Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar)
Abstract
1. Introduction
2. Materials and Methods
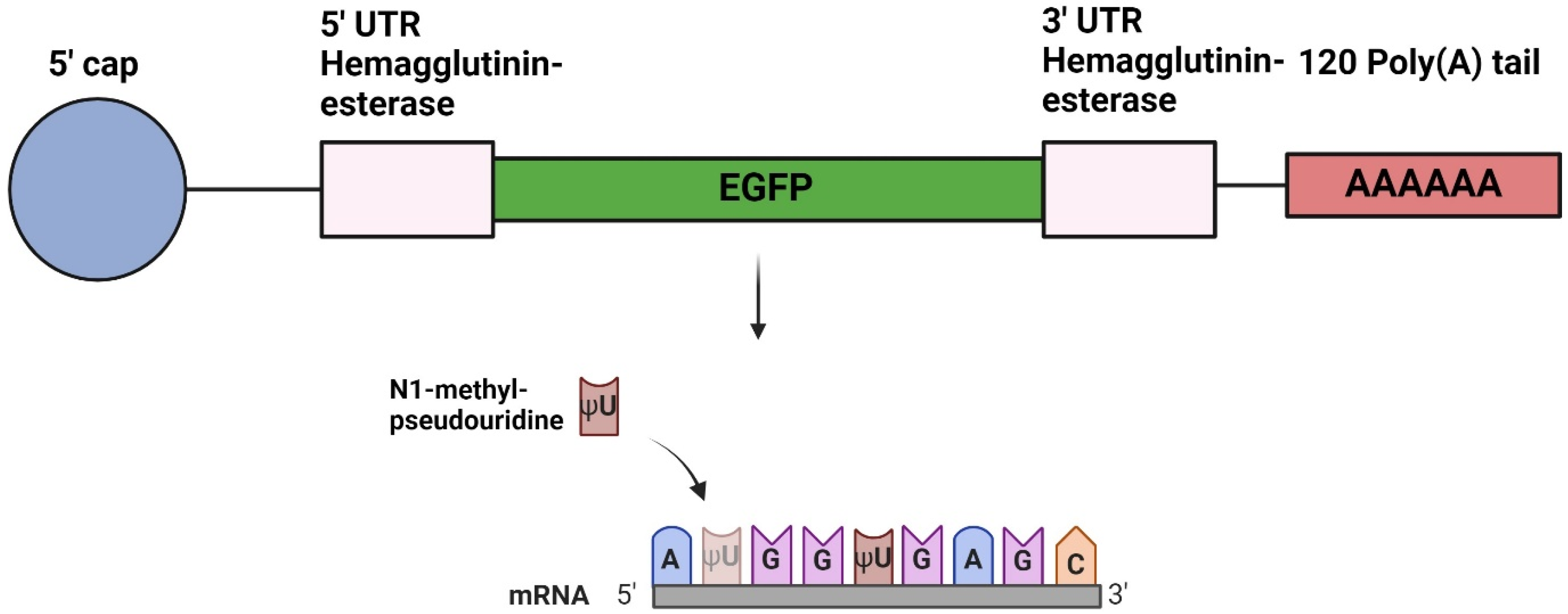
2.1. Design of DNA Plasmids
2.2. In Vitro Transcription of mRNA
2.3. mRNA-LNP Formulation
2.4. mRNA-LNP Characterization
2.5. In Vitro Expression
2.6. In Vitro Cytotoxicity of mRNA-LNPs
2.7. Injection of mRNA-LNPs in Salmon
2.8. Immunohistochemistry
3. Results
3.1. Production of mRNAs and LNP Encapsulation
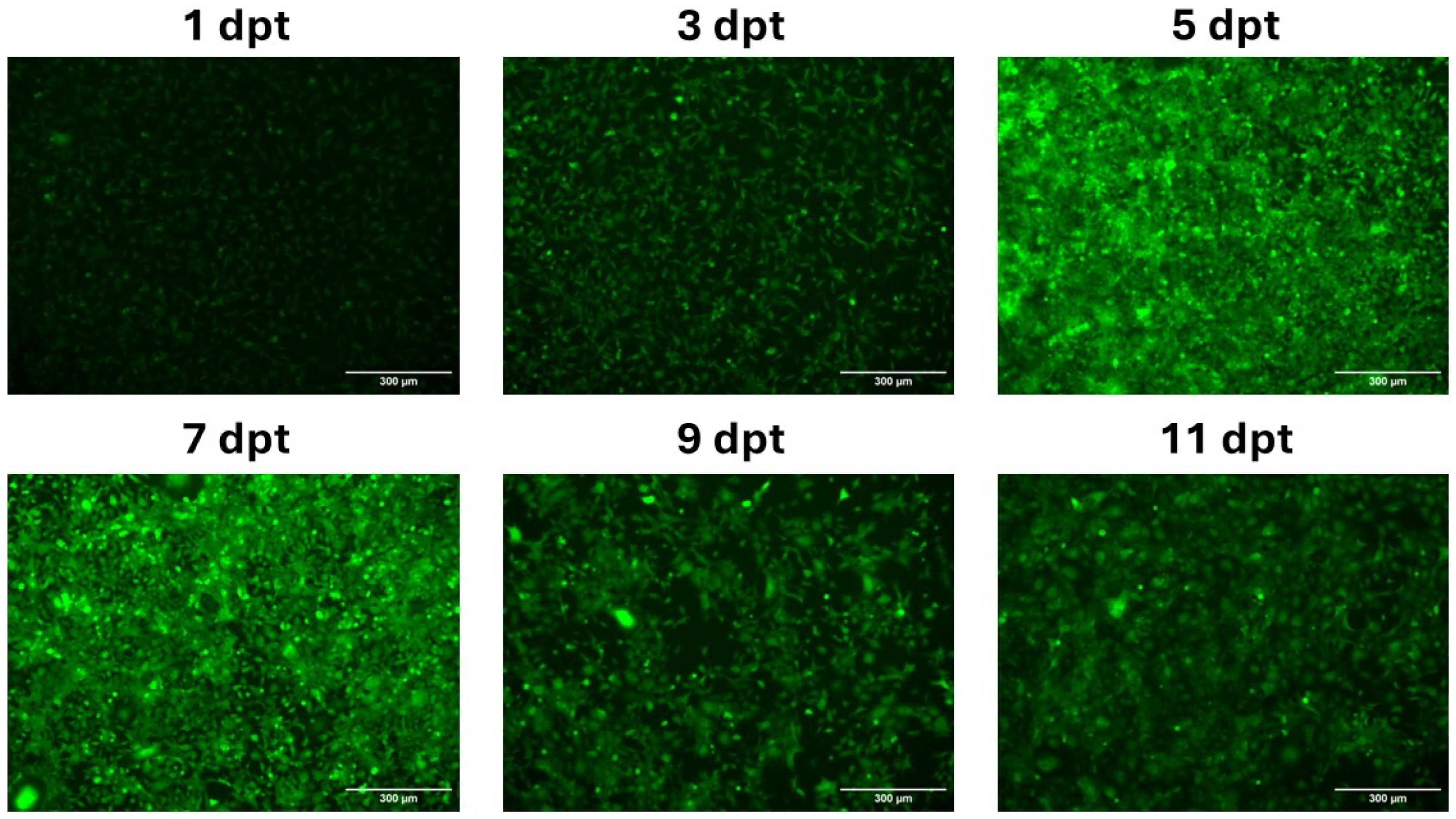
3.2. In Vitro Expression of mRNA-LNPs in CHH-1 Cells
3.3. Evaluation of mRNA-LNP Cytotoxicity
3.4. In Vivo Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Snieszko, S.F. A bacterial disease of carp in central Europe. Progress. Fish-Cult. 1940, 7, 12–15. [Google Scholar] [CrossRef]
- Gudding, R.; Van Muiswinkel, W.B. A history of fish vaccination: Science-based disease prevention in aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Midtlyng, P.J.; Reitan, L.J.; Lillehaug, A.; Ramstad, A. Protection, immune responses and side effects in atlantic salmon (Salmo salar L.) vaccinated against furunculosis by different procedures. Fish Shellfish Immunol. 1996, 6, 599–613. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Hølvold, L.B.; Myhr, A.I.; Dalmo, R.A. Strategies and hurdles using DNA vaccines to fish. Vet. Res. 2014, 45, 21. [Google Scholar] [CrossRef]
- Lu, S. Immunogenicity of DNA vaccines in humans: It takes two to tango. Hum. Vaccin. 2008, 4, 449–452. [Google Scholar] [CrossRef]
- Khan, F.H. The Elements of Immunology; Pearson Education India: Delhi, India, 2009. [Google Scholar]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the bnt162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Houseley, J.; Tollervey, D. The many pathways of RNA degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef]
- Scheel, B.; Braedel, S.; Probst, J.; Carralot, J.P.; Wagner, H.; Schild, H.; Jung, G.; Rammensee, H.G.; Pascolo, S. Immunostimulating capacities of stabilized RNA molecules. Eur. J. Immunol. 2004, 34, 537–547. [Google Scholar] [CrossRef]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA is an endogenous ligand for toll-like receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef]
- Jeeva, S.; Kim, K.H.; Shin, C.H.; Wang, B.Z.; Kang, S.M. An update on mRNA-based viral vaccines. Vaccines 2021, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Kauffman, K.J.; McClellan, R.L.; Appel, E.A.; Dorkin, J.R.; Tibbitt, M.W.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery. Adv. Mater. 2016, 28, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent t follicular helper and germinal center b cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Nita-Lazar, M.; Giomarelli, B.; Ahmed, H.; Du, S.; Cammarata, M.; Parrinello, N.; Bianchet, M.A.; Amzel, L.M. Structural and functional diversity of the lectin repertoire in teleost fish: Relevance to innate and adaptive immunity. Dev. Comp. Immunol. 2011, 35, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.A.; Litman, G.W. The phylogenetic origins of natural killer receptors and recognition: Relationships, possibilities, and realities. Immunogenetics 2011, 63, 123–141. [Google Scholar] [CrossRef]
- Sunyer, J.O.; Zarkadis, I.K.; Lambris, J.D. Complement diversity: A mechanism for generating immune diversity? Immunol. Today 1998, 19, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, A.; Nagao, K.; Kasai, H.; Hara, Y.; Umeda, M. Changes in the physicochemical properties of fish cell membranes during cellular senescence. Biosci. Biotechnol. Biochem. 2020, 84, 583–593. [Google Scholar] [CrossRef]
- Thompson, K.D.; Henderson, R.J.; Tatner, M.F. A comparison of the lipid composition of peripheral blood cells and head kidney leucocytes of atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 83–92. [Google Scholar] [CrossRef]
- Hatit, M.Z.C.; Lokugamage, M.P.; Dobrowolski, C.N.; Paunovska, K.; Ni, H.; Zhao, K.; Vanover, D.; Beyersdorf, J.; Peck, H.E.; Loughrey, D.; et al. Species-dependent in vivo mRNA delivery and cellular responses to nanoparticles. Nat. Nanotechnol. 2022, 17, 310–318. [Google Scholar] [CrossRef]
- Parot, J.; Mehn, D.; Jankevics, H.; Markova, N.; Carboni, M.; Olaisen, C.; Hoel, A.D.; Sigfúsdóttir, M.S.; Meier, F.; Drexel, R.; et al. Quality assessment of LNP-RNA therapeutics with orthogonal analytical techniques. J. Control. Release 2024, 367, 385–401. [Google Scholar] [CrossRef]
- Lannan, C.N.; Winton, J.R.; Fryer, J.L. Fish cell lines: Establishment and characterization of nine cell lines from salmonids. In Vitro 1984, 20, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Linares-Fernández, S.; Moreno, J.; Lambert, E.; Mercier-Gouy, P.; Vachez, L.; Verrier, B.; Exposito, J.Y. Combining an optimized mRNA template with a double purification process allows strong expression of in vitro transcribed mRNA. Mol. Ther. Nucleic Acids 2021, 26, 945–956. [Google Scholar] [CrossRef]
- Yu, M.; Song, W.; Tian, F.; Dai, Z.; Zhu, Q.; Ahmad, E.; Guo, S.; Zhu, C.; Zhong, H.; Yuan, Y.; et al. Temperature- and rigidity-mediated rapid transport of lipid nanovesicles in hydrogels. Proc. Natl. Acad. Sci. USA 2019, 116, 5362–5369. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Jha, B.K.; Silverman, R.H.; Weissman, D.; Karikó, K. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011, 39, 9329–9338. [Google Scholar] [CrossRef] [PubMed]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef]
- Svitkin, Y.V.; Cheng, Y.M.; Chakraborty, T.; Presnyak, V.; John, M.; Sonenberg, N. N1-methyl-pseudouridine in mRNA enhances translation through eif2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res. 2017, 45, 6023–6036. [Google Scholar] [CrossRef] [PubMed]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ t cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef] [PubMed]
- Bowen, L.; von Biela, V.R.; McCormick, S.D.; Regish, A.M.; Waters, S.C.; Durbin-Johnson, B.; Britton, M.; Settles, M.L.; Donnelly, D.S.; Laske, S.M.; et al. Transcriptomic response to elevated water temperatures in adult migrating yukon river chinook salmon (Oncorhynchus tshawytscha). Conserv. Physiol. 2020, 8, coaa084. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust t follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef] [PubMed]
- Naderi Sohi, A.; Kiani, J.; Arefian, E.; Khosrojerdi, A.; Fekrirad, Z.; Ghaemi, S.; Zim, M.K.; Jalili, A.; Bostanshirin, N.; Soleimani, M. Development of an mRNA-LNP vaccine against SARS-CoV-2: Evaluation of immune response in mouse and rhesus macaque. Vaccines 2021, 9, 1007. [Google Scholar] [CrossRef]
- Hassett, K.J.; Rajlic, I.L.; Bahl, K.; White, R.; Cowens, K.; Jacquinet, E.; Burke, K.E. mRNA vaccine trafficking and resulting protein expression after intramuscular administration. Mol. Ther. Nucleic Acids 2024, 35, 102083. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahl, L.O.S.; Hak, S.; Braaen, S.; Molska, A.; Rodà, F.; Parot, J.; Wessel, Ø.; Fosse, J.H.; Bjørgen, H.; Borgos, S.E.; et al. Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines 2024, 12, 788. https://doi.org/10.3390/vaccines12070788
Dahl LOS, Hak S, Braaen S, Molska A, Rodà F, Parot J, Wessel Ø, Fosse JH, Bjørgen H, Borgos SE, et al. Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines. 2024; 12(7):788. https://doi.org/10.3390/vaccines12070788
Chicago/Turabian StyleDahl, Lars Ole Sti, Sjoerd Hak, Stine Braaen, Alicja Molska, Francesca Rodà, Jeremie Parot, Øystein Wessel, Johanna Hol Fosse, Håvard Bjørgen, Sven Even Borgos, and et al. 2024. "Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar)" Vaccines 12, no. 7: 788. https://doi.org/10.3390/vaccines12070788
APA StyleDahl, L. O. S., Hak, S., Braaen, S., Molska, A., Rodà, F., Parot, J., Wessel, Ø., Fosse, J. H., Bjørgen, H., Borgos, S. E., & Rimstad, E. (2024). Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines, 12(7), 788. https://doi.org/10.3390/vaccines12070788








