The Immunomodulatory Function of Assembled Composite Nanopolypeptide Containing Bursal-Derived BP7 (CNPB7) in Promoting the Mucosal Immune Response within Poultry Immunization
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide, and Cells
2.2. MTT Assay
2.3. Preparation and Optimization of Composite Nanoparticle CNPB7
2.4. Nanoparticle Size and Potential Determination of Nanoparticle CNPB7
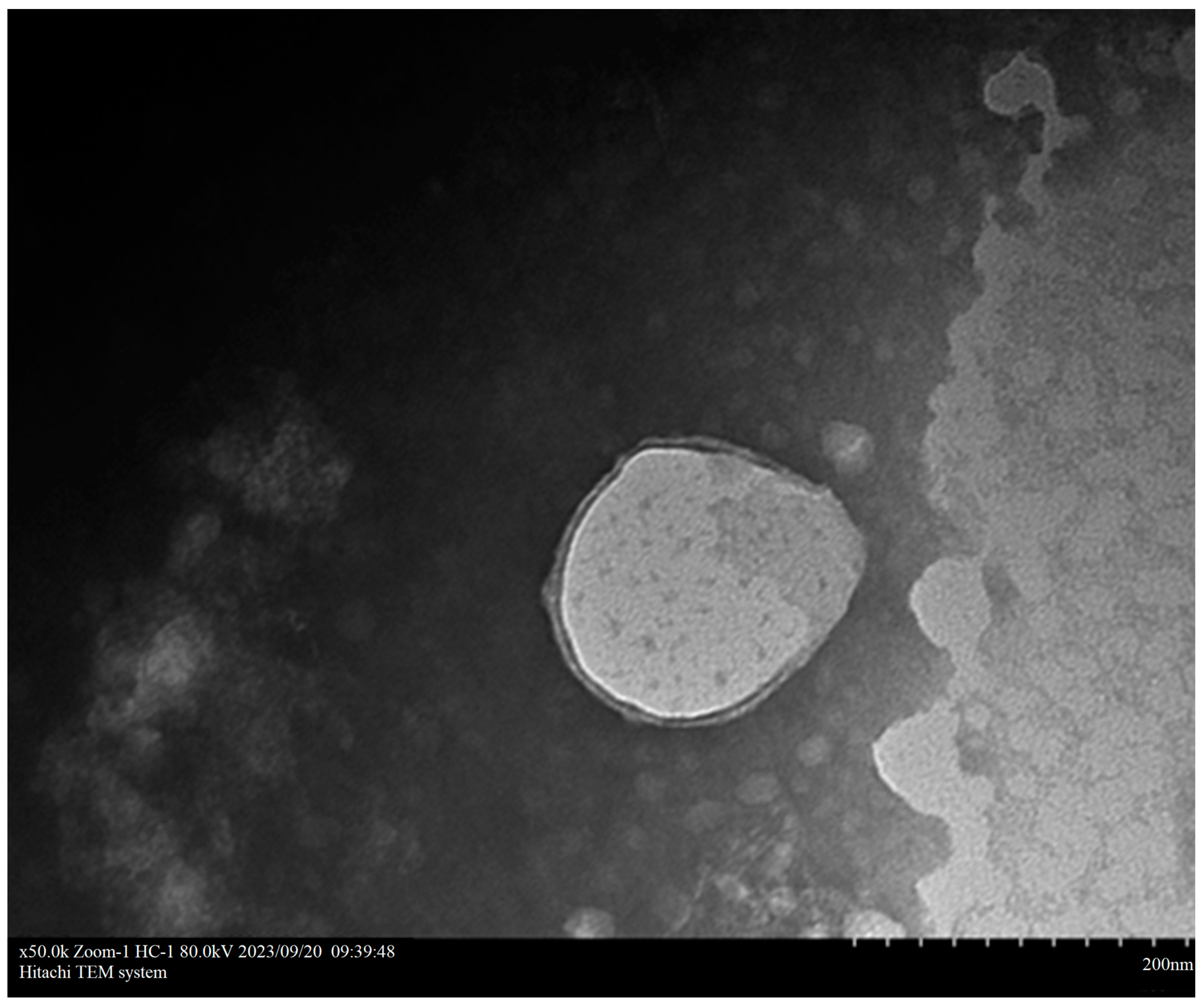
2.5. Transmission Electron Microscopy (TEM) of Nanoparticle CNPB7
2.6. Cell Counting Kit-8 (CCK8) Assay
2.7. The Nonspecific Innate Immune of Chicken Immunized with Nonopartical CNPB7
2.7.1. Chicken Immunization Only with Nanoparticle CNPB7
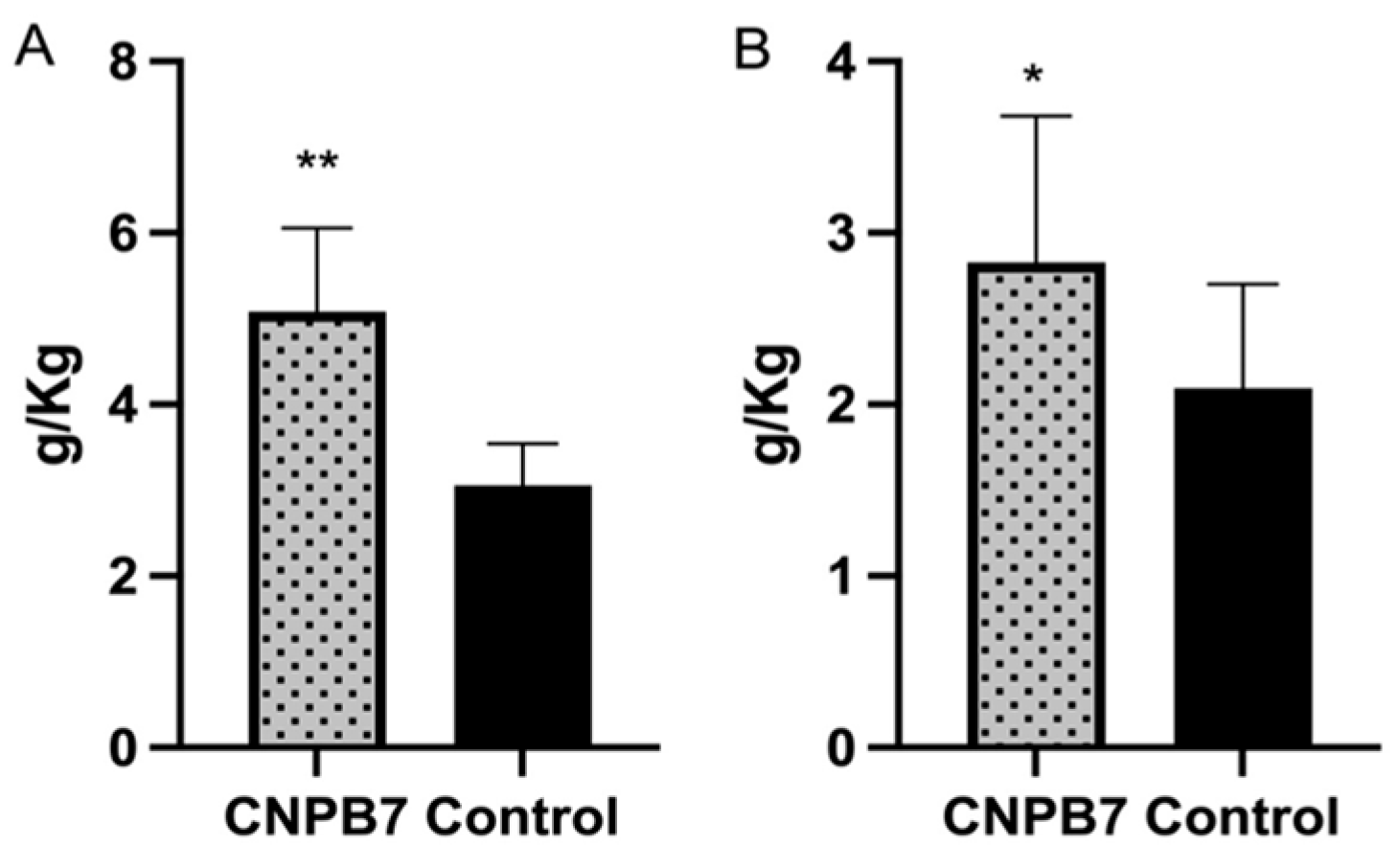
2.7.2. Immune Organ Index Detection of the Chicken Immunized with Nanoparticle CNPB7
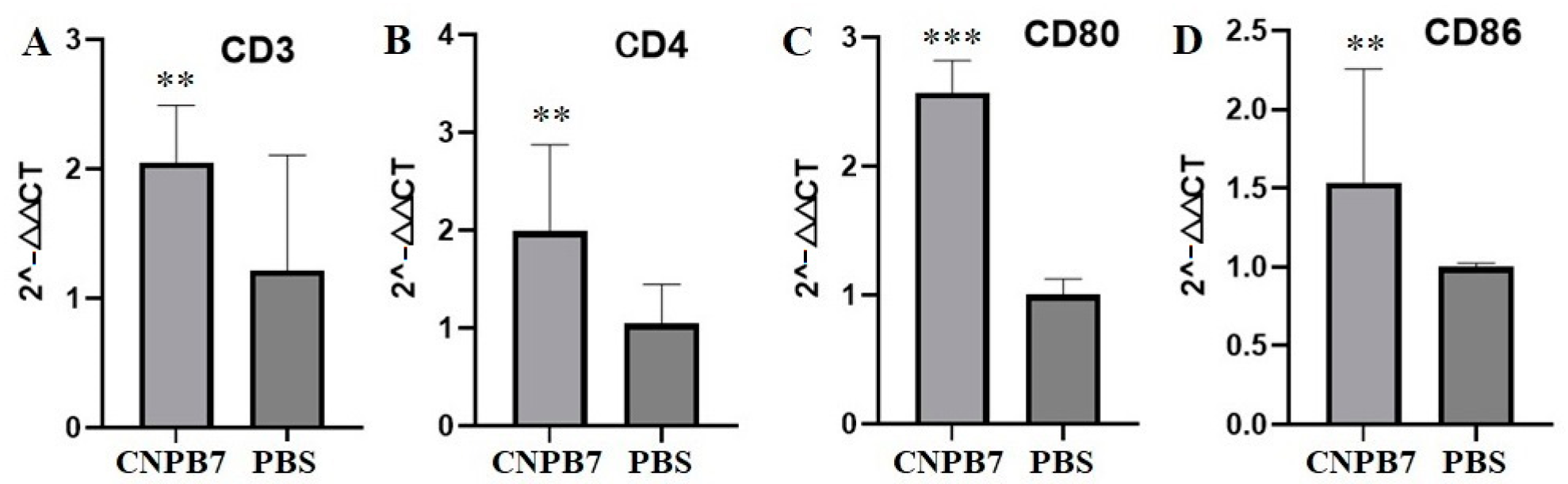
2.7.3. Immune Factors Detection of the Chicken Immunized with Nanoparticle CNPB7
2.8. The Specific Innate Immune of Chicken Immunized with Nanopartical CNPB7 and NDV-IBV Dual Vaccine
2.8.1. Vaccines Immunization Groups
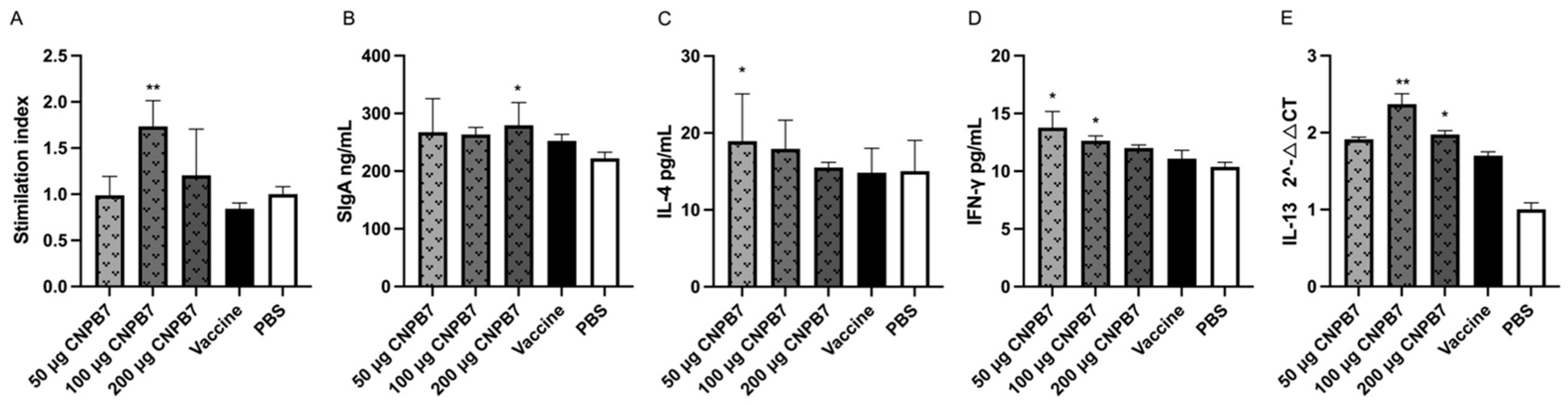
2.8.2. The Viabilities of Spleen Lymphocytes from the Immunized Chicken
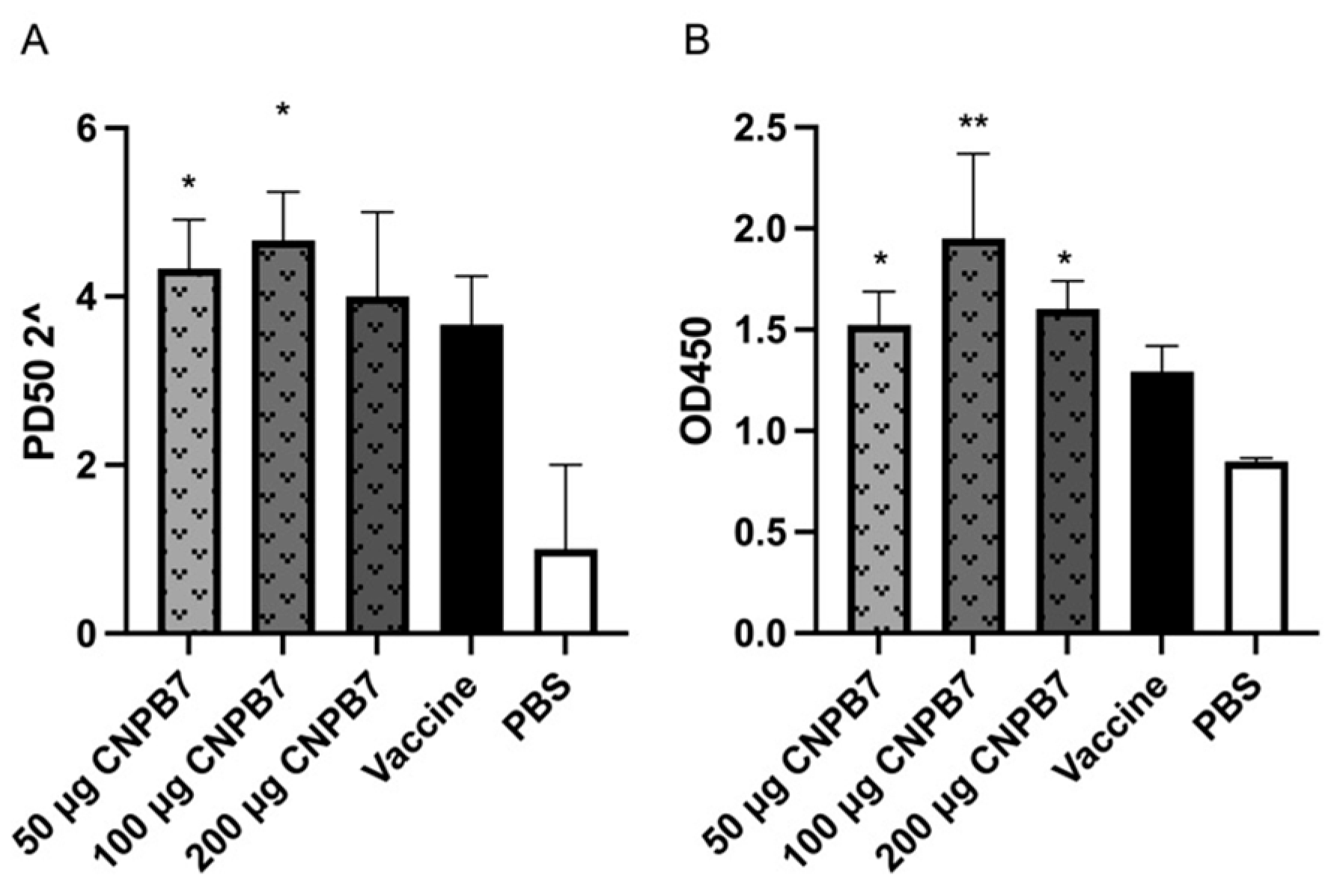
2.8.3. ELISA Detection for sIgA, Cytokines, and IBV Antibody Productions
2.8.4. Fluorescence Quantitative PCR for IL-13
2.8.5. Virus Neutralization Test for NDV Antibody
2.9. Data Analysis
3. Results
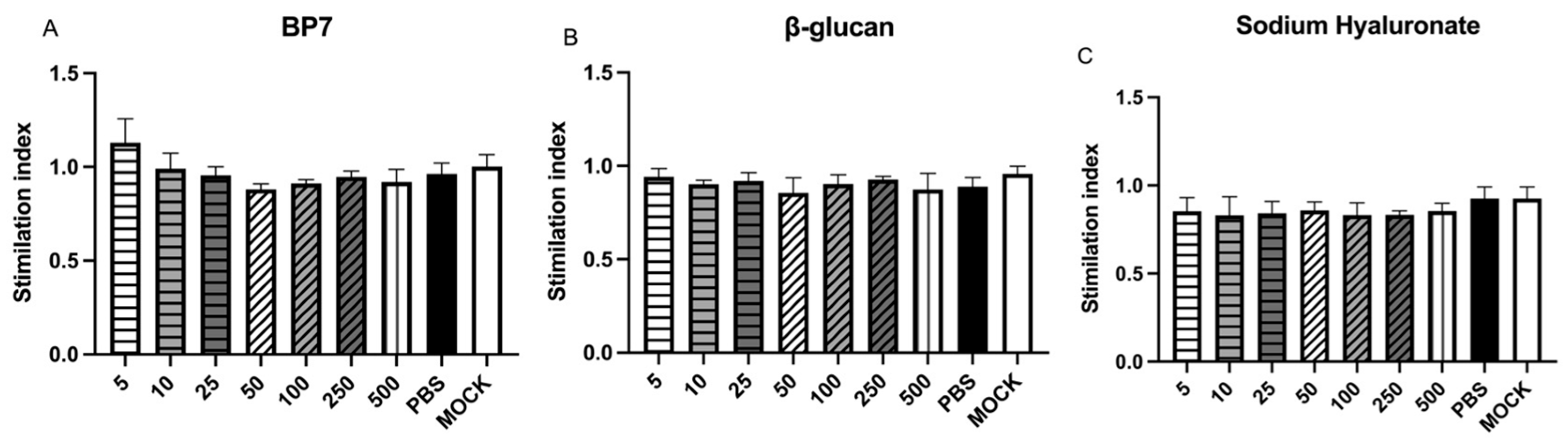
3.1. No Cytotoxicity of BP7, β-Glucan, and Hyaluronic Acid
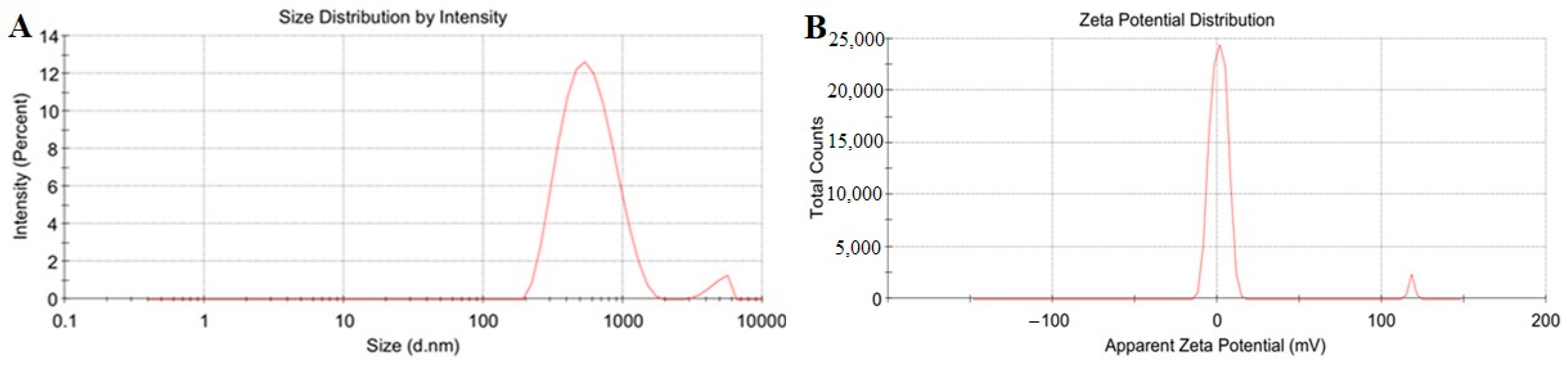
3.2. Determination and Characterization of Optimal Nanoparticles
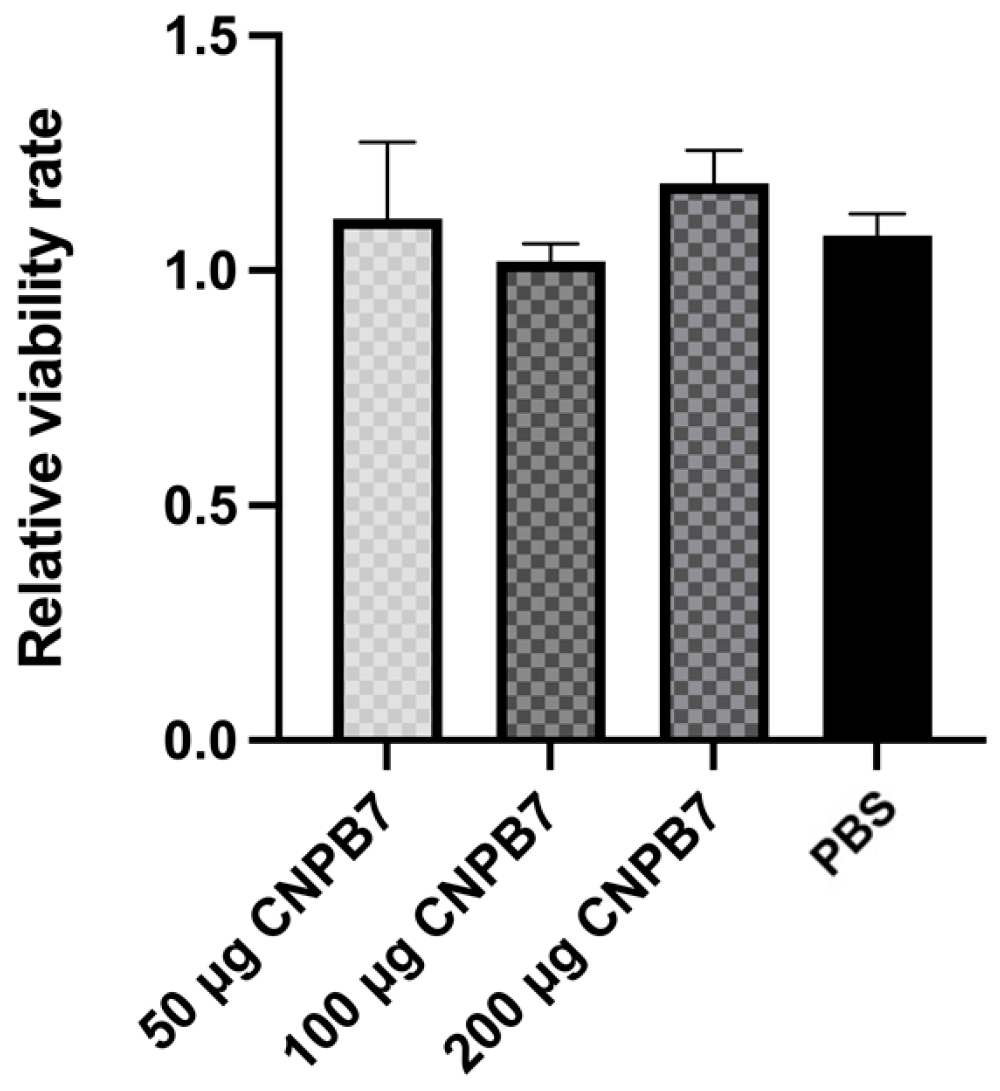
3.3. Nanoparticle CNPB7 Had No Cytotoxicity on DF-1 Cells
3.4. Nanoparticle CNPB7 Promoted Non-Specific Immune Response in Chickens
3.5. Nanoparticle CNPB7 Induced Various Biological Factor Expressions during Vaccination
3.6. Nanoparticle CNPB7 Induced Specific Antibody Production during Vaccination
4. Discussion
4.1. Characterization of Nanoparticle CNPB7
4.2. The Effect of Nanoparticle CNPB7 on Non-Specific Immune Function in Chickens
4.3. Evaluation of the Immune Efficacy of Nanoparticle CNPB7 during NDV-IBV Vaccination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine Adjuvants: Mechanisms and Platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-Age Vaccine Adjuvants, Their Development, and Future Perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.Z.; Miller, P.J. Newcastle Disease Vaccines—A Solved Problem or a Continuous Challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef]
- Falchieri, M.; Coward, V.J.; Reid, S.M.; Lewis, T.; Banyard, A.C. Infectious Bronchitis Virus: An Overview of the “Chicken Coronavirus”. J. Med. Microbiol. 2024, 73, 001828. [Google Scholar] [CrossRef] [PubMed]
- Dotiwala, F.; Upadhyay, A.K. Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens. Vaccines 2023, 11, 1585. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal Vaccines—Fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Ren, H.; Jia, W.; Xie, Y.; Yu, M.; Chen, Y. Adjuvant Physiochemistry and Advanced Nanotechnology for Vaccine Development. Chem. Soc. Rev. 2023, 52, 5172–5254. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.L.; Zheng, Y.; Zong, M.M.; Hao, S.S.; Zhou, G.F.; Cao, R.B.; Chen, P.Y.; Liu, Q.T. The Immunomodulatory Functions and Molecular Mechanism of a New Bursal Heptapeptide (BP7) in Immune Responses and Immature B Cells. Vet. Res. 2019, 50, 64. [Google Scholar] [CrossRef]
- Dong, X.; Bie, J.; Liu, X. Research Note: Isolation and Immunomodulatory Activity of Bursal Peptide, a Novel Peptide from Avian Immune System Developments. Poult. Sci. 2024, 103, 103–294. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Z.; Li, C.; Hao, S.; Lu, A.; Huang, X.; Feng, X. Bursal-Derived BP7 Induces the miRNA Molecular Basis of Chicken Macrophages and Promotes the Differentiation of B Cells. Vaccines 2022, 10, 1960. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785. [Google Scholar] [CrossRef]
- Huang, G.L.; Huang, H.L. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2023, 25, 766–772. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Moni, S.S.; Abdelwahab, S.I.; Jabeen, A.; Elmobark, M.E.; Aqaili, D.; Ghoal, G.; Oraibi, B.; Farasani, A.M.; Jerah, A.A.; Alnajai, M.M.A.; et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines 2023, 11, 1704. [Google Scholar] [CrossRef]
- Savelkoul, H.F.; Ferro, V.A.; Strioga, M.M.; Schijns, V.E. Choice and Design of Adjuvants for Parenteral and Mucosal Vaccines. Vaccines 2015, 3, 148–171. [Google Scholar] [CrossRef] [PubMed]
- Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef]
- Colaço, M.; Costa, J.P.; Borges, O. Glucan Particles: Choosing the Appropriate Size to Use as a Vaccine Adjuvant. Methods Mol. Biol. 2022, 24, 269–280. [Google Scholar]
- Bowerman, C.J.; Nilsson, B.L. A Reductive Trigger for Peptide Self-Assembly and Hydrogelation. J. Am. Chem. Soc. 2010, 132, 9526–9527. [Google Scholar] [CrossRef]
- Silvestre, F.; Santos, C.; Silva, V.; Ombredane, A.; Pinheiro, W.; Andrade, L.; Garcia, M.; Pacheco, T.; Joanitti, G.; Luz, G.; et al. Pharmacokinetics of Curcumin Delivered by Nanoparticles and the Relationship with Antitumor Efficacy: A Systematic Review. Pharmaceuticals 2023, 16, 943. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Shimogawa, R.; Liu, Y.; Zhang, L.; Foucher, A.C.; Routh, P.K.; Stach, E.A.; Frenkel, A.I.; Knecht, M.R. Biomimetic Control over Bimetallic Nanoparticle Structure and Activity via Peptide Capping Ligand Sequence. ACS Nano 2024, 18, 3286–3294. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, W.; Li, Q.; Wang, K.; Wang, Y.; Lv, F.; Chen, X.; Peng, X.; Wang, Y.; Li, J.; et al. Effects of Caulis Spatholobi Polysaccharide on Immunity, Intestinal Mucosal Barrier Function, and Intestinal Microbiota in Cyclophosphamide-Induced Immunosuppressive Chickens. Front. Vet. Sci. 2022, 9, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Andoh, K.; Suenaga, K.; Sakaguchi, M.; Yamazaki, K.; Honda, T. Decreased Neutralizing Antigenicity in IBV S1 Protein Expressed from Mammalian Cells. Virus Res. 2015, 208, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.V.; Canlon, B.; Cederroth, C.R. High-Quality RNA Extraction of the Mammalian Cochlea for qRT-PCR and Transcriptome Analyses. Hear. Res. 2015, 325, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chumbe, A.; Izquierdo-Lara, R.; Calderón, K.; Fernández-Díaz, M.; Vakharia, V.N. Development of a Novel Newcastle Disease Virus (NDV) Neutralization Test Based on Recombinant NDV Expressing Enhanced Green Fluorescent Protein. Virol. J. 2017, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J.; Yang, J.Y. Peptide-Directed Self-Assembly of Hydrogels. Acta Biomater. 2009, 5, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Radu, L.C.; Yang, J.; Kopeček, J. Self-Assembling Diblock Copolymers of Poly[N-(2-Hydroxypropyl)Methac yramide] and a β-Sheet Peptide. Macromol. Biosci. 2009, 9, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle Size and Surface Charge Affect Particle Uptake by Human Dendritic Cells in an In Vitro Model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Nature 2010, 468, 933–943. [Google Scholar] [CrossRef]
- Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Nonatelli, F.; Villanueva, H.; Barainka, M.; Zheleva, A.; van Santen, H.M.; Pastor, F. Modulating T Cell Responses by Targeting CD3. Cancers 2023, 15, 1189. [Google Scholar] [CrossRef]
- Ngoenkam, J.; Schamel, W.W.; Pongcharoen, S. Selected Signalling Proteins Recruited to the T-Cell Receptor-CD3 Complex. Immunology 2018, 153, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Steier, Z.; Kim, E.J.Y.; Aylard, D.A.; Robey, E.A. The CD4 Versus CD8 T Cell Fate Decision: A Multiomics-Informed Perspective. Annu. Rev. Immunol. 2024, 42, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Künzli, M.; Masopust, D. CD4(+) T Cell Memory. Nat. Immunol. 2023, 24, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Waters, E.; Rowshanravan, B.; Hinze, C.; Williams, C.; Janman, D.; Fox, T.A.; Booth, C.; Pesenacker, A.M.; Halliday, N.; et al. Differences in CD80 and CD86 Transendocytosis Reveal CD86 as a Key Target for CTLA-4 Immune Regulation. Nat. Immunol. 2022, 23, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Trzupek, D.; Dunstan, M.; Cutler, A.J.; Lee, M.; Godfrey, L.; Jarvis, L.; Rainbow, D.B.; Aschenbrenner, D.; Jones, J.L.; Uhlig, H.H.; et al. Discovery of CD80 and CD86 as Recent Activation Markers on Regulatory T Cells by Protein-RNA Single-Cell Analysis. Genome Med. 2020, 12, 55. [Google Scholar] [CrossRef]
- La Gruta, N.L.; Gras, S.; Daley, S.R.; Thomas, P.G.; Rossjohn, J. Understanding the Drivers of MHC Restriction of T Cell Receptors. Nat. Rev. Immunol. 2018, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Corse, E.; Gottschalk, R.A.; Allison, J.P. Strength of TCR-Peptide/MHC Interactions and In Vivo T Cell Responses. J. Immunol. 2011, 186, 5039–5045. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, A.D.; Nussenzweig, M.C. Immunology: Fifty Years of B Lymphocytes. Nature 2015, 517, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Yang, H.X.; He, J.; Yan, B.C.; Feng, T.; Liu, J.K. Steccherins A-D, Chamigrane-Type Sesquiterpenes from the Fungus Steccherinum Ochraceum with Selective Inhibition on B Lymphocyte Proliferation. Phytochemistry 2023, 10, 214. [Google Scholar] [CrossRef]
- Lopes, P.D.; Okino, C.H.; Fernando, F.S. Inactivated Infectious Bronchitis Virus Vaccine Encapsulated in Chitosan Nanoparticles Induces Mucosal Immune Responses and Effective Protection against Challenge. Vaccine 2018, 36, 2630–2636. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, Y.W.; Chen, G. Biological Evaluation of N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as a Carrier for the Delivery of Live Newcastle Disease Vaccine. Carbohydr. Polym. 2016, 149, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A. The Regulation of IgA Class Switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, C.; Buela, K.A.; Pizarro, T.T. Cytokine-Mediated Regulation of Innate Lymphoid Cell Plasticity in Gut Mucosal Immunity. Front. Immunol. 2020, 11, 585319. [Google Scholar] [CrossRef]
- Kubo, M. The Role of IL-4 Derived from Follicular Helper T (TFH) Cells and Type 2 Helper T (TH2) Cells. Int. Immunol. 2021, 33, 717–722. [Google Scholar] [CrossRef]
- Wynn, T.A. IL-13 Effector Functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef]
- Burke, J.D.; Young, H.A. IFN-γ: A Cytokine at the Right Time, Is in the Right Place. Semin. Immunol. 2019, 43, 101–280. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-γ Signaling and Response: The Barricade to Tumor Immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Golenbock, D.T. Beyond empiricism: Informing vaccine development through innate immunity research. Cell 2012, 148, 1284–1292. [Google Scholar] [CrossRef]
- Tam, E.H.; Peng, Y.; Cheah, M.X.Y.; Yan, C.; Xiao, T. Neutralizing Antibodies to Block Viral Entry and for Identification of Entry Inhibitors. Antivir. Res. 2024, 224, 105834. [Google Scholar] [CrossRef]
- Abela, I.A.; Kadelka, C.; Trkola, A. Correlates of Broadly Neutralizing Antibody Development. Curr. Opin. HIV AIDS 2019, 14, 279–285. [Google Scholar] [CrossRef]
- Ossendorp, F.; Ho, N.I.; Van Montfoort, N. How B Cells Drive T-Cell Responses: A Key Role for Cross-Presentation of Antibody-Targeted Antigens. Adv. Immunol. 2023, 160, 37–57. [Google Scholar] [PubMed]
| Primers | Sequences 5′-3′ |
|---|---|
| CD3-F | GGACGCTCCCACCATATCAG |
| CD3-R | AAGCTCGTGACATGAGTCCC |
| CD4-F | TGTGGAACTGTCACCTCGTG |
| CD4-R | CACATGCATGCAAGGCCAAT |
| CD80-F | TGTGACCCTCTTTGTCACCG |
| CD80-R | GGAATCCACGGATTTCGGGT |
| CD86-F | ACCAGCAAGCTGAATATCCCA |
| CD86-R | GACTAGCGGCACTGAGACAA |
| IL-13-F | GCTGGACAACATGACCGACTG |
| IL-13-R | GCAAGAAGTTCCGCAGGTAGATC |
| β-actin-F | GAGAAATTGT GCGTGACATCA |
| β-actin-R | CCTGAACCTCTCATTGCCA |
| Procedure | Conditions |
|---|---|
| Pre denaturation | 95 °C, 30 S |
| Cycle condition | 95 °C 5 S, 60 °C 20 S, 40 cycles |
| Melt curve | 95 °C 15 S, 60 °C 60 S, 95 °C 0 S |
| NAOH (%) | Sodium Tripolyphosphat (%) | Tween 20 (%) | Tween 80 (%) | Magnetic Stirring Time (h) | Particle Size (nm) | PDI | ZP (mV) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 0 | 2 | 952.9 | 0.825 | −13.3 |
| 1 | 0 | 1 | 0 | 4 | 643.9 | 0.608 | −14.4 |
| 0 | 1 | 0 | 1 | 2 | 643.2 | 0.547 | −19.2 |
| 0 | 1 | 2 | 0 | 4 | 545.4 | 0.488 | −16.9 |
| 0 | 1 | 0 | 1 | 4 | 514.9 | 0.298 | −26.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Hu, J.; Yin, G.; Cai, Y.; Gao, Z.; Liu, Y.; Zhong, M.; Wang, R.; Feng, X. The Immunomodulatory Function of Assembled Composite Nanopolypeptide Containing Bursal-Derived BP7 (CNPB7) in Promoting the Mucosal Immune Response within Poultry Immunization. Vaccines 2024, 12, 834. https://doi.org/10.3390/vaccines12080834
Guo X, Hu J, Yin G, Cai Y, Gao Z, Liu Y, Zhong M, Wang R, Feng X. The Immunomodulatory Function of Assembled Composite Nanopolypeptide Containing Bursal-Derived BP7 (CNPB7) in Promoting the Mucosal Immune Response within Poultry Immunization. Vaccines. 2024; 12(8):834. https://doi.org/10.3390/vaccines12080834
Chicago/Turabian StyleGuo, Xinyu, Jianing Hu, Guihu Yin, Yiqin Cai, Zichen Gao, Ye Liu, Meng Zhong, Ruiying Wang, and Xiuli Feng. 2024. "The Immunomodulatory Function of Assembled Composite Nanopolypeptide Containing Bursal-Derived BP7 (CNPB7) in Promoting the Mucosal Immune Response within Poultry Immunization" Vaccines 12, no. 8: 834. https://doi.org/10.3390/vaccines12080834
APA StyleGuo, X., Hu, J., Yin, G., Cai, Y., Gao, Z., Liu, Y., Zhong, M., Wang, R., & Feng, X. (2024). The Immunomodulatory Function of Assembled Composite Nanopolypeptide Containing Bursal-Derived BP7 (CNPB7) in Promoting the Mucosal Immune Response within Poultry Immunization. Vaccines, 12(8), 834. https://doi.org/10.3390/vaccines12080834







