Abstract
Measles and rubella remain global health threats, despite the availability of safe and effective vaccines. Estimates of population immunity are crucial for achieving elimination goals and assessing the impact of vaccination programs, yet conducting well-designed serosurveys can be challenging, especially in resource-limited settings. In this review, we provide a comprehensive assessment of 130 measles and rubella studies published from January 2014 to January 2024. Methodologies and design aspects of serosurveys varied greatly, including sample size, assay type, and population demographics. Most studies utilized enzyme immunoassays for IgG detection. Sample sizes showed diverse sampling methods but favored convenience sampling despite its limitations. Studies spanned 59 countries, predominantly including adults, and revealed disparities in seroprevalence across demographics, regions, and notably among migrants and women. Age-related declines in antibodies were observed, particularly among infants, and correlations between vaccination status and seropositivity varied. We conclude with an outlook on measles and rubella serosurveillance, emphasizing the need for proper survey design and the advantages of standardized, multiplex serology assays.
Keywords:
immune surveillance; serosurvey; seroprevalence; measles; rubella; multiplex assay; IgG antibody 1. Introduction
1.1. Measles and Rubella: Epidemiology, Disease Burden, and Elimination Efforts
Measles and rubella continue to pose significant public health challenges despite the availability of effective vaccines and global efforts towards their elimination. Measles, caused by the measles virus, is a highly contagious disease characterized by fever, cough, coryza, conjunctivitis, and a distinctive rash, which can lead to severe health complications, particularly in unvaccinated people [1]. Rubella, often milder in children and adults, can have devastating consequences if contracted during early pregnancy, leading to congenital rubella syndrome (CRS) in the developing fetus. This syndrome can include a range of severe birth defects or fetal deaths [2]. Two doses of the measles-containing vaccine (MCV) and a single dose of the rubella-containing vaccine (RCV) can provide lifelong protection [3,4,5]. Moreover, high levels of measles and rubella antibodies within a population contribute to herd immunity, which helps protect individuals who are unable to be vaccinated (e.g., infants, pregnant women, and immunocompromised individuals) by reducing the overall transmission of the viruses. Despite the availability of effective vaccines for measles, the incidence of measles doubled between 2017 and 2018, a trend that continued into 2019, leading several countries to lose their measles elimination status [6]. In 2022, measles incidence trended upwards, with an estimated 136,200 deaths and 9.2 million cases, marking a 43% increase in mortality and an 18% increase in cases compared with 2021 [7]. Rubella cases dropped by 48% between 2012 and 2019 and continued to decline into 2020. However, CRS cases increased between 2012 and 2022, which can be attributed to the initiation of CRS surveillance in several large countries [8]. It is estimated that 32,000–100,000 cases of CRS occur annually, depending on the estimate model used, underscoring the persistent public health challenge posed by rubella infection [5,9,10,11].
To facilitate concerted efforts towards elimination, the Measles and Rubella Initiative devised the Global Measles and Rubella Strategic Plan 2012–2020, aligning with the WHO’s Global Vaccine Action Plan 2011–2020 [12]. As part of this strategic plan, measles and rubella elimination was targeted in at least five WHO regions by the end of 2020 through the implementation of five core strategies [8,13]. Ultimately, complete elimination goals were not met by 2020 [14], though some progress was made. Between 2000 and 2019, global measles-containing vaccine first dose (MCV1) coverage increased from 72% to 86% [7]. Global routine measles-containing vaccine second dose (MCV2) coverage has increased steadily, from 42% in 2010 to 69% in 2018. By the end of 2019, 178 WHO member states had taken the critical step of introducing MCV2, underscoring the collective commitment towards advancing immunization initiatives and progress towards measles elimination [15]. Between 2012 and 2022, the number of countries providing rubella-containing vaccine (RCV) increased from 132 to 175, and verification of rubella elimination was documented in 98 (51%) of 194 WHO member states [8]. Unfortunately, the disruption of routine vaccination programs and supplemental immunization activities (SIAs) during the COVID-19 pandemic led to a decline in MCV1 coverage to 81% in 2021, the lowest since 2008 [7,16,17]. MCV1 coverage has begun to recover but remains alarmingly low (83% in 2022) [7]. Despite the fact that immunization averted approximately 23 million deaths between 2010 and 2018, the continued transmission of measles remains a potent reminder of the importance of sustaining robust immunization programs and surveillance systems to prevent outbreaks.
To continue efforts towards elimination, the Measles and Rubella Strategic Framework (MRSF) 2021–2030 was developed to provide a comprehensive guide on supporting global, regional, and national efforts. MRSF emphasizes strategic priorities such as integrating efforts into primary health care and universal health coverage, enhancing surveillance systems, ensuring vaccine supply, and promoting research and innovation. This framework aligns with the Immunization Agenda 2030 (IA2030) [18]. IA2030 outlines a global strategy to enhance health through immunization, in which measles and rubella elimination play a critical role, positioning measles as a “tracer” for the effectiveness of immunization programs. Measles outbreaks may indicate gaps in routine childhood immunization coverage [19]. Regardless, achieving high uniform vaccination coverage for measles and rubella remains a challenge, particularly in resource-constrained regions, and continues to impede progress towards elimination. Even for countries that have good overall coverage rates, immunization coverage in-country can vary greatly due to regional healthcare disparities, inadequate vaccine supply chain, political or social instability, or inadequate monitoring and surveillance [20]. Collectively, these challenges underscore the critical importance of enhancing surveillance systems and leveraging serosurveillance tools to monitor and address gaps in immunity. This approach can help guide the design of effective vaccination strategies aimed at preventing outbreaks and advancing toward the goal of measles and rubella elimination.
1.2. Antibody Response and Immune Memory to Measles and Rubella
The WHO currently recognizes eight clades of measles (A-H); however, there is only one serotype [21]. For rubella, there are thirteen different genotypes divided into two clades (1a, 1B, 1C, 1D, 1E, 1F, 1G, 1h, 1i, 1j, 2A, 2B, and 2C); however, like measles, there is only one serotype [22]. Upon initial exposure to a virus, either through disease or vaccination, the immune system initiates a primary immune response, leading to the production of various classes of antibodies. Immunoglobulin M (IgM) antibodies are prevalent early during infection. During measles infection, IgM levels peak a few days after the onset of the rash and decrease in the subsequent weeks. Similarly, during a rubella infection, IgM is typically detectable within two to five days after rash onset and remains such for one to three months post-infection. IgM levels decline as B cells undergo class switching to produce immunoglobulin G (IgG) antibodies. Measles IgG levels peak one to two weeks after the rash onset, although low levels are detectable within a few days after the rash appears [23,24]. Anti-rubella IgG becomes detectable shortly after IgM, peaking around one to two weeks after the rash appears [25]. IgG antibodies serve as unique biomarkers of prior exposure (either by vaccination or infection) to measles and rubella, and they typically persist for life [23]. The differentiation of B cells into memory B cells is crucial for producing high-affinity IgG antibodies and establishing a sustained antibody-mediated immune response post-exposure. Additionally, memory B cells can terminally differentiate into long-lived, antibody-producing plasma cells, usually conferring protection during subsequent re-exposure to a pathogen [26]. Measles and rubella vaccines are live, attenuated viruses that cause a similar infection to wild-type viruses, although usually without symptoms. In general, the antibody response is less robust to immunization than to disease and results in lower antibody titers. However, antibody tests cannot distinguish between disease-induced and vaccination-induced antibodies.
In addition to antibody production following infection with a wild-type or vaccine virus, pathogen-specific antibodies can be transferred from one person to another in two ways. The first is prophylactic administration of pooled human immune globulin with sufficient pathogen-specific antibodies by intramuscular, intravenous, or subcutaneous injection [27]. This is a rare procedure, and the antibody duration is only a few months, so this seroprevalence is irrelevant on a population scale. The second method of antibody transfer occurs during pregnancy, when a mother transfers her own antibodies through the placenta to her baby. The titer achieved in the infant is dependent on the mother’s titer and is typically roughly two-fold higher. On average, infants whose mothers have natural infection-induced immunity have higher titers than infants whose mothers have vaccine-induced immunity [28,29]. Infants of mothers who have no measles antibodies do not receive this transplacental protection. The duration of antibody protection among infants is directly related to the titer at birth and is decreasing across infant populations because of increasing proportions of mothers who have vaccine-induced or no measles immunity [29,30,31]. Because of the low levels of maternally derived measles antibodies and the severity of measles in infants, seroprevalence studies in infants represent a special case of serosurveys and need to be analyzed separately, typically by months of age.
In general, the measles seroprevalence in a population is highly age-dependent. Almost all infants are born with measles antibodies (provided their mother was previously vaccinated or infected), so the seroprevalence in newborns is close to 100%. However, titers in infants decrease rapidly, and, in many settings, infant titers are near zero by 4 to 6 months of age [32,33,34,35]. This is not a major risk in countries with elimination status because the likelihood of exposure to measles is very low. However, in measles-endemic countries, early infant susceptibility has led to increases in measles incidence among infants [36]. After maternal antibodies are depleted, measles seroprevalence in infants begins to increase as they are either vaccinated or infected with measles. Unfortunately, it is not possible to distinguish between maternally transferred, disease-induced, or vaccine-induced antibodies using serological testing.
The detection of IgG in serum does not necessarily indicate the presence of protective, neutralizing antibodies [37]. Functional, or neutralizing, antibodies can bind specific epitopes required for viral entry into host cells, thus preventing subsequent infections. Therefore, neutralizing antibodies are reliable indicators of protective immunity [38]. For measles, a neutralizing antibody titer of 120 milli-International Units (mIU)/mL is well established to protect against disease, although there has been some debate surrounding this in recent years [39]. The accepted correlate of protection for rubella is 10 IU/mL [40]. IgG antibodies that lack neutralizing activity (e.g., binding antibodies) are also produced during an immune response. While binding antibodies are pathogen-specific and may have other effector functions, they lack the ability to neutralize an infectious virus. Serological surveys typically assess the overall prevalence of IgG antibodies against specific pathogens in the population, irrespective of antibody activity [26].
In addition to humoral immunity, exposure to a pathogen triggers a cellular immune response. Broadly, CD4+ T cells provide essential help for several immune functions, such as affinity maturation of B cells and antibody isotype switching, while CD8+ T cells commonly exert antiviral effects by producing cytokines that suppress virus replication and eliminate infected cells. In the response to measles virus infection, T cells are considered essential for viral clearance [41]. Individuals with low or undetectable measles antibody levels have been shown to be somewhat protected against clinical measles, suggesting a significant role for cellular immunity [42]. However, the correlation between measles-specific antibody levels and the T cell immune response is poor [43]. Attempts to establish a cellular correlate of protection for rubella have failed to date [40], and no association between markers of humoral and cellular immunity has been identified [44]. Although seroprotective antibody titers for measles and rubella have been established, these correlates of protection should not be used to assess or infer the protection conferred by the cellular immune response. Additional studies are needed to establish correlates of protection specific to the cellular immune response.
1.3. Serosurveillance
Serosurveys are critical for understanding population-level immunity and disease burden estimates for infectious diseases. Typically, serosurveys do this by monitoring the presence of pathogen-specific IgG antibodies. For vaccine-preventable diseases (VPDs), well-designed serological surveillance studies can aid in assessing the effectiveness of vaccination programs and help identify gaps in immunity. For non-VPDs or for VPDs in countries where vaccination is not established, serosurveillance data can estimate disease burden or changes in disease exposure [45]. Unfortunately, serosurveys for estimating the disease burden of VPD can be complicated in areas with vaccination, as disease- and vaccine-induced antibodies are measured jointly and cannot be distinguished. These data can collectively guide public health decisions on vaccination policies and strategies to achieve and maintain elimination. For measles and rubella, serological surveys have been instrumental in global efforts towards elimination by evaluating immunity to identify susceptible populations or age groups, estimating disease burden in areas without vaccination, and assessing the impact of public health policies and campaigns [46]. Rubella serosurveys have also provided insights into true estimates of CRS disease burden [47].
Serosurveys often utilize enzyme immunoassays (EIAs) to assess the presence of IgG antibodies. Some EIAs and other IgG binding assays measure the total IgG antibody concentration, while others only provide a qualitative result, indicating the presence or absence of pathogen-specific IgG. EIAs can vary greatly in their sensitivity and specificity, depending on the antigen used (e.g., purified protein or whole virus lysate) as well as the assay format (e.g., direct, indirect, sandwich, or competitive EIA). These differences in assay performance can impact the ability to detect antibodies, particularly at low concentrations. One drawback of EIAs is the inability to distinguish neutralizing antibodies since these assays measure IgG binding to specific epitopes, regardless of their neutralization capacity [48]. For measles, the most abundantly produced antibodies target measles nucleoprotein, making it a common target in commercially available assays; however, nucleoprotein-specific antibodies are generally non-neutralizing; thus, total IgG titers do not correlate well with neutralization titers [48]. For rubella, the E1 glycoprotein is commonly used as the target for commercial test kits and is a target of neutralizing antibodies. EIA tests that provide a qualitative titer for rubella are designed to correspond with a protective level of antibodies; however, many studies have shown a lack of standardization [49,50,51,52,53]. Over time, antibody levels induced by measles and rubella vaccination may decrease [54,55]. As antibody levels wane, they may fall below the detection threshold for EIA assays that have poor sensitivity.
While EIAs are considered the standard methods for serosurveys, several studies have shown that EIAs and other immunostaining assays are less sensitive than plaque reduction neutralization tests (PRNTs), especially in the context of low antibody levels [56,57,58]. For measles, the plaque reduction neutralization test (PRNT) is considered the gold standard for assessing neutralizing immunity [59], while the immunocolorimetric neutralization assay is used for rubella [60]. A study by Tischer et al. demonstrated disparate positivity rates for measles between EIA, indirect immunofluorescence tests (IFT), and PRNT when serum samples were tested by each method [58]. The samples were collected from patients with known measles vaccination status but showed equivocal or negative results when initially tested with EIA several years after vaccination. Subsequent re-testing of these samples with IFT and PRNT showed positive rates of 81% for IFT and 94% for PRNT [58]. This discrepancy suggests that some EIAs may lack sufficient sensitivity to detect vaccine-induced antibodies several years post-immunization, posing potential challenges for serosurveys. However, applying neutralization assays in large-scale serosurveys is impractical due to technical difficulty, high cost, and labor-intensive processes.
Bead-based immunoassays have been around for more than two decades [61] and utilize antigen-bound beads to detect pathogen-specific IgG. Moreover, bead-based assays are often multiplexed, allowing for the simultaneous detection of antibodies to multiple pathogens in a single test. Bead-based immunoassays have been utilized for serological surveys for a variety of pathogens, including measles and rubella, and generally have robust performance characteristics, are cost-effective, and are resource-efficient [62]. A study by the National Institute of Public Health and the Environment in the Netherlands indicated a multiplexed bead-based assay (MBA) had higher sensitivity for measles and rubella IgG than individual ELISA [63]. MBA data exhibited strong correlations with ELISA data for both measles and rubella, with no cross-interference among antigens observed. Notably, MBA also demonstrated a higher correlation with PRNT than EIA [56,64]. In conclusion, MBAs present a compelling alternative to conventional EIA for detecting multiple target antibodies simultaneously in large-scale immunosurveillance studies.
2. Literature Review of Measles and Rubella Serosurveys (January 2014–January 2024)
2.1. Measles and Rubella Serosurvey Study Selection and Characteristics
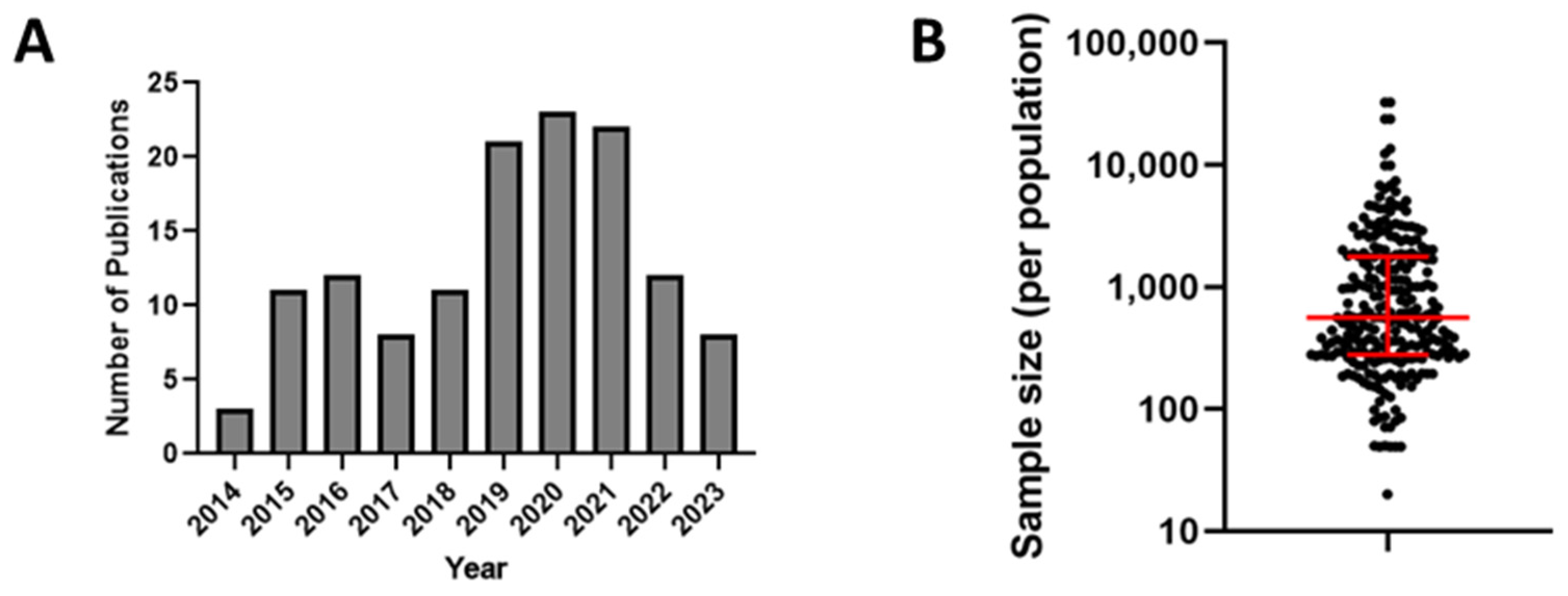
To understand the scope of measles and rubella seroprevalence surveys over the last decade, we conducted an online search using OVID (Medline, Embase, and Global Health), Cochrane Library, CINAHL, and Scopus. The search was constrained to the last 10 years (January 2014 through January 2024) and included the following keywords: ‘rubella’ and ‘measles’ combined with ‘serosurvey’, ‘seroprevalence’, ‘immunity’, and ‘population immunity’. All search sources were last consulted on 9 January 2024. Any duplicate articles returned by the search were removed, leaving 492 results. We further excluded references that were not in English or where a full-length, peer-reviewed primary research paper or relevant seroprevalence information were not found. Additionally, any studies that focused specifically on individuals with HIV or other chronic health conditions were also excluded. This resulted in a total of 130 published studies, of which 30 described serosurveys for measles, 25 for rubella, and 75 for both. We focused our analysis on distinct populations (e.g., age, sex, occupation, or pregnancy status) and reported seroprevalence for each population individually within the paper. We also grouped studies by year of publication, country, WHO region, and vaccination status. When appropriate, medians were used to calculate the central tendency of a research outcome. Records not showing these data items were considered missing or unavailable. We did not discriminate between studies using residual samples and studies using a priori study design for estimating some outcomes in our review (e.g., overall seroprevalence). Therefore, we acknowledge that this might influence these estimations, although the extent of such influence is unknown. Supplementary Tables S1 and S2 list each population described for each published study. A 2016 review of measles and rubella seroprevalence studies published between 1998 and June 2014 found a total of 68 studies for measles and 58 for rubella [65]. In comparison, there seems to be a notable increase in the publication rate of such studies (Figure 1A).
Figure 1.
(A) Distribution of the number of publications by year included in the review. (B) Plot of serosurvey sample sizes for all included studies for measles and rubella. Red lines represent the median and interquartile range.
2.2. Serosurvey Methodology
Of the 130 studies, 93 (71%) used EIAs to estimate IgG seroprevalence, with the most commonly used kits being Enzygnost (Siemens, Marburg, Germany, n = 28) and EUROIMMUN (EUROIMMUN, Lubeck, Germany, n = 20). In 25 (19%) studies, automated chemiluminescent immunoassay (CLIA), chemiluminescent microparticle immunoassay (CMIA), or enzyme-linked fluorescent assay (ELFA) technologies were utilized, with DiaSorin Liaison or Liaison XL (DiaSorin, Saluggia, Italy, n = 15) being the most common. Additionally, Luminex-based MBAs were used in seven of the studies (5%). The remaining studies utilized other methods such as the hemagglutination inhibition test, rapid immunochromatographic test, particle agglutination assay, or the specific method was not indicated. Notably, one study compared the performance of the Enzygnost and EUROIMMUN IgG EIAs in a serosurvey of rubella antibodies in pregnant women [66]. In that study, the authors observed a strong qualitative (96.3%) and quantitative (mean titer difference: 0.8 IU/mL) agreement between the two tests, indicating that results between certain EIA tests may be comparable. However, the sensitivity of certain EIA kits may still cause underestimates of seroprevalence. A study in Singapore using the Enzygnost EIA as the primary test method performed further testing on all samples that were negative for measles-specific IgG and a random sampling of equivocal specimens for additional testing. Subsequent testing by PRNT revealed that approximately 75% of the samples initially categorized as measles IgG-negative, along with all randomly selected samples categorized as measles IgG-equivocal, were actually seropositive. This had a significant impact on the estimated measles seroprevalence [67].
Sample sizes varied greatly across the 130 studies, ranging from sample sizes as small as 20 persons to as large as 32,502 individuals. In some instances, the seroprevalence was only reported for specific subgroups and not for the entire study population. As a result, some studies reported seroprevalence estimates for a very small number of people, with 24 (18%) studies reporting seroprevalence for groups of <200 individuals. Overall, the median sample population size for all studies was 561, and 63 (48%) studies surveyed populations of at least 1000 individuals (Figure 1B). Only 37 (28%) studies described the use of a priori sample size analysis to determine the appropriate sample size, as recommended by the WHO Guidelines for the assessment of measles and rubella seroprevalence. Other studies (n = 39) described the use of sampling methods, such as multistage random cluster sampling. Most studies (n = 92) did not specify a specific statistical method for participant selection. Instead, samples in those studies were obtained from residual blood donor or patient serum specimens, as well as from pre-employment or prenatal samples. Alternatively, many studies that actively recruited participants used targeted enrollment strategies, such as all students enrolled in a particular university program. The use of such opportunistic sampling is common due to its convenience and can provide local data on seroprevalence but cannot be generalized to an entire population.
2.3. Serosurvey Demographics
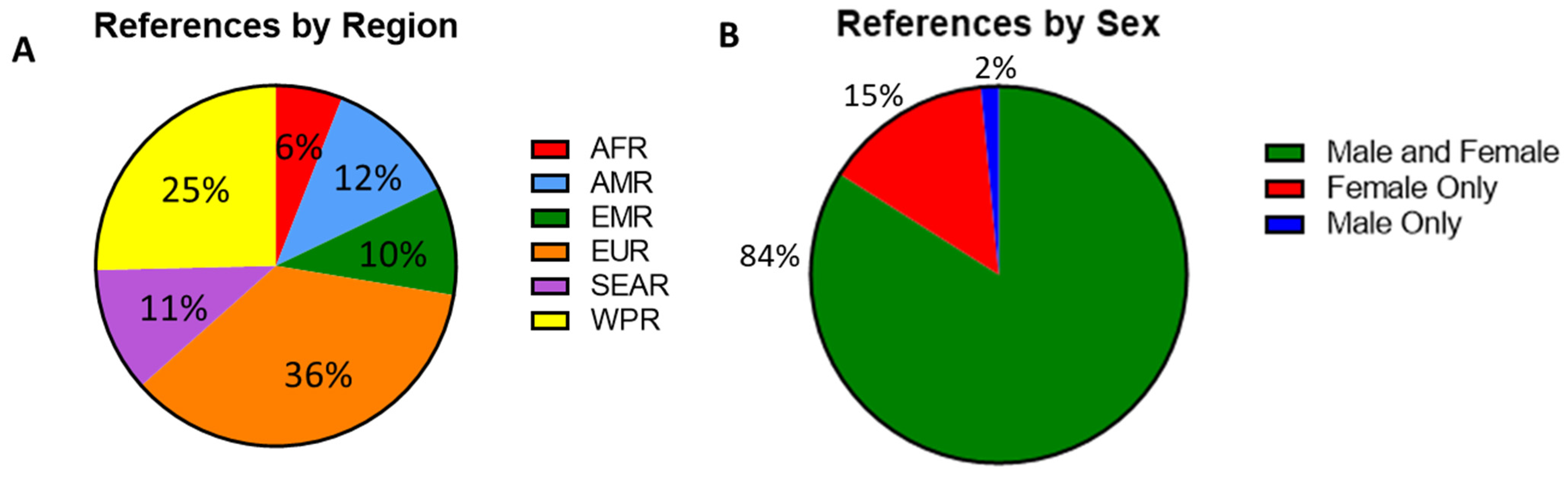
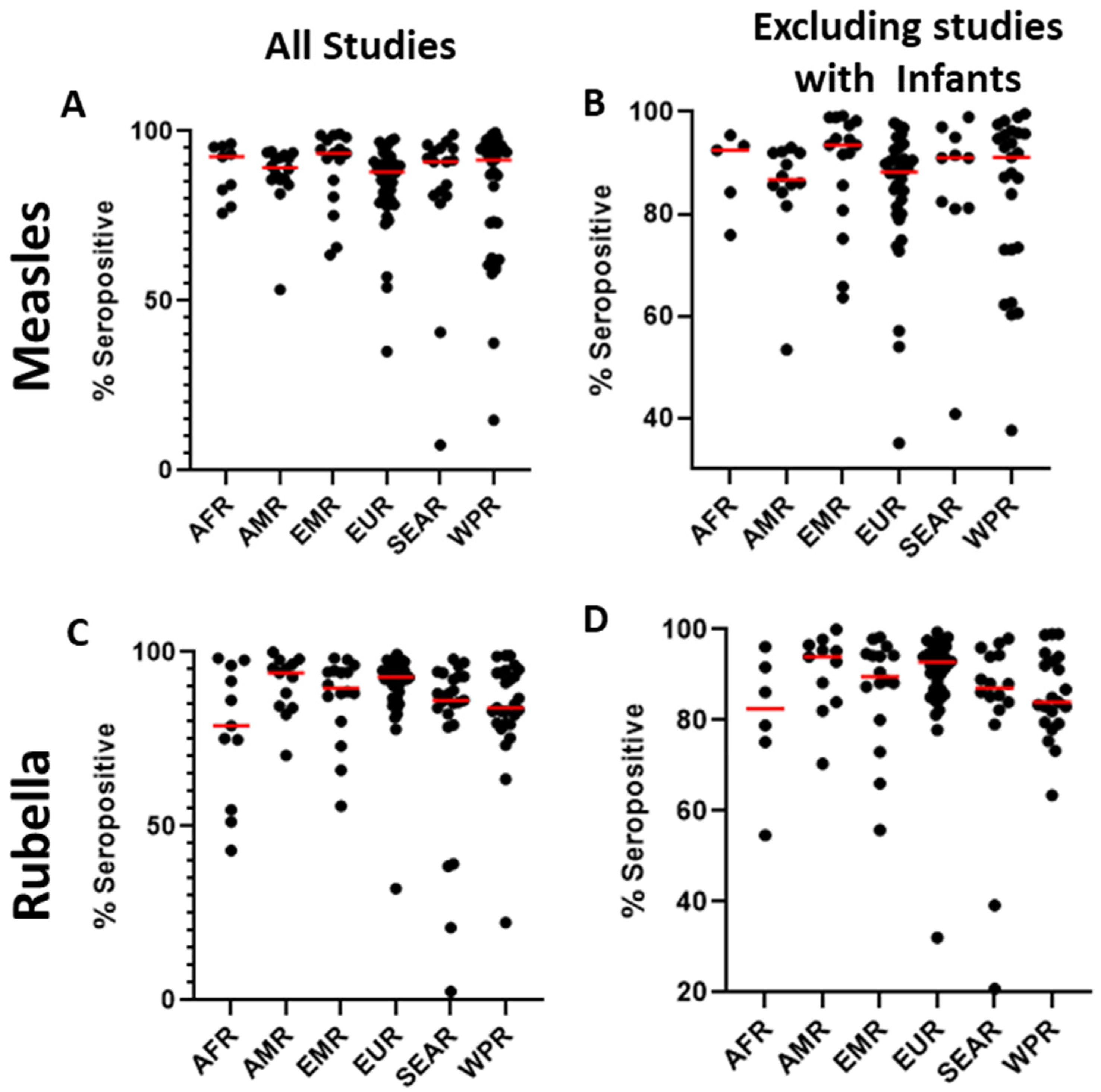
The serosurveys included a total of 59 countries; countries in the European WHO region (EUR) were most represented (n = 48), followed by the Western Pacific region (WPR, n = 34). In contrast, only eight studies reported data from the African region (AFR) (Figure 2A). Among the WHO regions represented in the included studies, the median measles seropositivity ranged from 88% to 93.5% (Table 1, Figure 3A). However, these estimates are certainly influenced by limited sampling in certain regions and differences among the populations studied. In particular, studies including infants at birth or early in life might have skewed the data because of waning maternal antibodies or a lack of exposure in the infants. When studies including infants were not considered, the median measles seropositivity ranged from 86.8% to 93.5% (Table 2, Figure 3B). For rubella, the median seropositivity ranged from 78.9% to 94% (Table 1, Figure 3C). When excluding studies with infants, the range is 82.6% to 94% (Table 2, Figure 3D).
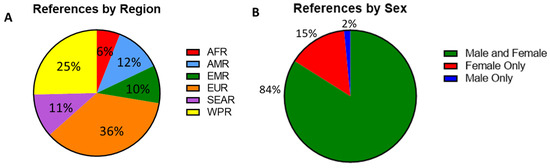
Figure 2.
(A) Percent of serosurvey studies by region, percentages indicating the proportion of all included studies. African region (AFR, n = 8), American region (AMR, n = 16), Eastern Mediterranean region (EMR, n = 13), European region (EUR, n = 48), Southeast Asian region (SEAR, n = 15), and Western Pacific region (WPR, n = 34). (B) A breakdown of gender distribution among participants with percentages indicating the proportion of all included studies. Male and female (n = 110), female only (n = 19) and male only (n = 2).

Table 1.
Overall median seropositivity by WHO region for measles or rubella based on studies included in this review.
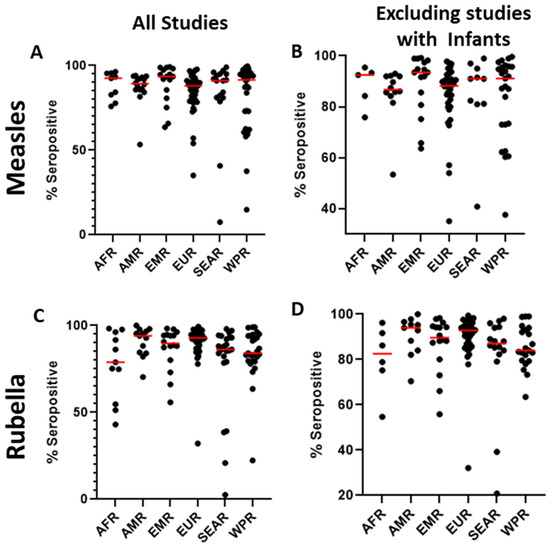
Figure 3.
Distribution of seroprevalence for measles (A) and rubella (C) for all studies included in the literature review and the distribution of seroprevalence for measles (B) and rubella (D) excluding studies that included infants. Red line represents the median.

Table 2.
Median seropositivity by WHO region for measles or rubella based on studies included in this review, excluding studies with data from infants.
Twelve studies described seroprevalence in asylum seekers, refugees, international adoptees, or other immigrants or displaced peoples, and eleven of those studies examined the migration of persons into European countries from Africa, Asia, or elsewhere in Europe. Within those studies, the median seroprevalence reported in migrants into Europe (79.4% for measles and 85.1% for rubella) was lower than the median seroprevalence reported in other studies of non-migrants in the region (88.5% for measles and 93.5% for rubella). Only 13 surveys were conducted in countries listed by the UN as Least Developed Countries (LDC), and four of those studies were conducted in Zambia. Eighty-three (63%) studies were conducted in developed countries (UN Human Development index >0.8, 2021)—a significant percent increase compared to what was reported by Dimech and Mulders in 2016 [65]. Developed countries have a need to identify immunization gaps and document sufficient levels of population immunity as countries move towards elimination status.
Among the studies, 114 (87%) included adults (aged ≥18 years), 62 (47%) included adolescents (aged 15–17 years), 49 (37%) included children (aged 1–14 years), and 24 (18%) included infants (aged <1 year). Additionally, 110 (84%) of the studies included both male and female subjects. Only two studies (1%) exclusively included men, as these were cohorts of military recruits and professional sports players. Nineteen studies (15%) included only women. These studies focused on either pregnant women, women of childbearing age (WCBA), or women in universities or other workplaces (Figure 2B).
2.4. Overall Seroprevalence and Antibody Titers
The median measles seroprevalence reported in all studies was 89.87%, with a wide range of 7.5–99.6%. Only 16 (15%) of measles studies reported quantitative test results. Among those, the median average titer reported was 1153 mIU/mL (range = 77–25,300 mIU/mL). The median rubella seroprevalence reported was 90.5% (range = 2.5–100%). Only 17 rubella studies (17%) reported quantitative test results. Of those, the median average titer reported was 51 IU/mL (range = 4–240 IU/mL). The wide range of reported seroprevalence and antibody titers are significantly influenced by studies in older infants, which is discussed in further detail below. For this reason, the median seroprevalence (as opposed to the mean) likely gives a more accurate estimation of average seroprevalence and antibody titers globally and by region.
2.5. Age and Seroprevalence
Because the measles antibody is long lasting, seroprevalence in a particular birth cohort typically continues to increase as the cohort ages and experiences accumulated disease or vaccine-induced immunity. However, different birth cohorts experience different disease and immunization rates, and relative troughs in seroprevalence may be seen in some older cohorts. To assist in identifying immunity gaps, analysis of these patterns of seroprevalence is a major reason for serosurveys. In studies that analyzed age as a variable among adults, adolescents, and children younger than one year (n = 78), researchers found that measles and rubella seroprevalence varied by age. Specifically, 41 studies reported an increase in measles seroprevalence among older adults compared to younger participants, as expected, whereas 22 studies observed no age-related differences. Similarly, 30 studies noted a lower rubella antibody prevalence among younger participants than older ones, but 27 studies reported no clear relationship between age and rubella antibody seroprevalence.
The median measles seroprevalence reported for studies that surveyed only infants was 90%. Among the eight studies that reported measles seroprevalence for infants only, there was a wide range of seropositivity (7.5–98%). This is due to differences in the exact age of infants in the study, and the fact that maternally acquired antibody titers wane between birth and the receipt of the measles-containing vaccine. In the four studies that further divided measles seroprevalence in infants by month of age, they all reported declining seroprevalence over the first year of life, as expected [68,69,70,71]. Differences in the exact age group studied make comparisons between studies difficult. However, there are some notable similarities. Cho et al. found that while measles seroprevalence was 94.4% in Korean infants <1 month old, seroprevalence dropped to 27.3% in 4-month-olds. Almost no infants ≥5 months old tested positive for the measles antibody [68]. Similarly, Muthiah et al. reported seropositivity for measles of 95% in Sri Lankan newborns, 23.1% in 7–8-month-olds, and 0% in 11–12-month-olds [69].
Only two studies examined rubella seroprevalence in infants by months of age, but the same pattern was observed as with measles. Muthiah et al. reported rubella seropositivity of 95% in Sri Lankan newborns, 9.6% in 7–8-month-olds, and 0% in 11–12-month-olds [69]. Similarly, Bassal et al. reported 80.4% rubella seropositivity in Israeli infants aged 0–6 months, compared with only 13% in infants aged 6–11 months [72]. In these studies, although each age group contained relatively few individuals, these data on measles and rubella seroprevalence in infants may cumulatively indicate a large window of susceptibility, which is most concerning for infants living in endemic settings. Whether infants should be vaccinated against measles and rubella earlier than current WHO recommendations (9 months of age in countries where transmission is ongoing) is an area of active debate. However, there is currently insufficient evidence on the long-term efficacy of early vaccination [73]. Given the increases in infant seronegativity seen in many studies, it is critical that infants be protected from exposure to measles by decreasing incidence in older populations, while research is being conducted on the immunogenicity of vaccinating infants at ages earlier than 9 months.
2.6. Seroprevalence and Vaccination
Sixty-eight studies had vaccination records for participants or surveyed them as to their recollection of previous vaccinations. In 24 of these, seropositivity for measles and/or rubella was reported to be positively associated with vaccination status or an increasing number of doses. Five studies found no statistically significant relationship or, interestingly, that vaccination status was negatively associated with seropositivity [74,75,76,77,78]. Three studies found higher titers of measles and rubella IgG in non-vaccinated individuals compared to those that had been vaccinated, as has been previously reported for individuals following infection [65,69,79,80,81]. This phenomenon was observed in countries that have had circulating measles or rubella viruses during the lifetime of the study participants.
Several studies reported higher seropositivity estimates for measles or rubella among the population than the percentage of participants reporting vaccination. This can be easily attributed to a prior infection with measles or the rubella virus. However, 10 studies reported lower seropositivity than the percentage of individuals either with vaccination records or those that recalled vaccination or infection [82,83,84,85,86,87,88,89,90,91]. These were typically studies of students or employees with available vaccination records. While such single-site studies can only give information on local conditions, this pattern of lower seroprevalence was observed in multiple countries spanning four WHO regions (AMR, EMR, EUR, and WPR) and may suggest instances of vaccine failure or waning immunity in the populations surveyed.
Ten studies described changes in national vaccination programs over time [80,92,93,94,95,96,97,98,99,100]. The results were mixed as to whether programmatic changes resulted in increased or decreased seropositivity for measles and/or rubella. For example, Gorun et al. surveyed 6914 WCBA who had undergone TORCH screening. When dividing the cohort by year of birth and vaccination program eligibility, they found the lowest rubella seroprevalence rates among the youngest cohort—82.4% of those born from 1997 to 2004 who were eligible for the measles–mumps–rubella (MMR) vaccine through the family practice system. In comparison, the seroprevalence was 95.8% in those born in 1989–1994 who were eligible for the monovalent rubella vaccine distributed in schools [92]. Coppeta et al. found no change in measles seropositivity in students following the implementation of a National Plan of Vaccine Prevention (NPVP) in Italy [100].
The effectiveness of supplementary immunization activities (SIAs) on a national or regional level was assessed in seven studies [101,102,103,104,105,106,107]. Of those studies, six described overall higher measles and/or rubella seroprevalence in eligible populations following a campaign, while one showed inconsistent results. Ichimura et al. evaluated the effectiveness of 2015 and 2019 SIAs in East Sepik, Papua New Guinea, and concluded that the 2019 SIA resulted in a higher measles and rubella seroprevalence in the target age group than the 2015 SIA. However, the authors note that the small number of participants (n = 278) was a significant limitation. Studies assessing seroprevalence following changes in the national immunization program or the implementation of SIAs should ideally be significantly powered, have sufficient documentation of vaccination, and be conducted over an appropriate timescale in order to reliably draw definitive conclusions on the impact of these initiatives.
3. Discussion
Future Considerations for Measles and Rubella Serosurveillance
Serosurveillance studies provide useful estimates of population immunity and the impact of vaccination programs, which can subsequently inform country-specific priorities and public health initiatives. Countries that have verified elimination status for measles and/or rubella, as well as countries that are approaching elimination, must document that adequate levels of population immunity are maintained or achieved. Furthermore, it is important for countries to understand the presence of gaps in population immunity, such as specific age groups or vulnerable communities that may be susceptible to measles or rubella outbreaks upon the introduction of disease. However, for accurate population-level estimates to be obtained from serosurveys, studies must have been designed and conducted appropriately.
Studies can benefit from a priori study design, including sample size calculations and a delimitation of the study population to ensure it is representative. Although it is recognized that using residual samples from other studies is a practical means to conduct serosurveillance, the results from these studies have inherent limitations. Residual samples may not be wholly representative of the overall population, preventing the generalization of immunity estimates. Also, the number of residual specimens available for testing may be limited, thereby limiting statistical power and potentially undermining confidence in the resulting immunity estimates. Even studies that incorporate active sampling into their design should carefully balance population demographics and the geographical distribution of clinical sites as best as possible to ensure a representative sample is collected. Only with a robust study design that accounts for these aspects can we confidently extrapolate results to the population of interest.
Globally standardizing testing methods and interpreting results across laboratories pose challenges for both measles and rubella [25,57,108]. As noted in our literature search, only 16 (12%) measles studies and 17 (17%) rubella studies reported a titer enabling quantitative analysis of data between studies. Most of the reviewed studies reported only qualitative data, determining the seropositive/seronegative status of samples. Comparison of qualitative data is further complicated by use of multiple different EIA platforms, which may be calibrated differently. Most commercial EIAs are designed for single patient testing, not population-level analyses, and thus have relatively high assay cutoffs, which can impact test sensitivity. Antibody titers following vaccination against measles and rubella are generally lower than following infection [109,110], which may lead to an underestimation of seroprevalence in a vaccinated population if a test with insufficient sensitivity is used. This may lead to the unnecessary implementation of vaccination campaigns or other costly public health measures, highlighting the need for sufficiently sensitive, standardized testing methods for use in serosurveillance.
In a systematic review, MBA was found to have robust sensitivity [56]. In this study, MBA for measles-specific IgG was shown to have a median sensitivity of 95% (compared to PRNT; IQR, 89.8–95.0). This sensitivity was similar to the reported median sensitivity of the Enzygnost EIA (92.1%, IQR 82.3–95.7), a commercial EIA kit that was highly employed in serosurveillance studies but has since been discontinued. Furthermore, MBA has shown a strong correlation to “gold standard” assays for evaluating immunity to measles and rubella. Measles-specific MBA has exhibited a strong correlation with PRNT, which is notable considering that most measles-specific EIAs have a poor correlation with PRNT [64]. In the case of rubella, MBA data were found to have a strong correlation with quantitative ELISA, which is commonly used for the evaluation of humoral immunity against rubella [63].
Multiplex assays, like the MBA, can also be more cost-effective than some commonly used immunoassays as they allow for the simultaneous measurement of antibody concentrations for multiple pathogens using a low sample volume [64]. This is due to the increased sample throughput and the sparing of samples and antigens, in addition to the decrease in human labor [111]. For example, 100 samples can be easily tested simultaneously for measles and rubella IgG on MBA using ~75% fewer resources (e.g., assay plates, reagents) than would be needed to test with single-plex ELISA for both pathogens. MBA also requires significantly less (~10 fold) viral antigen than is needed for conventional ELISA to test an equivalent number of specimens for measles and rubella, further highlighting the cost-savings that can be realized with multiplex testing platforms.
The capability of MBA to measure antibody levels against multiple pathogens simultaneously has additional advantages for informing public health decisions beyond monitoring existing vaccination programs. Serological MBAs can be used to quantify the burden of endemic diseases for which a vaccine has yet to be introduced into the immunization program, enabling public health programs to make informed decisions on the need and strategies for the introduction of new vaccines. Multiplex serology assays also enable programs to better leverage limited resources and strategize coordinated surveys for multiple pathogens [112]. For example, blood samples are routinely collected for different programs such as demographic and health surveys, transmission assessment surveys, and indicator surveys for HIV [23]. Serosurveillance for measles, rubella, or other pathogens could be readily integrated with these programs using MBA to maximize the use of the collected samples and related resources.
MBA has been successfully employed by the Centers for Disease Control and Prevention (CDC) for measles and rubella serosurveillance in collaboration with numerous countries and international non-profit organizations. Recently, we assessed over 2500 dried blood spots (DBS) samples from the Democratic Republic of Congo to quantify measles and rubella seroprevalence in parallel with screening of diphtheria and tetanus antibodies [113]. As the Democratic Republic of Congo has not yet implemented rubella vaccination, this illustrates the utility of MBA to simultaneously enable monitoring of vaccination programs (measles, tetanus, diphtheria), while assessing endemic disease burden (rubella) in the same survey.
MBA is amenable to technology transfer between laboratories, enabling multiplex-based serosurveillance to be conducted in multiple countries. As part of a partnership initiative for strengthening integrated serosurveillance, the CDC and the Pan-American Health Organization (PAHO) established a collaborative effort with delegates from Brazil, Mexico, and Paraguay to pilot the introduction of the MBA in their respective countries as a means of monitoring population immunity and disease transmission for multiple diseases, including measles and rubella [114]. This initiative is intended to showcase the utility of integrated serosurveillance using the MBA, combining surveillance programs that are usually addressed separately but overlap within the same populations and geographic areas. As with any serosurvey, linking the results to vaccination records and historical reports of cases and outbreaks will be critical.
4. Conclusions
Serosurveys remain useful tools for supporting elimination efforts for measles and rubella, but only if implemented with the appropriate design considerations and utilizing sufficiently sensitive assays to generate meaningful estimates of population immunity. Complementing overall seroprevalence data with seroprevalence estimates stratified across relevant demographic groups, such as age cohorts and geographic areas, is key for targeting interventions and assessments of program impacts. Unfortunately, this granular approach can be challenging and costly to ensure that there is sufficient statistical power across different strata. The number of published serosurveillance studies detailing measles and rubella immunity estimates has steadily increased over the past decade, yet studies largely continue to implement sub-optimal survey designs using a variety of serological assays. These limitations were previously noted by Dimech and Mulders nearly a decade ago [65,115], and we reiterate the need here for updated, standardized guidelines for conducting seroprevalence studies in support of measles and rubella elimination. Moreover, a standardized serological assay with high sensitivity—such as the MBA—will be critical for accurately assessing population immunity for measles and rubella as more countries achieve elimination.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines12070816/s1, Supplementary Table S1: List of study populations of measles serosurveys included in literature review and Supplementary Table S2: List of study populations of rubella serosurveys included in literature review.
Author Contributions
Conceptualization—P.A.R. and S.N.C. developed the concept and scope of the review; Methodology—A.M.B., E.E.-B. and E.P.-R. developed methodology and criteria for the literature review; Formal analysis—A.M.B., E.E.-B., E.P.-R., N.A.E.G. and D.L.J. conducted formal analyses of the literature; Writing—Original Draft Preparation—A.M.B., E.E.-B., E.P.-R., N.A.E.G. and D.L.J. wrote the initial manuscript draft; Writing—Review and Editing—A.M.B., E.E.-B., P.A.R. and S.N.C. reviewed and edited the manuscript; Supervision—P.A.R. and S.N.C. supervised all aspects of the work. The views are those of the authors and do not necessarily represent those of the Centers of Disease Control and Prevention or the United States Government. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to acknowledge the editors of this special issue of Vaccines for extending the invitation to contribute. The authors would also like to express our sincere gratitude to Mark Papania and Jim Alexander for their insightful comments and critical reading of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hubschen, J.M.; Gouandjika-Vasilache, I.; Dina, J. Measles. Lancet 2022, 399, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.K.; Moss, W.J. Rubella. Lancet 2022, 399, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef]
- WHO. Rubella vaccines: WHO position paper—July 2020. Wkly. Epidemiol. Rec. 2020, 95, 306–324. [Google Scholar]
- Gastanaduy, P.A.; Goodson, J.L.; Panagiotakopoulos, L.; Rota, P.A.; Orenstein, W.A.; Patel, M. Measles in the 21st Century: Progress Toward Achieving and Sustaining Elimination. J. Infect. Dis. 2021, 224 (Suppl. 2), S420–S428. [Google Scholar] [CrossRef] [PubMed]
- Minta, A.A.; Ferrari, M.; Antoni, S.; Portnoy, A.; Sbarra, A.; Lambert, B.; Hatcher, C.; Hsu, C.H.; Ho, L.L.; Steulet, C.; et al. Progress Toward Measles Elimination—Worldwide, 2000–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.C.; Zimmerman, L.A.; Alexander, J.P., Jr.; Crowcroft, N.S.; O’Connor, P.M.; Knapp, J.K. Progress Toward Rubella and Congenital Rubella Syndrome Elimination—Worldwide, 2012–2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 162–167. [Google Scholar] [CrossRef]
- Vynnycky, E.; Knapp, J.K.; Papadopoulos, T.; Cutts, F.T.; Hachiya, M.; Miyano, S.; Reef, S.E. Estimates of the global burden of Congenital Rubella Syndrome, 1996–2019. Int. J. Infect. Dis. 2023, 137, 149–156. [Google Scholar] [CrossRef]
- Reef, S.E.; Icenogle, J.P.; Plotkin, S.A. The path to eradication of rubella. Vaccine 2023, 41, 7525–7531. [Google Scholar] [CrossRef]
- Otani, N.; Shima, M.; Ueda, T.; Nakajima, K.; Takesue, Y.; Yamamoto, T.; Okuno, T. Changes in the Epidemiology of Rubella: The Influence of Vaccine-Introducing Methods and COVID-19. Vaccines 2023, 11, 1358. [Google Scholar] [CrossRef]
- WHO. Global Measles and Rubella Strategic Plan: 2012–2020. Available online: http://apps.who.int/iris/bitstream/10665/44855/1/9789241503396_eng.pdf (accessed on 5 January 2024).
- Orenstein, W.A.; Cairns, L.; Hinman, A.; Nkowane, B.; Olive, J.M.; Reingold, A.L. Measles and Rubella Global Strategic Plan 2012-2020 midterm review report: Background and summary. Vaccine 2018, 36 (Suppl. 1), A35–A42. [Google Scholar] [CrossRef]
- MacDonald, N.; Mohsni, E.; Al-Mazrou, Y.; Kim Andrus, J.; Arora, N.; Elden, S.; Madrid, M.Y.; Martin, R.; Mahmoud Mustafa, A.; Rees, H.; et al. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine 2020, 38, 5364–5371. [Google Scholar] [CrossRef]
- WHO. National Immunization Coverage Scorecards Estimates for 2019. Available online: https://iris.who.int/bitstream/handle/10665/337665/9789240014398-eng.pdf (accessed on 5 January 2024).
- Guglielmi, G. Pandemic drives largest drop in childhood vaccinations in 30 years. Nature 2022, 608, 253. [Google Scholar] [CrossRef]
- WHO. Global Progress against Measles Threatened Amidst COVID-19 Pandemic. 2021. Available online: https://www.who.int/news/item/10-11-2021-global-progress-against-measles-threatened-amidst-covid-19-pandemic (accessed on 5 January 2024).
- WHO. Measles and Rubella Strategic Framework: 2021–2030. Available online: https://iris.who.int/bitstream/handle/10665/339801/9789240015616-eng.pdf?sequence=1 (accessed on 5 January 2024).
- WHO. Immunization Agenda 2030 (IA2030); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Lindstrand, A.; Cherian, T.; Chang-Blanc, D.; Feikin, D.; O’Brien, K.L. The World of Immunization: Achievements, Challenges, and Strategic Vision for the Next Decade. J. Infect. Dis. 2021, 224 (Suppl. 2), S452–S467. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Genetic diversity of wild-type measles viruses and the global measles nucleotide surveillance database (MeaNS). Wkly. Epidemiol. Rec. 2015, 90, 373–380. [Google Scholar]
- World Health Organization. Standardization of the nomenclature for genetic characteristics of wild-type rubella viruses. Wkly. Epidemiol. Rec. 2005, 80, 126–132. [Google Scholar]
- Arnold, B.F.; Scobie, H.M.; Priest, J.W.; Lammie, P.J. Integrated serologic surveillance of population immunity and disease transmission. Emerg. Infect. Dis. 2018, 24, 1188–1194. [Google Scholar] [CrossRef]
- El Mubarak, H.S.; Ibrahim, S.A.; Vos, H.W.; Mukhtar, M.M.; Mustafa, O.A.; Wild, T.F.; Osterhaus, A.D.; de Swart, R.L. Measles virus protein-specific IgM, IgA, and IgG subclass responses during the acute and convalescent phase of infection. J. Med. Virol. 2004, 72, 290–298. [Google Scholar] [CrossRef]
- Dimech, W.; Grangeot-Keros, L.; Vauloup-Fellous, C. Standardization of Assays That Detect Anti-Rubella Virus IgG Antibodies. Clin. Microbiol. Rev. 2016, 29, 163–174. [Google Scholar] [CrossRef]
- den Hartog, G.; van Binnendijk, R.; Buisman, A.M.; Berbers, G.A.M.; van der Klis, F.R.M. Immune surveillance for vaccine-preventable diseases. Expert. Rev. Vaccines 2020, 19, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Young, M.K. The indications and safety of polyvalent immunoglobulin for post-exposure prophylaxis of hepatitis A, rubella and measles. Hum. Vaccin. Immunother. 2019, 15, 2060–2065. [Google Scholar] [CrossRef]
- Maldonado, Y.A.; Lawrence, E.C.; DeHovitz, R.; Hartzell, H.; Albrecht, P. Early loss of passive measles antibody in infants of mothers with vaccine-induced immunity. Pediatrics 1995, 96, 447–450. [Google Scholar] [PubMed]
- Leuridan, E.; Van Damme, P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 2007, 25, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Waaijenborg, S.; Hahne, S.J.; Mollema, L.; Smits, G.P.; Berbers, G.A.; van der Klis, F.R.; de Melker, H.E.; Wallinga, J. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J. Infect. Dis. 2013, 208, 10–16. [Google Scholar] [CrossRef]
- Caceres, V.M.; Strebel, P.M.; Sutter, R.W. Factors determining prevalence of maternal antibody to measles virus throughout infancy: A review. Clin. Infect. Dis. 2000, 31, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Altinkaynak, S.; Ertekin, V.; Inandi, T. The duration of maternal measles antibodies in children. J. Trop. Pediatr. 2003, 49, 302–305. [Google Scholar] [CrossRef]
- Oyedele, O.O.; Odemuyiwa, S.O.; Ammerlaan, W.; Muller, C.P.; Adu, F.D. Passive immunity to measles in the breastmilk and cord blood of some nigerian subjects. J. Trop. Pediatr. 2005, 51, 45–48. [Google Scholar] [CrossRef]
- Shilpi, T.; Sattar, H.; Miah, M.R. Determining infants’ age for measles vaccination based on persistence of protective level of maternal measles antibody. Bangladesh Med. Res. Counc. Bull. 2009, 35, 101–104. [Google Scholar] [CrossRef]
- Devecioglu, E.; Gokcay, G.; Boran, P.; Eren, T.; Yilmaz, G.; Badur, S. Prevalence of maternal measles, rubella, mumps and varicella antibodies in the first six months of life. Mikrobiyoloji Bul. 2018, 52, 324–327. [Google Scholar] [CrossRef]
- Leuridan, E.; Sabbe, M.; Van Damme, P. Measles outbreak in Europe: Susceptibility of infants too young to be immunized. Vaccine 2012, 30, 5905–5913. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef] [PubMed]
- Cheedarla, N.; Hanna, L.E. Chapter 7—Functional and Protective Role of Neutralizing Antibodies (NAbs) against Viral Infections. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: San Diego, CA, USA, 2019; pp. 83–93. [Google Scholar] [CrossRef]
- Bolotin, S.; Hughes, S.L.; Gul, N.; Khan, S.; Rota, P.A.; Severini, A.; Hahne, S.; Tricco, A.; Moss, W.J.; Orenstein, W.; et al. What Is the Evidence to Support a Correlate of Protection for Measles? A Systematic Review. J. Infect. Dis. 2020, 221, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. The Immune Response in Measles: Virus Control, Clearance and Protective Immunity. Viruses 2016, 8, 282. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Kennedy, R.B.; Ovsyannikova, I.G.; Schaid, D.J.; Poland, G.A. Current perspectives in assessing humoral immunity after measles vaccination. Expert. Rev. Vaccines 2019, 18, 75–87. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.D.; de Swart, R.L. Evaluating measles vaccines: Can we assess cellular immunity? Expert Rev. Vaccines 2012, 11, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Tosh, P.K.; Kennedy, R.B.; Vierkant, R.A.; Jacobson, R.M.; Poland, G.A. Correlation between rubella antibody levels and cytokine measures of cell-mediated immunity. Viral Immunol. 2009, 22, 451–456. [Google Scholar] [CrossRef]
- Cutts, F.T.; Hanson, M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health 2016, 21, 1086–1098. [Google Scholar] [CrossRef]
- Patel, M.K.; Goodson, J.L.; Alexander, J.P., Jr.; Kretsinger, K.; Sodha, S.V.; Steulet, C.; Gacic-Dobo, M.; Rota, P.A.; McFarland, J.; Menning, L.; et al. Progress Toward Regional Measles Elimination—Worldwide, 2000–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1700–1705. [Google Scholar] [CrossRef]
- Shanmugasundaram, D.; Awasthi, S.; Dwibedi, B.; Geetha, S.; Jain, M.; Malik, S.; Patel, B.; Singh, H.; Tripathi, S.; Viswanathan, R.; et al. Burden of congenital rubella syndrome (CRS) in India based on data from cross-sectional serosurveys, 2017 and 2019-20. PLoS Negl. Trop. Dis. 2021, 15, e0009608. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Immunological Basis for Immunization Series, Module 7: Measles Update 2020. Available online: https://iris.who.int/bitstream/handle/10665/331533/9789241516655-eng.pdf?sequence=1 (accessed on 5 January 2024).
- Bouthry, E.; Furione, M.; Huzly, D.; Ogee-Nwankwo, A.; Hao, L.; Adebayo, A.; Icenogle, J.; Sarasini, A.; Revello, M.G.; Grangeot-Keros, L.; et al. Assessing Immunity to Rubella Virus: A Plea for Standardization of IgG (Immuno)assays. J. Clin. Microbiol. 2016, 54, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Dimech, W.; Panagiotopoulos, L.; Francis, B.; Laven, N.; Marler, J.; Dickeson, D.; Panayotou, T.; Wilson, K.; Wootten, R.; Dax, E.M. Evaluation of eight anti-rubella virus immunoglobulin g immunoassays that report results in international units per milliliter. J. Clin. Microbiol. 2008, 46, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Dimech, W.; Arachchi, N.; Cai, J.; Sahin, T.; Wilson, K. Investigation into low-level anti-rubella virus IgG results reported by commercial immunoassays. Clin. Vaccine Immunol. 2013, 20, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Enders, M.; Bartelt, U.; Knotek, F.; Bunn, K.; Strobel, S.; Dietz, K.; Enders, G. Performance of the Elecsys Rubella IgG assay in the diagnostic laboratory setting for assessment of immune status. Clin. Vaccine Immunol. 2013, 20, 420–426. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chapter 9: Manual for the Laboratory-Based Surveillance of Measles, Rubella, and Congenital Rubella Syndrome. 9 June 2018. Available online: https://www.who.int/publications/m/item/chapter-9-manual-for-the-laboratory-based-surveillance-of-measles-rubella-and-congenital-rubella-syndrome (accessed on 5 January 2024).
- Castineiras, A.C.P.; Sales, A.C.; Picone, C.M.; Diogo, C.L.; Rossi, A.D.; Galliez, R.M.; Ferreira, O.D.C., Jr.; Castineiras, T.; Lopes, M.H.; Sartori, A.M.C. The decline of measles antibody titers in previously vaccinated adults: A cross-sectional analysis. Rev. Inst. Med. Trop. Sao Paulo 2024, 66, e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Zhang, X.; Zhang, X.; Du, J.; Che, X.; Gu, W.; Wang, J.; Jiang, W.; Liu, Y. Do adolescents need a rubella vaccination campaign? Rubella serosurvey among healthy children in Hangzhou, China. Hum. Vaccines Immunother. 2023, 19, 2254536. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.S.; Hasan, A.Z.; Bolotin, S.; Crowcroft, N.S.; Cutts, F.T.; Joh, E.; Loisate, S.; Moss, W.J.; Osman, S.; Hayford, K. Comparison of measles IgG enzyme immunoassays (EIA) versus plaque reduction neutralization test (PRNT) for measuring measles serostatus: A systematic review of head-to-head analyses of measles IgG EIA and PRNT. BMC Infect. Dis. 2023, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.J.; Doblas, D.; Andrews, N. Comparison of plaque reduction neutralisation test (PRNT) and measles virus-specific IgG ELISA for assessing immunogenicity of measles vaccination. Vaccine 2008, 26, 6392–6397. [Google Scholar] [CrossRef]
- Tischer, A.; Gassner, M.; Richard, J.L.; Suter-Riniker, F.; Mankertz, A.; Heininger, U. Vaccinated students with negative enzyme immunoassay results show positive measles virus-specific antibody levels by immunofluorescence and plaque neutralisation tests. J. Clin. Virol. 2007, 38, 204–209. [Google Scholar] [CrossRef]
- Ratnam, S.; Gadag, V.; West, R.; Burris, J.; Oates, E.; Stead, F.; Bouilianne, N. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J. Clin. Microbiol. 1995, 33, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.D.; Pankratz, V.S.; Larrabee, B.R.; Ogee-Nwankwo, A.; Chen, M.H.; Icenogle, J.P.; Poland, G.A. High-throughput assay optimization and statistical interpolation of rubella-specific neutralizing antibody titers. Clin. Vaccine Immunol. 2014, 21, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Reslova, N.; Michna, V.; Kasny, M.; Mikel, P.; Kralik, P. xMAP Technology: Applications in Detection of Pathogens. Front. Microbiol. 2017, 8, 55. [Google Scholar] [CrossRef]
- Chan, Y.; Fornace, K.; Wu, L.; Arnold, B.F.; Priest, J.W.; Martin, D.L.; Chang, M.A.; Cook, J.; Stresman, G.; Drakeley, C. Determining seropositivity-A review of approaches to define population seroprevalence when using multiplex bead assays to assess burden of tropical diseases. PLoS Negl. Trop. Dis. 2021, 15, e0009457. [Google Scholar] [CrossRef]
- Smits, G.P.; van Gageldonk, P.G.; Schouls, L.M.; van der Klis, F.R.; Berbers, G.A. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin. Vaccine Immunol. 2012, 19, 396–400. [Google Scholar] [CrossRef]
- Coughlin, M.M.; Matson, Z.; Sowers, S.B.; Priest, J.W.; Smits, G.P.; van der Klis, F.R.M.; Mitchell, A.; Hickman, C.J.; Scobie, H.M.; Goodson, J.L.; et al. Development of a Measles and Rubella Multiplex Bead Serological Assay for Assessing Population Immunity. J. Clin. Microbiol. 2021, 59, e02716-20. [Google Scholar] [CrossRef] [PubMed]
- Dimech, W.; Mulders, M.N. A 16-year review of seroprevalence studies on measles and rubella. Vaccine 2016, 34, 4110–4118. [Google Scholar] [CrossRef]
- Viswanathan, R.; George, S.; Murhekar, M.V.; Abraham, A.M.; Singh, M.P.; Jadhav, S.M.; Nag, V.; Naik, S.; Raut, C.; Munivenkatappa, A.; et al. Comparison of two commercial ELISA kits for detection of rubella specific IgM in suspected congenital rubella syndrome cases and rubella IgG antibodies in a serosurvey of pregnant women. Diagn. Microbiol. Infect. Dis. 2019, 94, 243–247. [Google Scholar] [CrossRef]
- Ng, Y.; Chua, L.A.V.; Cui, L.; Ang, L.W.; Tee, N.W.S.; Lin, R.T.P.; Ma, S.; Lee, V.J.M. Seroprevalence of vaccine-preventable diseases among children and adolescents in Singapore: Results from the National Paediatric Seroprevalence Survey 2018. Int. J. Infect. Dis. 2020, 92, 234–240. [Google Scholar] [CrossRef]
- Cho, H.K.; Lee, H.; Kim, H.W.; Kim, S.S.; Kang, H.J.; Kim, I.T.; Kim, K.H. Seroprevalences of Specific IgG Antibodies to Measles, Mumps, and Rubella in Korean Infants. J. Korean Med. Sci. 2016, 31, 1957–1962. [Google Scholar] [CrossRef]
- Muthiah, N.; Galagoda, G.; Handunnetti, S.; Peiris, S.; Pathirana, S. Dynamics of maternally transferred antibodies against measles, mumps, and rubella in infants in Sri Lanka. Int. J. Infect. Dis. 2021, 107, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Khampanisong, P.; Pauly, M.; Nouanthong, P.; Vickers, M.A.; Virachith, S.; Xaydalasouk, K.; Black, A.P.; Muller, C.P.; Hubschen, J.M. Waning of maternal antibodies against measles suggests a large window of susceptibility in infants in lao people’s Democratic republic. Pathogens 2021, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Gioula, G.; Exindari, M.; Melidou, A.; Minti, F.; Sidiropoulou, E.; Dionisopoulou, S.; Kiriazi, M.; Tsintarakis, E.; Malisiovas, N. Seroprevalence of measles in Northern Greece. Acta Microbiol. Hell. 2017, 62, 145–150. [Google Scholar]
- Bassal, R.; Shohat, T.; Levin, T.; Pando, R.; Shinar, E.; Amichay, D.; Barak, M.; Ben-Dor, A.; Bar-Haim, A.; Mendelson, E.; et al. The Concordance between Mumps and Rubella Sero-Positivity among the Israeli Population in 2015. Vaccines 2022, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Carazo, S.; Billard, M.N.; Boutin, A.; De Serres, G. Effect of age at vaccination on the measles vaccine effectiveness and immunogenicity: Systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 251. [Google Scholar] [CrossRef] [PubMed]
- Kader, C.; Kara, M.; Gocmen, A.Y.; Erbay, A.; Polat, M.F. Antibodies against vaccine preventable diseases in pregnant women measles, mumps, rubella, varicella and tetanus in Yozgat, Turkey. Konuralp Tip. Derg. 2017, 9, 29–34. [Google Scholar] [CrossRef]
- Rasheed, M.A.U.; Hickman, C.J.; McGrew, M.; Sowers, S.B.; Mercader, S.; Hopkins, A.; Grimes, V.; Yu, T.; Wrammert, J.; Mulligan, M.J.; et al. Decreased humoral immunity to mumps in young adults immunized with MMR vaccine in childhood. Proc. Natl. Acad. Sci. USA 2019, 116, 19071–19076. [Google Scholar] [CrossRef]
- Yang, X.; Tang, T.; Yang, Z.; Liu, L.; Yuan, S.; Zhang, T. Evaluation of measles vaccination coverage in Lincang City, Yunnan Province, China. Hum. Vaccines Immunother. 2021, 17, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Freidl, G.S.; Tostmann, A.; Curvers, M.; Ruijs, W.L.M.; Smits, G.; Schepp, R.; Duizer, E.; Boland, G.; de Melker, H.; van der Klis, F.R.M.; et al. Immunity against measles, mumps, rubella, varicella, diphtheria, tetanus, polio, hepatitis A and hepatitis B among adult asylum seekers in the Netherlands, 2016. Vaccine 2018, 36, 1664–1672. [Google Scholar] [CrossRef]
- Adam, O.; Musa, A.; Kamer, A.; Sausy, A.; Tisserand, E.; Hubschen, J.M. Seroprevalence of measles, mumps, and rubella and genetic characterization of mumps virus in Khartoum, Sudan. Int. J. Infect. Dis. 2020, 91, 87–93. [Google Scholar] [CrossRef]
- Takemoto, K.; Nishimura, N.; Kozawa, K.; Hibino, H.; Kawaguchi, M.; Takeuchi, S.; Fujishiro, N.; Arai, S.; Gotoh, K.; Hosono, H.; et al. Time-Series Analysis Comparing the Prevalence of Antibodies against Nine Viral Species Found in Umbilical Cord Blood in Japan. Jpn. J. Infect. Dis. 2016, 69, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Vos, R.A.; Mollema, L.; van Binnendijk, R.; Veldhuijzen, I.K.; Smits, G.; Janga-Jansen, A.V.A.; Baboe-Kalpoe, S.; Hulshof, K.; van der Klis, F.R.M.; Melker, H.E. Seroepidemiology of Measles, Mumps and Rubella on Bonaire, St. Eustatius and Saba: The First Population-Based Serosurveillance Study in Caribbean Netherlands. Vaccines 2019, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Sato, T.A.; Katayama, A.; Higuchi, K.; Shichijo, K.; Tsuchiya, T.; Takayama, N.; Takeuchi, Y.; Abe, T.; Okabe, N.; et al. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch. Virol. 2001, 146, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Gadallah, M.; El Sayed, N.; Kandeel, A.; Moussa, I.; Mohsen, A.; Dewedar, S. Seroprevalence of rubella antibodies among adult Egyptian females aged 20–30 years. Is there a need for rubella vaccination? Cent. Eur. J. Public Health 2014, 22, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kader, C.; Erbay, A.; Akca, N.K.; Polat, M.F.; Polat, S. Immunity of nursing students to measles, mumps, rubella, and varicella in Yozgat, Turkey. Am. J. Infect. Control 2016, 44, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Zanella, B.; Boccalini, S.; Bonito, B.; Del Riccio, M.; Tiscione, E.; Bonanni, P.; Working Group DHS; Working Group AOUMeyer; Working Group AUSLTC; Bechini, A. Increasing Measles Seroprevalence in a Sample of Pediatric and Adolescent Population of Tuscany (Italy): A Vaccination Campaign Success. Vaccines 2020, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; Mokhtari-Azad, T.; Zahraei, S.M. Measles vaccination coverage and seroprevalence of anti-measles antibody in south-east Islamic Republic of Iran. East. Mediterr. Health J. 2015, 21, 396–402. [Google Scholar] [CrossRef]
- Estofolete, C.F.; Milhim, B.H.G.d.A.; Franca, C.C.G.d.; Silva, G.C.D.d.; Augusto, M.T.; Terzian, A.C.B.; Zini, N.; Durigon, E.L.; Oliveira, D.B.L.; Massad, E.; et al. Prevalence of measles antibodies in Sao Jose do Rio Preto, Sao Paulo, Brazil: A serological survey model. Sci. Rep. 2020, 10, 5179. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Akduman Alasehir, E. Seroepidemiology of hepatitis viruses, measles, mumps, rubella and varicella among healthcare workers and students: Should we screen before vaccination? J. Infect. Public Health 2020, 13, 480–484. [Google Scholar] [CrossRef]
- Odemis, I.; Kose, S.; Akbulut, I.; Albayrak, H. Seroprevalence of measles, mumps, rubella, and varicella zoster virus antibodies among healthcare students: Analysis of vaccine efficacy and cost-effectiveness. Rev. Esp. Quimioter. 2019, 32, 525–531. [Google Scholar]
- Chung, H.; Cho, S.K.; Joo, J.; Kim, S.K.; Kim, E.O.; Kim, M.J.; Chong, Y.P.; Choi, S.H.; Lee, S.O.; Kim, Y.S.; et al. Causes of a Low Measles Seroprevalence among Young Healthcare Workers in Korea. Infect. Chemother. 2023, 55, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Karantoni, H.; Petrikkos, P.; Georgota, P.; Katerelos, P.; Liona, A.; Tsagarakis, S.; Theodoridou, M.; Hatzigeorgiou, D. Vaccination coverage and immunity levels against vaccine-preventable diseases in male Air Force recruits in Greece. Vaccine 2020, 38, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.K.; Kwak, S.H.; Hong, M.J.; Kim, S.H. Seroprevalence of Measles in Healthcare Workers in South Korea. Infect. Chemother. 2019, 51, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Gorun, F.; Malita, D.; Ciohat, I.; Vilibic-Cavlek, T.; Feier, H.; Tabain, I.; Craina, M.; Cretu, O.; Navolan, D. Prevalence of Rubella Antibodies among Fertile Women in the West of Romania, 18 Years after the Implementation of Immunization. Vaccines 2021, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Holka, J.; Pawlak, K.; Ciepiela, O. Seroprevalence of IgG antibodies against measles in a selected Polish population—Do we need to be re-vaccinated? Cent. Eur. J. Immunol. 2019, 44, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Wanlapakorn, N.; Wasitthankasem, R.; Vichaiwattana, P.; Auphimai, C.; Yoocharoen, P.; Vongpunsawad, S.; Poovorawan, Y. Antibodies against measles and rubella virus among different age groups in Thailand: A population-based serological survey. PLoS ONE 2019, 14, e0225606. [Google Scholar] [CrossRef] [PubMed]
- Plans, P.; de Ory, F.; Campins, M.; Alvarez, E.; Paya, T.; Guisasola, E.; Compte, C.; Vellbe, K.; Sanchez, C.; Lozano, M.J.; et al. Prevalence of anti-rubella, anti-measles and anti-mumps IgG antibodies in neonates and pregnant women in Catalonia (Spain) in 2013: Susceptibility to measles increased from 2003 to 2013. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Mahallawi, W.H. Rubella Humoral Immunity Among the Saudi Population of Madinah in the Western Region of Saudi Arabia. Viral Immunol. 2022, 35, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Borocz, K.; Csizmadia, Z.; Markovics, A.; Farkas, N.; Najbauer, J.; Berki, T.; Nemeth, P. Application of a fast and cost-effective ‘three-in-one’ MMR ELISA as a tool for surveying anti-MMR humoral immunity: The Hungarian experience. Epidemiol. Infect. 2020, 148, e17. [Google Scholar] [CrossRef]
- Chua, Y.X.; Ang, L.W.; Low, C.; James, L.; Cutter, J.L.; Goh, K.T. An epidemiological assessment towards elimination of rubella and congenital rubella syndrome in Singapore. Vaccine 2015, 33, 3150–3157. [Google Scholar] [CrossRef]
- Nogareda, F.; Gunregjav, N.; Sarankhuu, A.; Munkhbat, E.; Ichinnorov, E.; Nymadawa, P.; Wannemuehler, K.; Mulders, M.N.; Hagan, J.; Patel, M.K. Measles and rubella IgG seroprevalence in persons 6 month-35 years of age, Mongolia, 2016. Vaccine 2020, 38, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Coppeta, L.; Biondi, G.; Lieto, P.; Pietroiusti, A. Evaluation of immunity to measles in a cohort of Medical Students in Rome, Italy. Vaccines 2019, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Zahraei, S.M.; Mokhtari-Azad, T.; Izadi, S.; Mohammadi, M.; Sabouri, A. Seroprevalence of anti-rubella and anti-measles antibodies in women at the verge of marriage in Iran. Vaccine 2020, 38, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Murhekar, M.V.; Gupta, N.; Hasan, A.Z.; Kumar, M.S.; Kumar, V.S.; Prosperi, C.; Sapkal, G.N.; Thangaraj, J.W.V.; Kaduskar, O.; Bhatt, V.; et al. Evaluating the effect of measles and rubella mass vaccination campaigns on seroprevalence in India: A before-and-after cross-sectional household serosurvey in four districts, 2018–2020. Lancet Glob. Health 2022, 10, e1655–e1664. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, M.; Miyano, S.; Mori, Y.; Vynnycky, E.; Keungsaneth, P.; Vongphrachanh, P.; Xeuatvongsa, A.; Sisouk, T.; Som-Oulay, V.; Khamphaphongphane, B.; et al. Evaluation of nationwide supplementary immunization in Lao People’s Democratic Republic: Population-based seroprevalence survey of anti-measles and anti-rubella IgG in children and adults, mathematical modelling and a stability testing of the vaccine. PLoS ONE 2018, 13, e0194931. [Google Scholar] [CrossRef]
- Carcelen, A.C.; Mutembo, S.; Matakala, K.H.; Chilumba, I.; Mulundu, G.; Monze, M.; Mwansa, F.D.; Moss, W.J.; Hayford, K. Impact of a Measles and Rubella Vaccination Campaign on Seroprevalence in Southern Province, Zambia. Am. J. Trop. Med. Hyg. 2021, 104, 2229–2232. [Google Scholar] [CrossRef]
- Miyano, S.; Vynnycky, E.; Pattamavone, C.; Ichimura, Y.; Mori, Y.; Nouanthong, P.; Phounphenghack, K.; Tengbriacheu, C.; Khamphaphongphane, B.; Franzel, L.; et al. Comparison of population-based measles-rubella immunoglobulin G antibody prevalence between 2014 and 2019 in Lao People’s Democratic Republic: Impacts of the national immunization program. Int. J. Infect. Dis. 2023, 129, 70–77. [Google Scholar] [CrossRef]
- Ichimura, Y.; Yamauchi, M.; Yoshida, N.; Miyano, S.; Komada, K.; Thandar, M.M.; Tiwara, S.; Mita, T.; Hombhanje, F.W.; Mori, Y.; et al. Effectiveness of immunization activities on measles and rubella immunity among individuals in East Sepik, Papua New Guinea: A cross-sectional study. IJID Reg. 2022, 3, 84–88. [Google Scholar] [CrossRef]
- Hayford, K.; Mutembo, S.; Carcelen, A.; Matakala, H.K.; Munachoonga, P.; Winter, A.; Wanyiri, J.W.; Searle, K.; Mwansa, F.D.; Mwiche, A.; et al. Measles and rubella serosurvey identifies rubella immunity gap in young adults of childbearing age in Zambia: The added value of nesting a serological survey within a post-campaign coverage evaluation survey. Vaccine 2019, 37, 2387–2393. [Google Scholar] [CrossRef]
- Cohen, B.J.; Audet, S.; Andrews, N.; Beeler, J.; on behalf of the WHO Working Group on Measles Plaque Reduction Neutralization Test. Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 2007, 26, 59–66. [Google Scholar] [CrossRef]
- Anichini, G.; Gandolfo, C.; Fabrizi, S.; Miceli, G.B.; Terrosi, C.; Gori Savellini, G.; Prathyumnan, S.; Orsi, D.; Battista, G.; Cusi, M.G. Seroprevalence to Measles Virus after Vaccination or Natural Infection in an Adult Population, in Italy. Vaccines 2020, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, S.; Osman, S.; Hughes, S.L.; Ariyarajah, A.; Tricco, A.C.; Khan, S.; Li, L.; Johnson, C.; Friedman, L.; Gul, N.; et al. In Elimination Settings, Measles Antibodies Wane After Vaccination but Not After Infection: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2022, 226, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- van Gageldonk, P.G.; van Schaijk, F.G.; van der Klis, F.R.; Berbers, G.A. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 2008, 335, 79–89. [Google Scholar] [CrossRef]
- Arnold, B.F.; Martin, D.L.; Juma, J.; Mkocha, H.; Ochieng, J.B.; Cooley, G.M.; Omore, R.; Goodhew, E.B.; Morris, J.F.; Costantini, V.; et al. Enteropathogen antibody dynamics and force of infection among children in low-resource settings. eLife 2019, 8, e45594. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; (Centers for Disease Control and Prevention, Atlanta, GA, USA). Personal communication, 2024.
- Pan American Health Organization. Multiplex Bead Assay for Integrated Serological Surveillance of Communicable Diseases in the Region of the Americas. Report of the Third Regional Meeting (Cuernavaca, 4–5 March 2020); Pan American Health Organization: Washington, DC, USA, 2020. [Google Scholar]
- Dimech, W.; Mulders, M.N. A review of testing used in seroprevalence studies on measles and rubella. Vaccine 2016, 34, 4119–4122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).