 March 2024).
- World Health Organization. Summary of Status of Development and Availability of Avian Influenza A(H7N9) Candidate Vaccine Viruses and Potency Testing Reagents. Available online: https://cdn.who.int/media/docs/default-source/influenza/cvvs/cvv-zoonotic---northern-hemisphere-2021-2022/summary_a_h7n9_cvv_nh2021_22_20210305.pdf?sfvrsn=ccc739b8_9 (accessed on 12 February 2024).
- Jackson, L.A.; Stapleton, J.T.; Walter, E.B.; Chen, W.H.; Rouphael, N.G.; Anderson, E.J.; Neuzil, K.M.; Winokur, P.L.; Smith, M.J.; Schmader, K.E.; et al. Immunogenicity and safety of varying dosages of a fifth-wave influenza A/H7N9 inactivated vaccine given with and without AS03 adjuvant in healthy adults. Vaccine 2024, 42, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Duan, S.; DeBeauchamp, J.; Zanin, M.; Kercher, L.; Sonnberg, S.; Fabrizio, T.; Jeevan, T.; Crumpton, J.-C.; Oshansky, C.; et al. The immune correlates of protection for an avian influenza H5N1 vaccine in the ferret model using oil-in-water adjuvants. Sci. Rep. 2017, 7, 44727. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, T.J.; Podolsky, K.A.; Chromikova, V.; Kirkpatrick, E.; Falconieri, V.; Meade, P.; Amanat, F.; Tan, J.; Tenoever, B.R.; Tan, G.S.; et al. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat. Microbiol. 2017, 2, 1415–1424. [Google Scholar] [CrossRef]
- Kubota-Koketsu, R.; Yunoki, M.; Okuno, Y.; Ikuta, K. Virus Neutralization by Human Intravenous Immunoglobulin against Influenza Virus Subtypes A/H5 and A/H7. Biologics 2021, 15, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Lee, E.-Y.; Byun, Y.H.; Jung, E.-J.; Lee, Y.J.; Lee, Y.H.; Lee, K.-H.; Lee, J.; Seong, B.L. Protective efficacy in mice of monovalent and trivalent live attenuated influenza vaccines in the background of cold-adapted A/X-31 and B/Lee/40 donor strains. Vaccine 2014, 32, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 1972, 70, 767–777. [Google Scholar] [CrossRef]
- Jang, H.; Ross, T.M. Hemagglutination Inhibition (HAI) antibody landscapes after vaccination with H7Nx virus like particles. PLoS ONE 2021, 16, e0246613. [Google Scholar] [CrossRef]


| A | H2 strain | ID | Log (2) HAI titer | |||||||||||||||
| 11 | 12 | 13 | 14 | 15 | 21 | 22 | 23 | 24 | 25 | 31 | 33 | 34 | 35 | 41 | 42 | |||
| TW/64 | 2.32 | 2.32 | 2.32 | 3.32 | 2.32 | 2.32 | 5.32 | 3.32 | 5.32 | 3.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | ||
| Duk/78 | 5.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 7.32 | 2.32 | 2.32 | 3.32 | 3.32 | 3.32 | 2.32 | ||
| Ma/NL/01 | 6.32 | 4.32 | 2.32 | 2.32 | 9.32 | 3.32 | 9.32 | 6.32 | 8.32 | 6.32 | 7.32 | 3.32 | 8.32 | 8.32 | 3.32 | 2.32 | ||
| Tur/CA/08 | 5.32 | 2.32 | 6.32 | 2.32 | 4.32 | 2.32 | 3.32 | 2.32 | 2.32 | 9.32 | 2.32 | 2.32 | 5.32 | 6.32 | 3.32 | 2.32 | ||
| Duk/Cam/13 | 5.32 | 3.32 | 2.32 | 2.32 | 7.32 | 2.32 | 7.32 | 4.32 | 6.32 | 6.32 | 5.32 | 2.32 | 6.32 | 7.32 | 2.32 | 2.32 | ||
| Qu/RI/16 | 5.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 7.32 | 2.32 | 2.32 | 3.32 | 3.32 | 2.32 | 2.32 | ||
| B | H5 strain | ID | Log (2) HAI titer | |||||||||||||||
| 11 | 12 | 13 | 14 | 15 | 21 | 22 | 23 | 24 | 25 | 31 | 33 | 34 | 35 | 41 | 42 | |||
| VN/04 | 5.32 | 5.32 | 5.32 | 6.32 | 5.32 | 5.32 | 6.32 | 6.32 | 4.32 | 4.32 | 4.32 | 5.32 | 5.32 | 5.32 | 5.32 | 2.32 | ||
| WS/05 | 5.32 | 5.32 | 5.32 | 5.32 | 6.32 | 5.32 | 6.32 | 6.32 | 6.32 | 6.32 | 4.32 | 6.32 | 7.32 | 7.32 | 5.32 | 2.32 | ||
| HB/10 | 3.32 | 3.32 | 5.32 | 2.32 | 7.32 | 3.32 | 5.32 | 6.32 | 2.32 | 5.32 | 4.32 | 2.32 | 7.32 | 7.32 | 4.32 | 3.32 | ||
| gy/Wa/14 | 2.32 | 4.32 | 2.32 | 2.32 | 2.32 | 2.32 | 5.32 | 6.32 | 2.32 | 7.32 | 5.32 | 5.32 | 8.32 | 8.32 | 4.32 | 2.32 | ||
| SC/14 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 5.32 | 2.32 | 4.32 | 3.32 | 2.32 | 5.32 | 5.32 | 2.32 | 2.32 | ||
| Ast/20 | 6.32 | 4.32 | 3.32 | 3.32 | 4.32 | 4.32 | 5.32 | 7.32 | 5.32 | 5.32 | 5.32 | 6.32 | 6.32 | 6.32 | 5.32 | 3.32 | ||
| C | H7 strain | ID | Log (2) HAI titer | |||||||||||||||
| 11 | 12 | 13 | 14 | 15 | 21 | 22 | 23 | 24 | 25 | 31 | 33 | 34 | 35 | 41 | 42 | |||
| Tur/Italy/00 | 3.32 | 2.32 | 2.32 | 2.32 | 5.32 | 2.32 | 2.32 | 3.32 | 2.32 | 9.32 | 2.32 | 2.32 | 4.32 | 4.32 | 3.32 | 2.32 | ||
| Blu/OH/04 | 5.32 | 2.32 | 2.32 | 2.32 | 4.32 | 2.32 | 3.32 | 2.32 | 2.32 | 8.32 | 2.32 | 2.32 | 5.32 | 5.32 | 3.32 | 2.32 | ||
| Duk/JX/09 | 6.32 | 2.32 | 2.32 | 2.32 | 5.32 | 2.32 | 3.32 | 3.32 | 2.32 | 9.32 | 2.32 | 2.32 | 5.32 | 5.32 | 3.32 | 2.32 | ||
| AH/13 | 9.32 | 2.32 | 2.32 | 3.32 | 6.32 | 2.32 | 3.32 | 4.32 | 5.32 | 11.32 | 3.32 | 2.32 | 6.32 | 7.32 | 6.32 | 2.32 | ||
| HN/16 | 7.32 | 2.32 | 3.32 | 3.32 | 6.32 | 3.32 | 6.32 | 3.32 | 4.32 | 7.32 | 3.32 | 2.32 | 4.32 | 4.32 | 7.32 | 2.32 | ||
| GD/16 | 6.32 | 2.32 | 2.32 | 2.32 | 2.32 | 2.32 | 3.32 | 2.32 | 3.32 | 8.32 | 2.32 | 2.32 | 4.32 | 4.32 | 4.32 | 2.32 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, P.; Mota, Y.C.; Richardson, R.A.; Ross, T.M. A Computationally Optimized Broadly Reactive Hemagglutinin and Neuraminidase Vaccine Boosts Antibody-Secreting Cells and Induces a Robust Serological Response, Preventing Lung Damage in a Pre-Immune Model. Vaccines 2024, 12, 706. https://doi.org/10.3390/vaccines12070706
Ge P, Mota YC, Richardson RA, Ross TM. A Computationally Optimized Broadly Reactive Hemagglutinin and Neuraminidase Vaccine Boosts Antibody-Secreting Cells and Induces a Robust Serological Response, Preventing Lung Damage in a Pre-Immune Model. Vaccines. 2024; 12(7):706. https://doi.org/10.3390/vaccines12070706
Chicago/Turabian StyleGe, Pan, Yailin Campos Mota, Robert A. Richardson, and Ted M. Ross. 2024. "A Computationally Optimized Broadly Reactive Hemagglutinin and Neuraminidase Vaccine Boosts Antibody-Secreting Cells and Induces a Robust Serological Response, Preventing Lung Damage in a Pre-Immune Model" Vaccines 12, no. 7: 706. https://doi.org/10.3390/vaccines12070706
APA StyleGe, P., Mota, Y. C., Richardson, R. A., & Ross, T. M. (2024). A Computationally Optimized Broadly Reactive Hemagglutinin and Neuraminidase Vaccine Boosts Antibody-Secreting Cells and Induces a Robust Serological Response, Preventing Lung Damage in a Pre-Immune Model. Vaccines, 12(7), 706. https://doi.org/10.3390/vaccines12070706







