Abstract
In patients with lung cancer (LC), understanding factors that impact the dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-spike antibody (SAb) titers over time is critical, but challenging, due to evolving treatments, infections, vaccinations, and health status. The objective was to develop a time-dependent regression model elucidating individual contributions of factors influencing SAb levels in LC patients using a prospective, longitudinal, multi-institutional cohort study initiated in January 2021. The study evaluated 296 LC patients—median age 69; 55% female; 50% stage IV. Blood samples were collected every three months to measure SAb levels using FDA-approved ELISA. Asymptomatic and unreported infections were documented through measurement of anti-nucleocapsid Ab levels (Meso Scale Discovery). Associations between clinical characteristics and titers were evaluated using a time-dependent linear regression model with a generalized estimating equation (GEE), considering time-independent variables (age, sex, ethnicity, smoking history, histology, and stage) and time-dependent variables (booster vaccinations, SARS-CoV-2 infections, cancer treatment, steroid use, and influenza vaccination). Significant time-dependent effects increasing titer levels were observed for prior SARS-CoV-2 infection (p < 0.001) and vaccination/boosters (p < 0.001). Steroid use (p = 0.043) and chemotherapy (p = 0.033) reduced titer levels. Influenza vaccination was associated with increased SAb levels (p < 0.001), independent of SARS-CoV-2 vaccine boosters. Prior smoking significantly decreased titers in females (p = 0.001). Age showed no association with titers. This GEE-based linear regression model unveiled the nuanced impact of multiple variables on patient anti-spike Ab levels over time. After controlling for the major influences of vaccine and SARS-CoV-2 infections, chemotherapy and steroid use were found to have negatively affected titers. Smoking in females significantly decreased titers. Surprisingly, influenza vaccinations were also significantly associated, likely indirectly, with improved SARS-CoV-2 titers.
1. Introduction
Prior to the availability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, initial studies showed that patients with lung cancer (LC) were at increased risk of serious complications of SARS-CoV-2 infection, including hospitalization and death [,,,,,,]. Increased mortality risk was associated with advanced age, male sex, smoking, number of comorbidities, performance status, and actively progressing cancer []. In another meta-analysis of cancer patients, factors significantly associated with mortality included age, male sex, hypertension, and diabetes, with LC having the highest case fatality rate (32.9%) among solid tumors [].
Post-vaccination studies indicated that most LC patients developed durable antibody responses to SARS-CoV-2 vaccinations. However, multiple studies investigating SARS-CoV-2 spike protein antibody (SAb) titers, specifically in LC patients after vaccination, revealed a small subpopulation that appeared to have compromised immune responses. Our previous work revealed a small but significant percentage of patients (5%) with no detectable titer levels after full vaccination, a phenomenon not observed in healthy controls []. Valanparambil et al. found that 25% of LC patients showed notably poorer antibody responses (p < 0.001) []. Others have reported similar results, finding minimal to moderate deficits in SAb titers in LC patients but overall high (>90%) seroconversion rates [,].
The underlying clinical, demographic, and tumor biologic factors contributing to this suboptimal titer response in this vulnerable cancer population have not been fully elucidated. Potential reasons include the immunosuppressive activity of LC therapeutics and ancillary steroid use, the underlying malignancy, and history of cigarette smoking. When analyzing these effects, a substantial complicating factor stems from the timing and severity of SARS-CoV-2 infections and the timing of vaccinations and boosters relative to cancer-related events, including changes in disease status, cancer treatments, and use of steroids. We hypothesized that an analytic approach that takes into account the timing of vaccinations, SARS-CoV-2 infections, and cancer treatments relative to plasma draws conducted to measure Sab titers would provide more precise information regarding the effect of these events on immune response. To address this, we developed a time-dependent linear regression model to analyze the effects of cancer treatments, steroid use, demographics, infections, vaccinations, and other factors in the period immediately before anti-spike antibody titer level measurements. We applied this model to our large, well-annotated cohort of LC patients who have undergone serial plasma analysis every three months to measure Sab titer levels.
2. Materials and Methods
2.1. Study Design and Participant Data Collection
A prospective observational study (Mount Sinai STUDY-20-01470) received Institutional Review Board approval on 11 November 2020. This ongoing study is enrolling patients with LC treated at the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai Hospital, NY; the University of Texas Southwestern Medical Center in Dallas, TX; and National Jewish Health in Denver, CO. Adult patients diagnosed with LC of any stage, histology, or treatment status were eligible, regardless of SARS-CoV-2 infection or vaccination status. We continuously collected and updated clinical and demographic information, including previous SARS-CoV-2 infection, COVID-19 severity, vaccination (influenza and SARS-CoV-2) status and dates, patient self-reported smoking status, age at enrollment, co-morbidities, and treatments. Inclusion criteria were limited to an age of 18 years or older, no other ongoing cancer types, and ability to comprehend the informed consent form (produced in English, Spanish, Chinese, and Bengali). For this study, enrollment was limited to patients who had contributed at least one blood draw and were fully vaccinated, defined as two weeks after completion of initial vaccination series (for mRNA vaccines—after the second dose). Patients enrolled to the master study were not required to have received any vaccinations or boosters (SARS-CoV-2, viral influenza, or otherwise) or to have had documented SARS-CoV-2 infections; however, this sub-study analysis was limited to those who were fully vaccinated with the initial series. A comparison of these cohorts is shown in Table 1. Further cohort details are provided in our initial publication [].

Table 1.
Demographics.
2.2. Blood Draws and Processing
Blood sample collection was planned at enrollment and every 3 months up to 24 months. For titer analyses, blood was drawn into ethylenediaminetetraacetic acid (EDTA) tubes using a double-spin approach, aliquoted, and stored at −80 °C.
2.3. SARS-CoV-2 Binding Antibody Assessment
Anti-spike antibodies to SARS-CoV-2 were measured using a well-established two step enzyme-linked immunosorbent assay (ELISA) [,,,], as previously reported for this cohort []. Anti-nucleocapsid antibody analysis was conducted in separately frozen aliquots using the methodology and dichotomizations previously reported [,].
2.4. Statistical Considerations
The primary objective of this longitudinal cohort study was to investigate the association of anti-spike antibody titers with clinical characteristics in patients with LC who have been fully vaccinated (defined as: two doses of mRNA vaccines, Moderna mRNA-1273 or Pfizer BNT162b2, or one dose of adenoviral vaccine, J&J Ad26.COV2.S). A time-dependent linear regression model with generalized estimating equations (GEE) [] was applied (described in detail below), where the exchangeable correlation structure was used for the modeling of within-patient titers (logarithm base 10). We considered age at enrollment, self-reported gender, smoking history, race/ethnicity, cancer stage, and histology as time-independent variables, whereas time interval after being fully vaccinated, the use of anti-inflammatory medicine/anti-cancer treatments (steroids, chemotherapy, targeted therapy, or immunotherapy) within 30 days prior to the titer measurements, the receipt of influenza vaccine/SARS-CoV-2 booster within 90 days prior to the titer measurements, and the infection with SARS-CoV-2 prior to the titer measurements were considered time-dependent variables. In the primary regression model, the two continuous variables (age and time interval after being fully vaccinated) were transformed by cubic B-splines. Forest plots and waterfall plots were employed to visualize the significance and magnitude of association between the titers and the clinical characteristics. A sensitivity analysis was also conducted to assess the reliability of the findings (Supplementary Tables S4 and S5). In the sensitivity analysis, we excluded 16 titer measurements from 16 patients that occurred when both the influenza vaccine and the SARS-CoV-2 booster were administered within 90 days prior to the measurements and the two vaccines were administered within 10 days of each other. Furthermore, to explore the relationship between the titers and the clinical characteristics in different subgroups, we performed subgroup analyses, where the analysis cohort was divided by gender, ethnicity, or histology. All data analyses were carried out using base R 4.0.3 and the R packages Hmisc 4.5-0, mice 3.16.0, geepack 1.3-2, splines 4.0.3, and ggplot2 3.3.3.
2.5. Generalized Estimating Equations (GEE)
Let be the -th measurement of log10(anti-S) after full vaccination of patient , ( varies with patients), and (patients). Denote and as the mean and the variance of , respectively. We assume and , where is the vector of covariates, where covariates can be time-dependent or independent, and is the vector of coefficients to show the effect magnitudes. Also, we assume the correlation of as , where is an identity matrix of dimension , and is a square matrix where all elements are 1 and its dimension is the same as . is the intra-patient correlation.
With the measurements and , and , the coefficient estimate for can be obtained by solving the equation , where , , and . It is well-known from Ref. [] that under mild regularity conditions, approximates when is large, where and is the covariance of . The estimate for is given by , where With and , the confidence intervals and p-values can be calculated.
3. Results
3.1. Patients
Between 1 January 2021 and 13 March 2023, 398 patients were enrolled. For this analysis, 296 patients who had at least one SARS-CoV-2 antibody titer measurement and had received the full vaccination series at the time of data cut-off were included (Supplemental Figure S1). Of these, 267 were from the Tisch Cancer Institute at the Mount Sinai Hospital in New York City, 27 were from the University of Texas Southwestern in Dallas, and 2 patients were from National Jewish Health in Denver, Colorado. The median age was 69 (IQR: 62–76), 55% were female, and 26% self-reported as never-smokers. There were no significant differences between the enrolled cohort and the analysis cohort. Additional patient characteristics such as tumor stage, histology, first vaccination type, and cancer therapy are summarized in Table 1. The number and vaccination type sequence over time are shown by category and frequency in Supplemental Table S1.
3.2. Anti-Spike Antibody Titers over Time
SARS-CoV-2 anti-Spike antibody (SAb) measurements over time are shown in Figure 1A as independent events with a trend line. In Figure 1B, longitudinal SAb timepoints for each individual patient are connected by lines. Data points are color-coded based on number of vaccinations received, with “zero” on the x-axis representing time of full vaccination (two weeks past first dose of J&J or second dose of an mRNA-based vaccine). In this population, where 75% (223/296) of patients received at least one booster vaccination and 40% (119/296) received multiple boosters, the trend line shows an upward trend approximately 1 year after initial vaccination.
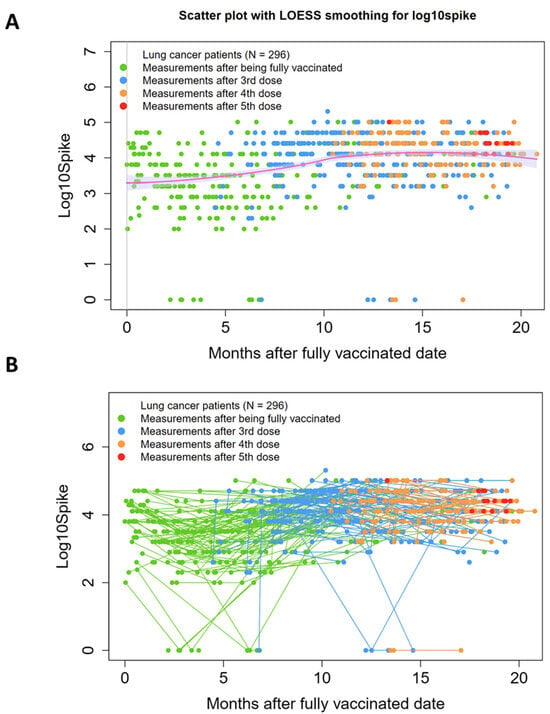
Figure 1.
SARS-CoV-2 anti-Spike antibody titers (SAb) measurements over time. (A) Scatter plot with LOESS smoothing, with measurements shown as independent events with a trend line. (B) Spaghetti plot, with data from individual patients linked by a straight connecting line. Data points are color-coded based on number of vaccinations received, with “zero” on the x-axis representing time of full vaccination (two weeks past first dose of J&J or second dose of an mRNA-based vaccine). Y-axis: Log10 of Sab titers. Individual patients who received boosters trend toward increased titers over time.
As we reported previously [], there was a small contingent of patients (n = 11, 5%) that showed at least one SAb reading of zero after full vaccination. In seven of these cases, subsequent booster vaccinations resulted in seroconversion to positive readings, with the exception of one patient, who maintained a zero titer through two boosters. Nucleocapsid antibody titers (anti-N) were also modest in these patients, with three achieving positivity by our pre-established cut-off []. Anti-S and anti-N levels over time for patients with at least one zero SAb reading after full vaccination are shown in Supplemental Figure S2A,B, respectively.
3.3. Model Findings
Variable considerations are presented in Table 2, with time dependence or time independence indicated. Variables that apply to all patients (age, gender, smoking history, ethnicity, cancer stage, histology, and time after full vaccination) are scored for completeness of data. Variable events which include specific cancer-related treatment interventions (steroid use, cancer therapies categorized as chemotherapy, targeted therapy, and immunotherapy), SARS-CoV-2 and influenza vaccinations, and SARS-CoV-2 infection are annotated for proportion of patients affected. SARS-CoV-2 infection rates were calculated as a product of clinically documented infections and/or nucleocapsid Ab positivity using previously described definitions []. Figure 2 displays the effects of antibody titers according to patient, cancer, and SARS-CoV-2 characteristics. The effect magnitude, p-value, and confidence intervals are shown in Table 3, with nonlinear effects of time after full vaccination and age detailed in Supplemental Table S3. In this cohort, steroid use (p = 0.043) and chemotherapy (p = 0.033), but not targeted or immune therapy, were significant contributors to decreased titers, whereas SARS-CoV-2 vaccination/boosters (p < 0.001), SARS-CoV-2 infection (p < 0.001), and surprisingly, influenza vaccination (p < 0.001) were independent significant contributors to increased titer levels. A history of tobacco smoking trended towards a decrease in SAb levels, but did not achieve significance in the general population (p = 0.089). Age in this LC population did not appear to influence titer levels (p = 0.819, a Chi-square test of df = 3 for the three components of age). In addition, time after full vaccination was associated with a population-level net increase in titer levels (p < 0.001, a Chi-square test of df = 3 for the three components of the time variable), peaking approximately sixteen months after full vaccination before trending downward.

Table 2.
Variables Considered in Model.
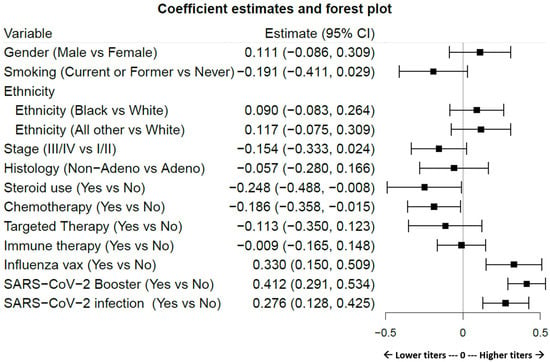
Figure 2.
Forest plot visualization of the magnitude with confidence intervals showing the effect of each variable on Sab titers. Estimates to the left of the zero line indicate decreasing effects on titer levels and to the right show increasing effects. Variable definitions and limits are articulated in Table 2. Table 3 shows numeric values. Significantly increased effects are observed subsequent to SARS-CoV-2 boosters, SARS-CoV-2 infections, and influenza vaccination. Significantly decreased effects are observed after chemotherapy and steroid use.

Table 3.
Impact of Variables.
The combined effects of multiple factors on SAb levels are estimated as a waterfall plot in Figure 3. Here, the individual and combined effects of smoking, steroid use, chemotherapy use, influenza vaccine, and SARS-CoV-2 vaccination/booster are shown in every possible combination, as indicated by the presence (Y) or absence (N) in the left columns. The waterfall plot is organized from most positive collective effect to most negative effect. The greatest positive effect on SAb levels resulted from a combination of recent SARS-CoV-2 booster vaccination, recent influenza vaccination, and an absence of smoking history, steroid use, and chemotherapy use. The greatest negative effect was observed in patients with a combined history of smoking, steroid use, receipt of recent chemotherapy, and temporal distance from vaccinations.
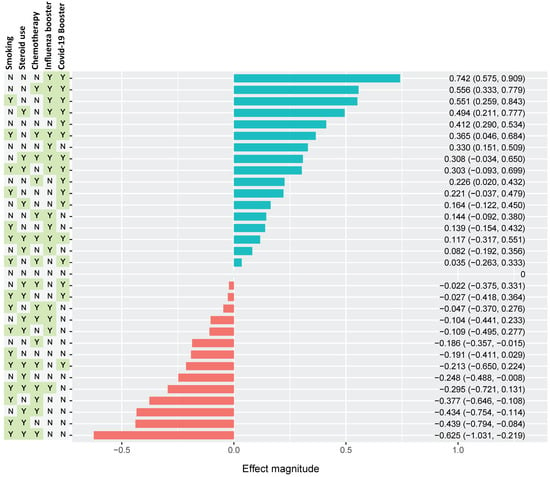
Figure 3.
Estimated combined effect of multiple variables on Sab titers. Selected variables include smoking history, steroid use, chemo use, influenza vaccination, and SARS-CoV-2 vaccination as yes (Y) or no (N). Effect magnitude is indicated as positive (teal) or negative (red) annotated for magnitude estimate and CIs. The variable groups are ordered vertically, with the most positive combination at the top and the most negative combination at the bottom (normalized to an “all zero” configuration). The most positive effect was observed in the N-N-N-Y-Y, which indicates a grouping of never-smokers with no recent steroid or chemo use, as well as recent receipt of both influenza and SARS-CoV-2 vaccines. The most negative effect occurs in group Y-Y-Y-N-N, with positive smoking history with recent use of both steroids and chemotherapy, but no recent receipt of vaccine boosters.
3.4. Differences by Gender
The distribution of analysis variables between females (n = 164) and males (n = 132) is shown in Supplemental Table S2. Females were more likely to be never-smokers (32% vs. 18%; p = 0.007) and more likely to be diagnosed with adenocarcinoma histology (74% vs. 60%; p = 0.008). Tumor stage and ethnicity did not significantly differ by gender. The effect estimate by forest plot is shown in Supplemental Figure S3 for each gender. In females, a significant negative effect from smoking on SAb levels was observed, which was not seen in males (who had an overall significantly increased smoking history). In contrast, the positive effect on SAb levels from influenza vaccination trended in females, but was significant in males. In males, the vaccination effect (both SARS-CoV-2 and influenza), along with SARS-CoV-2 infection, were more pronounced.
4. Discussion
As COVID-19 segues into an endemic problem, strategies now turn to identifying vulnerable populations, including cancer patients, that may respond suboptimally to vaccination and/or are at greater risk of severe COVID-related disease. Aspects of SARS-CoV-2 that make it particularly dangerous are its ability to evolve and rapidly proliferate new variants, as exemplified by its penchant for zoonotic infections []. With increased survival rates in advanced non-small cell LC patients due to modern therapies, ongoing studies necessarily evolved along with the pandemic to investigate potential interactions of these two diseases and implications for cancer therapies. However, such longitudinal studies are challenging due to the continuing, patient-specific evolution of both diseases. The principal obstacle stems from the highly variable timing of key events that serve as critical variables when studying the SARS-CoV-2—lung cancer juxtaposition. From the cancer care perspective, therapy, disease status, steroid use, and secondary interventions are constantly evolving. From the COVID-19 side, booster vaccinations, infections with emerging variants, and specific infection therapies occur sporadically. These time-dependent variables complicate the interpretation of SARS-CoV-2 antibody titer levels (vaccine- or infection-induced) already influenced by time-independent variables (ethnicity, age at diagnosis, gender, smoking history) and disease-specific variables (stage, histology).
To directly address this challenge, we developed a time-dependent linear regression model incorporating an estimating equation using an exchangeable correlation structure for modeling the association of within-participant titers. The model proved necessary in order to disentangle the effects of time-dependent variables on longitudinal SAb levels. This analysis revealed several key effects that were not previously discernable without an understanding of the events and interventions immediately preceding each titer reading. Obvious time-based effects on titer levels included booster shots and infections, both of which proved, as expected, to have a significant impact on SAb levels. By accounting for these large effects in a temporal context, we were able to explore the more subtle impact of other events and interventions.
One such feature was the negative effect of chemotherapy on titer levels. Modeling revealed a significant decline in patient SAb levels after chemotherapy treatment, which were not observed after targeted therapy or immunotherapy. In many cases, patients may have received combined treatment with chemotherapy and immune therapy; however, only chemotherapy had an impact on titer levels. The model assessed chemotherapy impact, if it was received within a 30-day window prior to titer measurement. Previous LC studies have been mixed in terms of identifying an effect of chemotherapy on titer levels. Poor seroconversion was linked to various cancer therapies in solid tumors, of which 10% were LC []. Others did not observe an effect in specific LC populations [,,]. Bowes et al. compared LC patients receiving radiotherapy to two separate control groups, showing significantly lower SAb levels in the treated group, although this group also had other concurrent immune suppressive conditions []. In our study, radiotherapy was not investigated.
Steroid use also emerged as a significant negative factor in regards to titer levels when evaluated in proximity to titer readings. In this population, steroids were sporadically used in response to acute conditions such as COPD exacerbation, radiation pneumonitis, or checkpoint inhibitor toxicity. Its modest but significant impact was unseen when using a static, population-based model. While we expected to see that steroid use could have an adverse effect on titer levels, its influence only became statistically apparent when using time-adjacent measurements, an indicator of this model’s effectiveness.
The effect of prior smoking was observed specifically and most strongly in females. Whether this was causative or associative with the significantly increased number of female never-smoker patients in this study, and the consequently significantly higher rate of adenocarcinoma, cannot be determined. Trontzas et al. observed an association between active smokers and lower post-vaccination anti-SARs-CoV-2 spike titer levels, but with no comparison of gender [].
An intriguing observation from this study was the effect of influenza vaccines on SARS-CoV-2 titer levels. This unexpected finding appears to be independent of whether the patients received this vaccination within the same time window with a SARS-CoV-2 booster. However, a sufficient number of patients also received those vaccinations at different times or received just one or the other, allowing for the effects to be measured independently. In the overall population and in the male-only subgroup, receipt of an influenza vaccination within 90 days prior to a titer reading had a significant positive effect on SAb levels. The underlying mechanism for this effect is currently being explored, and it certainly cannot be ruled out that it is associative rather than causative. For instance, patients in better health or with a better performance status may opt for receiving the influenza vaccination in addition to SARS-CoV-2 vaccine, creating an untestable bias. Future studies of cancer vaccines may consider inclusion of patient vaccination and infection history.
While the effects of SARS-CoV-2 infection and booster vaccinations are expected to increase SAb levels, as confirmed in this dataset, a modeling strategy that accounts for these large effects was nevertheless essential to investigate more subtle effects of other variables. To identify mild or asymptomatic infections, anti-SARS-CoV-2 nucleocapsid antibody levels were measured as a proxy for infection to supplement reported incidents. This analysis revealed a substantial and significant increase in infection rates for this study population compared to the documented infection rate alone, as described recently []. In this fully vaccinated population, we did not observe many severe complications from COVID-19, as was the case in patients prior to vaccination.
At the population level, the positive influences on titer levels from vaccination boosters and infections outweighed the negative effects from variables such as smoking history, chemotherapy, and steroid use, as well as the expected generalized decline of titer levels over time. This resulted in a net increase in population SAb levels for the first year or so before the levels plateaued and ultimately diminished approximately 16 months after full vaccination. Despite a subset of patients who initially did not respond to vaccination [], median anti-spike titer levels in this fully vaccinated LC population remained elevated more than 20 months after vaccination.
Factors that did not appear to significantly affect SAb levels in our population of patients with LC included age, tumor stage, histology, ethnicity, and gender. In the current study, the median age was 69, and a lack of representation from younger individuals (typical to LC cohorts) may skew the results. Comparisons between tumor stages showed non-significant trends towards higher stages associated with lower SAb levels, but significance was not observed in the general population (comparison of Stage I/II versus III/IV: p = 0.090), but trended in the male-only subgroup (p = 0.054). Patients of Caucasian descent represented 47% of the analysis dataset, with African Americans representing 20%, but no significant distinctions were observed in terms of variable effects on SAb levels between race/ethnicity (p = 0.407).
Beyond these clinically important immediate concerns involving vaccine-induced protection against SARS-CoV-2 infection, there is a significant knowledge gap regarding factors that influence B and T cell immunologic responses in LC patients in general. With the advent of mRNA vaccines directed against tumor acquired neo-antigens [], it is important to develop statistical approaches, such as the model developed and used here, as well as baseline data to help guide these studies. It will be of great interest to see if these anti-SARS-CoV-2 results are mirrored by those for anti-neoepitope vaccines, illustrating the need to prospectively plan for the long-term serial collection of multi-factorial data in such studies.
5. Conclusions
Advanced modeling that takes into account the timing of key events relative to longitudinal measurements of SARS-CoV-2 antibody levels provides a more detailed and granular assessment of variables that can positively or negatively impact serology. These studies revealed the expected positive effects of SARS-CoV-2 infection and booster vaccinations on Sab titer levels, as well as the negative effects of steroid use and chemotherapy treatment. Additionally, an unanticipated positive impact of influenza vaccination was observed on patient titers. Future studies, both specifically in the context of COVID-19 and LC, as well as for all types of other health studies that rely on the long-term serial collection of multi-factorial data, could benefit from similar modeling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12070713/s1, Figure S1: Consort plot; Figure S2: “zero “ titer patients. A. Spaghetti plot showing longitudinal SAb levels in the subset of patients who had at least one “zero” titer reading after full vaccination (n = 11). B. The same patient set with graph showing anti-nucleocapsid Ab titers.; Figure S3: Forest plots with estimated combined effect of multiple variables on SAb levels dichotomized by sex. Table S1. Patient-level differences in the sequence of administration of vaccinations by type in the entire cohort. Table S2. Population-level differences between females and males in the analysis cohort (n = 296). Statistical tests used: Fisher’s exact test for discrete data; Wilcoxon test for continuous data. Table S3. Effects of Time after fully vaccinated and Age in main analysis. Table S4. Sensitivity analysis. Table S5. Effects of Time after fully vaccinated and Age in sensitivity analysis.
Author Contributions
Conceptualization, P.C.M., A.M., J.C.K., J.D.M., P.A.B.J., and Y.S.; methodology, C.-Y.H., A.M.R., Y.H., R.M.V., F.K., and Y.S.; validation, P.C.M., C.-Y.H., and F.K.; formal analysis, P.C.M., C.-Y.H., A.M.R., Y.H., A.G.-S., and Y.S.; investigation, P.C.M., C.-Y.H., A.M.R., J.E.G., J.C., R.M.V., N.R., J.M.C., C.R., D.E.G., A.M., R.A., P.A.B.J., A.G.-S., and F.R.H.; resources, R.B., B.N., S.B., and F.R.H.; data curation, P.C.M., A.M.R., J.E.G., J.C., S.T., R.B., B.N., S.B., C.R., D.E.G., and J.C.K.; writing—original draft, P.C.M. and Y.S.; writing—review and editing, P.C.M., C.-Y.H., A.M.R., J.E.G., J.C., Y.H., S.T., R.M.V., N.R., R.B., B.N., J.M.C., S.B., C.R., D.E.G., A.M., J.C.K., R.A., J.D.M., P.A.B.J., A.G.-S., F.K., F.R.H., and Y.S.; supervision, P.C.M.; project administration, P.C.M., J.D.M., A.G.-S., and F.R.H.; funding acquisition, P.C.M. and F.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NCI SeroNET grant U54CA260560. We would like to acknowledge support from the Mount Sinai Biorepository and Pathology CoRE.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Mount Sinai Tisch Cancer Institute (Mount Sinai STUDY-20-01470, 11 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Access to data generated by this study may be requested in accordance with SeroNET and Mount Sinai policies.
Conflicts of Interest
PCM: Consulting: Guardant Health, Vivace Therapeutics, Amgen. JDM: royalties from the NCI and UTSW for distribution of human cell lines. CR: consulting fees from AstraZeneca, Daiichi Sankyo, Regeneron and Novocure; Honoraria:Bristol-Myers Squibb (BMS), Novartis, Invitae, Guardant Health, Novocure, COR2ED, Regeneron, Bayer, Boehringer Ingelheim, Abbvie, Thermo Fisher; is on data safety monitoring or advisory boards for Roche, Invitae, Novartis, Novocure, Janssen, EMD Serono, Bayer, Regeneron; Scientific advisory board member of Imagene; is a board member or has leadership roles in International Society of Liquid Biopsy (ISLB), The European School of Oncology (ESO), International Association for Study of Lung Cancer (IASLC), and Oncology Latin American Association (OLA); and reports financial or nonfinancial interests from Lung Cancer Research Foundation (LCRF-Pfizer), National Foundation for Cancer Research (NCRF), DaEG:consulting fees from Catalyst Pharmaceuticals; U.S. patent 11,747,345; pending patents 17 /045,482, 63/386,387, 63/382,972, and 63/382,257; research funding from AstraZeneca, Karyopharm, and Novocure; participating in advisory boards for Astra-Zeneca, Daiichi-Sankyo, Elevation Oncology, Janssen Scientific Affairs, Jazz Pharmaceuticals, Regeneron Pharmaceuticals, and Sanofi; stock shares in Gilead; and serving as co-founder and Chief Medical Officer of OncoSeer Diagnostics, Inc. 5he Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines influenza virus vaccines and influenza virus therapeutics which list Florian Krammer as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, CastleVax, to develop SARS-CoV-2 vaccines. Florian Krammer is a co-founder and scientific advisory board member of CastleVax. Florian Krammer has consulted for Merck, Curevac, Seqirus, GSK and Pfizer and is currently consulting for 3rd Rock Ventures, Gritstone and Avimex. The Krammer laboratory is collaborating with Dynavax on influenza vaccine development. 6AM:serves on advisory boards for AstraZeneca, Bayer, Boehringer Ingleheim, Exact Sciences, Novartis and Takeda. All honoraria are paid to LUNGevity Foundation. 7FRsHerves on advisory boards for AstraZeneca, BMS, Genentech, Abbvie, Novocure, Nextcure, Daiichi, Regeneron, Merus, Oncohost, G1 Therapeutics. hYushas nothing to disclose. Nio: onsulting rfor AsraZeneca, Regeneron Pharmaceuticals; EMD Serono as a lung cancer expert. ThB:consultant/ advisory board member for AstraZeneca, Takeda, Merus, Mirati, and Novocure. PAB: Leadership: Verastem; Honoraria: CStone Pharmaceuticals, Ascentage Pharma, VieCure, Genentech/Roche; Consulting or Advisory Role: CStone Pharmaceuticals, Ascentage Pharma, Genentech/Roche, Ipsen . he A.G.-S. laboratory has received research support from GSK, Pfizer, Senhwa Biosciences, Kenall Manufacturing, Blade Therapeutics, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC, Model Medicines, Atea Pharma, Applied Biological Laboratories and Merck, outside of the reported work. A.G.-S. has consulting agreements for the following companies involving cash and/or stock: Castlevax, Amovir, Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Pagoda, Accurius, Esperovax, Applied Biological Laboratories, Pharmamar, CureLab Oncology, CureLab Veterinary, Synairgen, Paratus, Pfizer and Prosetta, outside of the reported work. A.G.-S. has been an invited speaker in meeting events organized by Seqirus, Janssen, Abbott, Astrazeneca and Novavax. A.G.-S. is inventor on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections and cancer, owned by the Icahn School of Medicine at Mount Sinai, New York, outside of the reported work.
References
- Garassino, M.C.; Whisenant, J.G.; Huang, L.-C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.T.; Curley, H.M.; Fittall, M.W.T.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Turpin, A.; Ray-Coquard, I.; Le Malicot, K.; Thariat, J.; Ahle, G.; Neuzillet, C.; Paoletti, X.; Aldabbagh, K.; Michel, P.; et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 2020, 141, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rizvi, H.; Egger, J.V.; Preeshagul, I.R.; Wolchok, J.D.; Hellmann, M.D. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 2020, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Tagliamento, M.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Saini, K.S.; de Azambuja, E.; Punie, K.; Westphalen, C.B.; Morgan, G.; Pronzato, P.; et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103365. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Meshulami, N.; Russo, A.; Krammer, F.; García-Sastre, A.; Mack, P.C.; Gomez, J.E.; Bhardwaj, N.; Benyounes, A.; Sirera, R.; et al. Lung Cancer and Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Identifying Important Knowledge Gaps for Investigation. J. Thorac. Oncol. 2021, 17, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, H.; He, T.; Labbe, K.E.; Hernandez, A.V.; Chen, H.; Velcheti, V.; Stebbing, J.; Wong, K.-K. Clinical Characteristics and Outcomes of COVID-19–Infected Cancer Patients: A Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2020, 113, 371–380. [Google Scholar] [CrossRef]
- Mack, P.C.; Gomez, J.E.; Rodilla, A.M.; Carreño, J.M.; Hsu, C.-Y.; Rolfo, C.; Meshulami, N.; Moore, A.; Brody, R.I.; King, J.C.; et al. Longitudinal COVID-19-vaccination-induced antibody responses and Omicron neutralization in patients with lung cancer. Cancer Cell 2022, 40, 575–577. [Google Scholar] [CrossRef]
- Valanparambil, R.M.; Carlisle, J.; Linderman, S.L.; Akthar, A.; Millett, R.L.; Lai, L.; Chang, A.; McCook-Veal, A.A.; Switchenko, J.; Nasti, T.H.; et al. Antibody Response to COVID-19 mRNA Vaccine in Patients with Lung Cancer After Primary Immunization and Booster: Reactivity to the SARS-CoV-2 WT Virus and Omicron Variant. J. Clin. Oncol. 2022, 40, 3808–3816. [Google Scholar] [PubMed]
- Hernandez, A.; Boigues, M.; Felip, E.; Cucurull, M.; Notario, L.; Pous, A.; Torres, P.; Benitez, M.; Rodriguez, M.; Quirant, B.; et al. Immune Response and Effects of COVID-19 Vaccination in Patients with Lung Cancer—COVID Lung Vaccine Study. Cancers 2022, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Uryu, K.; Takeda, T.; Kunimatsu, Y.; Shiotsu, S.; Uchino, J.; Hirai, S.; Yamada, T.; Okada, A.; Hasegawa, Y.; et al. Safety and Immunogenicity of mRNA Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 in Patients with Lung Cancer Receiving Immune Checkpoint Inhibitors: A Multicenter Observational Study in Japan. J. Thorac. Oncol. 2022, 17, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Tan, J.; Jiang, K.; Hernandez, M.M.; Fabre, S.; Amanat, F.; Teo, C.; Arunkumar, G.A.; McMahon, M.; Capuano, C.; et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature 2021, 590, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef] [PubMed]
- Żak, M.M.; Stock, A.; Stadlbauer, D.; Zhang, W.; Cummings, K.; Marsiglia, W.; Zargarov, A.; Amanat, F.; Tamayo, M.; Cordon-Cardo, C. Development and characterization of a quantitative ELISA to detect anti-SARS-CoV-2 spike antibodies. Heliyon 2021, 7, e08444. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, A.M.; Valanparambil, R.M.; Mack, P.C.; Hsu, C.Y.; Cagan, J.; Tavolacci, S.C.; Carreño, J.M.; Brody, R.; Moore, A.; King, J.C. Longitudinal nucleocapsid antibody testing reveals undocumented SARS-CoV-2 infections in patients with lung cancer. Cancer Cell 2023, 41, 1838–1840. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.-Y. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef]
- Pappas, G.; Vokou, D.; Sainis, I.; Halley, J.M. SARS-CoV-2 as a Zooanthroponotic Infection: Spillbacks, Secondary Spillovers, and Their Importance. Microorganisms 2022, 10, 2166. [Google Scholar] [CrossRef]
- Buttiron Webber, T.; Provinciali, N.; Musso, M.; Ugolini, M.; Boitano, M.; Clavarezza, M.; D’Amico, M.; Defferrari, C.; Gozza, A.; Briata, I.M. Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur. J. Cancer 2021, 159, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gounant, V.; Ferré, V.M.; Soussi, G.; Charpentier, C.; Flament, H.; Fidouh, N.; Collin, G.; Namour, C.; Assoun, S.; Bizot, A.; et al. Efficacy of Severe Acute Respiratory Syndrome Coronavirus-2 Vaccine in Patients with Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients with Minimal Serologic Response After Two Vaccine Doses. J. Thorac. Oncol. 2021, 17, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Bowes, C.L.; Naranbhai, V.; St Denis, K.J.; Lam, E.C.; Bertaux, B.; Keane, F.K.; Khandekar, M.J.; Balazs, A.B.; Iafrate, J.A.; Gainor, J.F. Heterogeneous immunogenicity of SARS-CoV-2 vaccines in cancer patients receiving radiotherapy. Radiother. Oncol. 2022, 166, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Trontzas, I.P.; Vathiotis, I.; Economidou, C.; Petridou, I.; Gomatou, G.; Grammoustianou, M.; Tsamis, I.; Syrigos, N.; Anagnostakis, M.; Fyta, E.; et al. Assessment of Seroconversion after SARS-CoV-2 Vaccination in Patients with Lung Cancer. Vaccines 2022, 10, 618. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).