Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens
Abstract
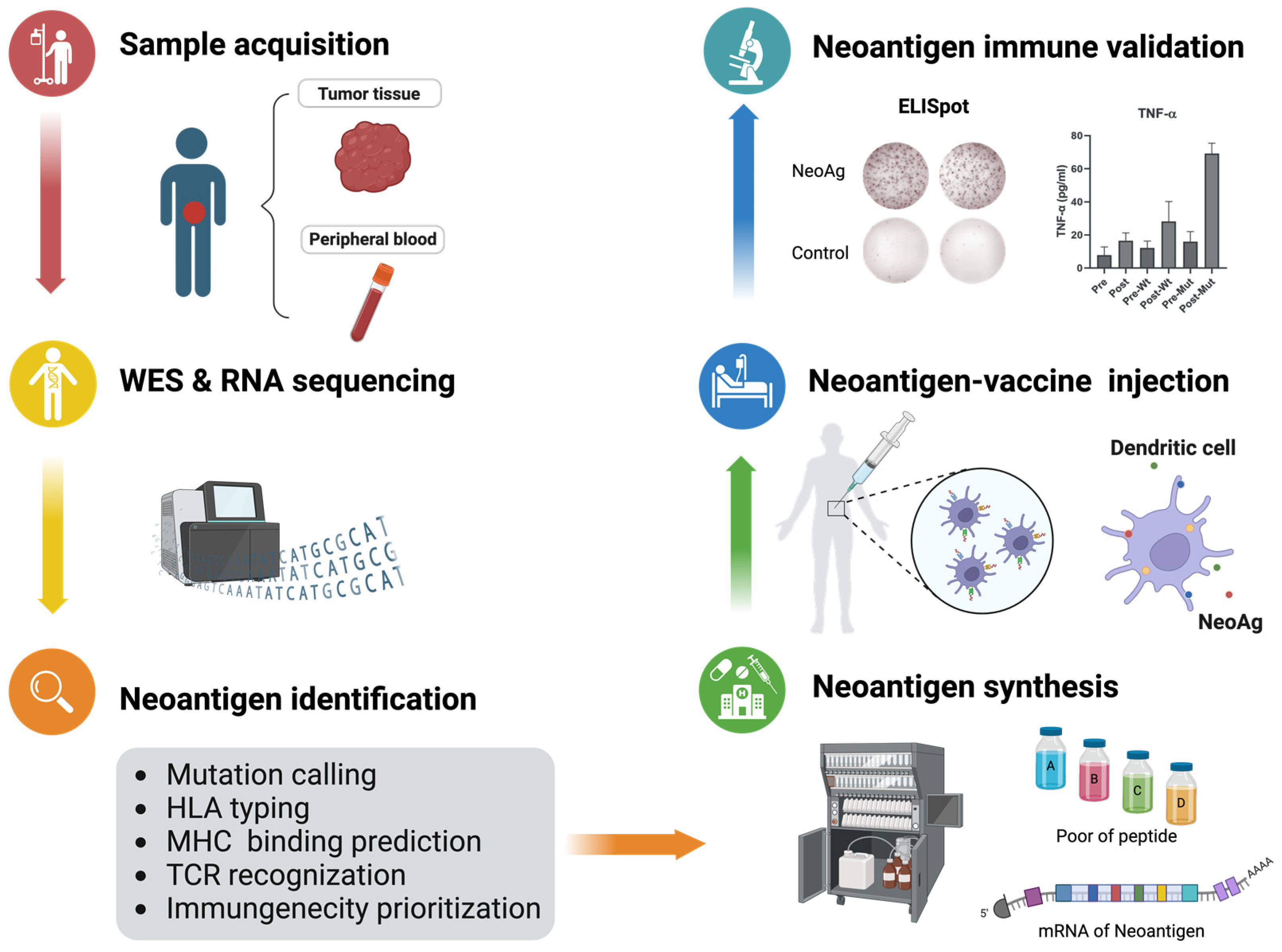
:1. Introduction
2. Prediction and Selection of Neoantigens
2.1. Source of Neoantigen
2.2. Prediction of Neoantigen
3. Presentation by MHC Molecular
4. Recognition by TCR
5. Neoantigen and Immunotherapies
5.1. Neoantigen-Based Cancer Vaccines
5.1.1. mRNA-Based Vaccines
5.1.2. Peptide-Based Vaccines
5.1.3. DC-Based Vaccines
5.2. TCR-T
5.3. Combination Therapies
6. Shared Vaccine
6.1. A Special Class of Neoantigen: Shared Neoantigen
6.1.1. KRAS
6.1.2. TP53
6.1.3. IDH1
6.1.4. EGFR
6.1.5. PIK3CA
6.1.6. ALK
| Gene | Frequency | Neoantigen Peptides | MHC Restriction | Immunotherapy |
|---|---|---|---|---|
| KRAS | 93% PDAC 50% CRC | G12D-YKLVVVGADGVGKSALTI G12R-YKLVVVGARGVGKSALTI | / | Neoantigen vaccines [132] |
| G12D-GADGVGKSA(L) | HLA-C*08:02 | TCR-T therapy [140,148] | ||
| TP53 | 20% BRCA | R175H-HMTEVVRHC | HLA-A*02:01 | TCR-T therapy [145], bispecific antibody [152] |
| IDH1 | 70% GL | R132H-GWVKPIIIGHHAYGDQYRAT | HLA-DRB1*01:01 | Neoantigen vaccines [154] |
| EGFR | 60% NSCLC | L858R-KITDFGRAK | HLA-A*11:01 | Neoantigen vaccine [156] |
| T790M-LTSTVQLIM | HLA-C*C15:02 | |||
| PIK3A | 24% BRCA 20% CESC 16% COAD | H1047L-ALHGGWTTK | HLA-A*03:01 | TCR-T therapy [160] |
| ALK | 5% NSCLC | AMLDLLHVA | HLA-A*02:01 | Neoantigen vaccines [161] |
| IVRCIGVSL RPRPSQPSSL VPRKNITLI | HLA-B*07:02 |
6.2. Tumor Associated Antigens
7. Challenges and Prospects
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in Cancer Immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor Neoantigens: From Basic Research to Clinical Applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An Immunogenic Personal Neoantigen Vaccine for Patients with Melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA Mutanome Vaccines Mobilize Poly-Specific Therapeutic Immunity against Cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen Vaccine Generates Intratumoral T Cell Responses in Phase Ib Glioblastoma Trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-Small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e24. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Q.; Zhang, R.; Xie, L.; Shu, Y.; Gao, S.; Wang, P.; Su, X.; Qin, Y.; Wang, Y.; et al. Personalized Neoantigen Pulsed Dendritic Cell Vaccine for Advanced Lung Cancer. Signal Transduct. Target. Ther. 2021, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Govindan, R.; Balogh, K.N.; Spigel, D.R.; Garon, E.B.; Bushway, M.E.; Poran, A.; Sheen, J.H.; Kohler, V.; Esaulova, E.; et al. Personalized Neoantigen Vaccine NEO-PV-01 with Chemotherapy and Anti-PD-1 as First-Line Treatment for Non-Squamous Non-Small Cell Lung Cancer. Cancer Cell 2022, 40, 1010–1026.e11. [Google Scholar] [CrossRef]
- Khattak, A.; Carlino, M.; Meniawy, T.; Ansstas, G.; Medina, T.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Abstract CT001: A Personalized Cancer Vaccine, mRNA-4157, Combined with Pembrolizumab versus Pembrolizumab in Patients with Resected High-Risk Melanoma: Efficacy and Safety Results from the Randomized, Open-Label Phase 2 mRNA-4157-P201/Keynote-942 Trial. Cancer Res. 2023, 83, CT001. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA Neoantigen Vaccines Stimulate T Cells in Pancreatic Cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer Vaccines: The next Immunotherapy Frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Pearlman, A.H.; Hwang, M.S.; Konig, M.F.; Hsiue, E.H.-C.; Douglass, J.; DiNapoli, S.R.; Mog, B.J.; Bettegowda, C.; Pardoll, D.M.; Gabelli, S.B.; et al. Targeting Public Neoantigens for Cancer Immunotherapy. Nat. Cancer 2021, 2, 487–497. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively Personalized Vaccination Trial for Newly Diagnosed Glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef]
- Schmidt, J.; Smith, A.R.; Magnin, M.; Racle, J.; Devlin, J.R.; Bobisse, S.; Cesbron, J.; Bonnet, V.; Carmona, S.J.; Huber, F.; et al. Prediction of Neo-Epitope Immunogenicity Reveals TCR Recognition Determinants and Provides Insight into Immunoediting. Cell Rep. Med. 2021, 2, 100194. [Google Scholar] [CrossRef]
- Wells, D.K.; van Buuren, M.M.; Dang, K.K.; Hubbard-Lucey, V.M.; Sheehan, K.C.F.; Campbell, K.M.; Lamb, A.; Ward, J.P.; Sidney, J.; Blazquez, A.B.; et al. Key Parameters of Tumor Epitope Immunogenicity Revealed Through a Consortium Approach Improve Neoantigen Prediction. Cell 2020, 183, 818–834.e13. [Google Scholar] [CrossRef]
- Kumar, H.; Luo, R.; Wen, J.; Yang, C.; Zhou, X.; Kim, P. FusionNeoAntigen: A Resource of Fusion Gene-Specific Neoantigens. Nucleic Acids Res. 2024, 52, D1276–D1288. [Google Scholar] [CrossRef]
- Greaves, M. A Causal Mechanism for Childhood Acute Lymphoblastic Leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef]
- Schram, A.M.; Chang, M.T.; Jonsson, P.; Drilon, A. Fusions in Solid Tumours: Diagnostic Strategies, Targeted Therapy, and Acquired Resistance. Nat. Rev. Clin. Oncol. 2017, 14, 735–748. [Google Scholar] [CrossRef]
- Dai, X.; Theobard, R.; Cheng, H.; Xing, M.; Zhang, J. Fusion Genes: A Promising Tool Combating against Cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2018, 1869, 149–160. [Google Scholar] [CrossRef]
- Gao, Q.; Liang, W.-W.; Foltz, S.M.; Mutharasu, G.; Jayasinghe, R.G.; Cao, S.; Liao, W.-W.; Reynolds, S.M.; Wyczalkowski, M.A.; Yao, L.; et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018, 23, 227–238.e3. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Starck, S.R.; Shastri, N. Nowhere to Hide: Unconventional Translation Yields Cryptic Peptides for Immune Surveillance. Immunol. Rev. 2016, 272, 8–16. [Google Scholar] [CrossRef]
- Wright, B.W.; Yi, Z.; Weissman, J.S.; Chen, J. The Dark Proteome: Translation from Noncanonical Open Reading Frames. Trends Cell Biol. 2022, 32, 243–258. [Google Scholar] [CrossRef]
- Cuevas, M.V.R.; Hardy, M.-P.; Hollý, J.; Bonneil, É.; Durette, C.; Courcelles, M.; Lanoix, J.; Côté, C.; Staudt, L.M.; Lemieux, S.; et al. Most Non-Canonical Proteins Uniquely Populate the Proteome or Immunopeptidome. Cell Rep. 2021, 34, 108815. [Google Scholar] [CrossRef]
- Ouspenskaia, T.; Law, T.; Clauser, K.R.; Klaeger, S.; Sarkizova, S.; Aguet, F.; Li, B.; Christian, E.; Knisbacher, B.A.; Le, P.M.; et al. Unannotated Proteins Expand the MHC-I-Restricted Immunopeptidome in Cancer. Nat. Biotechnol. 2022, 40, 209–217. [Google Scholar] [CrossRef]
- Kahles, A.; Lehmann, K.-V.; Toussaint, N.C.; Hüser, M.; Stark, S.G.; Sachsenberg, T.; Stegle, O.; Kohlbacher, O.; Sander, C.; Cancer Genome Atlas Research Network; et al. Comprehensive Analysis of Alternative Splicing Across Tumors from 8705 Patients. Cancer Cell 2018, 34, 211–224.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, L.-L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhu, X.; Ye, S.; Zhang, J.; Liao, J.; Zhang, N.; Zeng, X.; Wang, J.; Yang, B.; Zhang, Y.; et al. Tumour Circular RNAs Elicit Anti-Tumour Immunity by Encoding Cryptic Peptides. Nature 2024, 625, 593–602. [Google Scholar] [CrossRef]
- Baralle, F.E.; Giudice, J. Alternative Splicing as a Regulator of Development and Tissue Identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Jiao, B.; Nie, J.; Li, X.; Wang, W.; Wang, H. The Roles of Alternative Splicing in Tumor-Immune Cell Interactions. Curr. Cancer Drug Targets 2020, 20, 729–740. [Google Scholar] [CrossRef]
- Zarling, A.L.; Polefrone, J.M.; Evans, A.M.; Mikesh, L.M.; Shabanowitz, J.; Lewis, S.T.; Engelhard, V.H.; Hunt, D.F. Identification of Class I MHC-Associated Phosphopeptides as Targets for Cancer Immunotherapy. Proc. Natl. Acad. Sci. USA 2006, 103, 14889–14894. [Google Scholar] [CrossRef]
- Malaker, S.A.; Penny, S.A.; Steadman, L.G.; Myers, P.T.; Loke, J.C.; Raghavan, M.; Bai, D.L.; Shabanowitz, J.; Hunt, D.F.; Cobbold, M. Identification of Glycopeptides as Posttranslationally Modified Neoantigens in Leukemia. Cancer Immunol. Res. 2017, 5, 376–384. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.-G.; et al. Cross-Reactivity between Tumor MHC Class I-Restricted Antigens and an Enterococcal Bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Kalaora, S.; Nagler, A.; Nejman, D.; Alon, M.; Barbolin, C.; Barnea, E.; Ketelaars, S.L.C.; Cheng, K.; Vervier, K.; Shental, N.; et al. Identification of Bacteria-Derived HLA-Bound Peptides in Melanoma. Nature 2021, 592, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rousseau, B.; Qiu, K.; Huang, G.; Zhang, Y.; Su, H.; Le Bihan-Benjamin, C.; Khati, I.; Artz, O.; Foote, M.B.; et al. Killing Tumor-Associated Bacteria with a Liposomal Antibiotic Generates Neoantigens That Induce Anti-Tumor Immune Responses. Nat. Biotechnol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Lybaert, L.; Lefever, S.; Fant, B.; Smits, E.; De Geest, B.; Breckpot, K.; Dirix, L.; Feldman, S.A.; Van Criekinge, W.; Thielemans, K.; et al. Challenges in Neoantigen-Directed Therapeutics. Cancer Cell 2023, 41, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising Targets for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Ishii, K.J.; Schliehe, C. Challenges in Developing Personalized Neoantigen Cancer Vaccines. Nat. Rev. Immunol. 2024, 24, 213–227. [Google Scholar] [CrossRef]
- Lang, F.; Schrörs, B.; Löwer, M.; Türeci, Ö.; Sahin, U. Identification of Neoantigens for Individualized Therapeutic Cancer Vaccines. Nat. Rev. Drug Discov. 2022, 21, 261–282. [Google Scholar] [CrossRef]
- Bjerregaard, A.-M.; Nielsen, M.; Hadrup, S.R.; Szallasi, Z.; Eklund, A.C. MuPeXI: Prediction of Neo-Epitopes from Tumor Sequencing Data. Cancer Immunol. Immunother. 2017, 66, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mardis, E.R.; Maher, C.A. INTEGRATE-Neo: A Pipeline for Personalized Gene Fusion Neoantigen Discovery. Bioinformatics 2017, 33, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Schenck, R.O.; Lakatos, E.; Gatenbee, C.; Graham, T.A.; Anderson, A.R.A. NeoPredPipe: High-Throughput Neoantigen Prediction and Recognition Potential Pipeline. BMC Bioinform. 2019, 20, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wei, Z.; Zhang, Z.; Zhang, B.; Zhu, C.; Chen, K.; Chuai, G.; Qu, S.; Xie, L.; Gao, Y.; et al. pTuneos: Prioritizing Tumor Neoantigens from next-Generation Sequencing Data. Genome Med. 2019, 11, 67. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Tang, L.; Gong, Y.; Wei, Z.; Zhang, G.; Wang, F.; Liu, Q.; Yu, J. ASNEO: Identification of Personalized Alternative Splicing Based Neoantigens with RNA-Seq. Aging 2020, 12, 14633–14648. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Rieder, D.; Haider, M.; Trajanoski, Z.; Finotello, F. NeoFuse: Predicting Fusion Neoantigens from RNA Sequencing Data. Bioinformatics 2020, 36, 2260–2261. [Google Scholar] [CrossRef] [PubMed]
- Hundal, J.; Kiwala, S.; McMichael, J.; Miller, C.A.; Xia, H.; Wollam, A.T.; Liu, C.J.; Zhao, S.; Feng, Y.-Y.; Graubert, A.P.; et al. pVACtools: A Computational Toolkit to Identify and Visualize Cancer Neoantigens. Cancer Immunol. Res. 2020, 8, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wang, S.; Xu, L.; Zhou, Q.; Singla, N.; Gao, J.; Manna, S.; Pop, L.; Xie, Z.; Chen, M.; et al. Tumor Neoantigenicity Assessment with CSiN Score Incorporates Clonality and Immunogenicity to Predict Immunotherapy Outcomes. Sci. Immunol. 2020, 5, eaaz3199. [Google Scholar] [CrossRef]
- Diao, K.; Chen, J.; Wu, T.; Wang, X.; Wang, G.; Sun, X.; Zhao, X.; Wu, C.; Wang, J.; Yao, H.; et al. Seq2Neo: A Comprehensive Pipeline for Cancer Neoantigen Immunogenicity Prediction. Int. J. Mol. Sci. 2022, 23, 11624. [Google Scholar] [CrossRef]
- Rieder, D.; Fotakis, G.; Ausserhofer, M.; René, G.; Paster, W.; Trajanoski, Z.; Finotello, F. nextNEOpi: A Comprehensive Pipeline for Computational Neoantigen Prediction. Bioinformatics 2022, 38, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Chuwdhury, G.S.; Guo, Y.; Chiang, C.-L.; Lam, K.-O.; Kam, N.-W.; Liu, Z.; Dai, W. ImmuneMirror: A Machine Learning-Based Integrative Pipeline and Web Server for Neoantigen Prediction. Brief. Bioinform. 2024, 25, bbae024. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved Predictions of MHC Antigen Presentation by Concurrent Motif Deconvolution and Integration of MS MHC Eluted Ligand Data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Schmidt, J.; Croce, G.; Guillaume, P.; Bobisse, S.; Genolet, R.; Queiroz, L.; Cesbron, J.; Racle, J.; Harari, A. Improved Predictions of Antigen Presentation and TCR Recognition with MixMHCpred2.2 and PRIME2.0 Reveal Potent SARS-CoV-2 CD8+ T-Cell Epitopes. Cell Syst. 2023, 14, 72–83.e5. [Google Scholar] [CrossRef] [PubMed]
- Racle, J.; Guillaume, P.; Schmidt, J.; Michaux, J.; Larabi, A.; Lau, K.; Perez, M.A.S.; Croce, G.; Genolet, R.; Coukos, G.; et al. Machine Learning Predictions of MHC-II Specificities Reveal Alternative Binding Mode of Class II Epitopes. Immunity 2023, 56, 1359–1375.e13. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, T.J.; Rubinsteyn, A.; Laserson, U. MHCflurry 2.0: Improved Pan-Allele Prediction of MHC Class I-Presented Peptides by Incorporating Antigen Processing. Cell Syst. 2020, 11, 42–48.e7. [Google Scholar] [CrossRef] [PubMed]
- Sarkizova, S.; Klaeger, S.; Le, P.M.; Li, L.W.; Oliveira, G.; Keshishian, H.; Hartigan, C.R.; Zhang, W.; Braun, D.A.; Ligon, K.L.; et al. A Large Peptidome Dataset Improves HLA Class I Epitope Prediction across Most of the Human Population. Nat. Biotechnol. 2020, 38, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Reynisson, B.; Barra, C.; Buus, S.; Ternette, N.; Connelley, T.; Andreatta, M.; Nielsen, M. NNAlign_MA; MHC peptidome deconvolution for accurate MHC binding motif characterization and improved T-cell epitope predictions. Mol. Cell. Proteom. 2019, 18, 2459–2477. [Google Scholar] [CrossRef]
- Hao, Q.; Wei, P.; Shu, Y.; Zhang, Y.-G.; Xu, H.; Zhao, J.-N. Improvement of Neoantigen Identification Through Convolution Neural Network. Front. Immunol. 2021, 12, 682103. [Google Scholar] [CrossRef] [PubMed]
- Bulik-Sullivan, B.; Busby, J.; Palmer, C.D.; Davis, M.J.; Murphy, T.; Clark, A.; Busby, M.; Duke, F.; Yang, A.; Young, L.; et al. Deep Learning Using Tumor HLA Peptide Mass Spectrometry Datasets Improves Neoantigen Identification. Nat. Biotechnol. 2019, 37, 55–63. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Olsson, N.; Wagar, L.E.; Fast, E.; Liu, C.L.; Muftuoglu, Y.; Sworder, B.J.; Diehn, M.; Levy, R.; et al. Predicting HLA Class II Antigen Presentation through Integrated Deep Learning. Nat. Biotechnol. 2019, 37, 1332–1343. [Google Scholar] [CrossRef]
- Qu, W.; You, R.; Mamitsuka, H.; Zhu, S. DeepMHCI: An Anchor Position-Aware Deep Interaction Model for Accurate MHC-I Peptide Binding Affinity Prediction. Bioinformatics 2023, 39, btad551. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, A.P.; Fu, Y.; Gorbushin, N.; McHardy, R.; Djermani, B.A.; Copoiu, L.; Rooney, M.; Lang, M.; Tovchigrechko, A.; Şahin, U.; et al. LightMHC: A Light Model for pMHC Structure Prediction with Graph Neural Networks. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, B.; Guo, H.; Emani, P.; Clancy, T.; Jiang, C.; Gerstein, M.; Ning, X.; Cheng, C.; Min, M.R. Binding Peptide Generation for MHC Class I Proteins with Deep Reinforcement Learning. Bioinformatics 2023, 39, btad055. [Google Scholar] [CrossRef]
- You, R.; Qu, W.; Mamitsuka, H.; Zhu, S. DeepMHCII: A Novel Binding Core-Aware Deep Interaction Model for Accurate MHC-II Peptide Binding Affinity Prediction. Bioinformatics 2022, 38, i220–i228. [Google Scholar] [CrossRef] [PubMed]
- Hartout, P.; Počuča, B.; Méndez-García, C.; Schleberger, C. Investigating the Human and Nonobese Diabetic Mouse MHC Class II Immunopeptidome Using Protein Language Modeling. Bioinformatics 2023, 39, btad469. [Google Scholar] [CrossRef]
- Wang, G.; Wu, T.; Ning, W.; Diao, K.; Sun, X.; Wang, J.; Wu, C.; Chen, J.; Xu, D.; Liu, X.-S. TLimmuno2: Predicting MHC Class II Antigen Immunogenicity through Transfer Learning. Brief. Bioinform. 2023, 24, bbad116. [Google Scholar] [CrossRef]
- Zhang, L.; Udaka, K.; Mamitsuka, H.; Zhu, S. Toward More Accurate Pan-Specific MHC-Peptide Binding Prediction: A Review of Current Methods and Tools. Brief. Bioinform 2012, 13, 350–364. [Google Scholar] [CrossRef]
- Blaha, D.T.; Anderson, S.D.; Yoakum, D.M.; Hager, M.V.; Zha, Y.; Gajewski, T.F.; Kranz, D.M. High-Throughput Stability Screening of Neoantigen/HLA Complexes Improves Immunogenicity Predictions. Cancer Immunol. Res. 2019, 7, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Fenoy, E.; Harndahl, M.; Kristensen, A.B.; Nielsen, I.K.; Nielsen, M.; Buus, S. Pan-Specific Prediction of Peptide-MHC Class I Complex Stability, a Correlate of T Cell Immunogenicity. J. Immunol. 2016, 197, 1517–1524. [Google Scholar] [CrossRef]
- Dash, P.; Fiore-Gartland, A.J.; Hertz, T.; Wang, G.C.; Sharma, S.; Souquette, A.; Crawford, J.C.; Clemens, E.B.; Nguyen, T.H.O.; Kedzierska, K.; et al. Quantifiable Predictive Features Define Epitope-Specific T Cell Receptor Repertoires. Nature 2017, 547, 89–93. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, X.; Li, B. GIANA Allows Computationally-Efficient TCR Clustering and Multi-Disease Repertoire Classification by Isometric Transformation. Nat. Commun. 2021, 12, 4699. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, C.; Rubelt, F.; Scriba, T.J.; Davis, M.M. Analyzing the Mycobacterium Tuberculosis Immune Response by T-Cell Receptor Clustering with GLIPH2 and Genome-Wide Antigen Screening. Nat. Biotechnol. 2020, 38, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Jurtz, V.I.; Jessen, L.E.; Bentzen, A.K.; Jespersen, M.C.; Mahajan, S.; Vita, R.; Jensen, K.K.; Marcatili, P.; Hadrup, S.R.; Peters, B.; et al. NetTCR: Sequence-Based Prediction of TCR Binding to Peptide-MHC Complexes Using Convolutional Neural Networks. bioRxiv 2018. [Google Scholar] [CrossRef]
- Montemurro, A.; Schuster, V.; Povlsen, H.R.; Bentzen, A.K.; Jurtz, V.; Chronister, W.D.; Crinklaw, A.; Hadrup, S.R.; Winther, O.; Peters, B.; et al. NetTCR-2.0 Enables Accurate Prediction of TCR-Peptide Binding by Using Paired TCRα and β Sequence Data. Commun. Biol. 2021, 4, 1060. [Google Scholar] [CrossRef] [PubMed]
- Gielis, S.; Moris, P.; Bittremieux, W.; Neuter, N.D.; Ogunjimi, B.; Laukens, K.; Meysman, P. TCRex: Detection of Enriched T Cell Epitope Specificity in Full T Cell Receptor Sequence Repertoires. bioRxiv 2019. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, J.; Zheng, T.; Zhang, X.; Xiao, X.; Zhu, X.; Lai, X.; Liu, X. SETE: Sequence-Based Ensemble Learning Approach for TCR Epitope Binding Prediction. Comput. Biol. Chem. 2020, 87, 107281. [Google Scholar] [CrossRef] [PubMed]
- Springer, I.; Besser, H.; Tickotsky-Moskovitz, N.; Dvorkin, S.; Louzoun, Y. Prediction of Specific TCR-Peptide Binding From Large Dictionaries of TCR-Peptide Pairs. Front. Immunol. 2020, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Z.; Zhu, J.; Wang, Y.; Jiang, P.; Xiao, X.; Bernatchez, C.; Heymach, J.V.; Gibbons, D.L.; Wang, J.; et al. Deep Learning-Based Prediction of the T Cell Receptor-Antigen Binding Specificity. Nat. Mach. Intell. 2021, 3, 864–875. [Google Scholar] [CrossRef]
- Peng, X.; Lei, Y.; Feng, P.; Jia, L.; Ma, J.; Zhao, D.; Zeng, J. Characterizing the Interaction Conformation between T-Cell Receptors and Epitopes with Deep Learning. Nat. Mach. Intell. 2023, 5, 395–407. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Fan, Y.; Zhu, C.; Wei, Z.; Zhou, C.; Chuai, G.; Chen, Q.; Zhang, H.; Liu, Q. Pan-Peptide Meta Learning for T-Cell Receptor–Antigen Binding Recognition. Nat. Mach. Intell. 2023, 5, 236–249. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Yao, H. Accurate TCR-pMHC Interaction Prediction Using a BERT-Based Transfer Learning Method. Brief. Bioinform. 2024, 25, bbad436. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, B.; Lin, S.; Sun, H.; Mao, X.; Wang, M.; Chu, Y.; Hong, L.; Wei, D.-Q.; Li, M.; et al. TEPCAM: Prediction of T-Cell Receptor-Epitope Binding Specificity via Interpretable Deep Learning. Protein Sci. 2024, 33, e4841. [Google Scholar] [CrossRef]
- Yin, R.; Ribeiro-Filho, H.V.; Lin, V.; Gowthaman, R.; Cheung, M.; Pierce, B.G. TCRmodel2: High-Resolution Modeling of T Cell Receptor Recognition Using Deep Learning. Nucleic Acids Res. 2023, 51, W569–W576. [Google Scholar] [CrossRef] [PubMed]
- Kwee, B.P.Y.; Messemaker, M.; Marcus, E.; Oliveira, G.; Scheper, W.; Wu, C.J.; Teuwen, J.; Schumacher, T.N. STAPLER: Efficient Learning of TCR-Peptide Specificity Prediction from Full-Length TCR-Peptide Data. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jokinen, E.; Dumitrescu, A.; Huuhtanen, J.; Gligorijević, V.; Mustjoki, S.; Bonneau, R.; Heinonen, M.; Lähdesmäki, H. TCRconv: Predicting Recognition between T Cell Receptors and Epitopes Using Contextualized Motifs. Bioinformatics 2023, 39, btac788. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, M.; Cheng Li, S. TEINet: A Deep Learning Framework for Prediction of TCR-Epitope Binding Specificity. Brief. Bioinform. 2023, 24, bbad086. [Google Scholar] [CrossRef]
- Zhang, P.; Bang, S.; Cai, M.; Lee, H. Context-Aware Amino Acid Embedding Advances Analysis of TCR-Epitope Interactions. eLife 2023, 12, RP88837. [Google Scholar] [CrossRef]
- Pham, M.-D.N.; Nguyen, T.-N.; Tran, L.S.; Nguyen, Q.-T.B.; Nguyen, T.-P.H.; Pham, T.M.Q.; Nguyen, H.-N.; Giang, H.; Phan, M.-D.; Nguyen, V. epiTCR: A Highly Sensitive Predictor for TCR-Peptide Binding. Bioinformatics 2023, 39, btad284. [Google Scholar] [CrossRef]
- Croce, G.; Bobisse, S.; Moreno, D.L.; Schmidt, J.; Guillame, P.; Harari, A.; Gfeller, D. Deep Learning Predictions of TCR-Epitope Interactions Reveal Epitope-Specific Chains in Dual Alpha T Cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Korpela, D.; Jokinen, E.; Dumitrescu, A.; Huuhtanen, J.; Mustjoki, S.; Lähdesmäki, H. EPIC-TRACE: Predicting TCR Binding to Unseen Epitopes Using Attention and Contextualized Embeddings. Bioinformatics 2023, 39, btad743. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P. Structure-Based Prediction of T Cell Receptor:Peptide-MHC Interactions. eLife 2023, 12, e82813. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, R.; Dong, X.; Suo, S.; Zhao, Z. Prediction and Characterization of T Cell Response by Improved T Cell Receptors to Antigen Specificity with Interpretable Deep Learning. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhang, P.; Bang, S.; Lee, H. PiTE: TCR-Epitope Binding Affinity Prediction Pipeline Using Transformer-Based Sequence Encoder. Pac. Symp. Biocomput. 2023, 28, 347–358. [Google Scholar] [PubMed]
- Wu, J.; Qi, M.; Zhang, F.; Zheng, Y. TPBTE: A Model Based on Convolutional Transformer for Predicting the Binding of TCR to Epitope. Mol. Immunol. 2023, 157, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qian, X.; Tong, Y.; Li, F.; Wang, K.; Zhang, X.; Liu, T.; Wang, J. AttnTAP: A Dual-Input Framework Incorporating the Attention Mechanism for Accurately Predicting TCR-Peptide Binding. Front. Genet. 2022, 13, 942491. [Google Scholar] [CrossRef]
- Jian, Y.; Kruus, E.; Min, M.R. T-Cell Receptor-Peptide Interaction Prediction with Physical Model Augmented Pseudo-Labeling. In Proceedings of the 28th ACM SIGKDD Conference on Knowledge Discovery and Data Mining, Washington, DC, USA, 14–18 August 2022; Association for Computing Machinery: New York, NY, USA, 2022; pp. 3090–3097. [Google Scholar]
- Cai, M.; Bang, S.; Zhang, P.; Lee, H. ATM-TCR: TCR-Epitope Binding Affinity Prediction Using a Multi-Head Self-Attention Model. Front. Immunol. 2022, 13, 893247. [Google Scholar] [CrossRef]
- van Rooij, N.; van Buuren, M.M.; Philips, D.; Velds, A.; Toebes, M.; Heemskerk, B.; van Dijk, L.J.A.; Behjati, S.; Hilkmann, H.; El Atmioui, D.; et al. Tumor Exome Analysis Reveals Neoantigen-Specific T-Cell Reactivity in an Ipilimumab-Responsive Melanoma. J. Clin. Oncol. 2013, 31, e439–e442. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Robbins, P.F.; Lu, Y.-C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining Exomic Sequencing Data to Identify Mutated Antigens Recognized by Adoptively Transferred Tumor-Reactive T Cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef]
- Gubin, M.M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.P.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.D.; Krebber, W.-J.; et al. Checkpoint Blockade Cancer Immunotherapy Targets Tumour-Specific Mutant Antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Viveiros, P.; Lopes, G.; Sukhadia, B.; Sheikh, M.M.; Saravia, D.; Florou, V.; Sokol, E.S.; Frampton, G.M.; Chalmers, Z.R.; et al. Clinical and Immunological Implications of Frameshift Mutations in Lung Cancer. J. Thorac. Oncol. 2019, 14, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Asmann, Y.; Cattaneo, L.; Braggio, E.; Keats, J.; Auclair, D.; Lonial, S.; MMRF CoMMpass Network; Russell, S.J.; Stewart, A.K. High Somatic Mutation and Neoantigen Burden Are Correlated with Decreased Progression-Free Survival in Multiple Myeloma. Blood Cancer J. 2017, 7, e612. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting Neoantigens to Augment Antitumour Immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Zhou, T.; Xia, X.; Han, C.; Liu, Z.; Li, Q.; Shu, Y.; Xu, H. Novel Insights into HBV-hepatocellular Carcinoma at Single-cell Sequencing. MedComm Oncol. 2023, 2, e60. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer Immunotherapy. A Dendritic Cell Vaccine Increases the Breadth and Diversity of Melanoma Neoantigen-Specific T Cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA Vaccine–Induced Neoantigen-Specific T Cell Immunity in Patients with Gastrointestinal Cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.-J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, Heterologous Chimpanzee Adenovirus and Self-Amplifying mRNA Neoantigen Vaccine for Advanced Metastatic Solid Tumors: Phase 1 Trial Interim Results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised Neoantigen Therapy mRNA-4157 (V940) plus Pembrolizumab versus Pembrolizumab Monotherapy in Resected Melanoma (KEYNOTE-942): A Randomised, Phase 2b Study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Rappaport, A.R.; Kyi, C.; Lane, M.; Hart, M.G.; Johnson, M.L.; Henick, B.S.; Liao, C.-Y.; Mahipal, A.; Shergill, A.; Spira, A.I.; et al. A Shared Neoantigen Vaccine Combined with Immune Checkpoint Blockade for Advanced Metastatic Solid Tumors: Phase 1 Trial Interim Results. Nat. Med. 2024, 30, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Gane, E.J.; Marron, T.U.; Perales-Linares, R.; Yan, J.; Cooch, N.; Shu, D.H.; Fertig, E.J.; Kagohara, L.T.; Bartha, G.; et al. Personalized Neoantigen Vaccine and Pembrolizumab in Advanced Hepatocellular Carcinoma: A Phase 1/2 Trial. Nat. Med. 2024, 30, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Mason, P.W.; Geall, A.; Mandl, C.W. RNA-Based Vaccines. Vaccine 2012, 30, 4414–4418. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Wang, H.; Li, M.; Deng, J.; Zhu, A.; Ma, L.; Li, W.; et al. The Nano Delivery Systems and Applications of mRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-Based Therapeutic Cancer Vaccine: Current Trends in Clinical Application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Wang, L.; Liu, B. Personalized Neoantigen Vaccination with Synthetic Long Peptides: Recent Advances and Future Perspectives. Theranostics 2020, 10, 6011–6023. [Google Scholar] [CrossRef]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than One Reason to Rethink the Use of Peptides in Vaccine Design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Bijker, M.S.; van den Eeden, S.J.F.; Franken, K.L.; Melief, C.J.M.; Offringa, R.; van der Burg, S.H. CD8+ CTL Priming by Exact Peptide Epitopes in Incomplete Freund’s Adjuvant Induces a Vanishing CTL Response, Whereas Long Peptides Induce Sustained CTL Reactivity. J. Immunol. 2007, 179, 5033–5040. [Google Scholar] [CrossRef]
- Bijker, M.S.; van den Eeden, S.J.F.; Franken, K.L.; Melief, C.J.M.; van der Burg, S.H.; Offringa, R. Superior Induction of Anti-Tumor CTL Immunity by Extended Peptide Vaccines Involves Prolonged, DC-Focused Antigen Presentation. Eur. J. Immunol. 2008, 38, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Leet, D.E.; Allesøe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal Neoantigen Vaccines Induce Persistent Memory T Cell Responses and Epitope Spreading in Patients with Melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and Nanotechnology in Drug Development: Focus on Ocular Targets. Int. J. Nanomed. 2013, 8, 495–503. [Google Scholar] [CrossRef]
- Mi, Y.; Hagan, C.T., 4th; Vincent, B.G.; Wang, A.Z. Emerging Nano-/Microapproaches for Cancer Immunotherapy. Adv. Sci. 2019, 6, 1801847. [Google Scholar] [CrossRef]
- Melief, C.J.M.; van der Burg, S.H. Immunotherapy of Established (Pre)Malignant Disease by Synthetic Long Peptide Vaccines. Nat. Rev. Cancer 2008, 8, 351–360. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer Vaccine Nanodiscs for Personalized Cancer Immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-Based Programming of Lymph-Node Targeting in Molecular Vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef]
- Pant, S.; Wainberg, Z.A.; Weekes, C.D.; Furqan, M.; Kasi, P.M.; Devoe, C.E.; Leal, A.D.; Chung, V.; Basturk, O.; VanWyk, H.; et al. Lymph-Node-Targeted, mKRAS-Specific Amphiphile Vaccine in Pancreatic and Colorectal Cancer: The Phase 1 AMPLIFY-201 Trial. Nat. Med. 2024, 30, 531–542. [Google Scholar] [CrossRef]
- Steinman, R.M. Decisions about Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-Z.; Zhao, X.; Song, X.-R. Ex Vivo Pulsed Dendritic Cell Vaccination against Cancer. Acta Pharmacol. Sin. 2020, 41, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Leisegang, M.; Park, J.-H.; Ren, L.; Kato, T.; Ikeda, Y.; Harada, M.; Kiyotani, K.; Lengyel, E.; Fleming, G.F.; et al. Induction of Neoantigen-Specific Cytotoxic T Cells and Construction of T-Cell Receptor-Engineered T Cells for Ovarian Cancer. Clin. Cancer Res. 2018, 24, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.N.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A Pilot Trial Using Lymphocytes Genetically Engineered with an NY-ESO-1-Reactive T-Cell Receptor: Long-Term Follow-up and Correlates with Response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor Regression in Patients with Metastatic Synovial Cell Sarcoma and Melanoma Using Genetically Engineered Lymphocytes Reactive with NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1-Specific TCR-Engineered T Cells Mediate Sustained Antigen-Specific Antitumor Effects in Myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Leidner, R.; Sanjuan Silva, N.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.-P.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-Engineered T Cell Therapy in Solid Tumors: State of the Art and Perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Simoni, Y.; Zhuang, S.; Gabel, A.; Ma, S.; Chee, J.; Islas, L.; Cessna, A.; Creaney, J.; Bradley, R.K.; et al. Characterization of Neoantigen-Specific T Cells in Cancer Resistant to Immune Checkpoint Therapies. Proc. Natl. Acad. Sci. USA 2021, 118, e2025570118. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G.; et al. A Bi-Adjuvant Nanovaccine That Potentiates Immunogenicity of Neoantigen for Combination Immunotherapy of Colorectal Cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef]
- Bryan Iorgulescu, J.; Braun, D.; Oliveira, G.; Keskin, D.B.; Wu, C.J. Acquired Mechanisms of Immune Escape in Cancer Following Immunotherapy. Genome Med. 2018, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.-C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.-M.; Wu, J.; et al. Pancreatic Cancer Genomes Reveal Aberrations in Axon Guidance Pathway Genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A Comprehensive Survey of Ras Mutations in Cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Lo, W.; Parkhurst, M.; Robbins, P.F.; Tran, E.; Lu, Y.-C.; Jia, L.; Gartner, J.J.; Pasetto, A.; Deniger, D.; Malekzadeh, P.; et al. Immunologic Recognition of a Shared P53 Mutated Neoantigen in a Patient with Metastatic Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 534–543. [Google Scholar] [CrossRef]
- Kim, S.P.; Vale, N.R.; Zacharakis, N.; Krishna, S.; Yu, Z.; Gasmi, B.; Gartner, J.J.; Sindiri, S.; Malekzadeh, P.; Deniger, D.C.; et al. Adoptive Cellular Therapy with Autologous Tumor-Infiltrating Lymphocytes and T-Cell Receptor-Engineered T Cells Targeting Common P53 Neoantigens in Human Solid Tumors. Cancer Immunol. Res. 2022, 10, 932–946. [Google Scholar] [CrossRef]
- Hsiue, E.H.-C.; Wright, K.M.; Douglass, J.; Hwang, M.S.; Mog, B.J.; Pearlman, A.H.; Paul, S.; DiNapoli, S.R.; Konig, M.F.; Wang, Q.; et al. Targeting a Neoantigen Derived from a Common TP53 Mutation. Science 2021, 371, eabc8697. [Google Scholar] [CrossRef]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Biological Role and Therapeutic Potential of IDH Mutations in Cancer. Cancer Cell 2018, 34, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.; Bunse, L.; Pusch, S.; Sahm, F.; Wiestler, B.; Quandt, J.; Menn, O.; Osswald, M.; Oezen, I.; Ott, M.; et al. A Vaccine Targeting Mutant IDH1 Induces Antitumour Immunity. Nature 2014, 512, 324–327. [Google Scholar] [CrossRef]
- da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR Mutations and Lung Cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef]
- Li, F.; Deng, L.; Jackson, K.R.; Talukder, A.H.; Katailiha, A.S.; Bradley, S.D.; Zou, Q.; Chen, C.; Huo, C.; Chiu, Y.; et al. Neoantigen Vaccination Induces Clinical and Immunologic Responses in Non-Small Cell Lung Cancer Patients Harboring EGFR Mutations. J. Immunother. Cancer 2021, 9, e002531. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Mitsudomi, T. Not All Epidermal Growth Factor Receptor Mutations in Lung Cancer Are Created Equal: Perspectives for Individualized Treatment Strategy. Cancer Sci. 2016, 107, 1179–1186. [Google Scholar] [CrossRef]
- González-Galarza, F.F.; Takeshita, L.Y.C.; Santos, E.J.M.; Kempson, F.; Maia, M.H.T.; da Silva, A.L.S.; Teles e Silva, A.L.; Ghattaoraya, G.S.; Alfirevic, A.; Jones, A.R.; et al. Allele Frequency Net 2015 Update: New Features for HLA Epitopes, KIR and Disease and HLA Adverse Drug Reaction Associations. Nucleic Acids Res. 2015, 43, D784–D788. [Google Scholar] [CrossRef]
- Bailey, M.H.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.C.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 173, 371–385.e18. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.S.; Ma, J.; Klatt, M.G.; Dündar, F.; Bandlamudi, C.; Razavi, P.; Wen, H.Y.; Weigelt, B.; Zumbo, P.; Fu, S.N.; et al. Immunogenicity and Therapeutic Targeting of a Public Neoantigen Derived from Mutated PIK3CA. Nat. Med. 2022, 28, 946–957. [Google Scholar] [CrossRef]
- Mota, I.; Patrucco, E.; Mastini, C.; Mahadevan, N.R.; Thai, T.C.; Bergaggio, E.; Cheong, T.-C.; Leonardi, G.; Karaca-Atabay, E.; Campisi, M.; et al. ALK Peptide Vaccination Restores the Immunogenicity of ALK-Rearranged Non-Small Cell Lung Cancer. Nat. Cancer 2023, 4, 1016–1035. [Google Scholar] [CrossRef]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic Vaccines for Cancer: An Overview of Clinical Trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Adotévi, O.; Vernerey, D.; Jacoulet, P.; Meurisse, A.; Laheurte, C.; Almotlak, H.; Jacquin, M.; Kaulek, V.; Boullerot, L.; Malfroy, M.; et al. Safety, Immunogenicity, and 1-Year Efficacy of Universal Cancer Peptide–Based Vaccine in Patients With Refractory Advanced Non–Small-Cell Lung Cancer: A Phase Ib/Phase IIa De-Escalation Study. J. Clin. Oncol. 2023, 41, 373–384. [Google Scholar] [CrossRef]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A Phase 1/2 Trial of an Immune-Modulatory Vaccine against IDO/PD-L1 in Combination with Nivolumab in Metastatic Melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and Therapeutic Relevance of Cancer-Associated Fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Luo, H.; Xia, X.; Huang, L.-B.; An, H.; Cao, M.; Kim, G.D.; Chen, H.-N.; Zhang, W.-H.; Shu, Y.; Kong, X.; et al. Pan-Cancer Single-Cell Analysis Reveals the Heterogeneity and Plasticity of Cancer-Associated Fibroblasts in the Tumor Microenvironment. Nat. Commun. 2022, 13, 6619. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef]
- Mao, W.; Zhou, T.; Zhang, F.; Qian, M.; Xie, J.; Li, Z.; Shu, Y.; Li, Y.; Xu, H. Pan-Cancer Single-Cell Landscape of Drug-Metabolizing Enzyme Genes. Pharmacogenet. Genom. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- San Lucas, F.A.; Allenson, K.; Bernard, V.; Castillo, J.; Kim, D.U.; Ellis, K.; Ehli, E.A.; Davies, G.E.; Petersen, J.L.; Li, D.; et al. Minimally Invasive Genomic and Transcriptomic Profiling of Visceral Cancers by Next-Generation Sequencing of Circulating Exosomes. Ann. Oncol. 2016, 27, 635–641. [Google Scholar] [CrossRef]
- Weber, Z.T.; Collier, K.A.; Tallman, D.; Forman, J.; Shukla, S.; Asad, S.; Rhoades, J.; Freeman, S.; Parsons, H.A.; Williams, N.O.; et al. Modeling Clonal Structure over Narrow Time Frames via Circulating Tumor DNA in Metastatic Breast Cancer. Genome Med. 2021, 13, 89. [Google Scholar] [CrossRef]
- Bao, H.; Min, L.; Bu, F.; Wang, S.; Meng, J. Recent Advances of Liquid Biopsy: Interdisciplinary Strategies toward Clinical Decision-Making. Interdiscip. Med. 2023, 1, e20230021. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, L.; Zhao, Q.; Zheng, Z.-H.; Chen, Z.-N.; Bian, H.; Yang, X.; Lu, H.-Y.; Lin, P.; Chen, X.; et al. Cysteine Carboxyethylation Generates Neoantigens to Induce HLA-Restricted Autoimmunity. Science 2023, 379, eabg2482. [Google Scholar] [CrossRef]


| Name | Short Description | Format of Input | Ref. |
|---|---|---|---|
| ImmuneMirror | Integrative Pipeline and Web Server | DNA, RNA-seq | [52] |
| nextNEOpi | Comprehensive pipeline for neoantigen prediction from raw sequencing data | DNA, RNA-seq | [51] |
| Seq2Neo | A one-stop solution for neoepitope features prediction | DNA, RNA-seq | [50] |
| QBRC | neoantigen calling pipeline from somatic mutation | DNA, RNA-seq | [49] |
| pVACtools | A Computational Toolkit, including pVACseq, pVACfuse, pVACviz and pVACvector | VCF | [48] |
| NeoFuse | Gene fusion-derived neoantigen | RNA-seq | [47] |
| ASNEO | Alternative Splicing-derived neoantigen | RNA-seq | [46] |
| Name | MHC Class | Short Description | Ref. |
|---|---|---|---|
| DeepMHCI | I | An anchor position-aware deep interaction model, with a great performance on non-9-mer peptides | [62] |
| LightMHC | I | A Light Model for pMHC Structure Prediction with Graph Neural Networks | [63] |
| PepPPO | I | Characterize binding motif via generating repertoires of peptides presented by given MHC-I alleles | [64] |
| DeepMHCII | II | A novel binding core-aware deep interaction model for accurate MHC-II peptide binding affinity prediction | [65] |
| AEGIS | II | Apply natural language processing algorithms to identify the MHCII immunopeptidome in humans and a preclinincal mouse model | [66] |
| TLimmuno2 | II | A transfer learning-based, long short-term memory model | [67] |
| Name | Short Description | Ref. |
|---|---|---|
| TABR-BERT | A BERT-based transfer learning method | [82] |
| TEIM | TCR–Epitope Interaction Modelling at Residue Level predicted both pairwise residue distances and contact sites involved in the TCR–epitope interactions | [80] |
| PanPep | Pan-Peptide Meta Learning by combining the concepts of meta-learning and the neural Turing machine, particularly when confronted with unseen epitopes | [81] |
| TEPCAM | TCR-Epitope identification based on Cross-Attention and Multi-channel convolution | [83] |
| TCRmodel2 | An adapted AlphaFold framework for speedy, accurate modeling of both TCR–pMHC complexes and unbound TCRs | [84] |
| STAPLER | TCR-peptide specificity prediction from full-length TCR-peptide data | [85] |
| TCRconv | Using contextualized motifs to predicte recognition | [86] |
| TEINet | A deep learning framework utilizes transfer learning model | [87] |
| catELMo | Predicting binding between immune cells receptors and antigens based on protein sequence data | [88] |
| epiTCR | A Random Forest-based method dedicated to predicting the TCR-peptide interactions | [89] |
| MixTCRpred | A deep learning TCR-epitope interaction predictor | [90] |
| EPIC-TRACE | A new machine learning model that utilizes the both α and βTCR chains, epitope sequence, and MHC | [91] |
| TCRdock | Structural based prediction of TCR epitope specificity | [92] |
| DeepTR | Pan peptide-MHC class I binding prediction with a user-friendly web service | [93] |
| PiTE | A binding affinity prediction consists of two sequence encoders and a stack of linear layers | [94] |
| TPBTE | A model based on convolutional Transformer for Predicting the Binding of TCR to Epitope | [95] |
| AttnTAP | The bi-directional long short-term memory model for robust feature extraction of TCR sequences | [96] |
| PhyAugmentation | The deep neural network with physical modeling and data-augmented pseudo-labeling | [97] |
| ATM-TCR | Multi-head self-attention mechanism to capture biological contextual information | [98] |
| Trial (Format) | Cancer Type | Phase | Short Description | Ref. |
|---|---|---|---|---|
| NCT01970358 (SLP) | Melanoma | I/Ib | One of the pioneering works on personalized therapeutic neoantigens, demonstrating anti-tumor efficacy in combination with ICIs | [3] |
| NCT02035956 (mRNA) | Melanoma | I | One of the pioneering works on personalized therapeutic vaccines using TAA and neoantigens | [4] |
| NCT02287428 (SLP) | Glioblastoma | I/Ib | Demonstrates the feasibility of therapeutic neoantigen vaccines in immunologically ‘cold’ tumors | [5] |
| NCT02149225 (peptides) | Glioblastoma | I | Demonstrates the feasibility of therapeutic vaccines using TAA and neoantigens in immunologically ‘cold’ tumors | [13] |
| NCT02897765 (SLP) | Bladder Tumors, Melanoma, Lung Cancer | Ib | In the large cohort, neoantigen-specific CD4+ and CD8+ T cell responses were observed in all vaccinated patients | [6] |
| NCT02956551 (Dendritic cell) | Non-Small-Cell Lung | I | Provides new therapeutic opportunities for lung cancer treatment | [7] |
| NCT03639714 (GRT-C901 adenovirus-based/GRT-R902 RNA-based) | Non-Small Cell Lung Cancer, Colorectal Cancer, Gastroesophageal Adenocarcinoma, Urothelial Carcinoma | I/II | Demonstrate exceptional performance in the treatment of patients with advanced metastatic solid tumors | [110] |
| NCT03380871 (SLP) | Non-Small Cell Lung Cancer | Ib | Personalized neoantigen vaccine with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer | [8] |
| NCT03897881 (mRNA) | Melanoma | IIb | Reported clinical benefit by prolonged recurrence free survival (RFS) in patients with resected melanoma comparing ICB plus neoantigen mRNA vaccine to ICB alone. | [9,111] |
| NCT04161755 (mRNA) | Pancreatic Cancer | I | Reported a prolonged RFS in vaccine-responder patients compared to non-responders, demonstrating that neoantigen vaccines offer effective treatment for PDAC | [10] |
| NCT03953235 (GRT-C903 adenovirus-based/GRT-R904 RNA-based) | Solid Tumor | I/II | Demonstrate good tolerability and initial therapeutic potential in advanced solid tumors through vaccines targeting shared neoantigens in combination with ICI | [112] |
| NCT04251117 (DNA) | Hepatocellular carcinoma | I/II | Demonstrate personalized therapeutic vaccines enhancing responses to PD-1 inhibitors through the induction of tumor-specific immunity | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.; Long, Y.; Yang, Y.; Deng, Y.; Ding, Z.; Yang, L.; Shu, Y.; Xu, H. Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens. Vaccines 2024, 12, 717. https://doi.org/10.3390/vaccines12070717
Hao Q, Long Y, Yang Y, Deng Y, Ding Z, Yang L, Shu Y, Xu H. Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens. Vaccines. 2024; 12(7):717. https://doi.org/10.3390/vaccines12070717
Chicago/Turabian StyleHao, Qing, Yuhang Long, Yi Yang, Yiqi Deng, Zhenyu Ding, Li Yang, Yang Shu, and Heng Xu. 2024. "Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens" Vaccines 12, no. 7: 717. https://doi.org/10.3390/vaccines12070717
APA StyleHao, Q., Long, Y., Yang, Y., Deng, Y., Ding, Z., Yang, L., Shu, Y., & Xu, H. (2024). Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens. Vaccines, 12(7), 717. https://doi.org/10.3390/vaccines12070717





