Effectively Evaluating a Novel Consensus Subunit Vaccine Candidate to Prevent the H9N2 Avian Influenza Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Generation of HA Gene-Based Recombinant Protein
2.3. Production of the rHA Vaccine Candidate
2.4. Chicken Vaccination Experiments and Viral Challenge Study
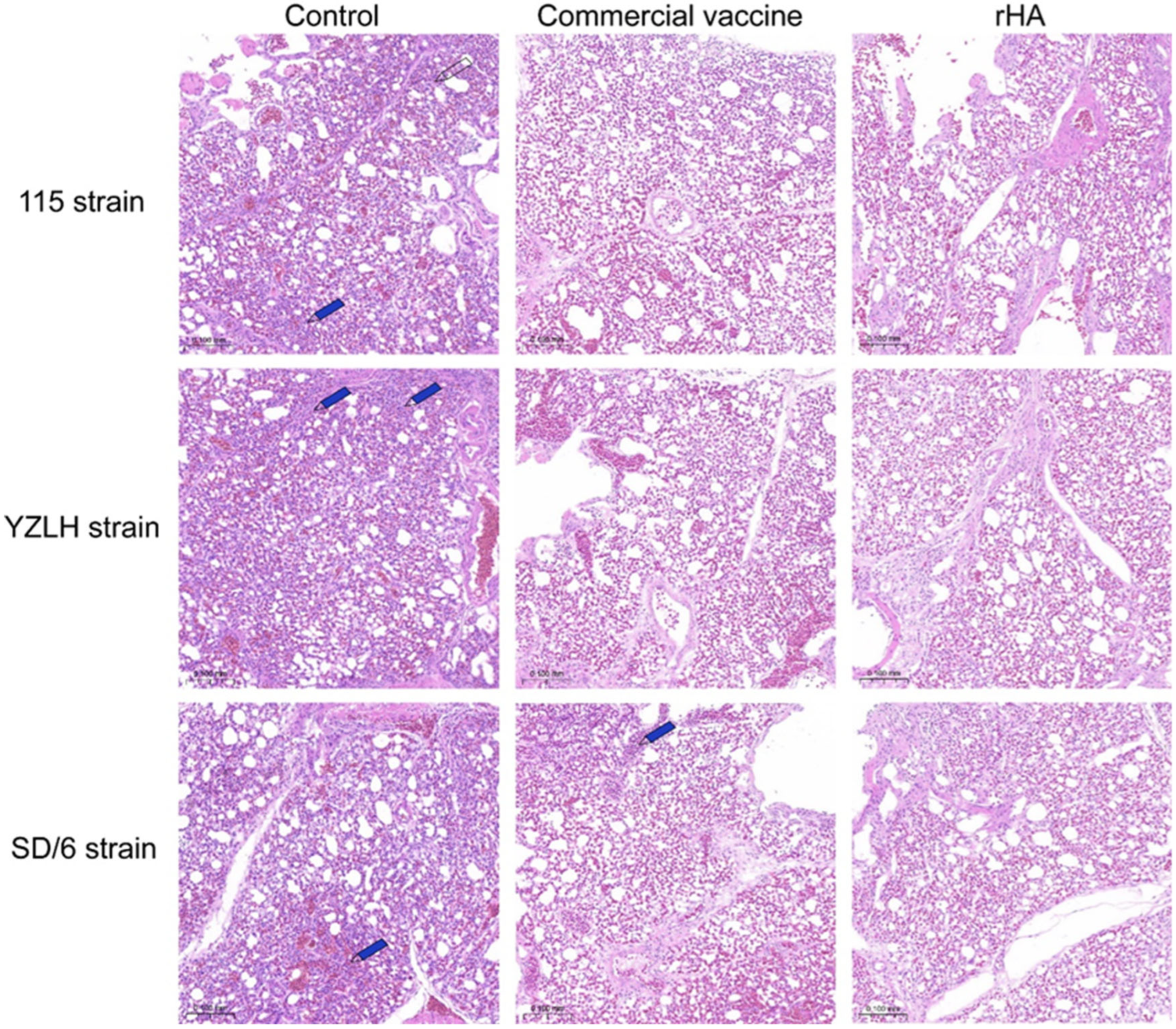
2.5. Body Weights and Histopathology
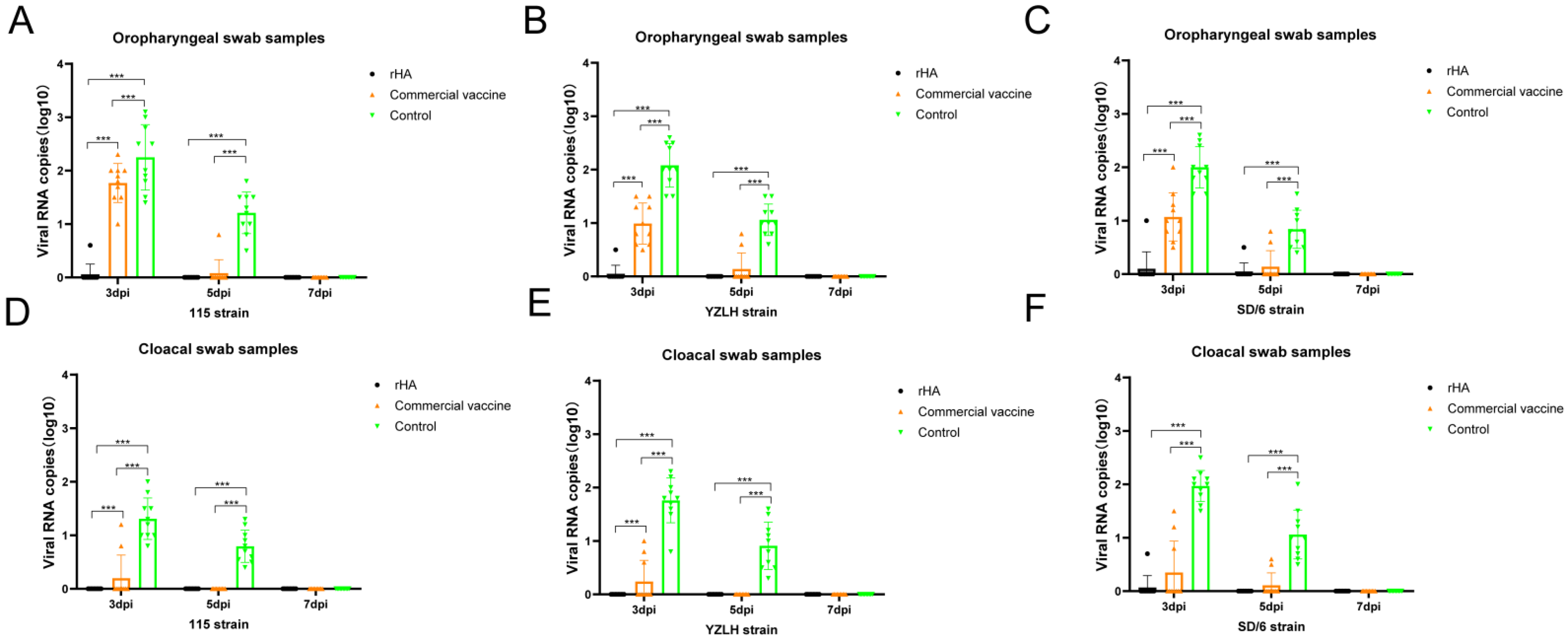
2.6. Viral Shedding
2.7. HI Assay
2.8. Statistical Analysis
3. Results
3.1. Successful Rescue and Characterization of rHA
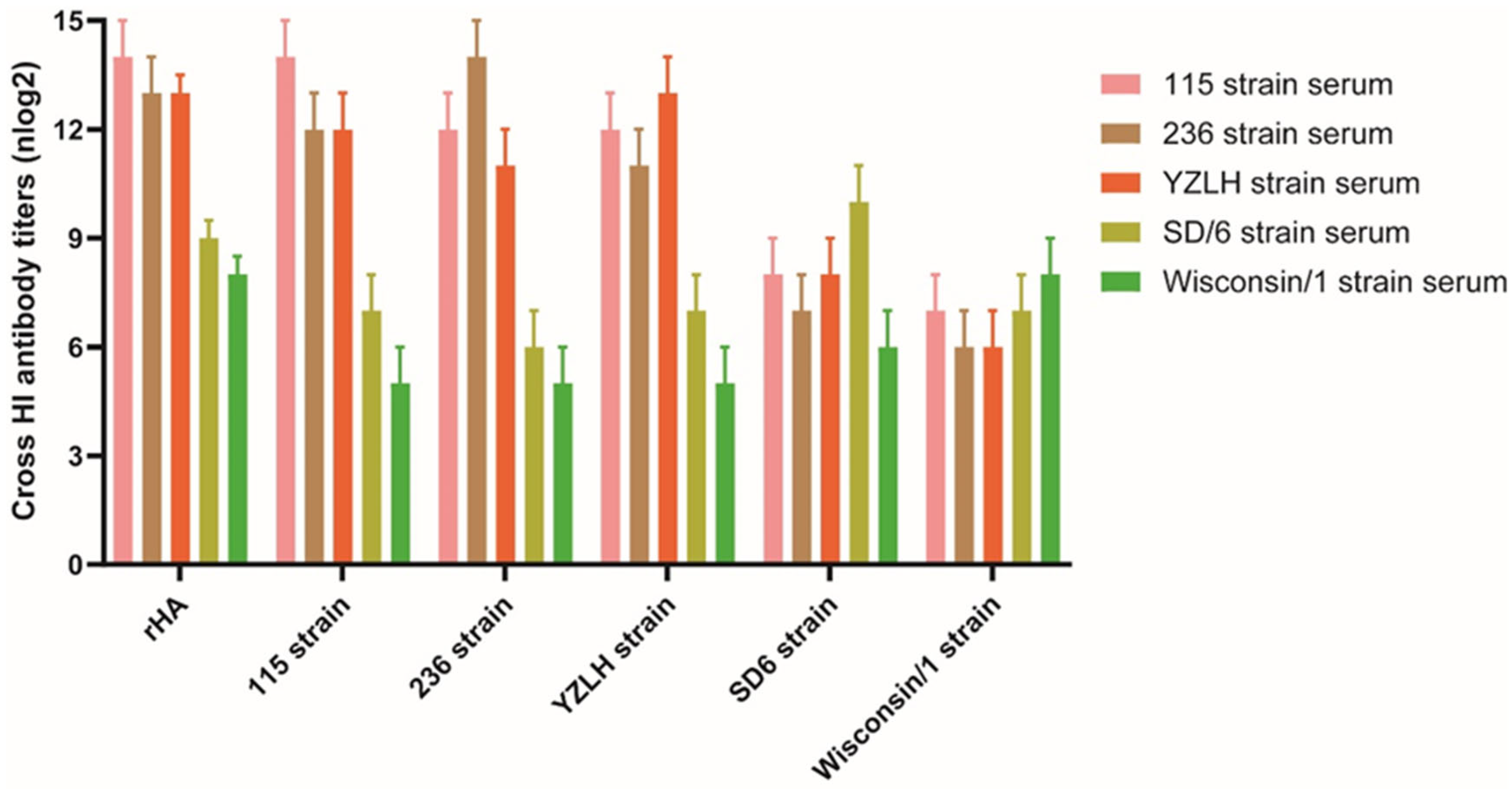
3.2. rHA Protein Displays Cross-Reactivity to Positive Sera against H9 Subtype AIVs
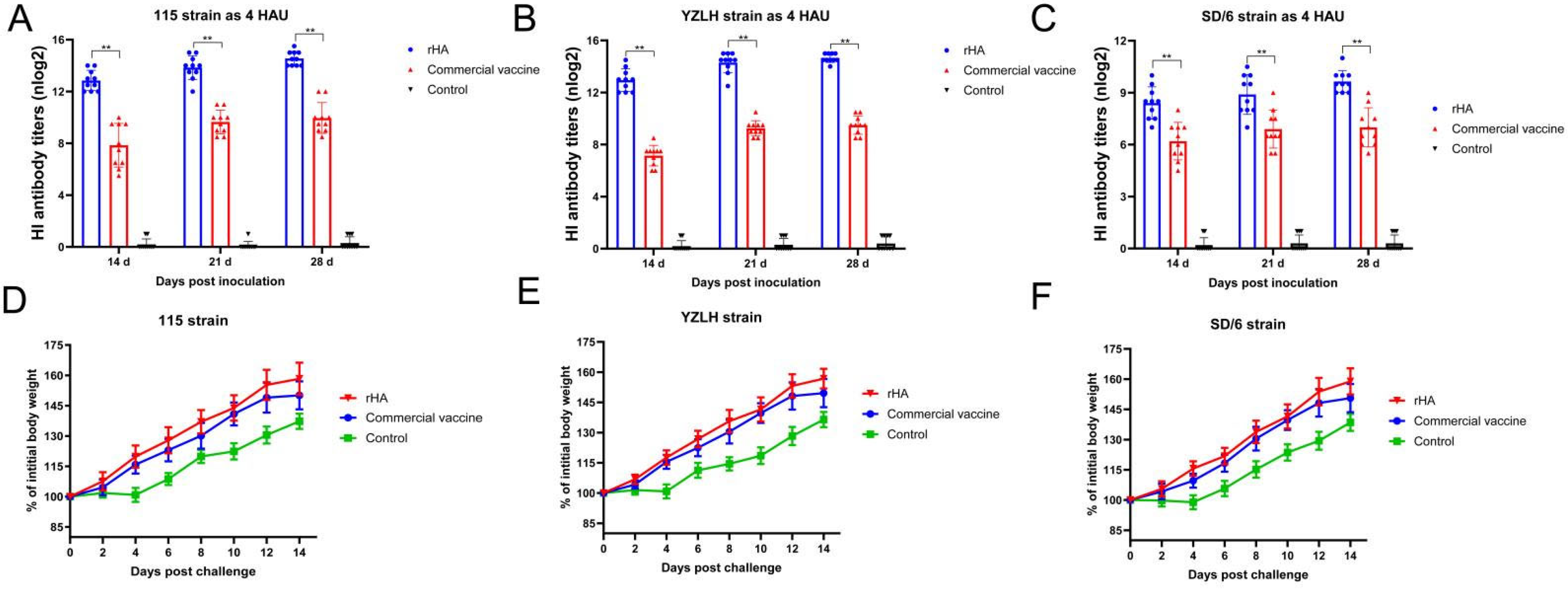
3.3. The rHA Subunit Vaccine Induces Potent Immune Responses
3.4. The rHA Subunit Vaccine Provides Significant Protection against the H9 Subtype AIV Challenge
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.T.; Linster, M.; Mendenhall, I.H.; Su, Y.C.F.; Smith, G.J.D. Avian influenza viruses in humans: Lessons from past outbreaks. Br. Med. Bull. 2019, 132, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Lycett, S.J.; Duchatel, F.; Digard, P. A brief history of bird flu. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180257. [Google Scholar] [CrossRef] [PubMed]
- Carnaccini, S.; Perez, D.R. H9 Influenza Viruses: An Emerging Challenge. Cold Spring Harb. Perspect. Med. 2020, 10, a038588. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Xu, L.; Wang, X.; Liu, X. Current situation of H9N2 subtype avian influenza in China. Vet. Res. 2017, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; James, J.; Sadeyen, J.R.; Mahmood, S.; Everest, H.J.; Chang, P.; Walsh, S.K.; Byrne, A.M.P.; Mollett, B.; Lean, F.; et al. Coinfection of Chickens with H9N2 and H7N9 Avian Influenza Viruses Leads to Emergence of Reassortant H9N9 Virus with Increased Fitness for Poultry and a Zoonotic Potential. J. Virol. 2022, 96, e0185621. [Google Scholar] [CrossRef] [PubMed]
- Pusch, E.A.; Suarez, D.L. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Vet. Sci. 2018, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Homme, P.J.; Easterday, B.C. Avian influenza virus infections. I. Characteristics of influenza A-turkey-Wisconsin-1966 virus. Avian Dis. 1970, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W.; Yousef, N.M.H.; Hilt, D.A. Further development and use of a molecular serotype identification test for infectious bronchitis virus. Avian Diseases 1997, 41, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Fellahi, S.; Nassik, S.; Maaroufi, I.; Tligui, N.S.; Touzani, C.D.; Rawi, T.; Delvecchio, A.; Ducatez, M.F.; El Houadfi, M. Pathogenesis of Avian Influenza Virus Subtype H9N2 in Turkeys and Evaluation of Inactivated Vaccine Efficacy. Avian Diseases 2021, 65, 46–51. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Lv, X.; Wang, Y.; Li, Y.; Li, M.; Liu, W.; Zhi, M.; Yang, X.; Fu, T.; et al. Novel Reassortant Avian Influenza A(H9N2) Virus Isolate in Migratory Waterfowl in Hubei Province, China. Front. Microbiol. 2020, 11, 220. [Google Scholar] [CrossRef]
- Bi, Y.; Li, J.; Li, S.; Fu, G.; Jin, T.; Zhang, C.; Yang, Y.; Ma, Z.; Tian, W.; Li, J.; et al. Dominant subtype switch in avian influenza viruses during 2016-2019 in China. Nat. Commun. 2020, 11, 5909. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, X.; Wang, X. The immune system of chicken and its response to H9N2 avian influenza virus. Vet. Q. 2023, 43, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, E.M.; Wan, H.Q.; Araya, Y.; Song, H.C.; Perez, D.R. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc. Natl. Acad. Sci. USA 2009, 106, 7565–7570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Qin, K.; Wang, J.J.; Pu, J.A.; Tang, Q.D.; Hu, Y.X.; Bi, Y.H.; Zhao, X.L.; Yang, H.C.; Shu, Y.L.; et al. High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proc. Natl. Acad. Sci. USA 2011, 108, 4164–4169. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, P.; Chu, J.; Zhu, J.; Li, Q.; Zhao, H.; Li, Y.; Xin, L.; Yang, X.; Xie, S.; et al. Characterization of four novel H5N6 avian influenza viruses with the internal genes from H5N1 and H9N2 viruses and experimental challenge of chickens vaccinated with current commercially available H5 vaccines. Transbound. Emerg. Dis. 2022, 69, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lloren, K.K.S.; Lee, T.; Kwon, J.J.; Song, M.S. Molecular Markers for Interspecies Transmission of Avian Influenza Viruses in Mammalian Hosts. Int. J. Mol. Sci. 2017, 18, 2706. [Google Scholar] [CrossRef] [PubMed]
- Um, S.; Siegers, J.Y.; Sar, B.; Chin, S.; Patel, S.; Bunnary, S.; Hak, M.; Sor, S.; Sokhen, O.; Heng, S.; et al. Human Infection with Avian Influenza A(H9N2) Virus, Cambodia, February 2021. Emerg. Infect. Dis. 2021, 27, 2742–2745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.S.; Li, L.J.; Zhu, Q.; Wang, Z.; Yuan, P.; Zhou, G.D.; Shi, W.J.; Chu, X.F.; Jiang, S.J.; Xie, Z.J. Co-infection of H9N2 influenza virus and contributes to the development of hemorrhagic pneumonia in mink. Vet. Microbiol. 2020, 240, 108542. [Google Scholar] [CrossRef]
- Liu, S.; Zhuang, Q.; Wang, S.; Jiang, W.; Jin, J.; Peng, C.; Hou, G.; Li, J.; Yu, J.; Yu, X.; et al. Control of avian influenza in China: Strategies and lessons. Transbound. Emerg. Dis. 2020, 67, 1463–1471. [Google Scholar] [CrossRef]
- Dong, J.Z.; Zhou, Y.; Pu, J.; Liu, L.T. Status and Challenges for Vaccination against Avian H9N2 Influenza Virus in China. Life 2022, 12, 1326. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, T.; Cui, Y.; Pu, X.; Li, J.; Xu, J.; Liu, G.; Jia, H.; Liu, D.; Song, S.; et al. Influenza H7N9 and H9N2 viruses: Coexistence in poultry linked to human H7N9 infection and genome characteristics. J. Virol. 2014, 88, 3423–3431. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hong, K.J.; Kim, H.; Nam, J.H. Influenza vaccines: Past, present, and future. Rev. Med. Virol. 2022, 32, e2243. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Zhang, J.; Huang, X.; Han, K.; Liu, Q.; Yang, J.; Zhang, L.; Li, Y. Development of an Inactivated Avian Influenza Virus Vaccine against Circulating H9N2 in Chickens and Ducks. Vaccines 2023, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, B.; Yao, J.; Ruan, W. A new H9 influenza virus mRNA vaccine elicits robust protective immunity against infection. Vaccine 2023, 41, 2905–2913. [Google Scholar] [CrossRef]
- Zhang, N.; Quan, K.; Chen, Z.; Hu, Q.; Nie, M.; Xu, N.; Gao, R.; Wang, X.; Qin, T.; Chen, S.; et al. The emergence of new antigen branches of H9N2 avian influenza virus in China due to antigenic drift on hemagglutinin through antibody escape at immunodominant sites. Emerg. Microbes Infect. 2023, 12, 2246582. [Google Scholar] [CrossRef]
- Zhu, S.; Nie, Z.; Che, Y.; Shu, J.; Wu, S.; He, Y.; Wu, Y.; Qian, H.; Feng, H.; Zhang, Q. The Chinese Hamster Ovary Cell-Based H9 HA Subunit Avian Influenza Vaccine Provides Complete Protection against the H9N2 Virus Challenge in Chickens. Viruses 2024, 16, 163. [Google Scholar] [CrossRef]
- Bissett, S.L.; Roy, P. Multi-Gene Recombinant Baculovirus Expression Systems: From Inception to Contemporary Applications. Viruses 2024, 16, 492. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Liu, J.; Wei, Y.; Wei, X. Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines 2023, 11, 1218. [Google Scholar] [CrossRef]
- Ishack, S.; Lipner, S.R. Bioinformatics and immunoinformatics to support COVID-19 vaccine development. J. Med. Virol. 2021, 93, 5209–5211. [Google Scholar] [CrossRef]
- Ullah, A.; Waqas, M.; Aziz, S.; Rahman, S.U.; Khan, S.; Khalid, A.; Abdalla, A.N.; Uddin, J.; Halim, S.A.; Khan, A.; et al. Bioinformatics and immunoinformatics approach to develop potent multi-peptide vaccine for coxsackievirus B3 capable of eliciting cellular and humoral immune response. Int. J. Biol. Macromol. 2023, 239, 124320. [Google Scholar] [CrossRef] [PubMed]
- Caceres, C.J.; Rajao, D.S.; Perez, D.R. Airborne Transmission of Avian Origin H9N2 Influenza A Viruses in Mammals. Viruses 2021, 13, 1919. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, Q.; Niu, S.; Huang, D.; Yan, D.; Teng, Q.; Li, X.; Beerens, N.; Forlenza, M.; de Jong, M.C.M.; et al. Efficacy of a recombinant turkey herpesvirus (H9) vaccine against H9N2 avian influenza virus in chickens with maternal-derived antibodies. Front. Microbiol. 2022, 13, 1107975. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lu, J.; Zhang, X.; Mei, M.; Feng, L.; Peng, D.; Hou, J.; Kang, S.M.; Liu, X.; Tang, Y. Single Dose of Consensus Hemagglutinin-Based Virus-Like Particles Vaccine Protects Chickens against Divergent H5 Subtype Influenza Viruses. Front. Immunol. 2017, 8, 1649. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ye, H.; Qiu, W.; Lin, H.; Chen, Y.; Zhang, H.; Liao, M. Phylogenetic classification of hemagglutinin gene of H9N2 avian influenza viruses isolated in China during 2012-2016 and evaluation of selected candidate vaccine strains. Poult. Sci. 2018, 97, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, Y.X.; Dong, M.Y.; Guo, Z.W.; Luo, Y.W.; Li, N.L.; Zhao, Y.; Li, M.; Lin, Y.; Xu, J.; et al. Evolution of prevalent H9N2 subtype of avian influenza virus during 2019 to 2022 for the development of a control strategy in China. Poult. Sci. 2023, 102, 102957. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Li, Y. Global genetic variation and transmission dynamics of H9N2 avian influenza virus. Transbound. Emerg. Dis. 2018, 65, 504–517. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Guo, Y.; Pei, Y.; Chen, X.; Lu, X.; Gao, R.; Chen, Y.; Gu, M.; Hu, J.; et al. Emergence of a new designated clade 16 with significant antigenic drift in hemagglutinin gene of H9N2 subtype avian influenza virus in eastern China. Emerg. Microbes Infect. 2023, 12, 2249558. [Google Scholar] [CrossRef] [PubMed]
- Song, C.L.; Liao, Z.H.; Shen, Y.; Wang, H.; Lin, W.C.; Li, H.; Chen, W.G.; Xie, Q.M. Assessing the efficacy of a recombinant H9N2 avian influenza virus-inactivated vaccine. Poult. Sci. 2020, 99, 4334–4342. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Elkashif, A.; Alhashimi, M.; Sambhara, S.; Mittal, S.K. Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines. Vaccines 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Fong Legaspi, S.L.; Schwartzman, L.M.; Gygli, S.M.; Sheng, Z.M.; Freeman, A.D.; Matthews, L.M.; Xiao, Y.; Ramuta, M.D.; Batchenkova, N.A.; et al. An inactivated multivalent influenza A virus vaccine is broadly protective in mice and ferrets. Sci. Transl. Med. 2022, 14, eabo2167. [Google Scholar] [CrossRef] [PubMed]
- Guthmiller, J.J.; Han, J.; Utset, H.A.; Li, L.; Lan, L.Y.; Henry, C.; Stamper, C.T.; McMahon, M.; O’Dell, G.; Fernandez-Quintero, M.L.; et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 2022, 602, 314–320. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Q.; Peng, P.; Li, R.; Li, J.; Wang, X.; Gu, M.; Hu, Z.; Hu, S.; Liu, X.; et al. Baculovirus-derived influenza virus-like particle confers complete protection against lethal H7N9 avian influenza virus challenge in chickens and mice. Vet. Microbiol. 2022, 264, 109306. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in Development and Application of Influenza Vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, T.; Wang, L.; Yang, Z.; Luo, C.; Li, M.; Luo, H.; Sun, C.; Yan, H.; Shu, Y. A mosaic influenza virus-like particles vaccine provides broad humoral and cellular immune responses against influenza A viruses. NPJ Vaccines 2023, 8, 132. [Google Scholar] [CrossRef]

| Challenge Virus | Vaccine | Oropharyngeal Swabs | Cloacal Swabs | ||||
|---|---|---|---|---|---|---|---|
| 3 d | 5 d | 7 d | 3 d | 5 d | 7 d | ||
| h9.4.2.5 clade of H9 AIV (115 strain) | rHA | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Commercial | 10/10 | 1/10 | 0/10 | 2/10 | 0/10 | 0/10 | |
| Control | 10/10 | 10/10 | 0/10 | 10/10 | 10/10 | 0/10 | |
| h9.4.2.5 clade of H9 AIV (YZLH strain) | rHA | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Commercial | 10/10 | 2/10 | 0/10 | 3/10 | 0/10 | 0/10 | |
| Control | 10/10 | 10/10 | 0/10 | 10/10 | 10/10 | 0/10 | |
| h9.4.2.3 clade of H9 AIV (SD/6 strain) | rHA | 1/10 | 1/10 | 0/10 | 1/10 | 0/10 | 0/10 |
| Commercial | 10/10 | 2/10 | 0/10 | 3/10 | 2/10 | 0/10 | |
| Control | 10/10 | 10/10 | 0/10 | 10/10 | 10/10 | 0/10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Wang, W.; Zhang, X.; Li, D.; Mei, M. Effectively Evaluating a Novel Consensus Subunit Vaccine Candidate to Prevent the H9N2 Avian Influenza Virus. Vaccines 2024, 12, 849. https://doi.org/10.3390/vaccines12080849
Wu Q, Wang W, Zhang X, Li D, Mei M. Effectively Evaluating a Novel Consensus Subunit Vaccine Candidate to Prevent the H9N2 Avian Influenza Virus. Vaccines. 2024; 12(8):849. https://doi.org/10.3390/vaccines12080849
Chicago/Turabian StyleWu, Qi, Weihua Wang, Xuehua Zhang, Ding Li, and Mei Mei. 2024. "Effectively Evaluating a Novel Consensus Subunit Vaccine Candidate to Prevent the H9N2 Avian Influenza Virus" Vaccines 12, no. 8: 849. https://doi.org/10.3390/vaccines12080849






