Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects, Study Population, and Kinetics
2.2. Indirect Enzyme Immunoassay (ELISA) Specific for SARS-CoV-2 RBD
2.3. Plaque Reduction Neutralization Test 50% (PRNT50)
2.4. Statistical Analysis
3. Results
3.1. Assessment of the Anti-SARS-CoV-2 Antibody Response: Primary and Booster Doses
3.1.1. Assessment of Total Anti-RBD Antibody Levels in Groups 1 (BNT162b2) and 2 (ChAdOx1)
3.1.2. Assessment of Total Anti-RBD Antibody Levels after the Second Booster with the Different Vaccines Administered
3.1.3. Assessment of Total Anti-RBD Antibody Levels between Group 1 (BNT162b2) and Group 2 (ChAdOx1)
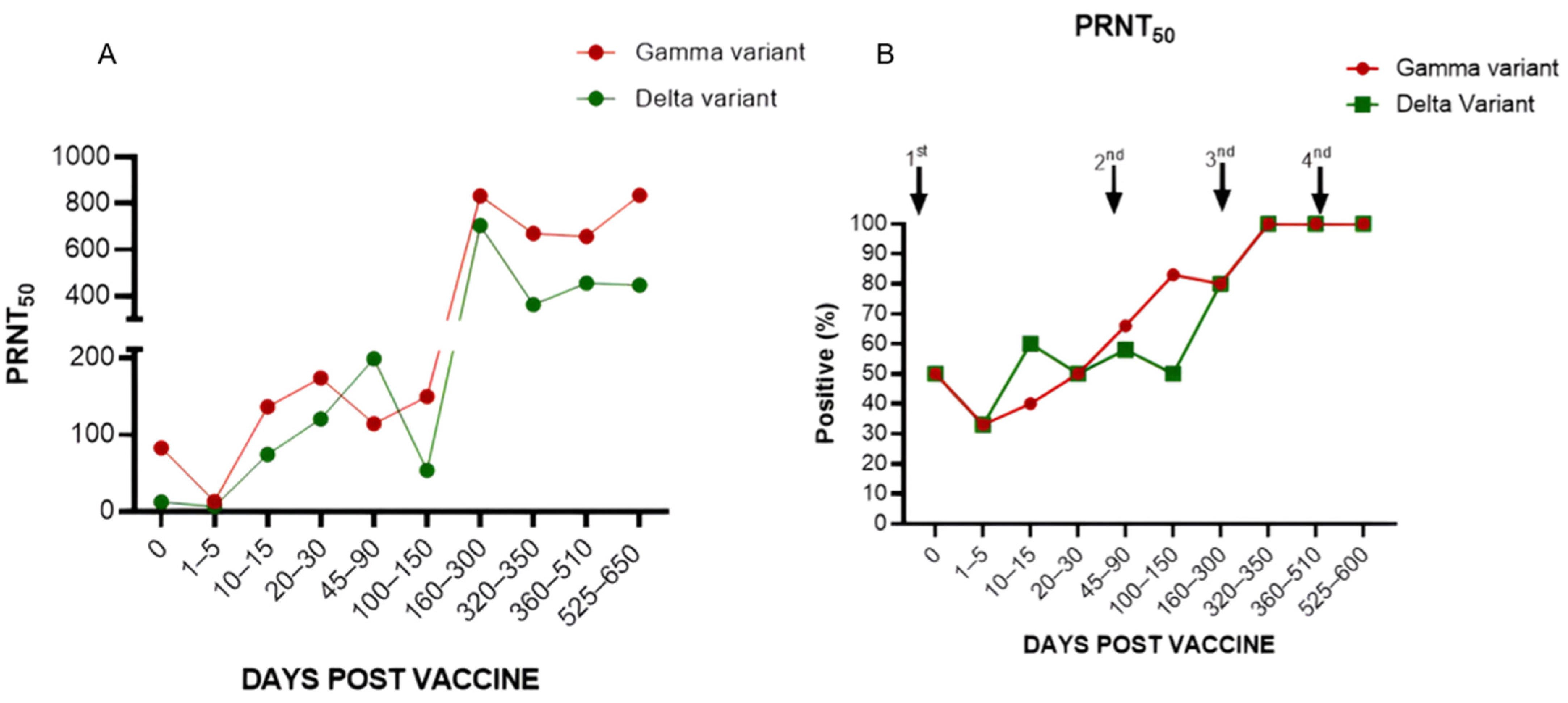
3.1.4. Levels of Neutralizing Antibodies in Individuals Who Received the ChadOx1 Vaccine in the Primary Vaccination Scheme, First Booster with BNT162b2, and Second Booster with CoronaVac (Group 2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.F.; Santiago, A.M.; de Brito, W.B.; Pereira, K.A.S.; de Brito, W.B.; da Silva Torres, M.K.; da Costa Lopes, J.; dos Santos, E.F.; da Costa, F.P.; de Sarges, K.M.L.; et al. Polymorphisms in the MBL2 Gene Are Associated with the Plasma Levels of MBL and the Cytokines IL-6 and TNF-α in Severe COVID-19. Front. Immunol. 2023, 14, 1151058. [Google Scholar] [CrossRef]
- Rodrigues, F.B.B.; da Silva, R.; Santos, E.F.d.; de Brito, M.T.F.M.; da Silva, A.L.S.; de Meira Leite, M.; Póvoa da Costa, F.; de Nazaré do Socorro de Almeida Viana, M.; de Sarges, K.M.L.; Cantanhede, M.H.D.; et al. Association of Polymorphisms of IL-6 Pathway Genes (IL6, IL6R and IL6ST) with COVID-19 Severity in an Amazonian Population. Viruses 2023, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Raskin, S. Genetics of COVID-19. J. Pediatr. 2021, 97, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Beavis, K.G.; Matushek, S.M.; Abeleda, A.P.F.; Bethel, C.; Hunt, C.; Gillen, S.; Moran, A.; Tesic, V. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for Detection of IgA and IgG Antibodies. J. Clin. Virol. 2020, 129, 104468. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Wilmet, A.; Beukinga, I.; Favresse, J.; Dogné, J.; Douxfils, J.; Blairon, L. Analytical and Clinical Validation of an ELISA for Specific SARS-CoV-2 IgG, IgA, and IgM Antibodies. J. Med. Virol. 2021, 93, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Padhi, A.K.; Tripathi, T. Can SARS-CoV-2 Accumulate Mutations in the S-Protein to Increase Pathogenicity? ACS Pharmacol. Transl. Sci. 2020, 3, 1023–1026. [Google Scholar] [CrossRef]
- Noureddine, F.Y.; Chakkour, M.; El Roz, A.; Reda, J.; Al Sahily, R.; Assi, A.; Joma, M.; Salami, H.; Hashem, S.J.; Harb, B.; et al. The Emergence of SARS-CoV-2 Variant(s) and Its Impact on the Prevalence of COVID-19 Cases in the Nabatieh Region, Lebanon. Med. Sci. 2021, 9, 40. [Google Scholar] [CrossRef]
- Martínez-Anaya, C.; Ramos-Cervantes, P.; Vidaltamayo, R. Coronavirus, Diagnosis and Epidemiological Strategies against COVID-19 in Mexico. Educ. Quim. 2020, 31, 12–22. [Google Scholar] [CrossRef]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 Pandemic on Maternal and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development. Arch. Med. Res. 2021, 52, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and Immunogenicity of Seven COVID-19 Vaccines as a Third Dose (Booster) Following Two Doses of ChAdOx1 NCov-19 or BNT162b2 in the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, P.D.B.M.; Almukhtar, D.M. Role Of Vaccines Against COVID-19 Pandemic. SLAS Discov. 2023, 28, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Lindsell, C.J.; Stewart, T.G.; Hernandez, A.F.; Collins, S.; McCarthy, M.W.; Jayaweera, D.; Gentile, N.; Castro, M.; Sulkowski, M.; et al. Inhaled Fluticasone Furoate for Outpatient Treatment of COVID-19. N. Engl. J. Med. 2023, 389, 1085–1095. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Neto, W.F.F.; da Silva, C.P.; Ribeiro, A.C.S.; Martins, L.C.; de Sousa, A.W.; Freitas, M.N.O.; Chiang, J.O.; Silva, F.A.; Dos Santos, E.B.; et al. Absence of Anti-RBD Antibodies in SARS-CoV-2 Infected or Naive Individuals Prior to Vaccination with CoronaVac Leads to Short Protection of Only Four Months Duration. Vaccines 2022, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Rawle, D.J.; Le, T.T.; Dumenil, T.; Yan, K.; Tang, B.; Nguyen, W.; Watterson, D.; Modhiran, N.; Hobson-Peters, J.; Bishop, C.; et al. ACE2-Lentiviral Transduction Enables Mouse SARS-CoV-2 Infection and Mapping of Receptor Interactions. PLoS Pathog. 2021, 17, e1009723. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- McLean, G.; Kamil, J.; Lee, B.; Moore, P.; Schulz, T.F.; Muik, A.; Sahin, U.; Türeci, Ö.; Pather, S. The Impact of Evolving SARS-CoV-2 Mutations and Variants on COVID-19 Vaccines. mBio 2022, 13, e02979-21. [Google Scholar] [CrossRef]
- Firouzabadi, N.; Ghasemiyeh, P.; Moradishooli, F.; Mohammadi-Samani, S. Update on the Effectiveness of COVID-19 Vaccines on Different Variants of SARS-CoV-2. Int. Immunopharmacol. 2023, 117, 109968. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 MRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 MRNA Vaccines Induce Persistent Human Germinal Centre Responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Marchevsky, N.; Kelly, S.; Felle, S.; Eldawi, A.; Rajasingam, R.; Mahmud, R.; Lambe, T.; Voysey, M.; Gonzalez, I.; et al. Immunogenicity, Safety and Reactogenicity of Heterologous (Third Dose) Booster Vaccination with a Full or Fractional Dose of Two Different COVID-19 Vaccines: A Phase 4, Single-Blind, Randomized Controlled Trial in Adults. Hum. Vaccines Immunother. 2023, 19, 2233400. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Tura, B.; Alberto, C.; Magliano, S.; Senna, K.; Macedo, L.S.; Santos, M.; Padilla, M.P.; Fernandes, R. Vacina Da Fiocruz [ChAdOx-1 (Vacina COVID-19 Recombinante)] e Da Pfizer/Wyeth [BNT162b2 (Vacina COVID-19)] Para Prevenção Da COVID-19; Ministério da Saúde: Brasília, Brazil, 2021. [Google Scholar]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. MRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus Homologous COVID-19 Booster Vaccination in Previous Recipients of Two Doses of CoronaVac COVID-19 Vaccine in Brazil (RHH-001): A Phase 4, Non-Inferiority, Single Blind, Randomised Study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Nanthapisal, S.; Puthanakit, T.; Jaru-Ampornpan, P.; Nantanee, R.; Sodsai, P.; Himananto, O.; Sophonphan, J.; Suchartlikitwong, P.; Hiransuthikul, N.; Angkasekwinai, P.; et al. A Randomized Clinical Trial of a Booster Dose with Low versus Standard Dose of AZD1222 in Adult after 2 Doses of Inactivated Vaccines. Vaccine 2022, 40, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wu, H.; Xu, J.; Liu, J. Immunogenicity and Safety of Heterologous versus Homologous Prime-Boost Schedules with an Adenoviral Vectored and MRNA COVID-19 Vaccine: A Systematic Review. Infect. Dis. Poverty 2022, 11, 53. [Google Scholar] [CrossRef]
- Deng, J.; Ma, Y.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Comparison of the Effectiveness and Safety of Heterologous Booster Doses with Homologous Booster Doses for SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10752. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Munro, A.P.S.; Wright, A.; Feng, S.; Janani, L.; Aley, P.K.; Babbage, G.; Baker, J.; Baxter, D.; Bawa, T.; et al. Persistence of Immune Responses after Heterologous and Homologous Third COVID-19 Vaccine Dose Schedules in the UK: Eight-Month Analyses of the COV-BOOST Trial. J. Infect. 2023, 87, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, R. MRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions. Vaccines 2023, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Fadlyana, E.; Setiabudi, D.; Kartasasmita, C.B.; Putri, N.D.; Rezeki Hadinegoro, S.; Mulholland, K.; Sofiatin, Y.; Suryadinata, H.; Hartantri, Y.; Sukandar, H.; et al. Immunogenicity and Safety in Healthy Adults of Full Dose versus Half Doses of COVID-19 Vaccine (ChAdOx1-S or BNT162b2) or Full-Dose CoronaVac Administered as a Booster Dose after Priming with CoronaVac: A Randomised, Observer-Masked, Controlled Trial in Indonesia. Lancet Infect. Dis. 2023, 23, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Suemori, K.; Tanaka, K.; Okamoto, A.; Murakami, A.; Miyamoto, H.; Takasuka, Y.; Yamashita, M.; Takenaka, K. Long-Term Transition of Antibody Titers in Healthcare Workers Following the First to Fourth Doses of MRNA COVID-19 Vaccine: Comparison of Two Automated SARS-CoV-2 Immunoassays. J. Infect. Chemother. 2023, 29, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Fukushima, W.; Nakagama, Y.; Kido, Y.; Kase, T.; Kondo, K.; Kaku, N.; Matsumoto, K.; Suita, A.; Mukai, E.; et al. Factors Impacting Antibody Kinetics, Including Fever and Vaccination Intervals, in SARS-CoV-2-Naïve Adults Receiving the First Four MRNA COVID-19 Vaccine Doses. Sci. Rep. 2024, 14, 7217. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Y.; Xue, J.H.; Xiao, Y.; Jia, Z.J.; Wu, M.J.; Liu, Y.Y.; Li, W.L.; Liang, X.M.; Yang, T.C. Response and Duration of Serum Anti-SARS-CoV-2 Antibodies after Inactivated Vaccination within 160 Days. Front. Immunol. 2021, 12, 786554. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ar Royan, H.; Tyas, I.I.; Nadira, A.; Abdi, I.F.; Anwar, S.; Husnah, M.; Ichsan, I.; Pranata, A.; Mudatsir, M.; et al. Waning Anti-SARS-CoV-2 Neutralizing Antibody in CoronaVac-Vaccinated Individuals in Indonesia. F1000Res 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Volpe, G.J.; Vessoni, S.C.S.; Soares, L.B.; Leite dos Santos Almeida, M.A.A.; Braga, P.E.; de Moraes, G.R.; Ferreira, N.N.; Garibaldi, P.M.M.; Kashima, S.; Fonseca, B.A.L.; et al. Antibody Response Dynamics to CoronaVac Vaccine and Booster Immunization in Adults and the Elderly: A Long-Term, Longitudinal Prospective Study. IJID Reg. 2023, 7, 222–229. [Google Scholar] [CrossRef]
- ANVISA. VOTO No. 214/2021/SEI/DIRE2/ANVISA: Avaliação Da Anvisa Sobre Doses de Reforço Para as Vacinas Contra a COVID-19; Ministério da Saúde: Brasília, Brazil, 2021. [Google Scholar]
- Ativação da Célula B e Produção de Anticorpos. In Imunologia Celular e Molecular; Abbas, A.K.; Lichtman, A.H.; Pillai, S. (Eds.) Elsevier Editora Ltd.: Rio de Janeiro, Brazil, 2019; pp. 1–565. [Google Scholar]
- Pinho, C.T.; Vidal, A.F.; Negri Rocha, T.C.; Oliveira, R.R.M.; da Costa Barros, M.C.; Closset, L.; Azevedo-Pinheiro, J.; Braga-da-Silva, C.; Silva, C.S.; Magalhães, L.L.; et al. Transmission Dynamics of SARS-CoV-2 Variants in the Brazilian State of Pará. Front. Public Health 2023, 11, 1186463. [Google Scholar] [CrossRef]
- Graninger, M.; Camp, J.V.; Aberle, S.W.; Traugott, M.T.; Hoepler, W.; Puchhammer-Stöckl, E.; Weseslindtner, L.; Zoufaly, A.; Aberle, J.H.; Stiasny, K. Heterogeneous SARS-CoV-2-Neutralizing Activities after Infection and Vaccination. Front. Immunol. 2022, 13, 888794. [Google Scholar] [CrossRef] [PubMed]
- Khaleeq, S.; Sengupta, N.; Kumar, S.; Patel, U.R.; Rajmani, R.S.; Reddy, P.; Pandey, S.; Singh, R.; Dutta, S.; Ringe, R.P.; et al. Neutralizing Efficacy of Encapsulin Nanoparticles against SARS-CoV2 Variants of Concern. Viruses 2023, 15, 346. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Saied, A.R.A.; Mitra, S.; Alhumaydhi, F.A.; Emran, T.B.; Wilairatana, P. Omicron Variant (B.1.1.529) and Its Sublineages: What Do We Know so Far amid the Emergence of Recombinant Variants of SARS-CoV-2? Biomed. Pharmacother. 2022, 154, 113522. [Google Scholar] [CrossRef] [PubMed]
- Wietschel, K.A.; Fechtner, K.; Antileo, E.; Abdurrahman, G.; Drechsler, C.A.; Makuvise, M.K.; Rose, R.; Voß, M.; Krumbholz, A.; Michalik, S.; et al. Non-Cross-Reactive Epitopes Dominate the Humoral Immune Response to COVID-19 Vaccination—Kinetics of Plasma Antibodies, Plasmablasts and Memory B Cells. Front. Immunol. 2024, 15, 1382911. [Google Scholar] [CrossRef] [PubMed]
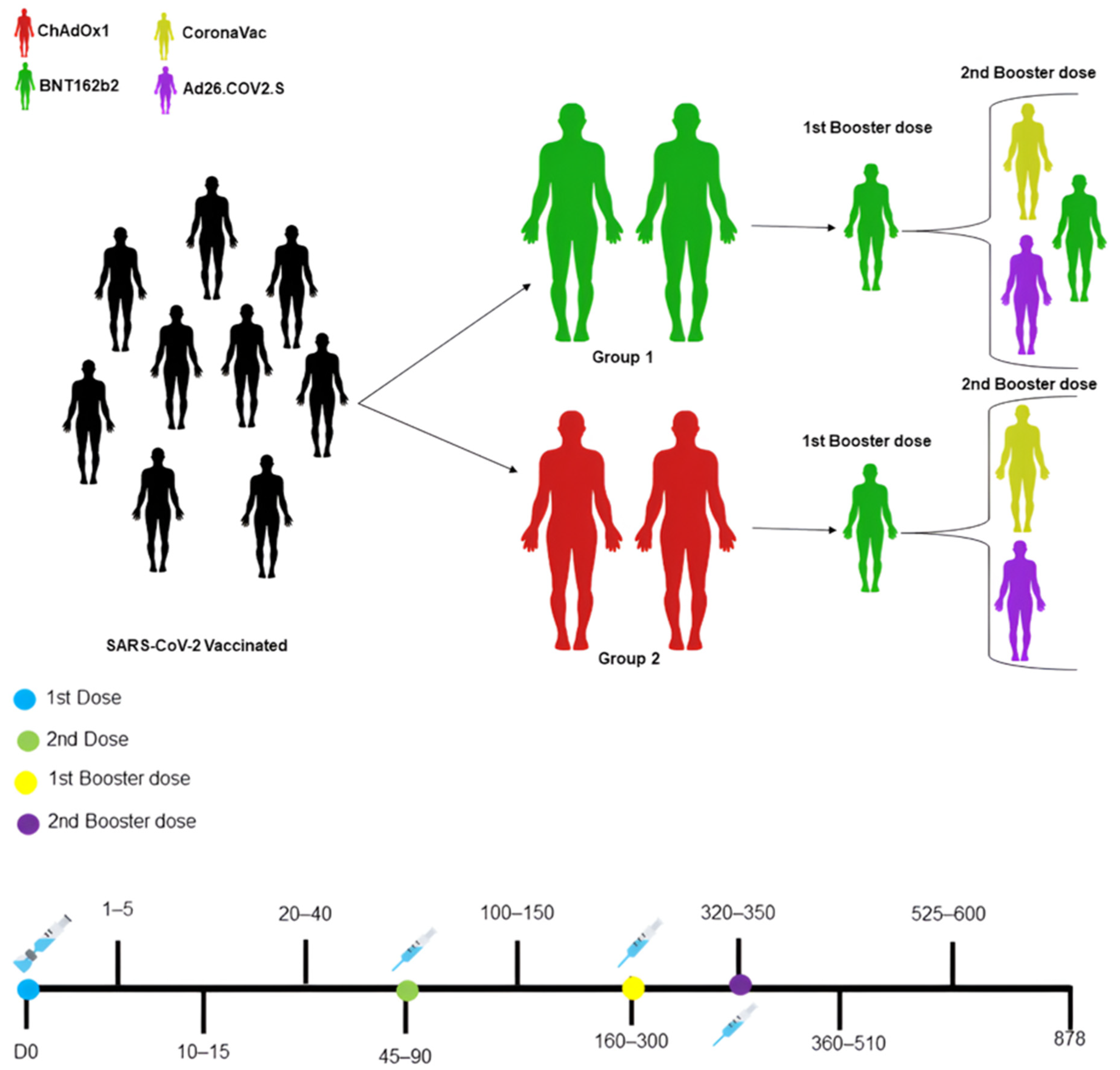
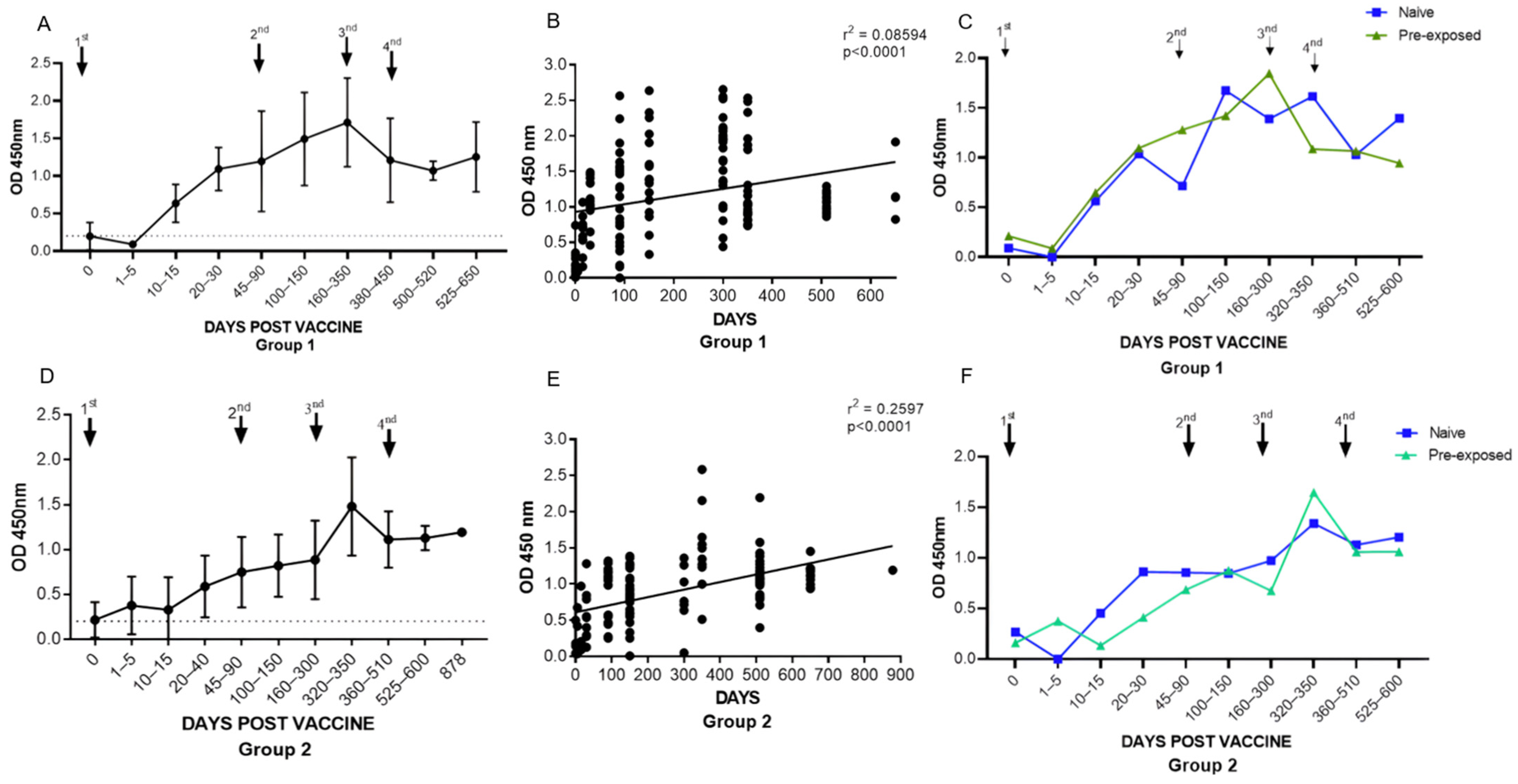
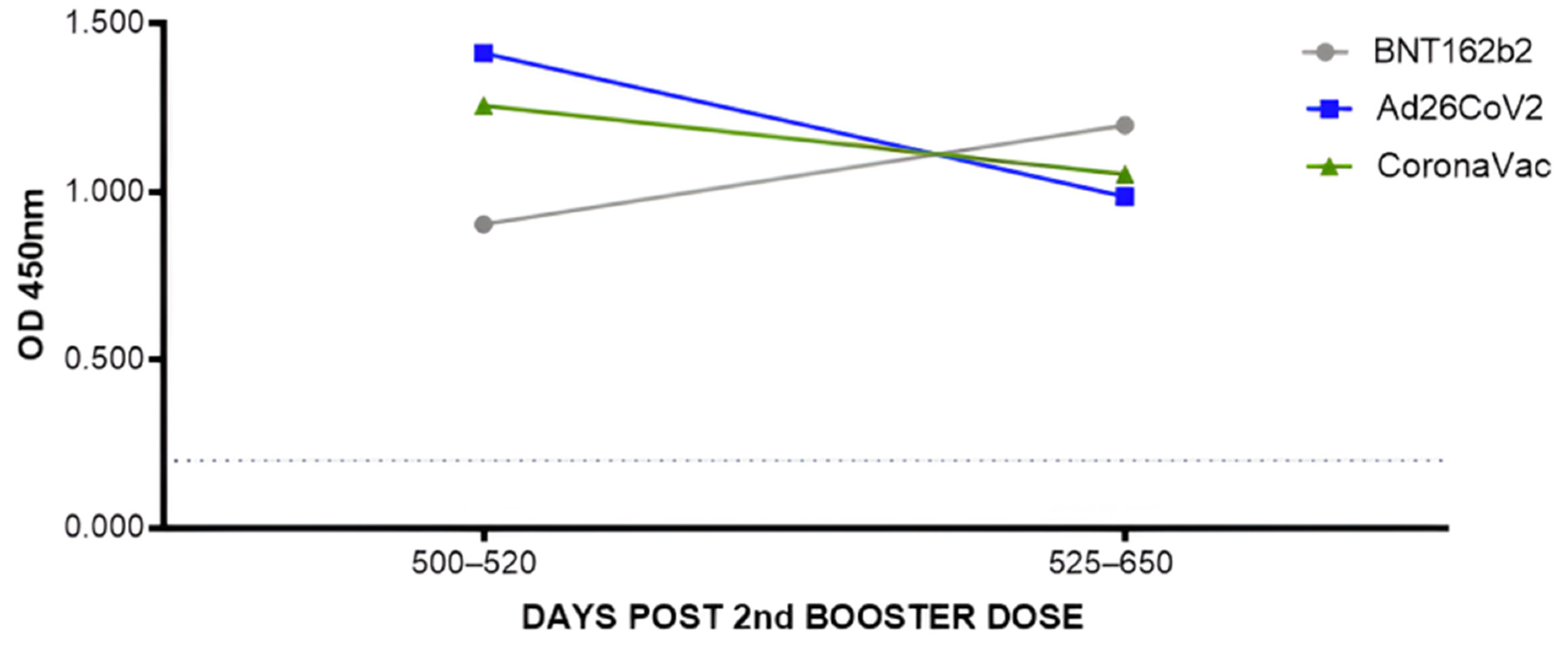

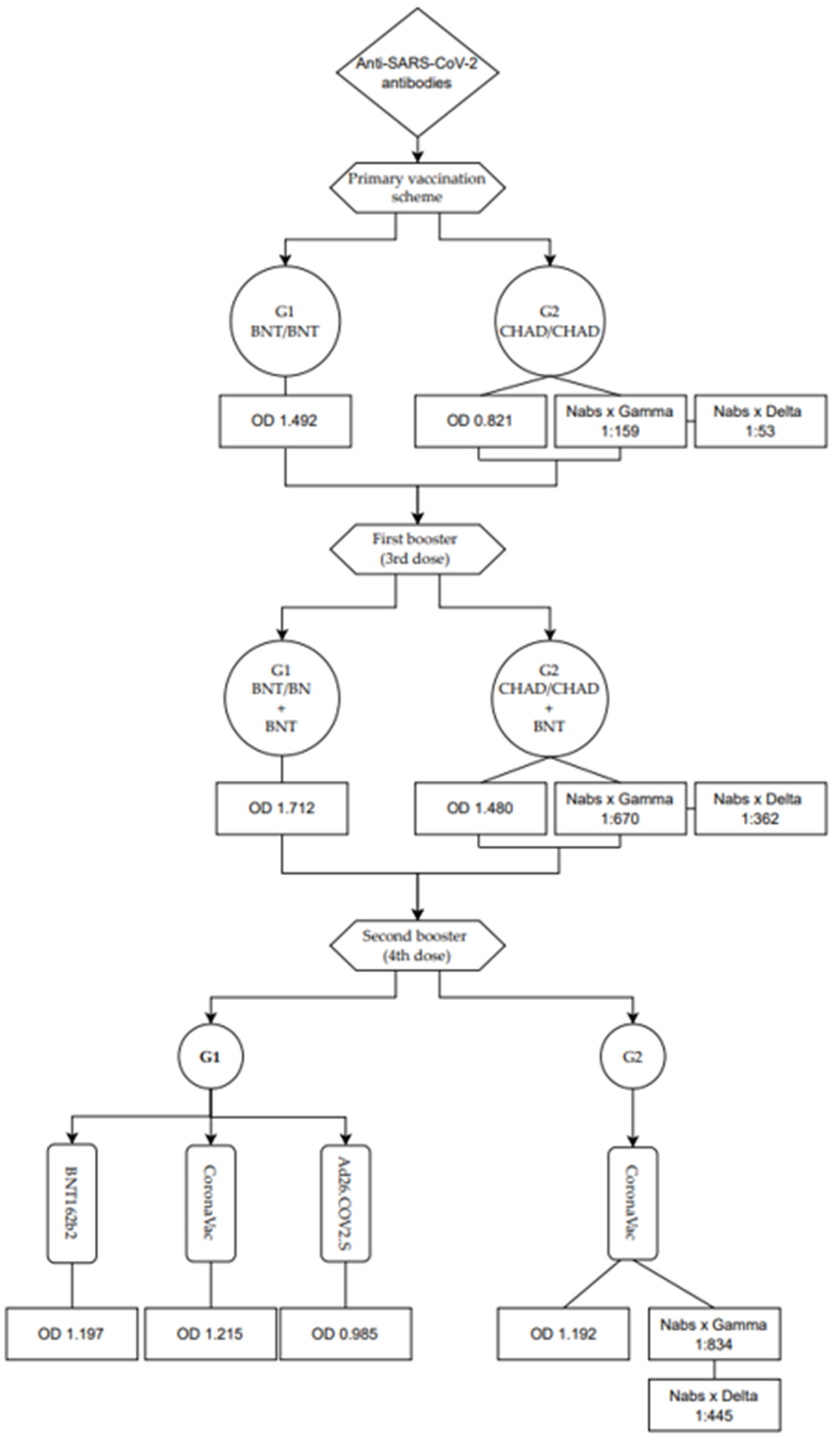
| Variables | Group 1 | Group 2 |
|---|---|---|
| Gender | ||
| Female | 12 (54.5%) | 12 (66.6%) |
| Male | 10 (45.4%) | 6 (33.3%) |
| Age Group | ||
| Means (±) | 39.8 (±7.5) | 39.6 (±12.4) |
| Median | 38.5 | 39.0 |
| Natural Infection | ||
| Symptomatic | 17 (77.2%) | 9 (50%) |
| Asymptomatic | 5 (22.7%) | 9 (50%) |
| Comorbidities | ||
| Psoriasis | - | 1 (5.5%) |
| Arterial Hypertension | - | 1 (5.5%) |
| Obesity | - | 1 (5.5%) |
| Type 2 diabetes | 1 (4.5%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, T.A.; Nogami, P.Y.; de Oliveira, C.F.; Neto, W.F.F.; da Silva, C.P.; Ribeiro, A.C.S.; de Sousa, A.W.; Freitas, M.N.O.; Chiang, J.O.; Silva, F.A.; et al. Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster. Vaccines 2024, 12, 792. https://doi.org/10.3390/vaccines12070792
do Nascimento TA, Nogami PY, de Oliveira CF, Neto WFF, da Silva CP, Ribeiro ACS, de Sousa AW, Freitas MNO, Chiang JO, Silva FA, et al. Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster. Vaccines. 2024; 12(7):792. https://doi.org/10.3390/vaccines12070792
Chicago/Turabian Styledo Nascimento, Tatiana A., Patricia Y. Nogami, Camille F. de Oliveira, Walter F. F. Neto, Carla P. da Silva, Ana Claudia S. Ribeiro, Alana W. de Sousa, Maria N. O. Freitas, Jannifer O. Chiang, Franko A. Silva, and et al. 2024. "Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster" Vaccines 12, no. 7: 792. https://doi.org/10.3390/vaccines12070792






