SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity
Abstract
:1. Respiratory Tract Infections and Mucosal Immune Responses
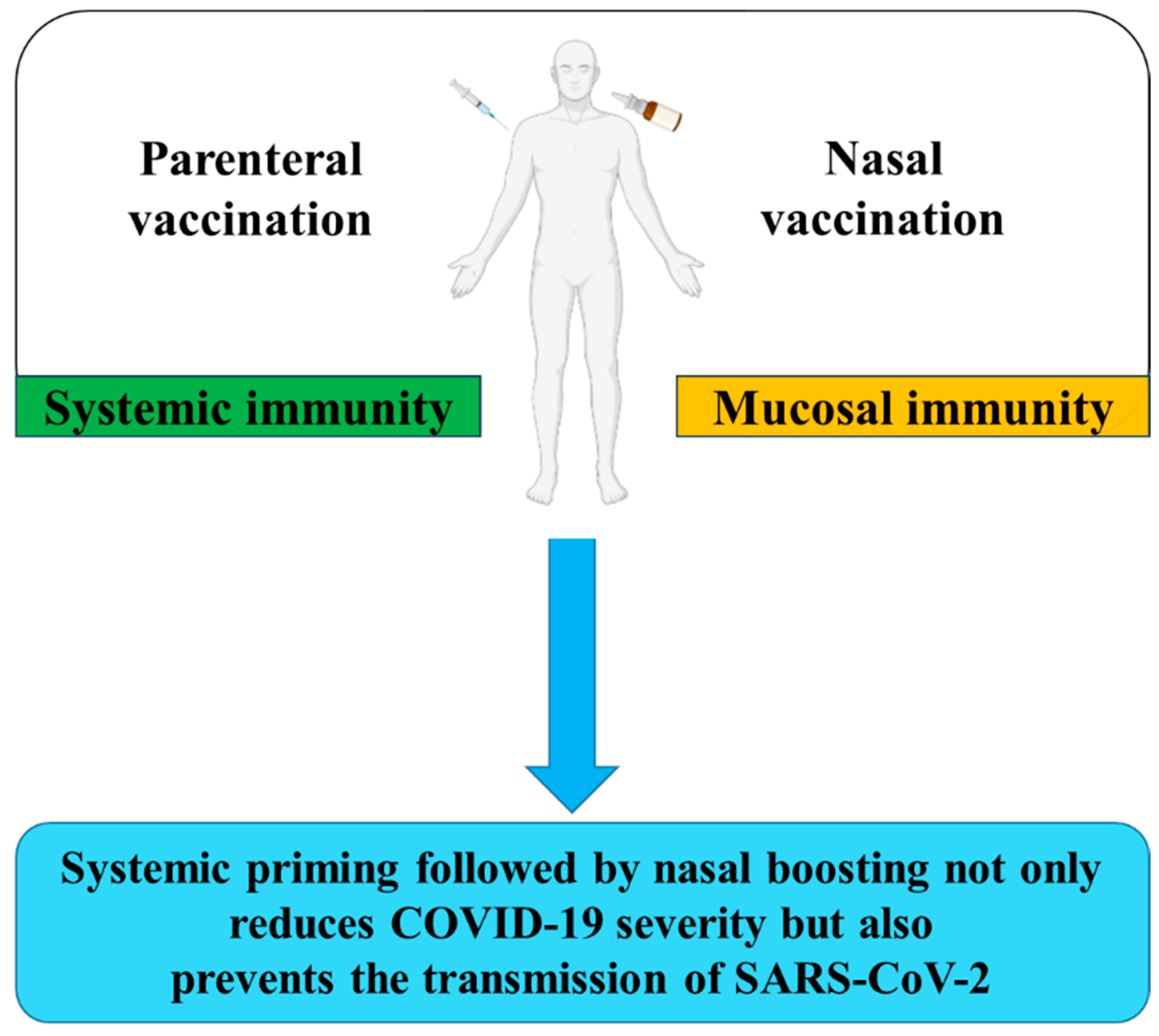
2. Mucosal Versus Systemic Vaccines
3. SARS-CoV-2
3.1. Pathogenicity of SARS-CoV-2
3.2. Virulence Factors of SARS-CoV-2
3.3. SARS-CoV-2 Variants
3.4. Immunity against SARS-CoV-2
4. Treatments against SARS-CoV-2 by Monoclonal Antibodies (mAbs) and Vaccination
4.1. Therapeutic mAbs
4.2. Vaccination against SARS-CoV-2
4.2.1. Systemic Vaccines against SARS-CoV-2
| Vaccine Type/ Platform | Expressed SARS-CoV-2 Component | Approved COVID-19 Vaccine | Developer | Dosage Number and Schedule | Reference |
|---|---|---|---|---|---|
| Nucleoside | Modified mRNA encoding S protein | COMIRNATY (BNT162b2) | BioNTech SE, Pfizer Inc. | Two doses, 3 weeks apart | [166] |
| Modified mRNA encoding S protein | Moderna vaccine (mRNA-1273) | Moderna | Two doses, 4 weeks apart | [167] | |
| Modified adenovirus vector | Encoding S protein | VAXZEVRIA (ChAdOx1-nCoV-19) | AstraZeneca, University of Oxford | Two doses given 4 to 12 weeks apart | [168] |
| Covishield (ChAdOx1 nCoV-19) | Serum Institute of India | Two doses given 12 weeks apart | [169] | ||
| Ad26CoV2.S | Johnson & Johnson | One time dose | [170] | ||
| CONVIDECIA (Ad5-nCoV) | CanSino Biologics Inc. | One time dose | [171] | ||
| Sputnik V | Gamaleya Research Institute of Epidemiology and Microbiology | Two doses given 3 weeks apart | [172] | ||
| Inactivated SARS-CoV-2 | BBIBP-CorV | Sinopharm | Two doses, 3 weeks apart | [173] | |
| CoviVac | Russian Academy of Sciences | Two doses given two weeks apart | [174] | ||
| CoronaVac | Sinovac Biotech Ltd. | Two doses given 2 weeks apart | [175] | ||
| COVAXIN (BBV152) | Bharat Biotech | Two doses given 4 weeks apart | [176] | ||
| VLA2001 | Valneva | Two doses given 4 weeks apart | [177] | ||
| Adjuvanted protein subunit | Dimeric RBD (with aluminium hydroxide) | ZF2001 | Chinese Academy of Sciences | Three doses given 30 days apart | [178] |
| Recombinant RBD fusion heterodimer of the Alpha and the Beta variants of SARS-CoV-2 (with an oil-in-water emulsion based on squalene (SQBA)) | PHH-1V (Bimervax) | HIPRA | Booster dose (for 16 years and older age group) | [179] | |
| Peptide Subunit | A peptide vaccine composed of three short peptides derived from SARS-CoV-2 spike protein (S454–478, S1181–1202, and S1191–1211) conjugated to SARS-CoV-2 nucleocapsid protein | EpiVacCorona | Vektor State Research Centre, Russia | Two doses, 3-4 weeks apart | [180] |
| Recombinant protein | S protein nanoparticle | NUVAXOVID (Nvx-CoV-2373) | Novavax | Two doses, 3 weeks apart | [181] |
| Covovax | Serum Institute of India | Two doses, 3 weeks apart | [182] | ||
4.2.2. Mucosal Vaccines against SARS-CoV-2
| Vaccine Type/ Platform | Expressed SARS-CoV-2 Component | Vaccine Name | Developer | Phase | Clinical Trial Identifier | Application | Reference |
|---|---|---|---|---|---|---|---|
| Viral Vector (Replicating) | live-attenuated influenza virus vector-based expressing SARS-CoV-2 RBD | dNS1-RBD | Beijing Wantai | Approved | ChiCTR2000037782 ChiCTR2000039715 ChiCTR2100048316 | Two intranasal doses, 14 or 21 days apart | [183] |
| Replication deficient Influenza A (CA4-DelNS1) virus expressing RBD domain of S protein | DelNS1-2019-nCoV-RBDOPT1 | University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | Phase 3 | ChiCTR2100051391 | Two intranasal doses, 14 days apart | [184] | |
| Respiratory Syncytial virus expressing S protein | MV-014-212 | Meissa Vaccines, Inc. | Phase 1 | NCT04798001 | Single intranasal dose, or 2 intranasal doses 36 days apart | [185] | |
| Human adenovirus serotype 5 expressing S protein and nucleocapsid | hAd5-S-Fusion + N-ETSD | ImmunityBio Inc. | Phase 1b | NCT04591717 | Single subcutaneous dose followed by single sublingual dose, 21 days apart | [186] | |
| Adenoviral vector expressing WA1 S protein | BBV154 | Bharat Biotech International Limited | Approved | NCT05522335 | Two intranasal doses, 28 days apart | [187] | |
| Viral Vector (Non-replicating) | Parainfluenza virus 5 expressing WA1 S protein | CVXGA1/PIV5-SARS-CoV-2 | CyanVac LLC | Phase 1 | NCT04954287 | Single intranasal dose | [188] |
| Adenoviral vector expressing S protein | SC-Ad6-1 | Tetherex Pharmaceuticals Corporation | Phase 1 | NCT04839042 | Single intranasal dose, or 2 intranasal doses one month apart | [189] | |
| Adenoviral vector expressing S protein | ChAdOx1/AZD1222 | University of Oxford and AstraZeneca Biopharmaceuticals | Phase 1 | NCT04816019 | Single intranasal dose | [190] | |
| Adenoviral vector expressing WA1 S protein | Ad5-nCoV-IH (Convidecia Air) | CanSinoBio | Approved | NCT04552366 | Two doses with different administration routes (2 intranasal doses, 1 intramuscular and 1 intranasal dose, 28 days apart | [191] | |
| Adenoviral vector | Sputnik V/Gam-COVIDVac | The Gamaleya Research Institute of Epidemiology and Microbiology | Approved | NCT04954092 NCT05248373 | Single intranasal dose | [192] | |
| Attenuated SARS-CoV-2 WA1 strain | CoviLiv | Codagenix/Serum Institute of India | Phase 3 | ISRCTN15779782 | Two intranasal doses, 28 days apart | [193] [194] | |
| Live attenuated virus | RBD adjuvanted with aluminium hydroxide | CIGB-669 (RBD + AgnHB) (Mambisa) | Center for Genetic Engineering and Biotechnology (CIGB) | Phase 2 | RPCEC00000345 | Two intranasal dose, 28 days apart, or One intramuscular dose followed by 2 intranasal doses, 28 days apart | [195] |
| Protein subunit | S protein encapsulated by an artificial cell membrane | ACM Biolabs ACM-SARS-CoV-2- beta ACM CpG vaccine candidate (ACM-001) | ACM Biolabs | Phase 1 | NCT05385991 | Single dose, after full vaccination with any registered and commercial SARS-CoV-2 vaccines | [196] |
| Recombinant S protein | RAZI-COV PARS | Razi Vaccine and Serum Research Institute | Approved | IRCT20201214049709N2 | Two intramuscular doses, followed by 1 intranasal dose | [197] |
5. Prime-Boost Vaccination against SARS-CoV-2
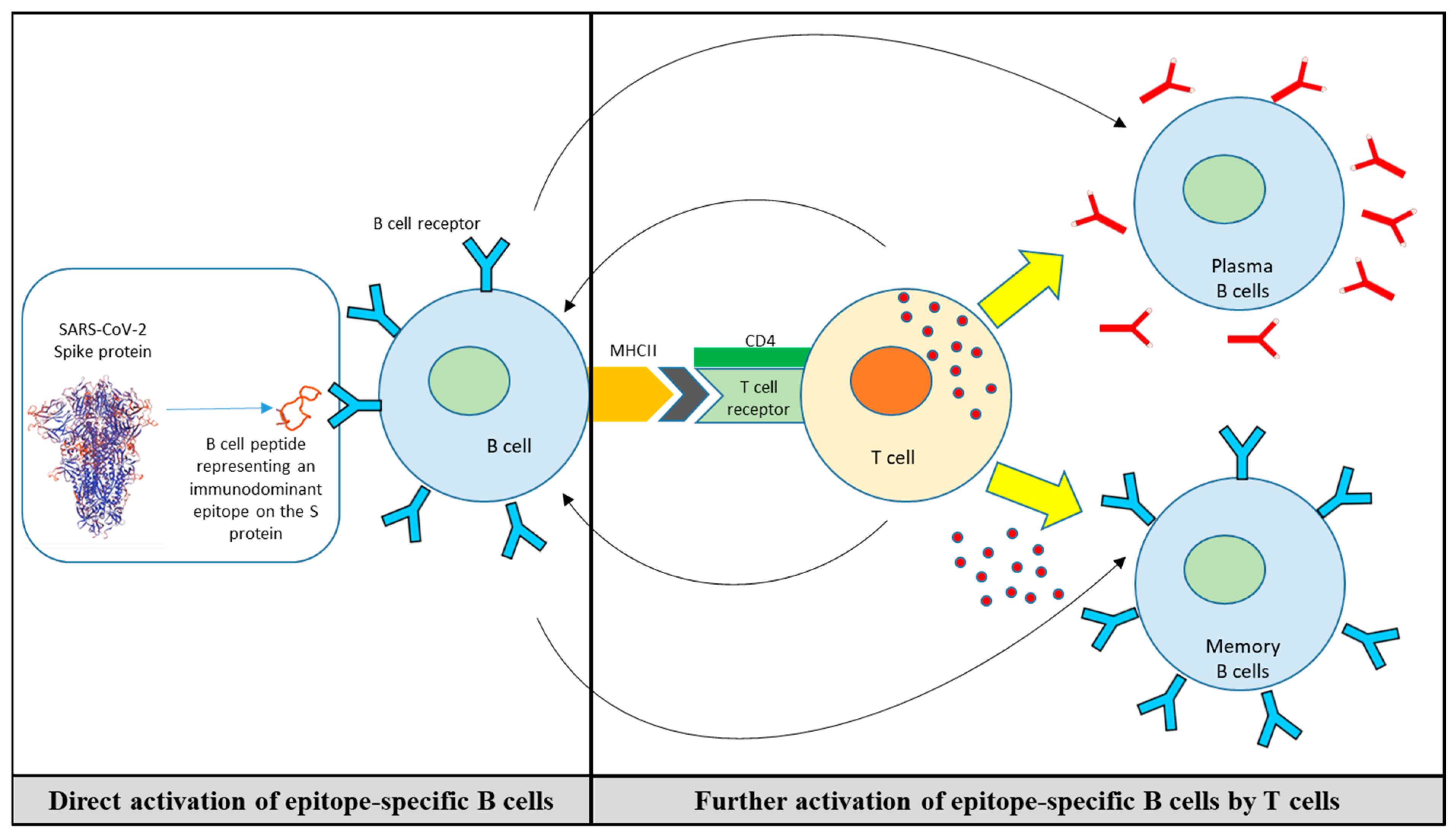
6. B-Cell Peptide/Mimotope-Based Vaccine
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CD | Cluster of differentiation |
| COVID-19 | coronavirus disease 2019 |
| ELISA | Enzyme-linked immunosorbent assay |
| EMA | European Medicine’s Agency |
| FDA | American and Druga Administration |
| IFN-γ | Interferon-gamma |
| IL | Interleukin 2 |
| RBD | Receptor-binding domain |
| RBM | Receptor-binding motif |
| SARS | Severe Acute Respiratory Syndrome |
| TFH | T follicular helper cells |
| Th1 | T helper cell type 1 |
| Th2 | T helper cell type 1 |
References
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Jin, X.; Ren, J.; Li, R.; Gao, Y.; Zhang, H.; Li, J.; Zhang, J.; Wang, X.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine 2021, 37, 100986. [Google Scholar] [CrossRef]
- Mettelman, R.C.; Allen, E.K.; Thomas, P.G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 2022, 55, 749–780. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, Y.; Wang, T.; Xie, F. Innate and adaptive immune response in SARS-CoV-2 infection-Current perspectives. Front. Immunol. 2022, 13, 1053437. [Google Scholar] [CrossRef]
- Brown, B.; Ojha, V.; Fricke, I.; Al-Sheboul, S.A.; Imarogbe, C.; Gravier, T.; Green, M.; Peterson, L.; Koutsaroff, I.P.; Demir, A.; et al. Innate and Adaptive Immunity during SARS-CoV-2 Infection: Biomolecular Cellular Markers and Mechanisms. Vaccines 2023, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Twigg, H.L., 3rd. Humoral immune defense (antibodies): Recent advances. Proc. Am. Thorac. Soc. 2005, 2, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory IgA: Designed for Anti-Microbial Defense. Front. Immunol. 2013, 4, 222. [Google Scholar] [CrossRef]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef]
- Wallace, L.E.; Liu, M.; Van Kuppeveld, F.J.M.; De Vries, E.; De Haan, C.A.M. Respiratory mucus as a virus-host range determinant. Trends Microbiol. 2021, 29, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Woodland, D.L. Vaccine Development. Viral Immunol. 2017, 30, 141. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, W.A.; Ahmed, R. Simply put: Vaccination saves lives. Proc. Natl. Acad. Sci. USA 2017, 114, 4031–4033. [Google Scholar] [CrossRef] [PubMed]
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, V.; Lowen, A.C.; Lakdawala, S.S. Block the Spread: Barriers to Transmission of Influenza Viruses. Annu. Rev. Virol. 2023, 10, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Azegami, T.; Yuki, Y.; Kiyono, H. Challenges in mucosal vaccines for the control of infectious diseases. Int. Immunol. 2014, 26, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Scent of a vaccine. Science 2021, 373, 397–399. [Google Scholar] [CrossRef]
- Bladh, O.; Aguilera, K.; Marking, U.; Kihlgren, M.; Greilert Norin, N.; Smed-Sorensen, A.; Sallberg Chen, M.; Klingstrom, J.; Blom, K.; Russell, M.W.; et al. Comparison of SARS-CoV-2 spike-specific IgA and IgG in nasal secretions, saliva and serum. Front. Immunol. 2024, 15, 1346749. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J. Harnessing Nasal Immunity with IgA to Prevent Respiratory Infections. Immuno 2022, 2, 571–583. [Google Scholar] [CrossRef]
- Tokunoh, N.; Tamiya, S.; Watanabe, M.; Okamoto, T.; Anindita, J.; Tanaka, H.; Ono, C.; Hirai, T.; Akita, H.; Matsuura, Y.; et al. A nasal vaccine with inactivated whole-virion elicits protective mucosal immunity against SARS-CoV-2 in mice. Front. Immunol. 2023, 14, 1224634. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.; Kett, V. Current prospects and future challenges for nasal vaccine delivery. Hum. Vaccin. Immunother. 2017, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, E.; Osaki, S.; Tsuchihashi, T.; Ushijima, H.; Tsukinoki, K. Point-of-Care Diagnostic Biosensors to Monitor Anti-SARS-CoV-2 Neutralizing IgG/sIgA Antibodies and Antioxidant Activity in Saliva. Biosensors 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Artenstein, M.S.; Bellanti, J.A.; Buescher, E.L. Identification of the Antiviral Substances in Nasal Secretions. Proc. Soc. Exp. Biol. Med. 1964, 117, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, J.A.; Artenstein, M.S.; Brandt, B.L.; Klutinis, B.S.; Buescher, E.L. Immunoglobulin responses in serum and nasal secretions after natural adenovirus infections. J. Immunol. 1969, 103, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, J.A.; Artenstein, M.S.; Buescher, E.L. Characterization of Virus Neutralizing Antibodies in Human Serum and Nasal Secretions. J. Immunol. 1965, 94, 344–351. [Google Scholar] [CrossRef]
- Bellanti, J.A.; Sanga, R.L.; Klutinis, B.; Brandt, B.; Artenstein, M.S. Antibody Responses in Serum and Nasal Secretions of Children Immunized with Inactivated and Attenuated Measles-Virus Vaccines. N. Engl. J. Med. 1969, 280, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.E.; Vidarsson, G. Antibodies and their receptors: Different potential roles in mucosal defense. Front. Immunol. 2013, 4, 200. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Jarlhelt, I.; Nielsen, S.K.; Jahn, C.X.H.; Hansen, C.B.; Perez-Alos, L.; Rosbjerg, A.; Bayarri-Olmos, R.; Skjoedt, M.O.; Garred, P. SARS-CoV-2 Antibodies Mediate Complement and Cellular Driven Inflammation. Front. Immunol. 2021, 12, 767981. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert. Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, G.M.; Finn, P.W.; Ward, E.S.; Dumont, J.; Dickinson, B.L.; Blumberg, R.S.; Lencer, W.I. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002, 196, 303–310. [Google Scholar] [CrossRef]
- Ogra, P.L.; Karzon, D.T. Poliovirus antibody response in serum and nasal secretions following intranasal inoculation with inactivated poliovaccine. J. Immunol. 1969, 102, 15–23. [Google Scholar] [CrossRef]
- Ogra, P.L.; Karzon, D.T.; Righthand, F.; MacGillivray, M. Immunoglobulin Response in Serum and Secretions after Immunization with Live and Inactivated Poliovaccine and Natural Infection. N. Engl. J. Med. 1968, 279, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Dotiwala, F.; Upadhyay, A.K. Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens. Vaccines 2023, 11, 1585. [Google Scholar] [CrossRef]
- Pabst, R. Mucosal vaccination by the intranasal route. Nose-associated lymphoid tissue (NALT)—Structure, function and species differences. Vaccine 2015, 33, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; St John, A.L. Promises and challenges of mucosal COVID-19 vaccines. Vaccine 2023, 41, 4042–4049. [Google Scholar] [CrossRef]
- Clement, M.; Humphreys, I.R. Cytokine-Mediated Induction and Regulation of Tissue Damage During Cytomegalovirus Infection. Front. Immunol. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Hillaire, M.L.B.; Rimmelzwaan, G.F.; Kreijtz, J. Clearance of influenza virus infections by T cells: Risk of collateral damage? Curr. Opin. Virol. 2013, 3, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Fang, J.; Yu, J.; Zhang, W.; He, Z.; Ye, L.; Wang, H. The role of CD4+ T cells in tumor and chronic viral immune responses. MedComm 2023, 4, e390. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, M.; Dimonte, S.; Humphreys, I.R. Mucosal T-cell responses to chronic viral infections: Implications for vaccine design. Cell Mol. Immunol. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kervevan, J.; Chakrabarti, L.A. Role of CD4+ T Cells in the Control of Viral Infections: Recent Advances and Open Questions. Int. J. Mol. Sci. 2021, 22, 523. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.R.; Flannery, B.; Ambrose, C.S.; Begue, R.E.; Caspard, H.; DeMarcus, L.; Fowlkes, A.L.; Kersellius, G.; Steffens, A.; Fry, A.M.; et al. Live Attenuated and Inactivated Influenza Vaccine Effectiveness. Pediatrics 2019, 143, e20182094. [Google Scholar] [CrossRef] [PubMed]
- Ilyushina, N.A.; Haynes, B.C.; Hoen, A.G.; Khalenkov, A.M.; Housman, M.L.; Brown, E.P.; Ackerman, M.E.; Treanor, J.J.; Luke, C.J.; Subbarao, K.; et al. Live attenuated and inactivated influenza vaccines in children. J. Infect. Dis. 2015, 211, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.; Brokstad, K.A.; Pathirana, R.D.; Bredholt, G.; Jul-Larsen, A.; Trieu, M.C.; Lartey, S.L.; Montomoli, E.; Tondel, C.; Aarstad, H.J.; et al. Live Attenuated Influenza Vaccine in Children Induces B-Cell Responses in Tonsils. J. Infect. Dis. 2016, 214, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.; Smith, I.; Sjursen, H.; Cox, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccin. Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef]
- Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Kawai, A.; Yamamoto, Y.; Nogimori, T.; Takeshita, K.; Yamamoto, T.; Yoshioka, Y. The Potential of Neuraminidase as an Antigen for Nasal Vaccines to Increase Cross-Protection against Influenza Viruses. J. Virol. 2021, 95, e0118021. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef]
- Lewis, D.J.; Huo, Z.; Barnett, S.; Kromann, I.; Giemza, R.; Galiza, E.; Woodrow, M.; Thierry-Carstensen, B.; Andersen, P.; Novicki, D.; et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 2009, 4, e6999. [Google Scholar] [CrossRef]
- Zheng, Z.; Diaz-Arevalo, D.; Guan, H.; Zeng, M. Noninvasive vaccination against infectious diseases. Hum. Vaccin. Immunother. 2018, 14, 1717–1733. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Williams, C.M.; Wijesundara, D.K.; Furuya, Y. Impact of Pre-Existing Immunity to Influenza on Live-Attenuated Influenza Vaccine (LAIV) Immunogenicity. Vaccines 2020, 8, 683. [Google Scholar] [CrossRef]
- Mok, D.Z.L.; Chan, K.R. The Effects of Pre-Existing Antibodies on Live-Attenuated Viral Vaccines. Viruses 2020, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Coelingh, K.L.; Wu, X.W.; Mallory, R.M.; Ambrose, C.S. An integrated multi-study analysis of serum HAI antibody responses to Ann Arbor strain live attenuated influenza vaccine in children and adults. Trials Vaccinol. 2014, 3, 150–153. [Google Scholar] [CrossRef]
- Zhou, H.; Ni, W.J.; Huang, W.; Wang, Z.; Cai, M.; Sun, Y.C. Advances in Pathogenesis, Progression, Potential Targets and Targeted Therapeutic Strategies in SARS-CoV-2-Induced COVID-19. Front. Immunol. 2022, 13, 834942. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Calina, D.; Falzone, L.; Petrakis, D.; Mitrut, R.; Siokas, V.; Pennisi, M.; Lanza, G.; Libra, M.; Doukas, S.G.; et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020, 146, 111769. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, X.; Wang, Z.; Chen, R.; Li, T.; Zeng, D.; Li, M. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res 2020, 286, 198043. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Zhu, G.; Zhang, Y.; Bi, Z.; Yu, Y.; Huang, B.; Fu, S.; Tan, Y.; Sun, J.; et al. Clinical Features of Maintenance Hemodialysis Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Clin. J. Am. Soc. Nephrol. 2020, 15, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Alefishat, E.; Jelinek, H.F.; Mousa, M.; Tay, G.K.; Alsafar, H.S. Immune response to SARS-CoV-2 variants: A focus on severity, susceptibility, and preexisting immunity. J. Infect. Public Health 2022, 15, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Elkoshi, Z. The Binary Model of Chronic Diseases Applied to COVID-19. Front. Immunol. 2021, 12, 716084. [Google Scholar] [CrossRef]
- Manjili, R.H.; Zarei, M.; Habibi, M.; Manjili, M.H. COVID-19 as an Acute Inflammatory Disease. J. Immunol. 2020, 205, 12–19. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Moghimi, N.; Di Napoli, M.; Biller, J.; Siegler, J.E.; Shekhar, R.; McCullough, L.D.; Harkins, M.S.; Hong, E.; Alaouieh, D.A.; Mansueto, G.; et al. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 infection. Curr. Neurol. Neurosci. Rep. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Dale, L. Neurological Complications of COVID-19: A Review of the Literature. Cureus 2022, 14, e27633. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Minotti, C.; McKenzie, C.; Dewandel, I.; Bekker, C.; Sturniolo, G.; Doni, D.; Giaquinto, C.; Van Der Zalm, M.M.; Dona, D. How does post COVID differ from other post-viral conditions in childhood and adolescence (0–20 years old)? A systematic review. EClinicalMedicine 2024, 68, 102436. [Google Scholar] [CrossRef] [PubMed]
- Pleasure, S.J.; Green, A.J.; Josephson, S.A. The Spectrum of Neurologic Disease in the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic Infection: Neurologists Move to the Frontlines. JAMA Neurol. 2020, 77, 679–680. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Simani, L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020, 413, 116832. [Google Scholar] [CrossRef]
- Caronna, E.; Alpuente, A.; Torres-Ferrus, M.; Pozo-Rosich, P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache 2021, 61, 1277–1280. [Google Scholar] [CrossRef]
- Orendacova, M.; Kvasnak, E. Possible Mechanisms Underlying Neurological Post-COVID Symptoms and Neurofeedback as a Potential Therapy. Front. Hum. Neurosci. 2022, 16, 837972. [Google Scholar] [CrossRef]
- Acharya, A.; Kevadiya, B.D.; Gendelman, H.E.; Byrareddy, S.N. SARS-CoV-2 Infection Leads to Neurological Dysfunction. J. Neuroimmune Pharmacol. 2020, 15, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Du, T.; Hong, W.; Chen, L.; Que, H.; Lu, S.; Peng, X. Neurological complications and infection mechanism of SARS-CoV-2. Signal Transduct. Target. Ther. 2021, 6, 406. [Google Scholar] [CrossRef] [PubMed]
- Molaverdi, G.; Kamal, Z.; Safavi, M.; Shafiee, A.; Mozhgani, S.H.; Ghobadi, M.Z.; Goudarzvand, M. Neurological complications after COVID-19: A narrative review. eNeurologicalSci 2023, 33, 100485. [Google Scholar] [CrossRef] [PubMed]
- Collantes, M.E.V.; Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G. Neurological Manifestations in COVID-19 Infection: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2021, 48, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Delorme, C.; Houot, M.; Rosso, C.; Carvalho, S.; Nedelec, T.; Maatoug, R.; Pitron, V.; Gassama, S.; Sambin, S.; Bombois, S.; et al. The wide spectrum of COVID-19 neuropsychiatric complications within a multidisciplinary centre. Brain Commun. 2021, 3, fcab135. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Palacios-Cena, D.; Gomez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Cabanes-Martinez, L.; Villadoniga, M.; Gonzalez-Rodriguez, L.; Araque, L.; Diaz-Cid, A.; Ruz-Caracuel, I.; Pian, H.; Sanchez-Alonso, S.; Fanjul, S.; Del Alamo, M.; et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin. Neurophysiol. 2020, 131, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Kannappan, S.; Kim, T.; Lee, J.H.; Lee, H.R.; Kim, K.K. Structural understanding of SARS-CoV-2 virus entry to host cells. Front. Mol. Biosci. 2023, 10, 1288686. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Spandidos, D.A.; Karteris, E. Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 21. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Jiang, S.; Du, L.; Shi, Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: Calling for developing therapeutic and prophylactic strategies. Emerg. Microbes Infect. 2020, 9, 275–277. [Google Scholar] [CrossRef]
- Finkelstein, M.T.; Mermelstein, A.G.; Parker Miller, E.; Seth, P.C.; Stancofski, E.D.; Fera, D. Structural Analysis of Neutralizing Epitopes of the SARS-CoV-2 Spike to Guide Therapy and Vaccine Design Strategies. Viruses 2021, 13, 134. [Google Scholar] [CrossRef]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-CoV-2. Front. Cell Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020, 588, 327–330. [Google Scholar] [CrossRef]
- Almagro, J.C.; Mellado-Sanchez, G.; Pedraza-Escalona, M.; Perez-Tapia, S.M. Evolution of Anti-SARS-CoV-2 Therapeutic Antibodies. Int. J. Mol. Sci. 2022, 23, 9763. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses 2023, 15, 167. [Google Scholar] [CrossRef]
- Zappa, M.; Verdecchia, P.; Angeli, F. Is the competition between variants the end of severe acute respiratory syndrome coronavirus 2 pandemic? A journey from Wuhan to XBB.1.16. Eur. J. Intern. Med. 2023, 113, 13–15. [Google Scholar] [CrossRef]
- Andre, M.; Lau, L.S.; Pokharel, M.D.; Ramelow, J.; Owens, F.; Souchak, J.; Akkaoui, J.; Ales, E.; Brown, H.; Shil, R.; et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology 2023, 12, 1267. [Google Scholar] [CrossRef]
- Lyngse, F.P.; Kirkeby, C.T.; Denwood, M.; Christiansen, L.E.; Molbak, K.; Moller, C.H.; Skov, R.L.; Krause, T.G.; Rasmussen, M.; Sieber, R.N.; et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat. Commun. 2022, 13, 5760. [Google Scholar] [CrossRef]
- Xia, H.; Zou, J.; Kurhade, C.; Cai, H.; Yang, Q.; Cutler, M.; Cooper, D.; Muik, A.; Jansen, K.U.; Xie, X.; et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe 2022, 30, 485–488.e483. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature 2022, 607, 351–355. [Google Scholar] [CrossRef]
- Li, D.; Duan, M.; Wang, X.; Gao, P.; Zhao, X.; Xu, K.; Gao, G.F. Neutralization of BQ.1, BQ.1.1, and XBB with RBD-Dimer Vaccines. N. Engl. J. Med. 2023, 388, 1142–1145. [Google Scholar] [CrossRef]
- Qu, P.; Xu, K.; Faraone, J.N.; Goodarzi, N.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Horowitz, J.C.; Mallampalli, R.K.; Saif, L.J.; et al. Immune Evasion, Infectivity, and Fusogenicity of SARS-CoV-2 Omicron BA.2.86 and FLip Variants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Jiang, S. SARS-CoV-2 evolution from the BA.2.86 to JN.1 variants: Unexpected consequences. Trends Immunol. 2024, 45, 81–84. [Google Scholar] [CrossRef]
- Sievers, B.L.; Cheng, M.T.K.; Csiba, K.; Meng, B.; Gupta, R.K. SARS-CoV-2 and innate immunity: The good, the bad, and the “goldilocks”. Cell Mol. Immunol. 2024, 21, 171–183. [Google Scholar] [CrossRef]
- Sunagar, R.; Singh, A.; Kumar, S. SARS-CoV-2: Immunity, Challenges with Current Vaccines, and a Novel Perspective on Mucosal Vaccines. Vaccines 2023, 11, 849. [Google Scholar] [CrossRef]
- Abebe, E.C.; Dejenie, T.A. Protective roles and protective mechanisms of neutralizing antibodies against SARS-CoV-2 infection and their potential clinical implications. Front. Immunol. 2023, 14, 1055457. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhang, X. Neutralizing antibodies for the prevention and treatment of COVID-19. Cell Mol. Immunol. 2021, 18, 2293–2306. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, D.K.; Lee, J.; Kim, Y.I.; Seo, J.M.; Kim, Y.G.; Jeong, J.H.; Kim, M.; Kim, J.I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.; Sahi, V.; Figueroa, A.; et al. Potent Neutralizing Antibodies Directed to Multiple Epitopes on SARS-CoV-2 Spike. bioRxiv 2020. [Google Scholar] [CrossRef]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e1021. [Google Scholar] [CrossRef]
- Wagner, A.; Guzek, A.; Ruff, J.; Jasinska, J.; Scheikl, U.; Zwazl, I.; Kundi, M.; Stockinger, H.; Farcet, M.R.; Kreil, T.R.; et al. Neutralising SARS-CoV-2 RBD-specific antibodies persist for at least six months independently of symptoms in adults. Commun. Med. 2021, 1, 13. [Google Scholar] [CrossRef]
- Joyner, M.J.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; Lesser, E.R.; et al. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv 2020. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Plonne, D.; Voss, M.; Ivanova, R.; Frodl, R.; Deininger, S. SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons. J. Clin. Virol. 2020, 130, 104542. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Abraham, J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020, 20, 401–403. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Belhadi, D.; Peiffer-Smadja, N.; Moschopoulos, C.D.; Lescure, F.X.; Janocha, H.; Karofylakis, E.; Yazdanpanah, Y.; Mentre, F.; Skevaki, C.; et al. Review of trials currently testing treatment and prevention of COVID-19. Clin. Microbiol. Infect. 2020, 26, 988–998. [Google Scholar] [CrossRef]
- Bloch, E.M.; Shoham, S.; Casadevall, A.; Sachais, B.S.; Shaz, B.; Winters, J.L.; van Buskirk, C.; Grossman, B.J.; Joyner, M.; Henderson, J.P.; et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020, 130, 2757–2765. [Google Scholar] [CrossRef]
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat. Microbiol. 2021, 6, 1233–1244. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Baden, L.; Cheng, V.C.C.; Edwards, K.M.; Gallagher, J.C.; Gandhi, R.T.; Muller, W.J.; Nakamura, M.M.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2022, 78, ciaa478. [Google Scholar] [CrossRef]
- Jin, H.; Geiger, C.; Jessop, N.; Pedotti, R.; Raposo, C.; Whitley, L.; Brown, J.S.; Muros-Le Rouzic, E. Anti-SARS-CoV-2 monoclonal antibodies for the treatment of mild-to-moderate COVID-19 in multiple sclerosis: A retrospective cohort study. Mult. Scler. Relat. Disord. 2023, 79, 104943. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Amadasi, S.; Zanella, I.; Degli Antoni, M.; Storti, S.; Tiecco, G.; Castelli, F. Monoclonal Antibodies against SARS-CoV-2: Current Scenario and Future Perspectives. Pharmaceuticals 2021, 14, 1272. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, L.; Xu, Z.; Wang, X.; Xie, Y.; Chai, Y.; Zheng, A.; Zhou, J.; Qiao, S.; Huang, M.; et al. An updated atlas of antibody evasion by SARS-CoV-2 Omicron sub-variants including BQ.1.1 and XBB. Cell Rep. Med. 2023, 4, 100991. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Giraud, E.; Bourret, J.; Donati, F.; Tran-Rajau, J.; Chiaravalli, J.; Lemoine, F.; Agou, F.; Simon-Loriere, E.; van der Werf, S.; et al. Enhanced neutralization escape to therapeutic monoclonal antibodies by SARS-CoV-2 omicron sub-lineages. iScience 2023, 26, 106413. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schafer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines 2023, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, N.; Ghasemiyeh, P.; Moradishooli, F.; Mohammadi-Samani, S. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int. Immunopharmacol. 2023, 117, 109968. [Google Scholar] [CrossRef]
- Rahman, M.M.; Masum, M.H.U.; Wajed, S.; Talukder, A. A comprehensive review on COVID-19 vaccines: Development, effectiveness, adverse effects, distribution and challenges. Virusdisease 2022, 33, 1–22. [Google Scholar] [CrossRef]
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. [Google Scholar] [CrossRef] [PubMed]
- Garner-Spitzer, E.; Wagner, A.; Kundi, M.; Stockinger, H.; Ohradanova-Repic, A.; Gebetsberger, L.; Schoetta, A.M.; Gudipati, V.; Huppa, J.B.; Kunert, R.; et al. SARS-CoV-2-Specific Antibody (Ab) Levels and the Kinetic of Ab Decline Determine Ab Persistence Over 1 Year. Front. Med. 2022, 9, 822316. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Hamada, K.; Jubishi, D.; Hashimoto, H.; Okamoto, K.; Hisasue, N.; Sunohara, M.; Saito, M.; Shinohara, T.; Yamashita, M.; et al. Waning cellular immune responses and predictive factors in maintaining cellular immunity against SARS-CoV-2 six months after BNT162b2 mRNA vaccination. Sci. Rep. 2023, 13, 9607. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Merzon, E.; Schaffer, A.A.; Shenhar, Y.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Magen, E.; Vinker, S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: Test negative design study. BMJ 2021, 375, e067873. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Vattiatio, G.; Lustig, A.; Maclaren, O.J.; Plank, M.J. Modelling the dynamics of infection, waning of immunity and re-infection with the Omicron variant of SARS-CoV-2 in Aotearoa New Zealand. Epidemics 2022, 41, 100657. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Garner-Spitzer, E.; Schotta, A.M.; Orola, M.; Wessely, A.; Zwazl, I.; Ohradanova-Repic, A.; Weseslindtner, L.; Tajti, G.; Gebetsberger, L.; et al. SARS-CoV-2-mRNA Booster Vaccination Reverses Non-Responsiveness and Early Antibody Waning in Immunocompromised Patients—A Phase Four Study Comparing Immune Responses in Patients with Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 889138. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Suarez, C.; Murillo-Zamora, E. Waning immunity to SARS-CoV-2 following vaccination or infection. Front. Med. 2022, 9, 972083. [Google Scholar] [CrossRef] [PubMed]
- Van Egeren, D.; Stoddard, M.; White, L.F.; Hochberg, N.S.; Rogers, M.S.; Zetter, B.; Joseph-McCarthy, D.; Chakravarty, A. Vaccines Alone Cannot Slow the Evolution of SARS-CoV-2. Vaccines 2023, 11, 853. [Google Scholar] [CrossRef]
- Huo, N.; Wu, S.; Wang, Y.; Wang, B.; Zhang, Z.; Zhang, J.; Song, X.; Hou, L.; Chen, W. Monovalent XBB.1.5 booster vaccination induces a broad spectrum of SARS-CoV-2 neutralizing antibodies. Emerg. Microbes Infect. 2024, 13, 2286260. [Google Scholar] [CrossRef]
- Rosenblum, H.G.; Wallace, M.; Godfrey, M.; Roper, L.E.; Hall, E.; Fleming-Dutra, K.E.; Link-Gelles, R.; Pilishvili, T.; Williams, J.; Moulia, D.L.; et al. Interim Recommendations from the Advisory Committee on Immunization Practices for the Use of Bivalent Booster Doses of COVID-19 Vaccines—United States, October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Carreno, J.M.; Singh, G.; Simon, V.; Krammer, F.; PVI Study Group. Bivalent COVID-19 booster vaccines and the absence of BA.5-specific antibodies. Lancet Microbe 2023, 4, e569. [Google Scholar] [CrossRef] [PubMed]
- Blankson, J.N. Bivalent COVID-19 Vaccines: Can the Original Antigenic Sin Be Forgiven? J. Infect. Dis. 2023, 227, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Palanica, A.; Jeon, J. Initial Mix-and-Match COVID-19 Vaccination Perceptions, Concerns, and Side Effects across Canadians. Vaccines 2022, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-Match COVID-19 Vaccinations (Heterologous Boost): A Review. Infect. Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Deming, M.E.; Lyke, K.E. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Barouch, D.H. SARS-CoV-2 Hybrid Immunity: The Best of Both Worlds. J. Infect. Dis. 2023, 228, 1311–1313. [Google Scholar] [CrossRef]
- Samoud, S.; Bettaieb, J.; Gdoura, M.; Kharroubi, G.; Ben Ghachem, F.; Zamali, I.; Ben Hmid, A.; Salem, S.; Gereisha, A.A.; Dellagi, M.; et al. Immunogenicity of Mix-and-Match CoronaVac/BNT162b2 Regimen versus Homologous CoronaVac/CoronaVac Vaccination: A Single-Blinded, Randomized, Parallel Group Superiority Trial. Vaccines 2023, 11, 1329. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, X.; Zhang, X.; Yao, H.; Wu, N. Immune interference in effectiveness of influenza and COVID-19 vaccination. Front. Immunol. 2023, 14, 1167214. [Google Scholar] [CrossRef]
- Schultz-Cherry, S.; McGargill, M.A.; Thomas, P.G.; Estepp, J.H.; Gaur, A.H.; Allen, E.K.; Allison, K.J.; Tang, L.; Webby, R.J.; Cherry, S.D.; et al. Cross-reactive Antibody Response to mRNA SARS-CoV-2 Vaccine After Recent COVID-19-Specific Monoclonal Antibody Therapy. Open Forum Infect. Dis. 2021, 8, ofab420. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Spinardi, J.R.; Srivastava, A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 2023, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Garner-Spitzer, E.; Auer, C.; Gattinger, P.; Zwazl, I.; Platzer, R.; Orola-Taus, M.; Pichler, P.; Amman, F.; Bergthaler, A.; et al. Breakthrough Infections in SARS-CoV-2-Vaccinated Multiple Myeloma Patients Improve Cross-Protection against Omicron Variants. Vaccines 2024, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.B.; Morawski, P.A.; Pruner, K.B.; Fahning, M.L.; Howard, C.A.; Franko, N.; Logue, J.; Eggenberger, J.; Stokes, C.; Golez, I.; et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell 2022, 185, 1588–1601.e1514. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Goel, A.; Katiyar, H.; Tiwari, P.; Mayank; Sana, A.; Khetan, D.; Bhadauria, D.S.; Raja, A.; Khokher, N.; et al. Durability of ChAdOx1 nCoV-19 (Covishield((R))) Vaccine Induced Antibody Response in Health Care Workers. Vaccines 2022, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. J&J’s one-shot COVID vaccine offers hope for faster protection. Nature 2021. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Piniaeva, A.N.; Ignatyev, G.M.; Gordeychuk, I.V.; Volok, V.P.; Rogova, Y.V.; Shishova, A.A.; Kovpak, A.A.; Ivin, Y.Y.; Antonova, L.P.; et al. Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies. Emerg. Microbes Infect. 2021, 10, 1790–1806. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Wressnigg, N.; Taucher, C.; Eder-Lingelbach, S.; Querton, B.; Krammer, M.; Lilja, A.; Hochreiter, R.; Hoffmann, M.; Pöhlmann, S.; Jaramillo, J.C. Effects of Homologous and Heterologous Booster Vaccinations of the Inactivated Dual-Adjuvanted Vaccine Vla2001 against COVID-19 Including Variants of Concern: A Phase 3 Randomized Clinical Trial. Int. J. Infect. Dis. 2023, 130, S25. [Google Scholar] [CrossRef]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer-Based COVID-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef]
- Prenafeta, A.; Bech-Sabat, G.; Moros, A.; Barreiro, A.; Fernandez, A.; Canete, M.; Roca, M.; Gonzalez-Gonzalez, L.; Garriga, C.; Confais, J.; et al. Preclinical evaluation of PHH-1V vaccine candidate against SARS-CoV-2 in non-human primates. iScience 2023, 26, 107224. [Google Scholar] [CrossRef]
- Shalash, A.O.; Toth, I.; Skwarczynski, M. The potential of developing a protective peptide-based vaccines against SARS-CoV-2. Drug Dev. Res. 2022, 83, 1251–1256. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Anez, G.; Adelglass, J.M.; Barrat Hernandez, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Gunale, B.; Kapse, D.; Kar, S.; Bavdekar, A.; Kohli, S.; Lalwani, S.; Meshram, S.; Raut, A.; Kulkarni, P.; Samuel, C.; et al. Safety and Immunogenicity of SARS-CoV-2 Recombinant Spike Protein Vaccine in Ch\ildren and Adolescents in India: A Phase 2-3 Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 911–920. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; et al. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2023, 11, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Tioni, M.F.; Jordan, R.; Pena, A.S.; Garg, A.; Wu, D.; Phan, S.I.; Weiss, C.M.; Cheng, X.; Greenhouse, J.; Orekov, T.; et al. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. NPJ Vaccines 2022, 7, 85. [Google Scholar] [CrossRef]
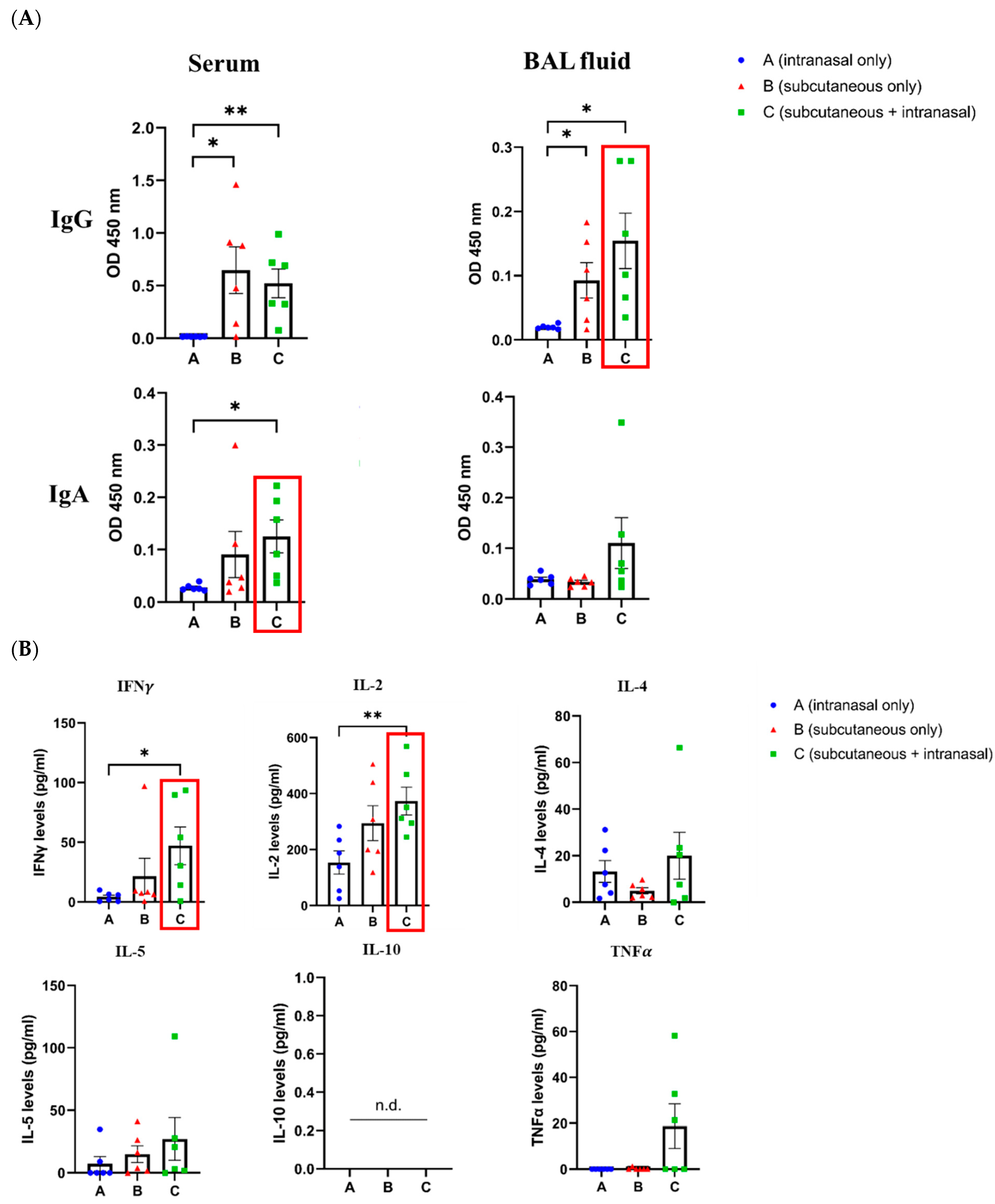
- Rice, A.; Verma, M.; Shin, A.; Zakin, L.; Sieling, P.; Tanaka, S.; Balint, J.; Dinkins, K.; Adisetiyo, H.; Morimoto, B.; et al. Intranasal plus subcutaneous prime vaccination with a dual antigen COVID-19 vaccine elicits T-cell and antibody responses in mice. Sci. Rep. 2021, 11, 14917. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin((R))). NPJ Vaccines 2023, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Li, K.; Rowe, D.K.; Diaz, M.C.H.; Griffin, E.F.; Beavis, A.C.; Johnson, S.K.; Padykula, I.; Jones, C.A.; Briggs, K.; et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 2021, 7, eabi5246. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Gordeychuk, I.V.; Dolzhikova, I.V.; Dzharullaeva, A.S.; Krasina, M.E.; Bayurova, E.O.; Grousova, D.M.; Kovyrshina, A.V.; Kondrashova, A.S.; Avdoshina, D.V.; et al. Immunogenicity and protectivity of intranasally delivered vector-based heterologous prime-boost COVID-19 vaccine Sputnik V in mice and non-human primates. Emerg. Microbes Infect. 2022, 11, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Song, Y.; Coleman, J.R.; Stawowczyk, M.; Tafrova, J.; Tasker, S.; Boltz, D.; Baker, R.; Garcia, L.; et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102775118. [Google Scholar] [CrossRef]
- Kaufmann, J.K.; Wyllie, K.; Zhao, Y.; Tea, L.; Tasker, S.; Yeolekar, L.R.; Dhere, R.; Mueller, S. 1938. CoviLiv™, a Novel Intranasal Live-Attenuated COVID-19 Vaccine Candidate, Induces Robust Humoral and Cellular Immunity in First-In-Human Clinical Trial CDX-CoV-001. Open Forum Infect. Dis. 2023, 10, ofad500.2469. [Google Scholar] [CrossRef]
- Guillen, G.; Limonta, M.; Muzio, V.; Lemos, G.; Hernández-Bernal, F.; Chinea, G.; Gonzalez-Roche, D.; Martin, A.; Bequet, M.; Marques, G.; et al. Cuban Vaccines Abdala and Mambisa against COVID-19. Int. J. Infect. Dis. 2023, 130, S9–S10. [Google Scholar] [CrossRef]
- Lam, J.H.; Shivhare, D.; Chia, T.W.; Chew, S.L.; Sinsinbar, G.; Aw, T.Y.; Wong, S.; Venkataraman, S.; Lim, F.W.I.; Vandepapeliere, P.; et al. Artificial Cell Membrane Polymersome-Based Intranasal Beta Spike Formulation as a Second Generation COVID-19 Vaccine. ACS Nano 2022, 16, 16757–16775. [Google Scholar] [CrossRef]
- Mohazzab, A.; Fallah Mehrabadi, M.H.; Es-Haghi, A.; Kalantari, S.; Mokhberalsafa, L.; Setarehdan, S.A.; Sadeghi, F.; Rezaei Mokarram, A.; Haji Moradi, M.; Razaz, S.H.; et al. Phase II, Safety and Immunogenicity of RAZI Cov Pars (RCP) SARS CoV-2 Vaccine in Adults Aged 18-70 Years; A Randomized, Double-Blind Clinical Trial. J. Pharm. Sci. 2023, 112, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Israelow, B.; Pena-Hernandez, M.A.; Suberi, A.; Zhou, L.; Luyten, S.; Reschke, M.; Dong, H.; Homer, R.J.; Saltzman, W.M.; et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 2022, 378, eabo2523. [Google Scholar] [CrossRef]
- Christensen, D.; Polacek, C.; Sheward, D.J.; Hanke, L.; Moliner-Morro, A.; McInerney, G.; Murrell, B.; Hartmann, K.T.; Jensen, H.E.; Jungersen, G.; et al. Protection against SARS-CoV-2 transmission by a parenteral prime-Intranasal boost vaccine strategy. EBioMedicine 2022, 84, 104248. [Google Scholar] [CrossRef] [PubMed]
- Rioux, M.; McNeil, M.; Francis, M.E.; Dawe, N.; Foley, M.; Langley, J.M.; Kelvin, A.A. The Power of First Impressions: Can Influenza Imprinting during Infancy Inform Vaccine Design? Vaccines 2020, 8, 546. [Google Scholar] [CrossRef]
- Dangi, T.; Sanchez, S.; Lew, M.H.; Awakoaiye, B.; Visvabharathy, L.; Richner, J.M.; Koralnik, I.J.; Penaloza-MacMaster, P. Pre-existing immunity modulates responses to mRNA boosters. Cell Rep. 2023, 42, 112167. [Google Scholar] [CrossRef]
- Pulendran, B.S.; Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Charalambous, B.M.; Feavers, I.M. Mimotope vaccines. J. Med. Microbiol. 2001, 50, 937–939. [Google Scholar] [CrossRef]
- Pashov, A.D.; Plaxco, J.; Kaveri, S.V.; Monzavi-Karbassi, B.; Harn, D.; Kieber-Emmons, T. Multiple antigenic mimotopes of HIV carbohydrate antigens: Relating structure and antigenicity. J. Biol. Chem. 2006, 281, 29675–29683. [Google Scholar] [CrossRef]
- Steward, M.W. The development of a mimotope-based synthetic peptide vaccine against respiratory syncytial virus. Biologicals 2001, 29, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Geysen, H.M.; Rodda, S.J.; Mason, T.J. A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol. Immunol. 1986, 23, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Potocnakova, L.; Bhide, M.; Pulzova, L.B. An Introduction to B-Cell Epitope Mapping and In Silico Epitope Prediction. J. Immunol. Res. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 1–18. [Google Scholar] [CrossRef]
- Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 Vaccine Pipeline: An Overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. [Google Scholar] [CrossRef]
- Wiedermann, U.; Davis, A.B.; Zielinski, C.C. Vaccination for the prevention and treatment of breast cancer with special focus on Her-2/neu peptide vaccines. Breast Cancer Res. Treat. 2013, 138, 1–12. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinic, M.; Wiedermann, U. Vaccination against Her-2/neu, with focus on peptide-based vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Steinberger, P.; Drinic, M.; Wiedermann, U. Emerging targets for anticancer vaccination: PD-1. ESMO Open 2021, 6, 100278. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Battin, C.; Linhares, A.D.; Karin, B.; AMbroye, K.; Drinic, M.; Garner-Spitzer, E.; Zielinski, C.; Kundi, M.; Steinberger, P.; et al. Identification of PD1 B cell mimotopes with functional PD1-PDL1 blocking capacity: New strategy for cancer immunotherapy. Cancer Res. 2019, 79, 4110. [Google Scholar] [CrossRef]
- Tobias, J.; Drinic, M.; Hogler, S.; Ambroz, K.; Baier, K.; Kodajova, P.; Tomasich, E.; Berghoff, A.S.; Schmid, A.; Garner-Spitzer, E.; et al. Active immunization with a Her-2/neu-targeting Multi-peptide B cell vaccine prevents lung metastases formation from Her-2/neu breast cancer in a mouse model. Transl. Oncol. 2022, 19, 101378. [Google Scholar] [CrossRef]
- Tobias, J.; Drinic, M.; Schmid, A.; Hladik, A.; Watzenbock, M.L.; Battin, C.; Garner-Spitzer, E.; Steinberger, P.; Kundi, M.; Knapp, S.; et al. Combined Vaccination with B Cell Peptides Targeting Her-2/neu and Immune Checkpoints as Emerging Treatment Option in Cancer. Cancers 2022, 14, 5678. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Jasinska, J.; Baier, K.; Kundi, M.; Ede, N.; Zielinski, C.; Wiedermann, U. Enhanced and long term immunogenicity of a Her-2/neu multi-epitope vaccine conjugated to the carrier CRM197 in conjunction with the adjuvant Montanide. BMC Cancer 2017, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; Cavallo, F.; Nanni, P.; Forni, G. Vaccines for tumour prevention. Nat. Rev. Cancer 2006, 6, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Lindstrom, A.R.; Lin, T.Y.; Lam, K.S.; Li, Y. Peptide-based materials for cancer immunotherapy. Theranostics 2019, 9, 7807–7825. [Google Scholar] [CrossRef]
- Malito, E.; Bursulaya, B.; Chen, C.; Lo Surdo, P.; Picchianti, M.; Balducci, E.; Biancucci, M.; Brock, A.; Berti, F.; Bottomley, M.J.; et al. Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197. Proc. Natl. Acad. Sci. USA 2012, 109, 5229–5234. [Google Scholar] [CrossRef]
- Kamboj, K.K.; King, C.L.; Greenspan, N.S.; Kirchner, H.L.; Schreiber, J.R. Immunization with Haemophilus influenzae type b-CRM(197) conjugate vaccine elicits a mixed Th1 and Th2 CD(4+) T cell cytokine response that correlates with the isotype of antipolysaccharide antibody. J. Infect. Dis. 2001, 184, 931–935. [Google Scholar] [CrossRef]
- Yang, H.; Cao, J.; Lin, X.; Yue, J.; Zieneldien, T.; Kim, J.; Wang, L.; Fang, J.; Huang, R.P.; Bai, Y.; et al. Developing an Effective Peptide-Based Vaccine for COVID-19: Preliminary Studies in Mice Models. Viruses 2022, 14, 449. [Google Scholar] [CrossRef]
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; Garcia-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Battin, C.; De Sousa Linhares, A.; Lebens, M.; Baier, K.; Ambroz, K.; Drinic, M.; Hogler, S.; Inic-Kanada, A.; Garner-Spitzer, E.; et al. A New Strategy Toward B Cell-Based Cancer Vaccines by Active Immunization with Mimotopes of Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 895. [Google Scholar] [CrossRef]



| Name | Date of Approval | Treatment | Targeting Epitope | Administration Route | Neutralization Activity against the SARS-CoV-2 Variant Delta (EC50, ng/mL) [135] * | Fold-Reduction of Neutrlization Activity (EC50, ng/mL) against Delta BA.2, BA.5, BA.2.75.2, XBB, BQ.1, and BQ.1.1 Variants (Fold-Reduction) [135] * | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BA.2 | BA.2.75.2 | BA.5 | BQ.1 | BQ.1.1 | XBB | ||||||
| Bebtelovimab | February 2022 | Therapeutic | RBD | Intravenous | 0.4 | ||||||
| Sotrovimab | May 2021 | Therapeutic | RBD | Intravenous | 98.6 | ||||||
| Casirivimab | November 2020 | Therapeutic Or Post-exposure prophylaxis | RBM | Intravenous or sub-cutaneous | 14.7 | ||||||
| Imdevimab | 20.1 | ||||||||||
| Cilgavimab | December 2021 | Pre-exposure prophylaxis | RBD | Intramuscular | 21.7 | ||||||
| Tixagevimab | 12.4 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobias, J.; Steinberger, P.; Wilkinson, J.; Klais, G.; Kundi, M.; Wiedermann, U. SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity. Vaccines 2024, 12, 795. https://doi.org/10.3390/vaccines12070795
Tobias J, Steinberger P, Wilkinson J, Klais G, Kundi M, Wiedermann U. SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity. Vaccines. 2024; 12(7):795. https://doi.org/10.3390/vaccines12070795
Chicago/Turabian StyleTobias, Joshua, Peter Steinberger, Joy Wilkinson, Gloria Klais, Michael Kundi, and Ursula Wiedermann. 2024. "SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity" Vaccines 12, no. 7: 795. https://doi.org/10.3390/vaccines12070795





