Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Live Attenuated Influenza Vaccine (LAIV)
2.3. Hemagglutination Inhibition (HI) Assay
2.4. Microneutralization Assay
2.5. ELLA
2.6. Avidity ELISA
2.7. Statistical Analysis
3. Results
3.1. Cohort Stratification
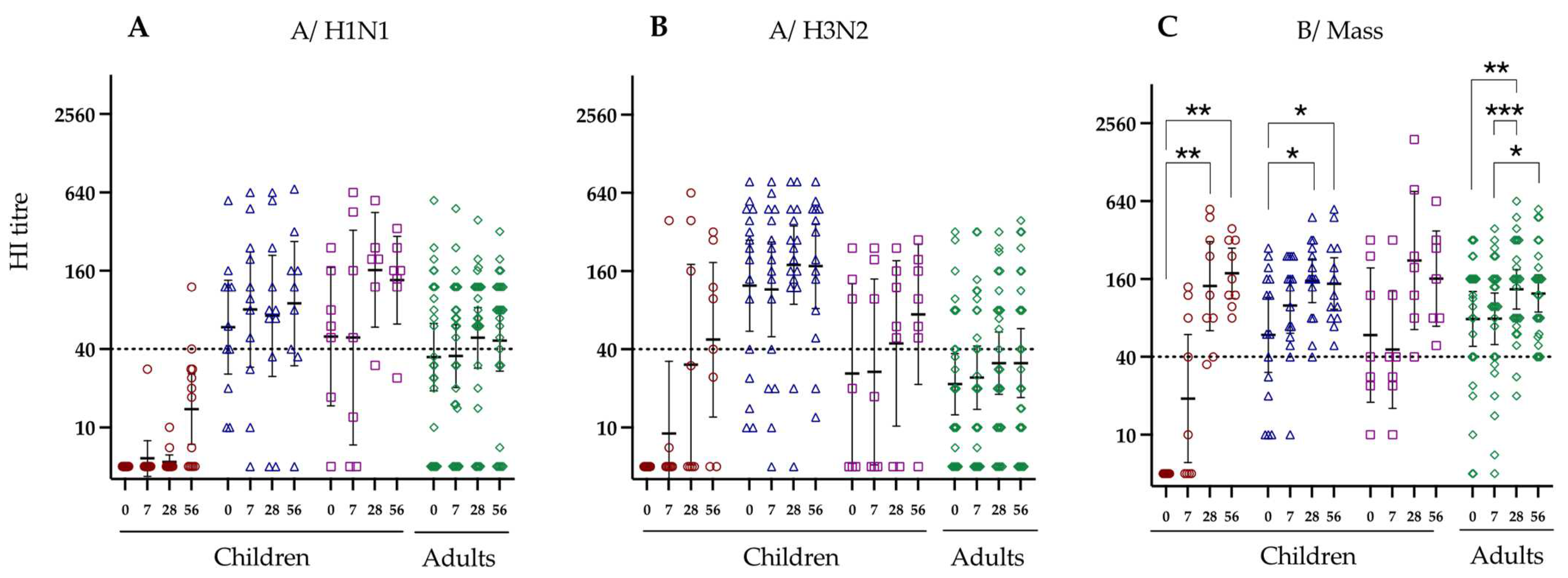
3.2. Hemagglutination Inhibition Responses to LAIV Vaccination
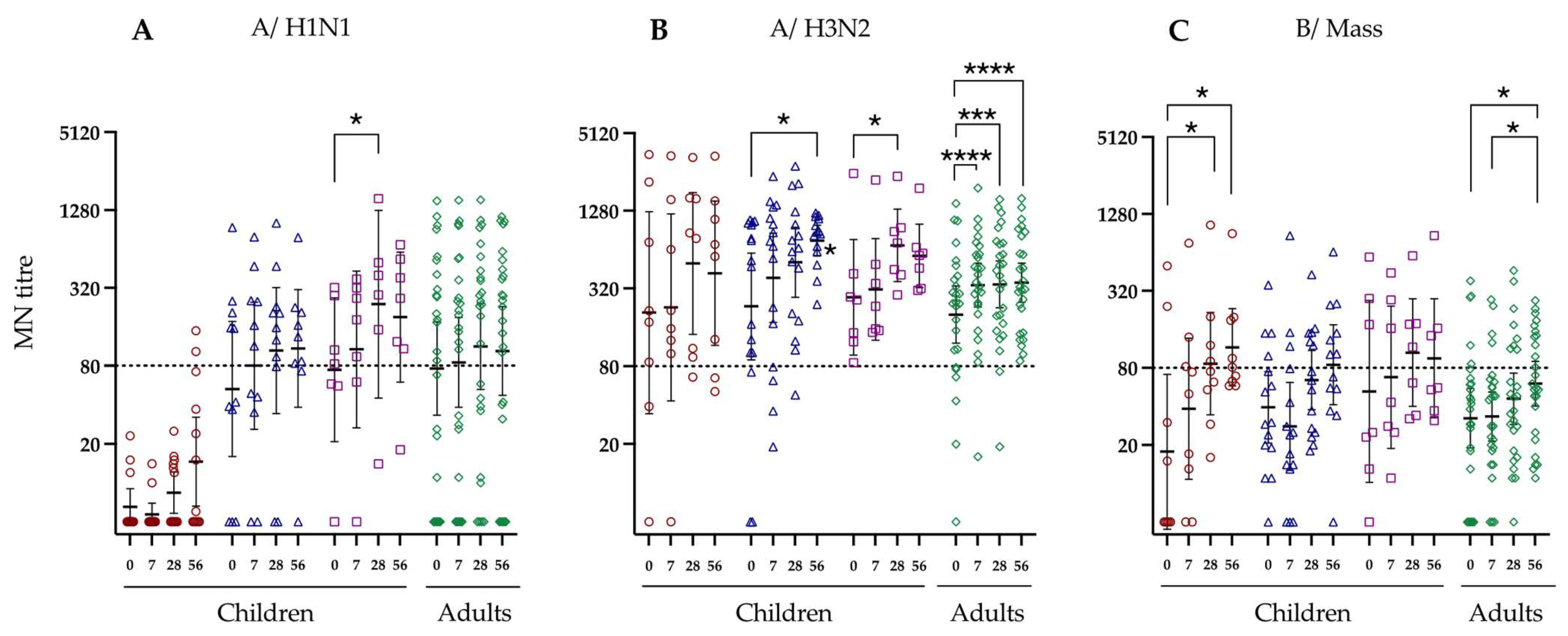
3.3. Neutralising Antibody Response to LAIV Vaccination
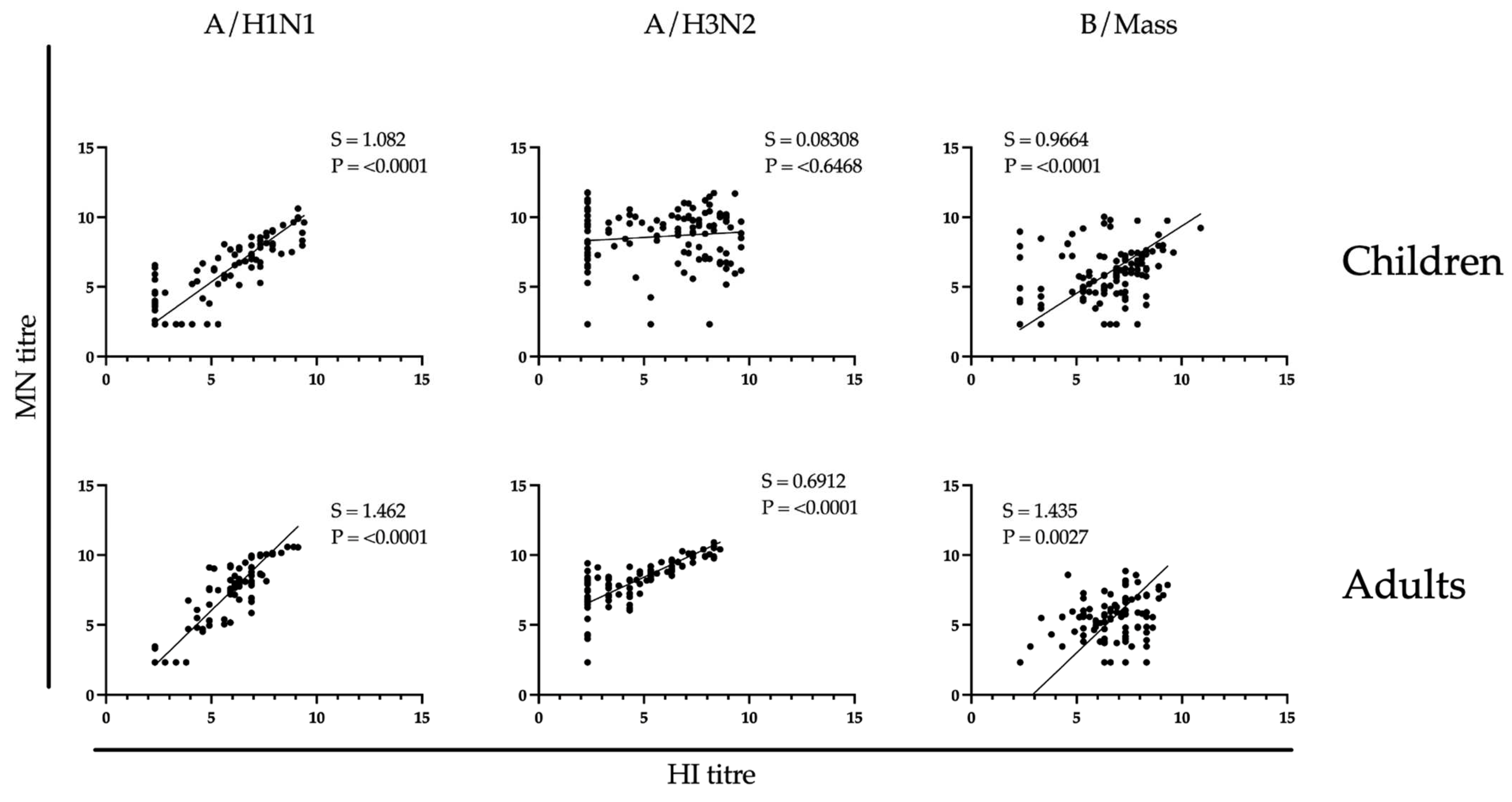
3.4. Correlation between Hemagglutination Inhibition and Microneutralizing Antibody Responses
3.5. Neuraminidase Inhibiting Antibodies
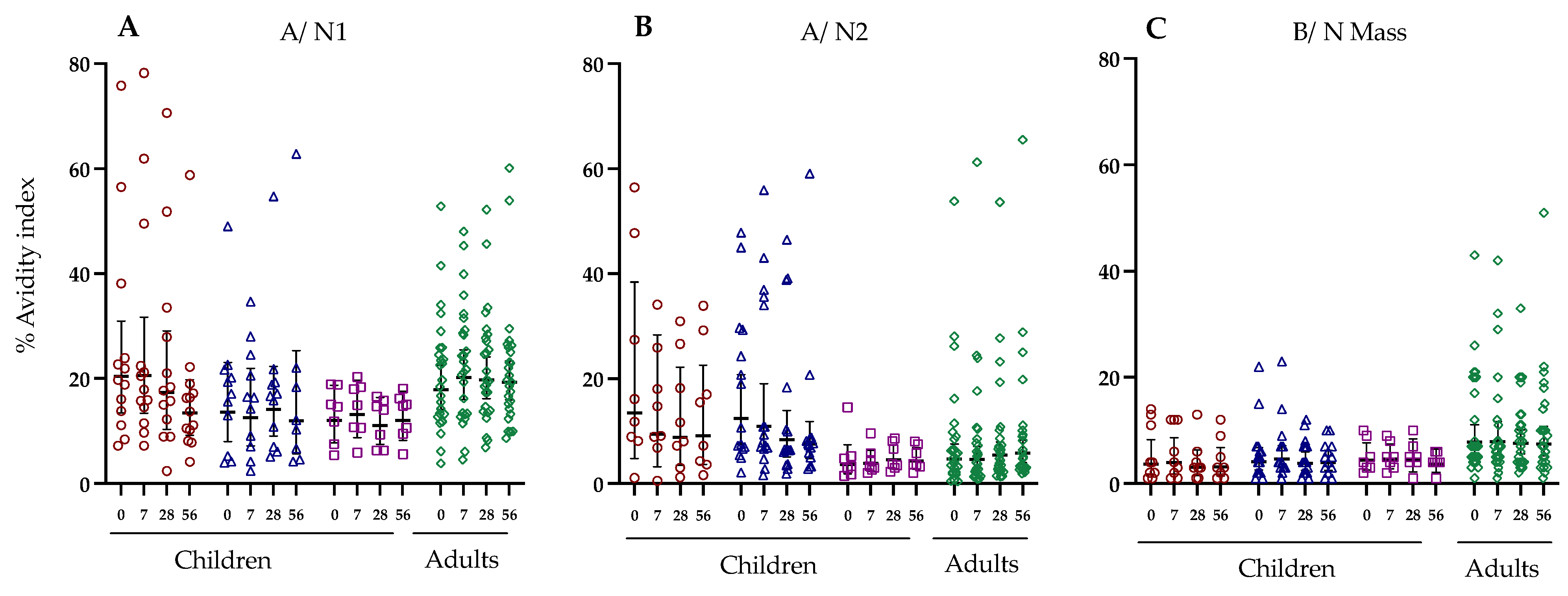
3.6. Antibody Avidity against Haemagglutinin and Neuraminidase Following LAIV Vaccination
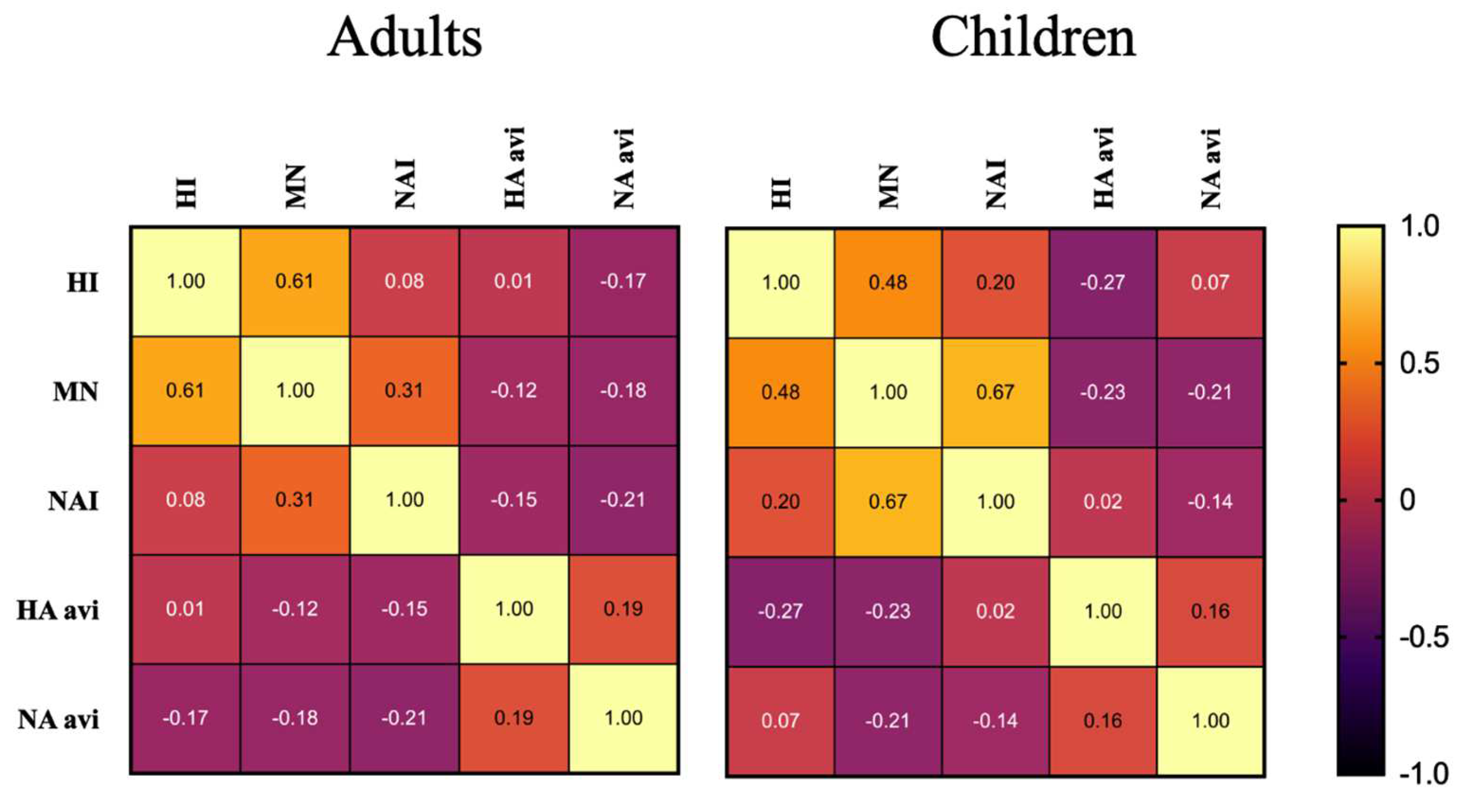
3.7. Cross Correlation of Immunological Responses in Children and Adults Following LAIV Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modeling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Factsheet about Seasonal Influenza. 14 June 2017. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet (accessed on 30 July 2024).
- Centers for Disease Control and Prevention. Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). 2024. Available online: https://www.cdc.gov/flu/prevent/nasalspray.htm (accessed on 30 July 2024).
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.V.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M. CAIV-T Comparative Efficacy Study Group Live attenuated versus inactivated influenza vaccine in infants and young children. New Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Crovari, P.; Wahn, U.; Klemola, T.; Schlesinger, Y.; Langussis, A.; Øymar, K.; Garcia, M.L.; Krygier, A.; Costa, H.; et al. Comparison of the Efficacy and Safety of Live Attenuated Cold-Adapted Influenza Vaccine, Trivalent, with Trivalent Inactivated Influenza Virus Vaccine in Children and Adolescents with Asthma. Pediatr. Infect. Dis. J. 2006, 25, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, S.; Vertruyen, A.; Arístegui, J.; Esposito, S.; McKeith, D.D.; Klemola, T.; Biolek, J.; Kühr, J.; Bujnowski, T.; Desgrandchamps, D.; et al. Superior Relative Efficacy of Live Attenuated Influenza Vaccine Compared with Inactivated Influenza Vaccine in Young Children with Recurrent Respiratory Tract Infections. Pediatr. Infect. Dis. J. 2006, 25, 870–879. [Google Scholar] [CrossRef]
- Ohmit, S.E.; Victor, J.C.; Rotthoff, J.R.; Teich, E.R.; Truscon, R.K.; Baum, L.L.; Rangarajan, B.; Newton, D.W.; Boulton, M.L.; Monto, A.S. Prevention of Antigenically Drifted Influenza by Inactivated and Live Attenuated Vaccines. New Engl. J. Med. New Engl. J. Med. 2006, 355, 2513–2522. [Google Scholar] [CrossRef]
- Ohmit, S.E.; Victor, J.C.; Teich, E.R.; Truscon, R.K.; Rotthoff, J.R.; Newton, D.W.; Campbell, S.A.; Boulton, M.L.; Monto, A.S. Prevention of Symptomatic Seasonal Influenza in 2005–2006 by Inactivated and Live Attenuated Vaccines. J. Infect. Dis. Online. Univ. Chic. Press J. Infect. Dis. 2008, 198, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S.; Ohmit, S.E.; Petrie, J.G.; Johnson, E.; Truscon, R.; Teich, E.; Rotthoff, J.; Boulton, M.; Victor, J.C. Comparative Efficacy of Inactivated and Live Attenuated Influenza Vaccines. N. Engl. J. Med. 2009, 361, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Development of Live Attenuated Influenza Vaccines|European Medicines Agency. (n.d.). Available online: https://www.ema.europa.eu/en/development-live-attenuated-influenza-vaccines (accessed on 30 July 2024).
- Brandenburg, B.; Koudstaal, W.; Goudsmit, J.; Klaren, V.; Tang, C.; Bujny, M.V.; Korse, H.J.W.M.; Kwaks, T.; Otterstrom, J.J.; Juraszek, J.; et al. Mechanisms of Hemagglutinin Targeted Influenza Virus Neutralization. PLoS ONE 2013, 8, e80034. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. 2007. Available online: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Guidance-for-Industry--Clinical-Data-Needed-to-Support-the-Licensure-of-Seasonal-Inactivated-Influenza-Vaccines.pdf (accessed on 30 July 2024).
- Wright, P.F.; Hoen, A.G.; Ilyushina, N.A.; Brown, E.P.; Ackerman, M.E.; Wieland-Alter, W.; Connor, R.I.; Jegaskanda, S.; Rosenberg-Hasson, Y.; Haynes, B.C.; et al. Correlates of Immunity to Influenza as Determined by Challenge of Children with Live, Attenuated Influenza Vaccine. Open Forum Infect. Dis. 2016, 3, ofw108. [Google Scholar] [CrossRef]
- Lartey, S.; Zhou, F.; Brokstad, K.A.; Mohn, K.G.-I.; A Slettevoll, S.; Pathirana, R.D.; Cox, R.J. Live-Attenuated Influenza Vaccine Induces Tonsillar Follicular T Helper Cell Responses That Correlate with Antibody Induction. J. Infect. Dis. 2019, 221, 21–32. [Google Scholar] [CrossRef]
- Roy, S.; Williams, C.M.; Wijesundara, D.K.; Furuya, Y. Impact of Pre-Existing Immunity to Influenza on Live-Attenuated Influenza Vaccine (LAIV) Immunogenicity. Vaccines 2020, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.-I.; Brokstad, K.A.; Pathirana, R.D.; Bredholt, G.; Jul-Larsen, Å.; Trieu, M.C.; Lartey, S.L.; Montemoli, E.; Tøndel, C.; Aarstad, H.J.; et al. Live Attenuated Influenza Vaccine in Children Induces B-Cell Responses in Tonsils. J. Infect. Dis. 2016, 214, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.D. Neuraminidase Is Important for the Initiation of Influenza Virus Infection in Human Airway Epithelium. J. Virol. 2004, 78, 12665–12667. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.L.; Khakpour, M.; Kilbourne, E.D. Protective Effects of Specific Immunity to Viral Neuraminidase on Influenza Virus Infection of Mice. J. Virol. 1968, 2, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Arifuzzaman, M.; Ahmad, S.M.; Hosen, M.I.; Rahman, M.A.; Rashu, R.; Sheikh, A.; Ryan, E.T.; Calderwood, S.B.; Qadri, F. Study of Avidity of Antigen-Specific Antibody as a Means of Understanding Development of Long-Term Immunological Memory after Vibrio cholerae O1 Infection. Clin. Vaccine Immunol. 2013, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Hahn, M.; Coyle, E.M.; King, L.R.; Lin, T.-L.; Treanor, J.; Sant, A.; Golding, H. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Verma, N.; Talaat, K.R.; Karron, R.A.; Golding, H. Immune Response Following H1N1pdm09 Vaccination: Differences in Antibody Repertoire and Avidity in Young Adults and Elderly Populations Stratified by Age and Gender. J. Infect. Dis. 2011, 205, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Monsalvo, A.C.; Batalle, J.P.; Lopez, M.F.; Krause, J.C.; Klemenc, J.; Hernandez, J.Z.; Maskin, B.; Bugna, J.; Rubinstein, C.; Aguilar, L.; et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 2010, 17, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.; Kawabata, H.; Krammer, F.; Carreño, J.M. An In Vitro Microneutralization Assay for Influenza Virus Serology. Curr. Protoc. 2022, 2, e465. [Google Scholar] [CrossRef]
- Martí, G.P.; Mohn, K.G.I.; Cox, R.J.; Brokstad, K.A. The Influence of Tonsillectomy on Total Serum Antibody Levels. Scand. J. Immunol. 2014, 80, 377–379. [Google Scholar] [CrossRef]
- Programme, G.I.; Serological Diagnosis of Influenza by Microneutralization Assay. 6 December 2010. Available online: https://www.who.int/publications/i/item/serological-diagnosis-of-influenza-by-microneutralization-assay (accessed on 30 July 2024).
- Walz, L.; Kays, S.K.; Zimmer, G.; von Messling, V. Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype. J. Virol. 2018, 92, 10-1128. [Google Scholar] [CrossRef]
- Veguilla, V.; Hancock, K.; Schiffer, J.; Gargiullo, P.; Lu, X.; Aranio, D.; Branch, A.; Dong, L.; Holiday, C.; Liu, F.; et al. Sensitivity and Specificity of Serologic Assays for Detection of Human Infection with 2009 Pandemic H1N1 Virus in U.S. Populations. J. Clin. Microbiol. 2011, 49, 2210–2215. [Google Scholar] [CrossRef]
- Monto, A.S.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Liu, M.; Zhong, W.; Levine, M.; Katz, J.M.; Ohmit, S.E. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. 2015. Available online: https://pubmed.ncbi.nlm.nih.gov/25858957/ (accessed on 30 July 2024).
- Weiss, C.D.; Wang, W.; Lu, Y.; Billings, M.; Eick-Cost, A.; Couzens, L.; Sanchez, J.L.; Hawksworth, A.W.; Seguin, P.; Myers, C.A.; et al. Neutralizing and Neuraminidase Antibodies Correlate with Protection against Influenza During a Late Season A/H3N2 Outbreak among Unvaccinated Military Recruits. 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/31840159/ (accessed on 30 July 2024).
- Ang, J.C.; Wang, B.; Wang, J.J.; Zeng, P.Y.F.; Krammer, F.; Ward, B.J.; Russell, M.L.; Loeb, M.; Miller, M.S. Comparative Immunogenicity of the 2014–2015 Northern Hemisphere Trivalent IIV and LAIV against Influenza A Viruses in Children. Vaccines 2019, 7, 87. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J.; Belshe, R.B. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir. Viruses 2010, 5, 67–75. [Google Scholar] [CrossRef]
- Heeringa, M.; Leav, B.; Smolenov, I.; Palladino, G.; Isakov, L.; Matassa, V. Comparability of Titers of Antibodies against Seasonal Influenza Virus Strains as Determined by Hemagglutination Inhibition and Microneutralization Assays. J. Clin. Microbiol. 2020, 58, 00750-20. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Singh, P.; Russell, M.L.; Bowdish, D.M.E.; Brewer, A.; Cyr, L.; Ward, B.J.; Loeb, M. Microneutralization Assay Titres Correlate with Protection against Seasonal Influenza H1N1 and H3N2 in Children. PLoS ONE 2015, 10, e0131531. [Google Scholar] [CrossRef]
- Bhat, Y.R. Influenza B infections in children: A review. World J. Clin. Pediatr. 2020, 9, 44–52. [Google Scholar] [CrossRef]
- Marshall, B.C.; Adler, S.P. Avidity maturation following immunization with two human cytomegalovirus (CMV) vaccines: A live attenuated vaccine (Towne) and a recombinant glycoprotein vaccine (GB/MF59). Viral Immunol. 2003, 16, 491–500. [Google Scholar] [CrossRef]
- Hedman, K.; Hietala, J.; Tiilikainen, A.; Hartikainen-Sorri, A.; Räihä, K.; Suni, J.; Väänänen, P.; Pietiläinen, M. Maturation of immunoglobulin G avidity after rubella vaccination studied by an enzyme linked immunosorbent assay (Avidity-ELISA) and by haemolysis typing. J. Med. Virol. 1989, 27, 293–298. [Google Scholar] [CrossRef]
- Yam, K.K.; Gupta, J.; Brewer, A.; Scheifele, D.W.; Halperin, S.; Ward, B.J. Unusual patterns of IGG avidity in some young children following two doses of the adjuvanted pandemic H1N1 (2009) influenza virus vaccine. Clin. Vaccine Immunol. 2013, 20, 459–467. [Google Scholar] [CrossRef]
- Eidem, S.; Tete, S.M.; Jul-Larsen, Å.; Hoschler, K.; Montomoli, E.; Brokstad, K.A.; Cox, R.J. Persistence and avidity maturation of antibodies to A(H1N1)pdm09 in healthcare workers following repeated annual vaccinations. Vaccine 2015, 33, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, A.; Ivan; Hung, I.F.N.; Xu, T.; Ip, W.C.T.; Wong, R.T.Y.; Ng, J.C.K.; Chan, J.F.W.; Chan, K.-H.; Yuen, K.-Y. High Titer and Avidity of Non neutralizing Antibodies against Influenza Vaccine Antigen Are Associated with Severe Influenza. Clin. Vaccine Immunol. 2012, 19, 1012–1018. [Google Scholar] [CrossRef]
- Aartse, A.; Mortier, D.; Mooij, P.; Hofman, S.; van Haaren, M.M.; Corcoran, M.; Hedestam, G.B.K.; Eggink, D.; Claireaux, M.; Bogers, W.M.J.M.; et al. Primary antibody response after influenza virus infection is first dominated by low-mutated HA-stem antibodies followed by higher-mutated HA-head antibodies. Front. Immunol. 2022, 13, 1026951. [Google Scholar] [CrossRef]
- Giurgea, L.T.; Cervantes-Medina, A.; Walters, K.-A.; Scherler, K.; Han, A.; Czajkowski, L.M.; Baus, H.A.; Hunsberger, S.; Klein, S.L.; Kash, J.C.; et al. Sex Differences in Influenza: The Challenge Study Experience. J. Infect. Dis. 2022, 225, 715–722. [Google Scholar] [CrossRef]


| Characteristics | ||||
|---|---|---|---|---|
| Groups | Children under 9 | Children 9 or above | Adults | |
| n | 24 | 7 | 26 | |
| Median Age (years) | 4 | 13 | 29 | |
| Age Range (years) | 3–7 | 11–17 | 18–59 | |
| Gender (%male) | 67% | 29% | 27% | |
| LAIV Doses | 2 | 1 | 1 | |
| Previous Pandemic Vaccination * | 25% | 43% | 50% | |
| Naïve | Primed | Age Primed | Age Primed | |
| A/H1N1 | 13 | 11 | 7 | 26 |
| A/H3N2 | 8 | 16 | ||
| B/Mass | 9 | 15 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoen, L.; Lartey, S.; Zhou, F.; Pathirana, R.D.; Krammer, F.; Mohn, K.G.-I.; Cox, R.J.; Brokstad, K.A. Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine. Vaccines 2024, 12, 864. https://doi.org/10.3390/vaccines12080864
Hoen L, Lartey S, Zhou F, Pathirana RD, Krammer F, Mohn KG-I, Cox RJ, Brokstad KA. Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine. Vaccines. 2024; 12(8):864. https://doi.org/10.3390/vaccines12080864
Chicago/Turabian StyleHoen, Lukas, Sarah Lartey, Fan Zhou, Rishi D. Pathirana, Florian Krammer, Kristin G. -I. Mohn, Rebecca J. Cox, and Karl A. Brokstad. 2024. "Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine" Vaccines 12, no. 8: 864. https://doi.org/10.3390/vaccines12080864







