Immunizing Mice with Influenza Virus-like Particles Expressing the Leishmania amazonensis Promastigote Surface Antigen Alleviates Inflammation in Footpad
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Parasites
2.2. Generation of Recombinant Baculovirus and VLPs
2.3. Confirmation of VLP Assembly, Antigen Expression, and Characterizing the VLPs
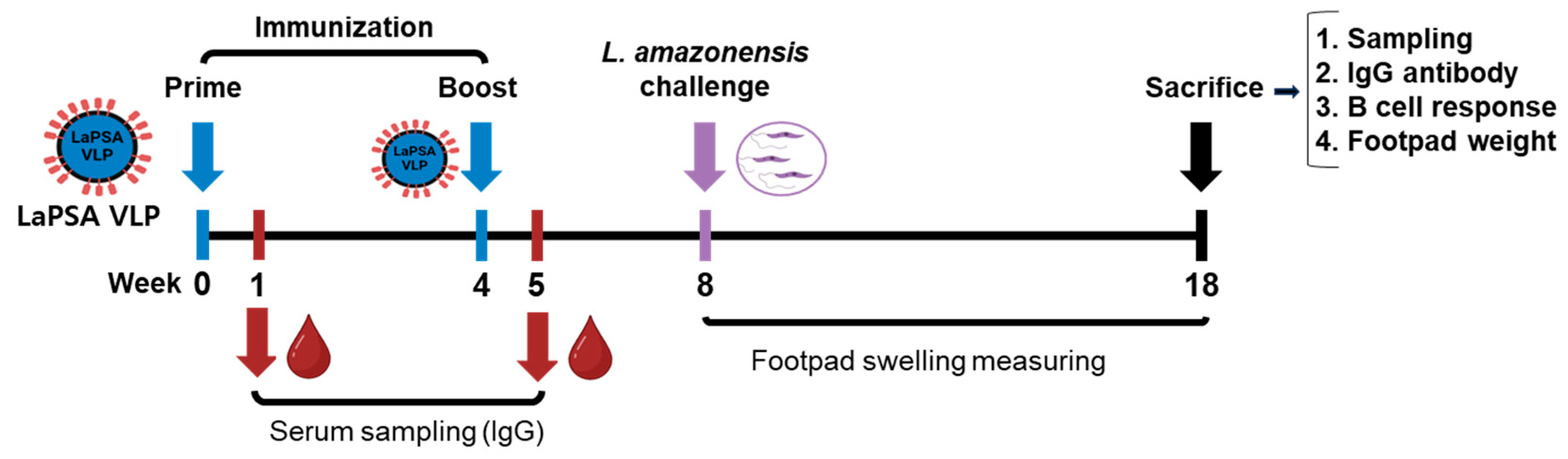
2.4. VLP Vaccine Immunization and L. amazonensis Infection in Mice
2.5. L. amazonensis-Specific IgG, IgG1 and IgG2a in Sera, IgG in Spleen, Popliteal Lymph Node (PLN), and Footpad Homogenates
2.6. B Cell Responses in PLN by Flow Cytometry
2.7. Footpad Thickness, Weight, and IFN-γ
2.8. Statistical Analysis
3. Results
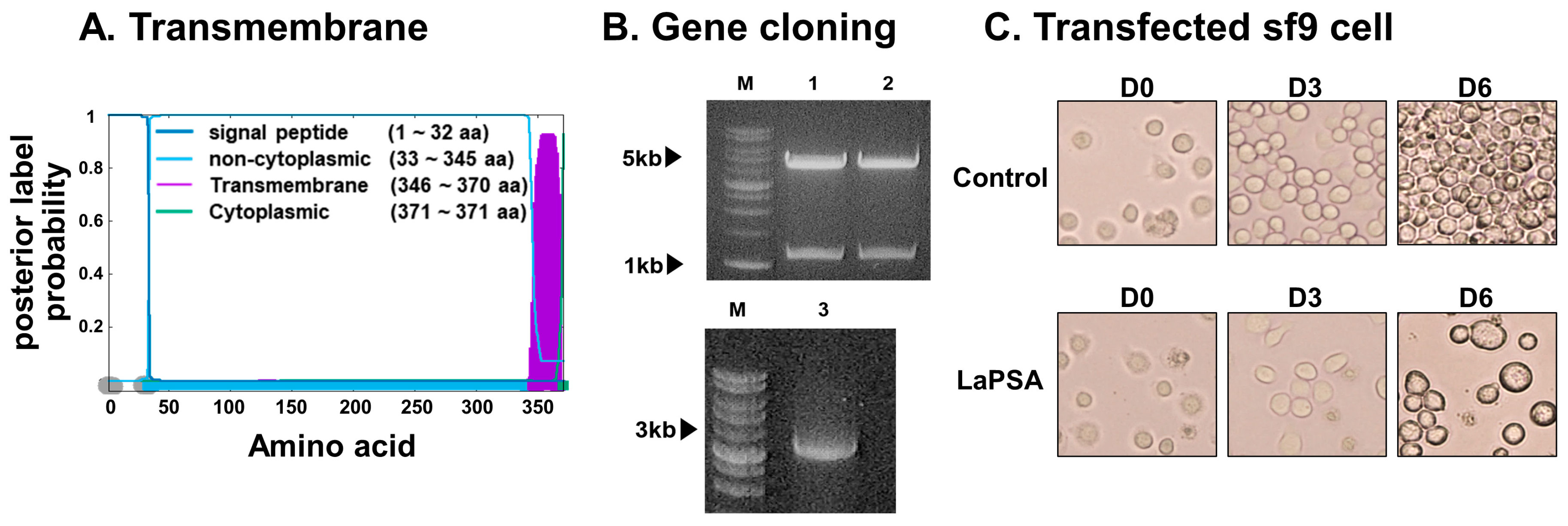
3.1. LaPSA Gene Cloning, VLP Assembly, and VLP Characterization
3.2. Animal Experimental Schedule
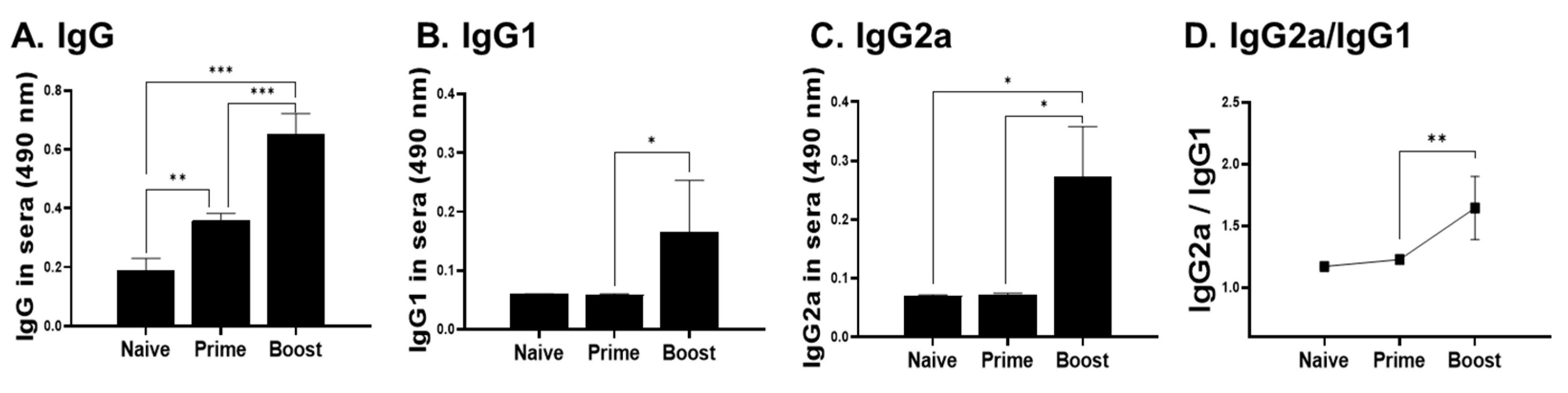
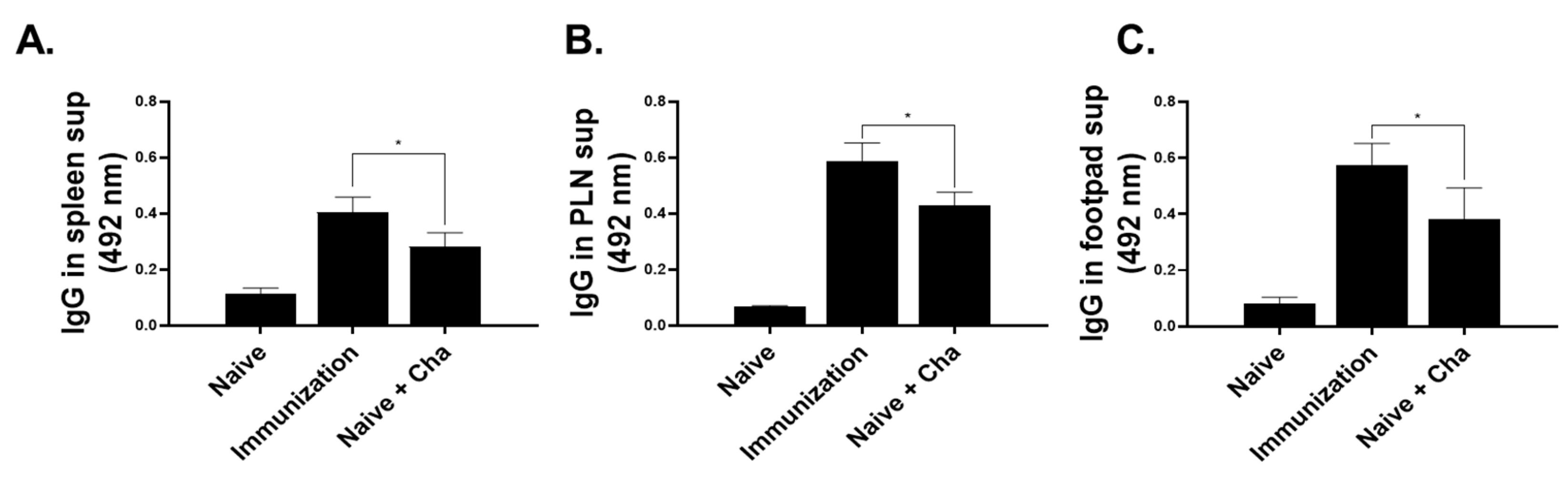
3.3. IgG Antibody Response in Sera, Spleen, PLN, and Footpad
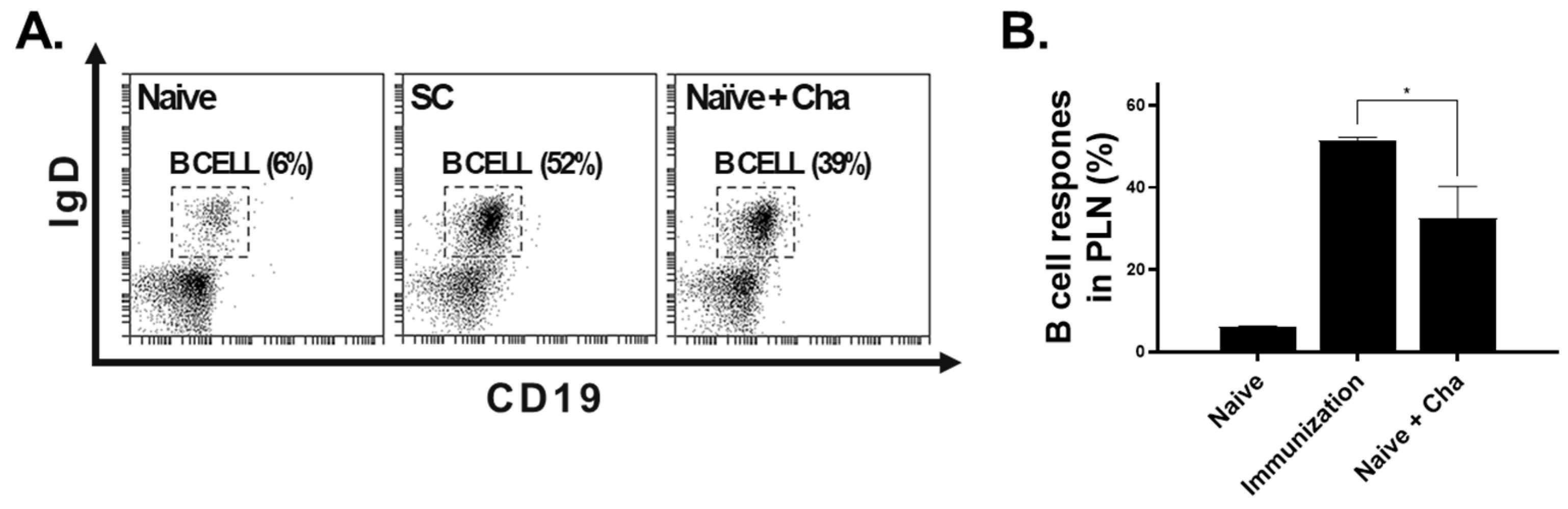
3.4. B Cell Response in the PLN
3.5. LaPSA-VLP Immunization Lessened the Severity of CL and Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R. The biology and control of phlebotomine sand flies. Clin. Dermatol. 1999, 17, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Madusanka, R.K.; Silva, H.; Karunaweera, N.D. Treatment of Cutaneous Leishmaniasis and Insights into Species-Specific Responses: A Narrative Review. Infect. Dis. Ther. 2022, 11, 695–711. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.; Reedijk, S.H.; Schallig, H.D. Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, J.A.; Nascimento, E.T.; Monteiro, G.R.; Fernandes, M.Z.; Ramos, A.M.; Wilson, M.E.; Pearson, R.D.; Jeronimo, S.M. Atypical American visceral leishmaniasis caused by disseminated Leishmania amazonensis infection presenting with hepatitis and adenopathy. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Tolezano, J.E.; Uliana, S.R.; Taniguchi, H.H.; Araújo, M.F.; Barbosa, J.A.; Barbosa, J.E.; Floeter-Winter, L.M.; Shaw, J.J. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral leishmaniasis from Araçatuba County, São Paulo State, Brazil. Vet. Parasitol. 2007, 149, 280–284. [Google Scholar] [CrossRef]
- Purse, B.V.; Masante, D.; Golding, N.; Pigott, D.; Day, J.C.; Ibañez-Bernal, S.; Kolb, M.; Jones, L. How will climate change pathways and mitigation options alter incidence of vector-borne diseases? A framework for leishmaniasis in South and Meso-America. PLoS ONE 2017, 12, e0183583. [Google Scholar] [CrossRef] [PubMed]
- Trájer, A.J.; Grmasha, R.A. The potential effects of climate change on the climatic suitability patterns of the Western Asian vectors and parasites of cutaneous leishmaniasis in the mid- and late twenty-first century. Theor. Appl. Climatol. 2024, 155, 1897–1914. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Bezemer, J.M.; van der Ende, J.; Limpens, J.; de Vries, H.J.C.; Schallig, H. Safety and efficacy of allylamines in the treatment of cutaneous and mucocutaneous leishmaniasis: A systematic review. PLoS ONE 2021, 16, e0249628. [Google Scholar] [CrossRef] [PubMed]
- Myung, K.S.; Beetham, J.K.; Wilson, M.E.; Donelson, J.E. Comparison of the post-transcriptional regulation of the mRNAs for the surface proteins PSA (GP46) and MSP (GP63) of Leishmania chagasi. J. Biol. Chem. 2002, 277, 16489–16497. [Google Scholar] [CrossRef] [PubMed]
- Handman, E.; Osborn, A.H.; Symons, F.; van Driel, R.; Cappai, R. The Leishmania promastigote surface antigen 2 complex is differentially expressed during the parasite life cycle. Mol. Biochem. Parasitol. 1995, 74, 189–200. [Google Scholar] [CrossRef]
- Beetham, J.K.; Donelson, J.E.; Dahlin, R.R. Surface glycoprotein PSA (GP46) expression during short- and long-term culture of Leishmania chagasi. Mol. Biochem. Parasitol. 2003, 131, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bras-Gonçalves, R.; Petitdidier, E.; Pagniez, J.; Veyrier, R.; Cibrelus, P.; Cavaleyra, M.; Maquaire, S.; Moreaux, J.; Lemesre, J.L. Identification and characterization of new Leishmania promastigote surface antigens, LaPSA-38S and LiPSA-50S, as major immunodominant excreted/secreted components of L. amazonensis and L. infantum. Infect. Genet. Evol. 2014, 24, 1–14. [Google Scholar] [CrossRef]
- Petitdidier, E.; Pagniez, J.; Pissarra, J.; Holzmuller, P.; Papierok, G.; Vincendeau, P.; Lemesre, J.L.; Bras-Gonçalves, R. Peptide-based vaccine successfully induces protective immunity against canine visceral leishmaniasis. NPJ Vaccines 2019, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Petitdidier, E.; Pagniez, J.; Papierok, G.; Vincendeau, P.; Lemesre, J.L.; Bras-Gonçalves, R. Recombinant Forms of Leishmania amazonensis Excreted/Secreted Promastigote Surface Antigen (PSA) Induce Protective Immune Responses in Dogs. PLoS Negl. Trop. Dis. 2016, 10, e0004614. [Google Scholar] [CrossRef]
- Chamakh-Ayari, R.; Bras-Gonçalves, R.; Bahi-Jaber, N.; Petitdidier, E.; Markikou-Ouni, W.; Aoun, K.; Moreno, J.; Carrillo, E.; Salotra, P.; Kaushal, H.; et al. In vitro evaluation of a soluble Leishmania promastigote surface antigen as a potential vaccine candidate against human leishmaniasis. PLoS ONE 2014, 9, e92708. [Google Scholar] [CrossRef]
- McMahon-Pratt, D.; Rodriguez, D.; Rodriguez, J.R.; Zhang, Y.; Manson, K.; Bergman, C.; Rivas, L.; Rodriguez, J.F.; Lohman, K.L.; Ruddle, N.H.; et al. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect. Immun. 1993, 61, 3351–3359. [Google Scholar] [CrossRef]
- Chu, K.B.; Quan, F.S. Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites. Curr. Top. Microbiol. Immunol. 2021, 433, 77–106. [Google Scholar] [CrossRef]
- Cecílio, P.; Pérez-Cabezas, B.; Fernández, L.; Moreno, J.; Carrillo, E.; Requena, J.M.; Fichera, E.; Reed, S.G.; Coler, R.N.; Kamhawi, S.; et al. Pre-clinical antigenicity studies of an innovative multivalent vaccine for human visceral leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005951. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.W.; Chu, K.B.; Eom, G.D.; Mao, J.; Kim, M.J.; Lee, H.; No, J.H.; Quan, F.S. Protective Humoral Immune Response Induced by Recombinant Virus-like Particle Vaccine Expressing Leishmania donovani Surface Antigen. ACS Infect. Dis. 2023, 9, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Latham, T.; Galarza, J.M. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 2001, 75, 6154–6165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.W.; Chu, K.B.; Eom, G.D.; Mao, J.; Quan, F.S. Vaccine efficacy induced by virus-like particles containing Leishmania donovani surface glycoprotein GP63. PLoS Negl. Trop. Dis. 2024, 18, e0012229. [Google Scholar] [CrossRef] [PubMed]
- Løvik, M.; Alberg, T.; Nygaard, U.C.; Samuelsen, M.; Groeng, E.C.; Gaarder, P.I. Popliteal lymph node (PLN) assay to study adjuvant effects on respiratory allergy. Methods 2007, 41, 72–79. [Google Scholar] [CrossRef]
- Troncoso, M.E.; Germanó, M.J.; Arrieta, V.J.; García Bustos, M.F.; Cifuente, D.; Cargnelutti, D.E.; Lozano, E.S. Antiparasitic Activity of Two Natural Terpenes from Salvia cuspidata against Leishmania amazonensis. J. Nat. Prod. 2023, 86, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Zhang, W.W.; Lypaczewski, P.; Cox, B.; Fultz, R.; Mishan, C.; Verma, C.; Huston, R.H.; et al. Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis. NPJ Vaccines 2022, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, A.; Baldwin, T.M.; Curtis, J.M.; Bengtsson, K.L.; Handman, E. Vaccination with recombinant Parasite Surface Antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine 1998, 16, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Carrión, J.; Anderson, C.; Alonso, C.; Sacks, D.; Soto, M. Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice. Infect. Immun. 2005, 73, 5842–5852. [Google Scholar] [CrossRef]
- Campos-Neto, A.; Porrozzi, R.; Greeson, K.; Coler, R.N.; Webb, J.R.; Seiky, Y.A.; Reed, S.G.; Grimaldi, G., Jr. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect. Immun. 2001, 69, 4103–4108. [Google Scholar] [CrossRef]
- Bertholet, S.; Goto, Y.; Carter, L.; Bhatia, A.; Howard, R.F.; Carter, D.; Coler, R.N.; Vedvick, T.S.; Reed, S.G. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine 2009, 27, 7036–7045. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.B.; Touihri, L.; Chtourou, Y.; Dellagi, K.; Bahloul, C. DNA based vaccination with a cocktail of plasmids encoding immunodominant Leishmania (Leishmania) major antigens confers full protection in BALB/c mice. Vaccine 2009, 27, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Maria Jesus, R.S.; Javier, E.O.; Maria del Rosario, G.M. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 2003, 21, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Laabs, E.M.; Wu, W.; Mendez, S. Vaccination with live Leishmania major and CpG DNA promotes interleukin-2 production by dermal dendritic cells and NK cell activation. Clin. Vaccine Immunol. 2009, 16, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Weigand, L.; Belkaid, Y.; Mendez, S. Immunomodulatory effects associated with a live vaccine against Leishmania major containing CpG oligodeoxynucleotides. Eur. J. Immunol. 2006, 36, 3238–3247. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, E.; Kochan, G.; Gherardi, M.M.; Esteban, M. MVA-LACK as a safe and efficient vector for vaccination against leishmaniasis. Microbes Infect. 2006, 8, 810–822. [Google Scholar] [CrossRef]
- Sánchez-Sampedro, L.; Gómez, C.E.; Mejías-Pérez, E.; Pérez-Jiménez, E.; Oliveros, J.C.; Esteban, M. Attenuated and replication-competent vaccinia virus strains M65 and M101 with distinct biology and immunogenicity as potential vaccine candidates against pathogens. J. Virol. 2013, 87, 6955–6974. [Google Scholar] [CrossRef]
- Stober, C.B.; Lange, U.G.; Roberts, M.T.; Alcami, A.; Blackwell, J.M. Heterologous priming-boosting with DNA and modified vaccinia virus Ankara expressing tryparedoxin peroxidase promotes long-term memory against Leishmania major in susceptible BALB/c Mice. Infect. Immun. 2007, 75, 852–860. [Google Scholar] [CrossRef]
- Rabienia, M.; Roudbari, Z.; Ghanbariasad, A.; Abdollahi, A.; Mohammadi, E.; Mortazavidehkordi, N.; Farjadfar, A. Exploring membrane proteins of Leishmania major to design a new multi-epitope vaccine using immunoinformatics approach. Eur. J. Pharm. Sci. 2020, 152, 105423. [Google Scholar] [CrossRef]
- Rashidi, S.; Faraji, S.N.; Mamaghani, A.J.; Hatam, S.; Kazemi, B.; Bemani, P.; Tabaei, S.J.S.; Hatam, G. Bioinformatics analysis for the purpose of designing a novel multi-epitope DNA vaccine against Leishmania major. Sci. Rep. 2022, 12, 18119. [Google Scholar] [CrossRef]
- Varotto-Boccazzi, I.; Epis, S.; Cattaneo, G.M.; Guerrini, N.; Manenti, A.; Rubolini, D.; Gabrieli, P.; Otranto, D.; Zuccotti, G.; Montomoli, E.; et al. Rectal Administration of Leishmania Cells Elicits a Specific, Th1-Associated IgG2a Response in Mice: New Perspectives for Mucosal Vaccination against Leishmaniasis, after the Repurposing of a Study on an Anti-Viral Vaccine Candidate. Trop. Med. Infect. Dis. 2023, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, A.; Baldwin, T.M.; Curtis, J.M.; Handman, E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J. Immunol. 1998, 160, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- McFall-Boegeman, H.; Huang, X. Mechanisms of cellular and humoral immunity through the lens of VLP-based vaccines. Expert Rev. Vaccines 2022, 21, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Vallado, M.; Mut-Martin, M.; García-Miss Mdel, R.; Dumonteil, E. Aluminium phosphate potentiates the efficacy of DNA vaccines against Leishmania mexicana. Vaccine 2005, 23, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.; Wang, V.; Sacks, D.L.; Seder, R.A.; Verthelyi, D. Prevention and treatment of cutaneous leishmaniasis in primates by using synthetic type D/A oligodeoxynucleotides expressing CpG motifs. Infect. Immun. 2005, 73, 4948–4954. [Google Scholar] [CrossRef] [PubMed]
- Verthelyi, D.; Gursel, M.; Kenney, R.T.; Lifson, J.D.; Liu, S.; Mican, J.; Klinman, D.M. CpG oligodeoxynucleotides protect normal and SIV-infected macaques from Leishmania infection. J. Immunol. 2003, 170, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, T.; Wolfram, M.; Patzer, S.I.; Ilg, T.; Wiese, M.; Overath, P. Subunit vaccination of mice against new world cutaneous leishmaniasis: Comparison of three proteins expressed in amastigotes and six adjuvants. Infect. Immun. 2000, 68, 1328–1336. [Google Scholar] [CrossRef]
- Diupotex, M.; Zamora-Chimal, J.; Cervantes-Sarabia, R.B.; Salaiza-Suazo, N.; Becker, I. Alpha-galactosylceramide as adjuvant induces protective cell-mediated immunity against Leishmania mexicana infection in vaccinated BALB/c mice. Cell. Immunol. 2023, 386, 104692. [Google Scholar] [CrossRef]
- Kang, H.J.; Chu, K.B.; Yoon, K.W.; Eom, G.D.; Mao, J.; Kim, M.J.; Lee, S.H.; Moon, E.K.; Quan, F.S. Neuraminidase in Virus-like Particles Contributes to the Protection against High Dose of Avian Influenza Virus Challenge Infection. Pathogens 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Pereira Silva Bezerra, I.; Amaral Abib, M.; Rossi-Bergmann, B. Intranasal but not subcutaneous vaccination with LaAg allows rapid expansion of protective immunity against cutaneous leishmaniasis. Vaccine 2018, 36, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Pratti, J.E.; Ramos, T.D.; Pereira, J.C.; da Fonseca-Martins, A.M.; Maciel-Oliveira, D.; Oliveira-Silva, G.; de Mello, M.F.; Chaves, S.P.; Gomes, D.C.; Diaz, B.L.; et al. Efficacy of intranasal LaAg vaccine against Leishmania amazonensis infection in partially resistant C57Bl/6 mice. Parasites Vectors 2016, 9, 534. [Google Scholar] [CrossRef]
- Liew, F.Y.; Hale, C.; Howard, J.G. Prophylactic immunization against experimental leishmaniasis. IV. Subcutaneous immunization prevents the induction of protective immunity against fatal Leishmania major infection. J. Immunol. 1985, 135, 2095–2101. [Google Scholar] [CrossRef]
- Pinheiro, R.O.; Pinto, E.F.; Lopes, J.R.; Guedes, H.L.; Fentanes, R.F.; Rossi-Bergmann, B. TGF-beta-associated enhanced susceptibility to leishmaniasis following intramuscular vaccination of mice with Leishmania amazonensis antigens. Microbes Infect. 2005, 7, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Vélez, I.D.; Gilchrist, K.; Arbelaez, M.P.; Rojas, C.A.; Puerta, J.A.; Antunes, C.M.; Zicker, F.; Modabber, F. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.L.; Melby, P.C. Animal models for the analysis of immune responses to leishmaniasis. Curr. Protoc. Immunol. 1998, 28, 19.2.1–19.2.20. [Google Scholar] [CrossRef]
- Wege, A.K.; Florian, C.; Ernst, W.; Zimara, N.; Schleicher, U.; Hanses, F.; Schmid, M.; Ritter, U. Leishmania major infection in humanized mice induces systemic infection and provokes a nonprotective human immune response. PLoS Negl. Trop. Dis. 2012, 6, e1741. [Google Scholar] [CrossRef]
- Hill, J.O.; North, R.J.; Collins, F.M. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect. Immun. 1983, 39, 1087–1094. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, G.-D.; Chu, K.B.; Yoon, K.-W.; Mao, J.; Kim, S.S.; Quan, F.-S. Immunizing Mice with Influenza Virus-like Particles Expressing the Leishmania amazonensis Promastigote Surface Antigen Alleviates Inflammation in Footpad. Vaccines 2024, 12, 793. https://doi.org/10.3390/vaccines12070793
Eom G-D, Chu KB, Yoon K-W, Mao J, Kim SS, Quan F-S. Immunizing Mice with Influenza Virus-like Particles Expressing the Leishmania amazonensis Promastigote Surface Antigen Alleviates Inflammation in Footpad. Vaccines. 2024; 12(7):793. https://doi.org/10.3390/vaccines12070793
Chicago/Turabian StyleEom, Gi-Deok, Ki Back Chu, Keon-Woong Yoon, Jie Mao, Sung Soo Kim, and Fu-Shi Quan. 2024. "Immunizing Mice with Influenza Virus-like Particles Expressing the Leishmania amazonensis Promastigote Surface Antigen Alleviates Inflammation in Footpad" Vaccines 12, no. 7: 793. https://doi.org/10.3390/vaccines12070793
APA StyleEom, G.-D., Chu, K. B., Yoon, K.-W., Mao, J., Kim, S. S., & Quan, F.-S. (2024). Immunizing Mice with Influenza Virus-like Particles Expressing the Leishmania amazonensis Promastigote Surface Antigen Alleviates Inflammation in Footpad. Vaccines, 12(7), 793. https://doi.org/10.3390/vaccines12070793






