Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design: Collection of Material and Laboratory Research Algorithm
2.2. VP1 Sequencing
2.3. High Throughput Sequencing
2.4. Classification of nOPV2 Isolates
2.5. Research Ethics
3. Results
3.1. VDPV2 Isolation
3.2. nOPV2 Isolation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutter, R.W.; Kew, O.M.; Cochi, S.L.; Aylward, R.B. Poliovirus Vaccine–Live. In Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 866–917. [Google Scholar]
- World Health Organization (WHO). Polio vaccines: WHO Psition Paper—June 2022. Wkly. Epidemiol. Rec. 2022, 97, 277–300. Available online: https://www.who.int/publications/i/item/WHO-WER9725-277-300 (accessed on 17 June 2024).
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-derived polioviruses. J. Infect. Dis. 2014, 210 (Suppl. S1), 283–293. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Meeting of the Strategic Advisory Group of Experts on Immunization, November 2012—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2013, 88, 1–16. Available online: https://iris.who.int/bitstream/handle/10665/242164/WER8901_1-19.PDF?sequence=1 (accessed on 17 June 2024).
- Macklin, G.R.; O’Reilly, K.M.; Grassly, N.C.; Edmunds, W.J.; Mach, O.; Santhana Gopala Krishnan, R.; Voorman, A.; Vertefeuille, J.F.; Abdelwahab, J.; Gumede, N.; et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science 2020, 368, 401–405. Available online: https://www.science.org/doi/10.1126/science.aba1238?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 17 June 2024). [CrossRef] [PubMed]
- Macklin, G.R.; Goel, A.K.; Mach, O.; Tallis, G.; Ahmed, J.A.; O’Reilly, K.M.; Grassly, N.C.; Diop, O.M. Epidemiology of type 2 vaccine-derived poliovirus outbreaks between 2016 and 2020. Vaccine 2023, 41, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Macklin, G.; Peak, C.; Eisenhawer, M.; Kurji, F.; Mach, O.; Konz, J.; Gast, C.; Bachtiar, N.S.; Bandyopadhyay, A.S.; Zipursky, S.; et al. Enabling accelerated vaccine roll-out for public health emergencies of international concern (PHEICs): Novel oral polio vaccine type 2 (nOPV2) experience. Vaccine 2022, 41 (Suppl. S1), A122–A127. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Zipursky, S. A novel tool to eradicate an ancient scourge: The novel oral polio vaccine type 2 story. Lancet Infect. Dis. 2023, 23, 67–71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Novel Oral Polio Vaccine Type 2 (nOPV2) Granted EUL Recommendation. 2021. Available online: https://polioeradication.org/news-post/novel-oral-polio-vaccine-type-2-nopv2-granted-interim-emergencyuse-listing-recommendation/ (accessed on 25 March 2024).
- World Health Organization (WHO). GPEI Press Release on nOPV2 Prequalification. Available online: https://polioeradication.org/news-post/gpei-press-release-on-nopv2-prequalification/ (accessed on 25 March 2024).
- O’Connor, P.; Huseynov, S.; Nielsen, C.F.; Saidzoda, F.; Saxentoff, E.; Sadykova, U.; Patrici Kormoss, P. Readiness for use of type 2 novel oral poliovirus vaccine in response to a type 2 circulating vaccine-derived poliovirus outbreak—Tajikistan, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 361–362. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7109a4.htm?s_cid=mm7109a4_w (accessed on 17 June 2024). [CrossRef] [PubMed]
- Mirzoev, A.; Macklin, G.R.; Zhang, Y.; Mainou, B.A.; Sadykova, U.; Olsavszky, V.S.; Huseynov, S.; Ruziev, M.; Saidzoda, F.; Bobokhonova, M.; et al. Assessment of serological responses following vaccination campaigns with type 2 novel oral polio vaccine: A population-based study in Tajikistan in 2021. Lancet Glob. Health 2022, 10, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Tsvirkun, O.V.; Tikhonova, N.T.; Turaeva, N.V.; Tishkova, F.K.; Ruziev, M.M.; Saidzoda, F.B.; Karpova, E.V.; Ivanova, O.E.; Kozlovskaya, L.I. The state of specific immunity of population of the Republic of Tajikistan to measles, rubella, poliomyelitis viruses. J. Microbiol. Epidemiol. Immunobiol. 2023, 100, 194–202. [Google Scholar] [CrossRef]
- Onischenko, G.G.; Ezhlova, E.B.; Melnikova, A.A.; Lazikova, G.F.; Demina, Y.V.; Frolova, N.V. Poliomyelitis in Tajikistan. Protection of Russia from emergence and spread of wild poliomyelitis virus. J. Microbiol. Epidemiol. Immunobiol. 2011, 2, 12–22. Available online: https://microbiol.crie.ru/jour/article/view/13454 (accessed on 17 June 2024). (In Russian).
- Yakovenko, M.L.; Gmyl, A.P.; Ivanova, O.E.; Eremeeva, T.P.; Ivanov, A.P.; Prostova, M.A.; Baykova, O.Y.; Isaeva, O.V.; Lipskaya, G.Y.; Shakaryan, A.K.; et al. The 2010 outbreak of poliomyelitis in Tajikistan: Epidemiology and lessons learnt. Eurosurveillance 2014, 19, 20706. [Google Scholar] [CrossRef]
- Ivanova, O.E.; Kozlovskaya, L.I.; Eremeeva, T.P.; Shakaryan, A.K.; Ivanov, A.P.; Baykova, O.Y.; Krasota, A.Y.; Shustova, E.Y.; Mustafina, A.N.; Morozova, N.S.; et al. Vaccine-associated paralytic poliomyelitis in a child: Fast transformation from Sabin-like virus to vaccine-derived poliovirus triggered an epidemiological response in two countries of the European region. Int. J. Infect. Dis. 2022, 125, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Romanenkova, N.I.; Bichurina, M.A.; Rozaeva, N.R.; Pogrebnaia, T.N. The role of epidemiologic surveillance of migrants in the system of poliomyelitis control. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 2012, 6, 27–31. Available online: https://microbiol.crie.ru/jour/article/view/13704 (accessed on 17 June 2024). (In Russian).
- Rospotrebnadzor, Sanitary and Epidemiological Requirements for Prevention of Infectious Diseases, Sanpin 3.3686-21. Available online: https://sanepidservice.ru/assets/files/sanpin-3.3686-21.pdf (accessed on 25 March 2024).
- World Health Organization (WHO). Circulating Vaccine-Derived Poliovirus Type 2 (cVDPV2)—Ukraine. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/circulating-vaccine-derived-poliovirus-type-2-(cvdpv2)-ukraine (accessed on 25 March 2024).
- World Health Organization (WHO). Comprehensive Outbreak Response Successfully Stops Spread of Polio in Tajikistan. Available online: https://www.who.int/europe/ru/news/item/28-04-2022-comprehensive-outbreak-response-successfully-stops-spread-of-polio-in-tajikistan (accessed on 25 March 2024).
- World Health Organization (WHO). Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://polioeradication.org/wp-content/uploads/2017/05/Polio_Lab_Manual04.pdf (accessed on 25 March 2024).
- Gerloff, N.; Sun, H.; Mandelbaum, M.; Maher, C.; Nix, W.A.; Zaidi, S.; Shaukat, S.; Seakamela, L.; Nalavade, U.P.; Sharma, D.K.; et al. Diagnostic Assay Development for Poliovirus eradication. J. Clin. Microbiol. 2018, 56, e01624-17. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.R.; Iber, J.C.; Chen, Q.; Ching, K.; Yang, S.J.; De, L.; Mandelbaum, M.D.; Emery, B.; Campagnoli, R.; Burns, C.C.; et al. Poliovirus serotype-specific VP1 sequencing primers. J. Virol. Methods 2011, 174, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Poplin, R.; Ruano-Rubio, V.; DePristo, M.A.; Fennell, T.J.; Carneiro, M.O.; Van der Auwera, G.A.; Kling, D.E.; Gauthier, L.D.; Levy-Moonshine, A.; Roazen, D.; et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 2017, 201178. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Burns, C.C.; Jorba, J.; Shulman, L.M.; Macadam, A.; Klapsa, D.; Majumdar, M.; Bullows, J.; Frolov, A.; Mate, R.; et al. Genetic characterization of novel oral polio vaccine type 2 viruses during initial use phase under emergency use listing—Worldwide, March–October 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 786–790. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7124a2-H.pdf (accessed on 17 June 2024). [CrossRef] [PubMed]
- World Health Organization (WHO). Standard Operating Procedures: Responding to a Poliovirus Event or Outbreak, 4th ed.; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://iris.who.int/bitstream/handle/10665/363627/9789240049154-eng.pdf (accessed on 25 March 2024).
- WHO. Polio Eradication Strategy 2022–2026: Delivering on a Promise; World Health Organization: Geneva, Switzerland, 2021; Available online: https://polioeradication.org/wp-content/uploads/2021/10/9789240031937-eng.pdf (accessed on 25 March 2024).
- Stehling-Ariza, T.; Wilkinson, A.L.; Diop, O.M.; Jorba, J.; Asghar, H.; Avagnan, T.; Grabovac, V.; Johnson, T.; Joshi, S.; Kfutwah, A.K.W.; et al. Surveillance to track progress toward poliomyelitis eradication—Worldwide, 2021–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 613–620. Available online: https://pubmed.ncbi.nlm.nih.gov/37289657/ (accessed on 17 June 2024). [CrossRef] [PubMed]
- Lee, S.E.; Greene, S.A.; Burns, C.C.; Tallis, G.; Wassilak, S.G.F.; Bolu, O. Progress toward poliomyelitis eradication—Worldwide, January 2021–March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 517–522. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10208367/ (accessed on 17 June 2024). [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Certification of poliomyelitis eradication—European Region, June 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 572–574. Available online: https://pubmed.ncbi.nlm.nih.gov/12139184/ (accessed on 17 June 2024).
- World Health Organization (WHO). Polio (Pol3) Immunization Coverage among 1-Year-Olds (%)—Russian Federation. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/polio-(pol3)-immunization-coverage-among-1-year-olds-(-) (accessed on 25 March 2024).
- Voorman, A.; Lyons, H.; Bennette, C.; Kovacs, S.; Makam, J.K.; Vertefeuille, J.F.; Tallis, G. Analysis of population immunity to poliovirus following cessation of trivalent oral polio vaccine. Vaccine 2023, 41 (Suppl. S1), 85–92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Variant Polio (cVDPV) Cases. Available online: https://polioeradication.org/wp-content/uploads/2024/02/weekly-polio-analyses-cVDPV-20240220.pdf (accessed on 25 March 2024).
- Van Damme, P.; De Coster, I.; Bandyopadhyay, A.S.; Revets, H.; Withanage, K.; De Smedt, P.; Suykens, L.; Oberste, M.S.; Weldon, W.C.; Costa-Clemens, S.A.; et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: A double-blind, single-centre phase 1 study. Lancet 2019, 394, 148–158. [Google Scholar] [CrossRef] [PubMed]
- De Coster, I.; Leroux-Roels, I.; Bandyopadhyay, A.S.; Gast, C.; Withanage, K.; Steenackers, K.; De Smedt, P.; Aerssens, A.; Leroux-Roels, G.; Oberste, M.S.; et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: Two clinical trials. Lancet 2021, 397, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Llorens, X.; Bandyopadhyay, A.S.; Gast, C.; De Leon, T.; DeAntonio, R.; Jimeno, J.; Caballero, M.I.; Aguirre, G.; Oberste, M.S.; Weldon, W.C.; et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: Two clinical trials. Lancet 2021, 397, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Mercer, L.; Gast, C.; De Leon, T.; Sáez-Llorens, X.; Fix, A.; Macadam, A.; Stephens, L.; Chumakov, K.; Smits, S.L.; et al. Evaluating stability of attenuated Sabin and two novel type 2 oral poliovirus vaccines in children. NPJ Vaccines 2022, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Bandyopadhyay, A.S.; Hoque, M.; Gast, C.; Yunus, M.; Jamil, K.M.; Mainou, B.A.; Konopka-Anstadt, J.L.; Hendley, W.S.; Vincent, A.; et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: A randomised, controlled, phase 2 clinical trial. Lancet 2023, 401, 131–139. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). GPEI Statement on cVDPV2 Detections in Burundi and Democratic Republic of the Congo. 16 March 2023. Available online: https://polioeradication.org/news-post/gpei-statement-on-cvdpv2-detections-in-burundi-and-democratic-republic-of-the-congo/ (accessed on 25 March 2024).
- Lopez Cavestany, R.; Eisenhawer, M.; Diop, O.M.; Verma, H.; Quddus, A.; Mach, O. The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens 2024, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Davlantes, E.; Jorba, J.; Henderson, E.; Bullard, K.; Deka, M.A.; Kfutwah, A.; Martin, J.; Bessaud, M.; Shulman, L.M.; Hawes, K.; et al. Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use—Six African Countries, 2021–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Bonilla, M.; Coulliette-Salmond, A.; Belgasmi, H.; Wong, K.; Sayyad, L.; Vega, E.; Grimoldi, F.; Oberste, M.S.; Rüttimann, R. Environmental Surveillance for Risk Assessment in the Context of a Phase 2 Clinical Trial of Type 2 Novel Oral Polio Vaccine in Panama. Viruses 2021, 13, 1355. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Health of Refugees and Migrants in the WHO European Region. Available online: https://www.who.int/europe/news-room/fact-sheets/item/the-health-of-refugees-and-migrants-in-the-who-european-region (accessed on 25 March 2024).
- Fougère, Y.; El Houss, S.; Suris, J.-C.; Rouvenaz-Defago, S.; Miletto, D.; Von der Weid, L.; Willen, F.; Williams-Smith, J.A.; Gehri, M.; Crisinel, P.A. Single doses of diphtheria-tetanus-pertussis and poliomyelitis vaccines are sufficient to generate a booster-type response to tetanus in most migrant children. Vaccine 2019, 37, 6441–6446. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, C.; Galanis, P.; Voulgari-Kokota, A.; Dikalioti, S.K.; Papachristidou, S.; Bozas, E.; Mentis, A.; Tsoumakas, K.; Pavlopoulou, I.D. Suboptimal Serologic Immunity Against Poliomyelitis Among New Migrant Children in Greece Calls for Organized Action. J. Immigr. Minor. Health 2023, 25, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hvass, A.M.F.; Wejse, C. High coverage of polio immunization program in refugees resettling in Denmark. A cross-sectional study of polio serology in newly arrived refugees. Expert Rev. Vaccines 2019, 18, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, L.; Colucci, M.E.; Capobianco, E.; Bracchi, M.T.; Zoni, R.; Palandri, L.; Affanni, P. Immunity status against poliomyelitis in young migrants: A seroprevalence study. Acta Biomed. 2019, 90, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Chironna, M.; Martinelli, D.; Sallustio, A.; Prato, R.; Germinario, C. Surveillance of poliovirus circulation among refugees in Italy, 2008–2011. J. Travel Med. 2012, 19, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Pogka, V.; Labropoulou, S.; Emmanouil, M.; Voulgari-Kokota, A.; Vernardaki, A.; Georgakopoulou, T.; Mentis, A.F. Laboratory Surveillance of Polio and Other Enteroviruses in High-Risk Populations and Environmental Samples. Appl. Environ. Microbiol. 2017, 83, e02872-16. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Neubauer, K.; Baillot, A.; Rieder, G.; Adam, M.; Diedrich, S. Stool screening of Syrian refugees and asylum seekers in Germany, 2013/2014: Identification of Sabin like polioviruses. Int. J. Med. Microbiol. 2015, 305, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.T.; Smith, M.; Carlyle, S.; Konopka-Anstadt, J.L.; Burns, C.C.; Konz, J.; Andino, R.; Macadam, A. Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature 2023, 619, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, D.; Pons-Salort, M.; Shaw, A.G.; Grassly, N.C. The role of genetic sequencing and analysis in the polio eradication programme. Virus Evol. 2020, 6, veaa040. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Majumdar, M.; Troman, C.; O’Toole, Á.; Benny, B.; Abraham, D.; Praharaj, I.; Kang, G.; Sharif, S.; Alam, M.M.; et al. Rapid and Sensitive Direct Detection and Identification of Poliovirus from Stool and Environmental Surveillance Samples by Use of Nanopore Sequencing. J. Clin. Microbiol. 2020, 58, e00920-20. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, H.; Tritama, E.; Wahid, R.; Anstadt, J.; Konz, J.; Chumakov, K.; Laassri, M. Improvement of the qmosRT-PCR Assay and Its Application for the Detection and Quantitation of the Three Serotypes of the Novel Oral Polio Vaccine in Stool Samples. Vaccines 2023, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
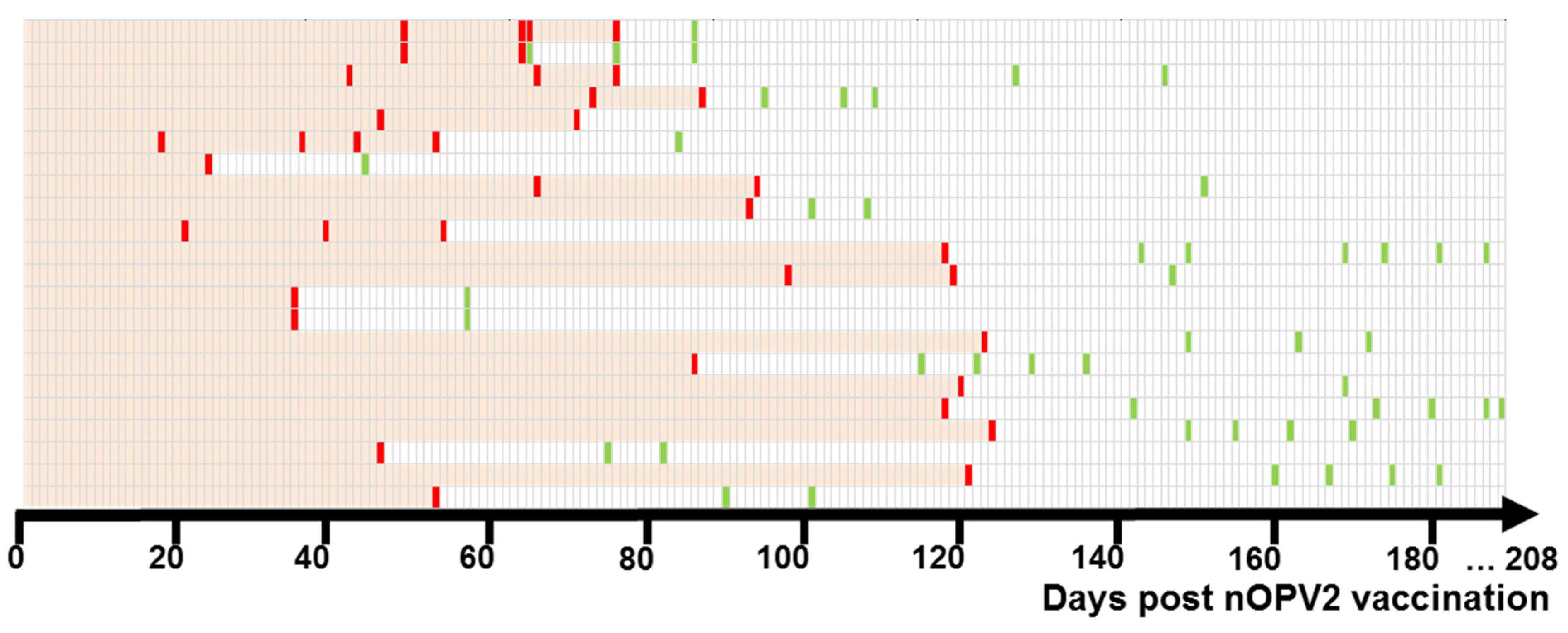

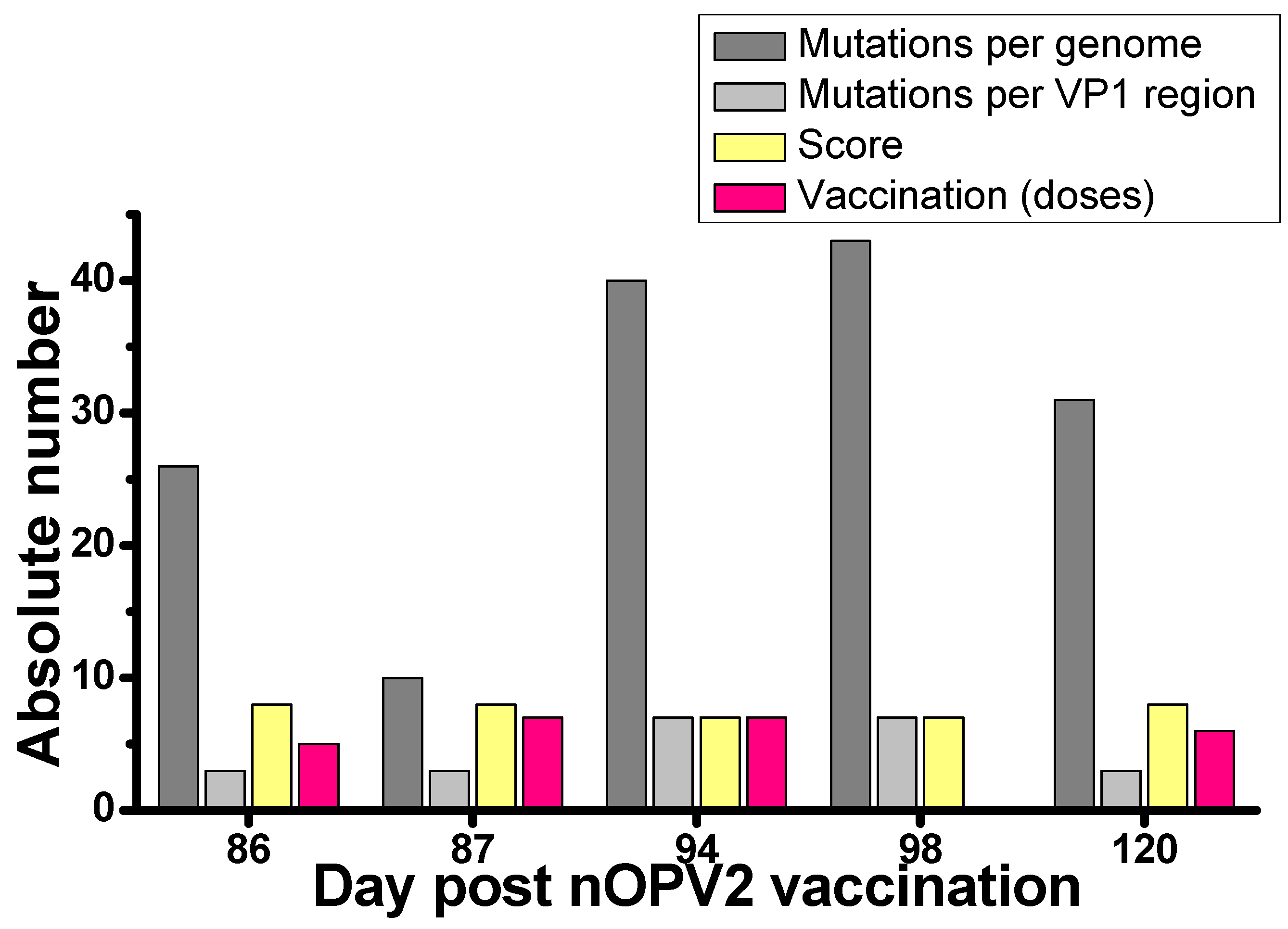
| Patient | Number of Polio Vaccine Doses/Date of Last Vaccination | Entry to Russia | Date of Sample Collection (year 2021) and Investigation Result | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/bOPV 9 Aug 2018 | 29 Jul 2021 | 3 Sep VDPV2 | 13 Sep VDPV2 | 1 Oct VDPV2 | 8 Oct VDPV2 | 15 Oct negative | 22 Oct VDPV2 | 29 Oct negative | 12 Nov negative | 17 Nov negative |
| 2 | 2/IPV 10 Dec 2020 | 20 Sep 2021 | 15 Oct VDPV2 | 27 Dec negative | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, O.E.; Eremeeva, T.P.; Baykova, O.Y.; Krasota, A.Y.; Yakovchuk, E.V.; Shustova, E.Y.; Malyshkina, L.P.; Mustafina, A.N.-I.; Mikhailova, Y.M.; Chirova, A.V.; et al. Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak. Vaccines 2024, 12, 718. https://doi.org/10.3390/vaccines12070718
Ivanova OE, Eremeeva TP, Baykova OY, Krasota AY, Yakovchuk EV, Shustova EY, Malyshkina LP, Mustafina AN-I, Mikhailova YM, Chirova AV, et al. Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak. Vaccines. 2024; 12(7):718. https://doi.org/10.3390/vaccines12070718
Chicago/Turabian StyleIvanova, Olga E., Tatiana P. Eremeeva, Olga Y. Baykova, Alexandr Y. Krasota, Elizaveta V. Yakovchuk, Elena Y. Shustova, Lyudmila P. Malyshkina, Aida N.-I. Mustafina, Yulia M. Mikhailova, Alina V. Chirova, and et al. 2024. "Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak" Vaccines 12, no. 7: 718. https://doi.org/10.3390/vaccines12070718
APA StyleIvanova, O. E., Eremeeva, T. P., Baykova, O. Y., Krasota, A. Y., Yakovchuk, E. V., Shustova, E. Y., Malyshkina, L. P., Mustafina, A. N.-I., Mikhailova, Y. M., Chirova, A. V., Cherepanova, E. A., Morozova, N. S., Gladkikh, A. S., Dolgova, A. S., Dedkov, V. G., Totolian, A. A., & Kozlovskaya, L. I. (2024). Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak. Vaccines, 12(7), 718. https://doi.org/10.3390/vaccines12070718








