Possible Paths to Measles Eradication: Conceptual Frameworks, Strategies, and Tactics
Abstract
1. Introduction
2. Disease Eradication
3. Measles Eradication
“A time-bound measles and rubella eradication goal should be set only when accelerated progress has been made, benchmarks that establish the conditions for a successful endgame to achieve eradication have been achieved, and there is evidence of a clear trajectory toward the goal. Setting an eradication goal when the endgame is in sight could catalyze a surge in commitment, effort, and resources to complete the task, thus heeding the call to go ‘‘big and fast” with measles and rubella eradication, and avoid a premature and drawn-out eradication effort with the potential for unmet goals, delayed milestones, and prolonged input of financial and human resources.”
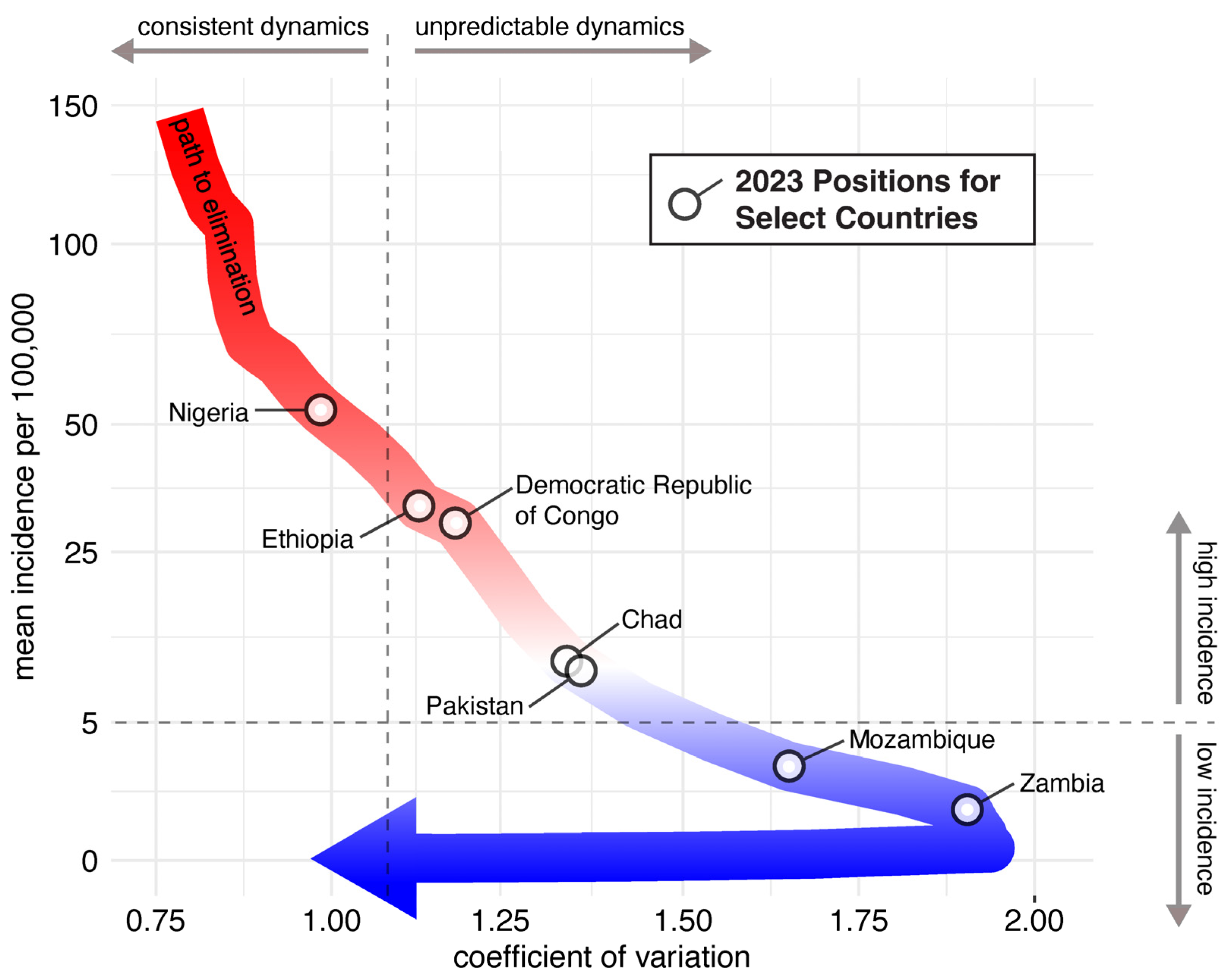
4. Infectious Disease Dynamics and Measles Elimination and Eradication
5. New Tools for Measles Eradication
6. Possible Paths to Measles Eradication
6.1. Incremental Path to Measles Eradication through Country-Wide and Regional Elimination
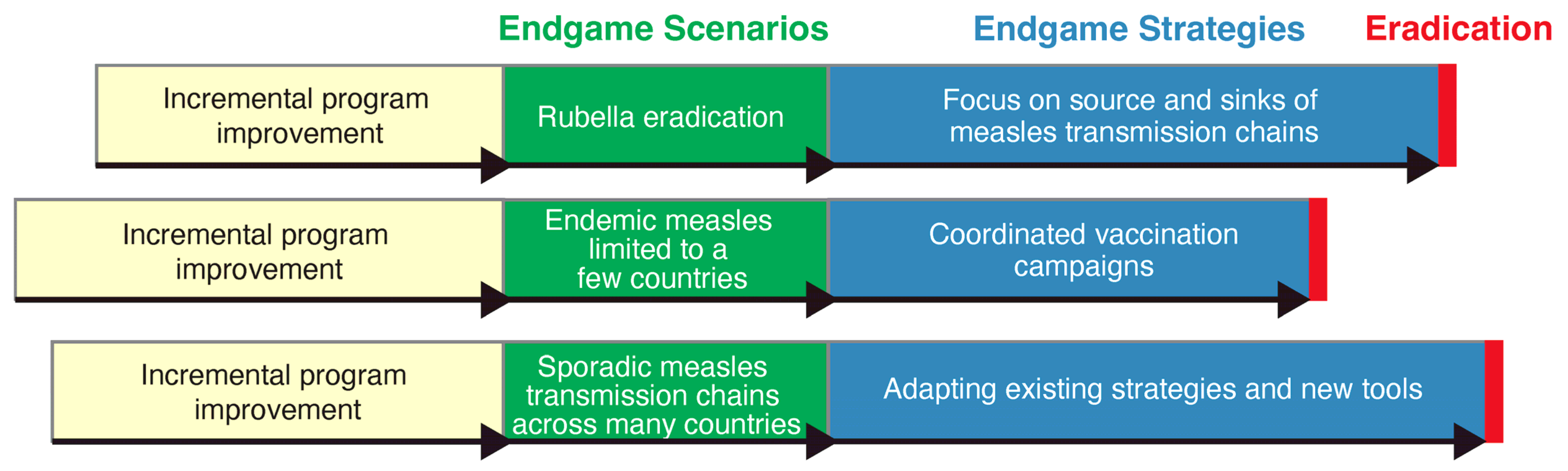
6.2. Phased Path to Measles Eradication through Different Endgame Scenarios and Strategies
6.3. Endgame Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UNICEF. COVID-19 Pandemic Leads to Major Backsliding on Childhood Vaccinations, New WHO, UNICEF Data Shows. 2021. Available online: https://www.unicef.org/press-releases/covid-19-pandemic-leads-major-backsliding-childhood-vaccinations-new-who-unicef-data (accessed on 29 April 2024).
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly. Epidemiol. Rec. 2017, 92, 205–227. [Google Scholar]
- Minta, A.A.; Ferrari, M.; Antoni, S.; Portnoy, A.; Sbarra, A.; Lambert, B.; Hatcher, C.; Hsu, C.H.; Ho, L.L.; Steulet, C.; et al. Progress toward measles elimination—Worldwide, 2000–2022. MMWR 2023, 72, 1262–1268. [Google Scholar] [CrossRef]
- De Quadros, C.A.; Andrus, J.K.; Danovaro-Holliday, M.C.; Castillo-Solórzano, C. Feasibility of global measles eradication after interruption of transmission in the Americas. Expert. Rev. Vaccines 2008, 7, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.; Leidner, A.J.; Gastañaduy, P.A. A review of measles outbreak cost estimates from the United States in the postelimination era (2004–2017): Estimates by perspective and cost type. Clin. Infect. Dis. 2020, 71, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.; Melnick, A.; Gastañaduy, P.A.; Kay, M.; Harbison, J.; Leidner, A.J.; Rice, S.; Asato, K.; Schwartz, L.; DeBolt, C. Societal costs of a measles outbreak. Pediatrics 2021, 147, e2020027037. [Google Scholar] [CrossRef] [PubMed]
- Stepan, N.L. Eradication. Riding the World of Diseases Forever; Cornell University Press: Ithica, NY, USA, 2011. [Google Scholar]
- Jenner, E. The Origin of Vaccine Inoculation; D.N. Shury: London, UK, 1801. [Google Scholar]
- Recommendations of the International Task Force for Disease Eradication. MMWR 1993, 42, 1–38.
- Cello, J.; Paul, A.V.; Wimmer, E. Chemical synthesis of poliovirus cDNA: Generation of infectious virus in the absence of natural template. Science 2002, 297, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Lederman, S.; Evans, D.H. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS ONE 2018, 13, e0188453. [Google Scholar] [CrossRef] [PubMed]
- Sencer, D.J.; Dull, H.B.; Langmuir, A.D. Epidemiologic basis for eradication of measles in 1967. Public Health Rep. 1967, 82, 253–256. [Google Scholar] [CrossRef]
- Albrecht, R.M. Can measles be eradicated? Science 1967, 156, 1029. [Google Scholar] [CrossRef]
- Moss, W.J.; Scott, S.; Mugala, N.; Ndhlovu, Z.; Beeler, J.A.; Audet, S.A.; Ngala, M.; Mwangala, S.; Nkonga-Mwangilwa, C.; Ryon, J.J.; et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: An observational study. J. Infect. Dis. 2007, 196, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Meeting of the International Task Force for Disease Eradication, November 2015. Wkly. Epidemiol. Rec. 2016, 91, 61–71.
- World Health Organization. Proceedings of the Global Technical Consultation to assess the feasibility of measles eradication, 28–30 July 2010. J. Infect. Dis. 2011, 204 (Suppl. S1), S4–S13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2010. Summary, conclusions and recommendations. Wkly. Ep Rec 2011, 86, 1–16. [Google Scholar]
- Orenstein, W.A.; Cairns, L.; Hinman, A.; Nkowane, B.; Olivé, J.M.; Reingold, A.L. Measles and Rubella Global Strategic Plan 2012–2020 Mid-Term Review. Vaccine 2018, 36, A35–A42. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J.; Shendale, S.; Lindstrand, A.; O’Brien, K.L.; Turner, N.; Goodman, T.; Kretsinger, K.; Measles, S.W.G.O.; Rubella, V.; Measles; et al. Feasibility assessment of measles and rubella eradication. Vaccine 2021, 39, 3544–3559. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D.L. “Herd immunity”: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R0) of measles: A systematic review. Lancet. Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Measles outbreak—Netherlands, April 1999–January 2000. MMWR 2000, 49, 299–303. [Google Scholar]
- Funk, S.; Knapp, J.K.; Lebo, E.; Reef, S.E.; Dabbagh, A.J.; Kretsinger, K.; Jit, M.; Edmunds, W.J.; Strebel, P.M. Combining serological and contact data to derive target immunity levels for achieving and maintaining measles elimination. BMC Med. 2019, 17, 180. [Google Scholar] [CrossRef]
- Truelove, S.A.; Graham, M.; Moss, W.J.; Metcalf, C.J.E.; Ferrari, M.J.; Lessler, J. Characterizing the impact of spatial clustering of susceptibility for measles elimination. Vaccine 2019, 37, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Afroj Moon, S.; Marathe, A.; Vullikanti, A. Are all underimmunized measles clusters equally critical? R. Soc. Open Sci. 2023, 10, 230873. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J. The theory of measles elimination: Implications for the design of elimination strategies. J. Infect. Dis. 2004, 189 (Suppl. S1), S27–S35. [Google Scholar]
- Cutts, F.T.; Dansereau, E.; Ferrari, M.J.; Hanson, M.; McCarthy, K.A.; Metcalf, C.J.E.; Takahashi, S.; Tatem, A.J.; Thakkar, N.; Truelove, S.; et al. Using models to shape measles control and elimination strategies in low- and middle-income countries: A review of recent applications. Vaccine 2020, 38, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.J.; Grenfell, B.T.; Strebel, P.M. Think globally, act locally: The role of local demographics and vaccination coverage in the dynamic response of measles infection to control. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2013, 368, 20120141. [Google Scholar] [CrossRef] [PubMed]
- Trentini, F.; Poletti, P.; Merler, S.; Melegaro, A. Measles immunity gaps and the progress towards elimination: A multi-country modelling analysis. Lancet. Infect. Dis. 2017, 17, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Shaman, J. Characteristics of measles epidemics in China (1951–2004) and implications for elimination: A case study of three key locations. PLoS Comput. Biol. 2019, 15, e1006806. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Grenfell, B.T.; Meyers, L.A. When individual behaviour matters: Homogeneous and network models in epidemiology. J. R. Soc. Interface 2007, 4, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.K.; Lambert, B.; Klein, D.; Klepac, P.; Papadopoulos, T.; Truelove, S.; Burgess, C.; Santos, H.; Knapp, J.K.; Reef, S.E.; et al. Feasibility of measles and rubella vaccination programmes for disease elimination: A modelling study. Lancet. Glob. Health 2022, 10, e1412–e1422. [Google Scholar] [CrossRef]
- Hao, L.; Glasser, J.W.; Su, Q.; Ma, C.; Feng, Z.; Yin, Z.; Goodson, J.L.; Wen, N.; Fan, C.; Yang, H.; et al. Evaluating vaccination policies to accelerate measles elimination in China: A meta-population modelling study. Int. J. Epidemiol. 2019, 48, 1240–1251. [Google Scholar] [CrossRef]
- McKee, A.; Ferrari, M.J.; Shea, K. Correlation between measles vaccine doses: Implications for the maintenance of elimination. Epidemiol. Infect. 2018, 146, 468–475. [Google Scholar] [CrossRef]
- Prada, J.M.; Metcalf, C.J.E.; Takahashi, S.; Lessler, J.; Tatem, A.J.; Ferrari, M. Demographics, epidemiology and the impact of vaccination campaigns in a measles-free world—Can elimination be maintained? Vaccine 2017, 35, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Glasser, J.W.; Feng, Z.; Omer, S.B.; Smith, P.J.; Rodewald, L.E. The effect of heterogeneity in uptake of the measles, mumps, and rubella vaccine on the potential for outbreaks of measles: A modelling study. Lancet. Infect. Dis. 2016, 16, 599–605. [Google Scholar] [CrossRef]
- Bharti, N.; Djibo, A.; Ferrari, M.J.; Grais, R.F.; Tatem, A.J.; McCabe, C.A.; Bjornstad, O.N.; Grenfell, B.T. Measles hotspots and epidemiological connectivity. Epidemiol. Infect. 2010, 138, 1308–1316. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Ma, C.; Hao, L.; Su, Q.; An, Z.; Ma, F.; Xie, S.; Xu, A.; Zhang, Y.; Ding, Z.; et al. Demographic transition and the dynamics of measles in six provinces in China: A modeling study. PLoS Med. 2017, 14, e1002255. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Z.; Duan, X.; Song, J.; Mao, N.; Cui, A.; Wang, C.; Du, H.; Wang, Y.; Li, F.; et al. Transmission pattern of measles virus circulating in China during 1993–2021: Genotyping evidence supports that China is approaching measles elimination. Clin. Infect. Dis. 2023, 76, e1140–e1149. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Antoni, S.; Nedelec, Y.; Sodha, S.; Menning, L.; Ogbuanu, I.U.; Gacic Dobo, M. The changing global epidemiology of measles, 2013–2018. J. Infect. Dis. 2020, 222, 1117–1128. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Age-related changes in the rate of disease transmission: Implications for the design of vaccination programmes. J. Hyg. (Lond) 1985, 94, 365–436. [Google Scholar] [CrossRef]
- Graham, M.; Suk, J.E.; Takahashi, S.; Metcalf, C.J.; Jimenez, A.P.; Prikazsky, V.; Ferrari, M.J.; Lessler, J. Challenges and opportunities in disease forecasting in outbreak settings: A case study of measles in Lola Prefecture, Guinea. Am. J. Trop. Med. Hyg. 2018, 98, 1489–1497. [Google Scholar] [CrossRef]
- Minetti, A.; Hurtado, N.; Grais, R.F.; Ferrari, M. Reaching hard-to-reach individuals: Nonselective versus targeted outbreak response vaccination for measles. Am. J. Epidemiol. 2014, 179, 245–251. [Google Scholar] [CrossRef][Green Version]
- Grout, L.; Conan, N.; Juan Giner, A.; Hurtado, N.; Fermon, F.; N’Goran, A.; Grellety, E.; Minetti, A.; Porten, K.; Grais, R.F. Local discrepancies in measles vaccination opportunities: Results of population-based surveys in Sub-Saharan Africa. BMC Public. Health 2014, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.B.; Masresha, B.G.; Moss, W.J.; Mulders, M.N.; Rota, P.A.; Omer, S.B.; Shefer, A.; Kriss, J.L.; Hanson, M.; Durrheim, D.N.; et al. Accelerating measles and rubella elimination through research and innovation—Findings from the Measles & Rubella Initiative research prioritization process, 2016. Vaccine 2019, 37, 5754–5761. [Google Scholar] [PubMed]
- Goodson, J.L.; Rota, P.A. Innovations in vaccine delivery: Increasing access, coverage, and equity and lessons learnt from measles and rubella elimination. Drug Deliv. Transl. Res. 2022, 12, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Warrener, L.; Slibinskas, R.; Chua, K.B.; Nigatu, W.; Brown, K.E.; Sasnauskas, K.; Samuel, D.; Brown, D. A point-of-care test for measles diagnosis: Detection of measles-specific IgM antibodies and viral nucleic acid. Bull. World Health Organ. 2011, 89, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Warrener, L.; Scobie, H.M.; Donadel, M.; Waku-Kouomou, D.; Mulders, M.N.; Rota, P.A. Rapid diagnostic tests to address challenges for global measles surveillance. Curr. Opin. Virol. 2020, 41, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Warrener, L.; Andrews, N.; Koroma, H.; Alessandrini, I.; Haque, M.; Garcia, C.C.; Matos, A.R.; Caetano, B.; Lemos, X.R.; Siqueira, M.M.; et al. Evaluation of a rapid diagnostic test for measles IgM detection; accuracy and the reliability of visual reading using sera from the measles surveillance programme in Brazil, 2015. Epidemiol. Infect. 2023, 151, e151. [Google Scholar] [CrossRef] [PubMed]
- Senin, A.; Noordin, N.M.; Sani, J.A.M.; Mahat, D.; Donadel, M.; Scobie, H.M.; Omar, A.; Chem, Y.K.; Zahari, M.I.; Ismail, F.; et al. A measles IgM rapid diagnostic test to address challenges with national measles surveillance and response in Malaysia. PLoS ONE 2024, 19, e0298730. [Google Scholar] [CrossRef] [PubMed]
- Hasso-Agopsowicz, M.; Crowcroft, N.; Biellik, R.; Gregory, C.J.; Menozzi-Arnaud, M.; Amorij, J.P.; Gilbert, P.A.; Earle, K.; Frivold, C.; Jarrahian, C.; et al. Accelerating the development of measles and rubella microarray patches to eliminate measles and rubella: Recent progress, remaining challenges. Front. Public Health 2022, 10, 809675. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Malvolti, S.; Cherian, T.; Mantel, C.; Biellik, R.; Jarrahian, C.; Menozzi-Arnaud, M.; Amorij, J.P.; Christiansen, H.; Papania, M.J.; et al. Estimating the future global dose demand for measles-rubella microarray patches. Front. Public Health 2022, 10, 1037157. [Google Scholar] [CrossRef]
- Malvolti, S.; Ko, M.; Menozzi-Arnaud, M.; Mantel, C.; Jarrahian, C.; Amorij, J.P.; Giersing, B.; Hasso-Agopsowicz, M. Exploring potential applications of measles and rubella microarray patches (MR-MAPs): Use case identification. Front. Public Health 2023, 11, 1165110. [Google Scholar] [CrossRef]
- Fu, H.; Abbas, K.; Malvolti, S.; Gregory, C.; Ko, M.; Amorij, J.P.; Jit, M. Impact and cost-effectiveness of measles vaccination through microarray patches in 70 low-income and middle-income countries: Mathematical modelling and early-stage economic evaluation. BMJ Glob. Health 2023, 8, e012204. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, N.; Zehrung, D.; Jarrahian, C.; Frivold, C.; Orubu, T.; Giersing, B. Potential use of microarray patches for vaccine delivery in low- and middle- income countries. Vaccine 2019, 37, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.C.; Moss, W.J. Measles and rubella microarray array patches to increase vaccination coverage and achieve measles and rubella elimination in Africa. Pan Afr. Med. J. 2020, 35, 3. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Goodson, J.L.; Rota, P.A.; Orenstein, W.A. A microneedle patch for measles and rubella vaccination: A game changer for achieving elimination. Curr. Opin. Virol. 2020, 41, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.; Bermingham, I.M.; Leelasena, I.; Hickling, J.; Young, P.R.; Muller, D.A.; Forster, A.H. Safety, tolerability, and immunogenicity of measles and rubella vaccine delivered with a high-density microarray patch: Results from a randomized, partially double-blinded, placebo-controlled phase I clinical trial. Vaccines 2023, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Adigweme, I.; Yisa, M.; Ooko, M.; Akpalu, E.; Bruce, A.; Donkor, S.; Jarju, L.B.; Danso, B.; Mendy, A.; Jeffries, D.; et al. A measles and rubella vaccine microneedle patch in The Gambia: A phase 1/2, double-blind, double-dummy, randomised, active-controlled, age de-escalation trial. Lancet 2024, 403, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Gastañaduy, P.A.; Goodson, J.L.; Panagiotakopoulos, L.; Rota, P.A.; Orenstein, W.A.; Patel, M. Measles in the 21st century: Progress toward achieving and sustaining elimination. J. Infect. Dis. 2021, 224, S420–S428. [Google Scholar] [CrossRef]
- De Quadros, C.A.; Hersh, B.S.; Nogueira, A.C.; Carrasco, P.A.; da Silveira, C.M. Measles eradication: Experience in the Americas. Bull. World Health Organ. 1998, 76 (Suppl. S2), 47–52. [Google Scholar] [PubMed]
- Patel, M.K.; Goodson, J.L.; Alexander, J.P., Jr.; Kretsinger, K.; Sodha, S.V.; Steulet, C.; Gacic-Dobo, M.; Rota, P.A.; McFarland, J.; Menning, L.; et al. Progress toward regional measles elimination—Worldwide, 2000–2019. MMWR 2020, 69, 1700–1705. [Google Scholar] [CrossRef]
- Graham, M.; Winter, A.K.; Ferrari, M.; Grenfell, B.; Moss, W.J.; Azman, A.S.; Metcalf, C.J.E.; Lessler, J. Measles and the canonical path to elimination. Science 2019, 364, 584–587. [Google Scholar] [CrossRef]
- Khanal, S.; Kassem, A.M.; Bahl, S.; Jayantha, L.; Sangal, L.; Sharfuzzaman, M.; Bose, A.S.; Antoni, S.; Datta, D.; Alexander, J.P., Jr. Progress toward measles elimination—South-East Asia Region, 2003–2020. MMWR 2022, 71, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; VanderEnde, K.; Dhawan, V.; Haldar, P.; Chatterjee, S.; Sharma, D.; Dzeyie, K.A.; Pattabhiramaiah, S.B.; Khanal, S.; Sangal, L.; et al. Progress toward measles and rubella elimination—India, 2005–2021. MMWR 2022, 71, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Khanal, S.; Sangal, L.; Tabassum, S.; Ungchusak, K.; Andrus, J. Measles and rubella elimination: Protecting children through immunization in South-East Asia Region (SEAR). Lancet Reg. Health Southeast. Asia 2023, 18, 100303. [Google Scholar] [CrossRef]
- Ma, C.; Rodewald, L.; Hao, L.; Su, Q.; Zhang, Y.; Wen, N.; Fan, C.; Yang, H.; Luo, H.; Wang, H.; et al. Progress toward measles elimination—China, January 2013-June 2019. MMWR 2019, 68, 1112–1116. [Google Scholar] [PubMed]
- Durrheim, D.N.; Xu, A.; Baker, M.G.; Hsu, L.Y.; Takashima, Y. China has the momentum to eliminate measles. Lancet Reg. Health West. Pac. 2023, 30, 100669. [Google Scholar] [CrossRef]
- Musa, N.; Ghoniem, A.; Hsu, C.H.; Mubarak, S.; Sume, G.; Sharifuzzaman, M.; Bak, J.; Hutin, Y.; Teleb, N.; Crowcroft, N.; et al. Progress toward measles elimination—World Health Organization Eastern Mediterranean Region, 2019–2022. MMWR 2024, 73, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Masresha, B.G.; Hatcher, C.; Lebo, E.; Tanifum, P.; Bwaka, A.M.; Minta, A.A.; Antoni, S.; Grant, G.B.; Perry, R.T.; O’Connor, P. Progress toward measles elimination—African Region, 2017-2021. MMWR 2023, 72, 985–991. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Measles and Rubella Strategic Framework 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Winter, A.K.; Moss, W.J. Rubella. Lancet 2022, 399, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.C.; Zimmerman, L.A.; Alexander, J.P., Jr.; Crowcroft, N.S.; O’Connor, P.M.; Knapp, J.K. Progress toward rubella and congenital rubella syndrome elimination—Worldwide, 2012-2022. MMWR 2024, 73, 162–167. [Google Scholar] [CrossRef]
- Plotkin, S.A. Rubella eradication: Not yet accomplished, but entirely feasible. J. Infect. Dis. 2021, 224, S360–S366. [Google Scholar] [CrossRef]
- Reef, S.E.; Icenogle, J.P.; Plotkin, S.A. The path to eradication of rubella. Vaccine 2023, 41, 7525–7531. [Google Scholar] [CrossRef] [PubMed]
- Grenfell, B.; Harwood, J. (Meta)population dynamics of infectious diseases. Trends Ecol. Evol. 1997, 12, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Grenfell, B.T. Understanding the persistence of measles: Reconciling theory, simulation and observation. Proc. Biol. Sci. 2002, 269, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Earn, D.J.; Rohani, P.; Grenfell, B.T. Persistence, chaos and synchrony in ecology and epidemiology. Proc. Biol. Sci. 1998, 265, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Nokes, D.J.; Swinton, J. Vaccination in pulses: A strategy for global eradication of measles and polio? Trends Microbiol. 1997, 5, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Zlojutro, A.; Khan, K.; Gardner, L. Measles resurgence in the USA: How international travel compounds vaccine resistance. Lancet. Infect. Dis. 2019, 19, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.; Dong, E.; Khan, K.; Sarkar, S. Persistence of US measles risk due to vaccine hesitancy and outbreaks abroad. Lancet. Infect. Dis. 2020, 20, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Feachem, R.G.A.; Chen, I.; Akbari, O.; Bertozzi-Villa, A.; Bhatt, S.; Binka, F.; Boni, M.F.; Buckee, C.; Dieleman, J.; Dondorp, A.; et al. Malaria eradication within a generation: Ambitious, achievable, and necessary. Lancet 2019, 394, 1056–1112. [Google Scholar] [CrossRef] [PubMed]
- Durrheim, D.N.; Andrus, J.K. The ethical case for global measles eradication-justice and the Rule of Rescue. Int. Health 2020, 12, 375–377. [Google Scholar] [CrossRef]
- Durrheim, D.N. Measles eradication-retreating is not an option. Lancet. Infect. Dis. 2020, 20, e138–e141. [Google Scholar] [CrossRef]
- Durrheim, D.N.; Crowcroft, N.S. The price of delaying measles eradication. Lancet Public. Health 2017, 2, e130–e131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolotin, S.; Osman, S.; Hughes, S.L.; Ariyarajah, A.; Tricco, A.C.; Khan, S.; Li, L.; Johnson, C.; Friedman, L.; Gul, N.; et al. In Elimination Settings, Measles antibodies wane after vaccination but not after infection: A systematic review and meta-analysis. J. Infect. Dis. 2022, 226, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Durrheim, D.N.; Andrus, J.K.; Pfaff, G.; Tabassum, S.; Bashour, H.; Githanga, D. Eradicating measles: A call for an exceptional coordinated global effort. J. Infect. Dis. 2019, 220, 1870–1872. [Google Scholar] [CrossRef] [PubMed]
| Scientific feasibility |
|
| Political will and popular support |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, A.K.; Moss, W.J. Possible Paths to Measles Eradication: Conceptual Frameworks, Strategies, and Tactics. Vaccines 2024, 12, 814. https://doi.org/10.3390/vaccines12070814
Winter AK, Moss WJ. Possible Paths to Measles Eradication: Conceptual Frameworks, Strategies, and Tactics. Vaccines. 2024; 12(7):814. https://doi.org/10.3390/vaccines12070814
Chicago/Turabian StyleWinter, Amy K., and William J. Moss. 2024. "Possible Paths to Measles Eradication: Conceptual Frameworks, Strategies, and Tactics" Vaccines 12, no. 7: 814. https://doi.org/10.3390/vaccines12070814
APA StyleWinter, A. K., & Moss, W. J. (2024). Possible Paths to Measles Eradication: Conceptual Frameworks, Strategies, and Tactics. Vaccines, 12(7), 814. https://doi.org/10.3390/vaccines12070814






