The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics
Abstract
:1. Introduction
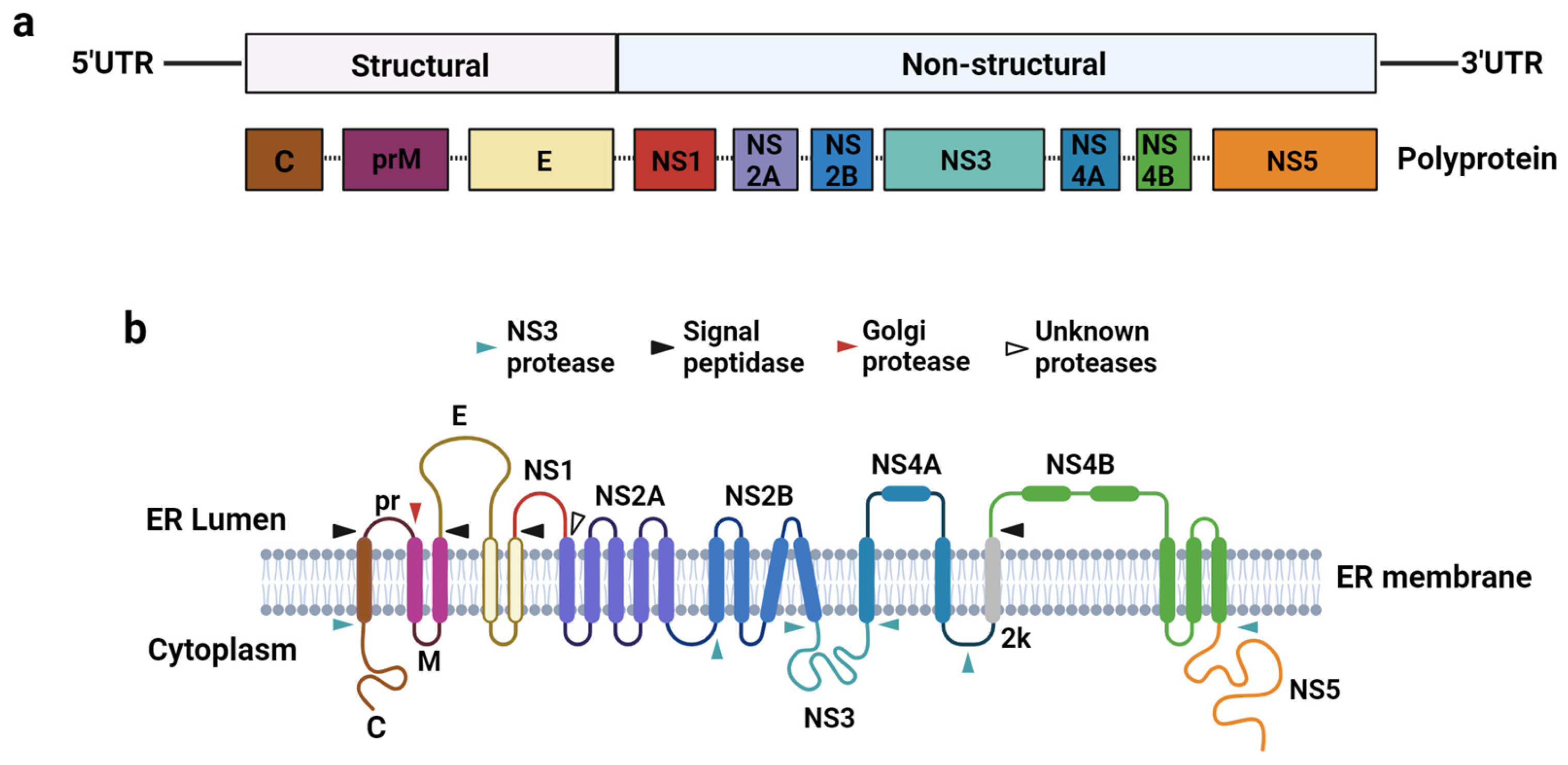
2. Molecular Biology of Flaviviruses
2.1. Flavivirus Genome Organisation and Replication Cycle
2.2. Innate and Adaptive Immune Responses against Flaviviruses during an Infection
2.2.1. Innate Immune Response to Flaviviral Infections
2.2.2. Adaptive Immunity in Response to Flaviviruses
2.3. Role of Non-Structural (NS) Proteins in Viral Replication and Immune Evasion
| Protein | Molecular Weight | Subcellular Location | Function(s) | Reference |
|---|---|---|---|---|
| NS1 | 46–55 kDa | Intracellular; cytoplasm; ER lumen; |
| [50,64,65,66,67] |
| Membrane-associated (dimer) |
| |||
| Secreted (hexamer) | ||||
| NS1′ | 52–53 kDa | Cytoplasm; ER lumen; secreted |
| [68,69] |
| NS2A | 22 kDa | ER membrane |
| [70,71] |
| NS2B | ~14 kDa | ER membrane |
| [72] |
| NS3 | ~70 kDa | Cytoplasm; nucleus |
| [73,74] |
| NS4A | 16 kDa | ER membrane |
| [75] |
| NS4B | 27 kDa | ER membrane |
| [76,77] |
| NS5 | ~100 kDa | Cytoplasm; nucleus |
| [78,79,80] |
3. Non-Structural Protein 5 (NS5) Is a Multi-Functional Protein Required for Viral RNA Replication and Inhibition of the Innate Immune Response
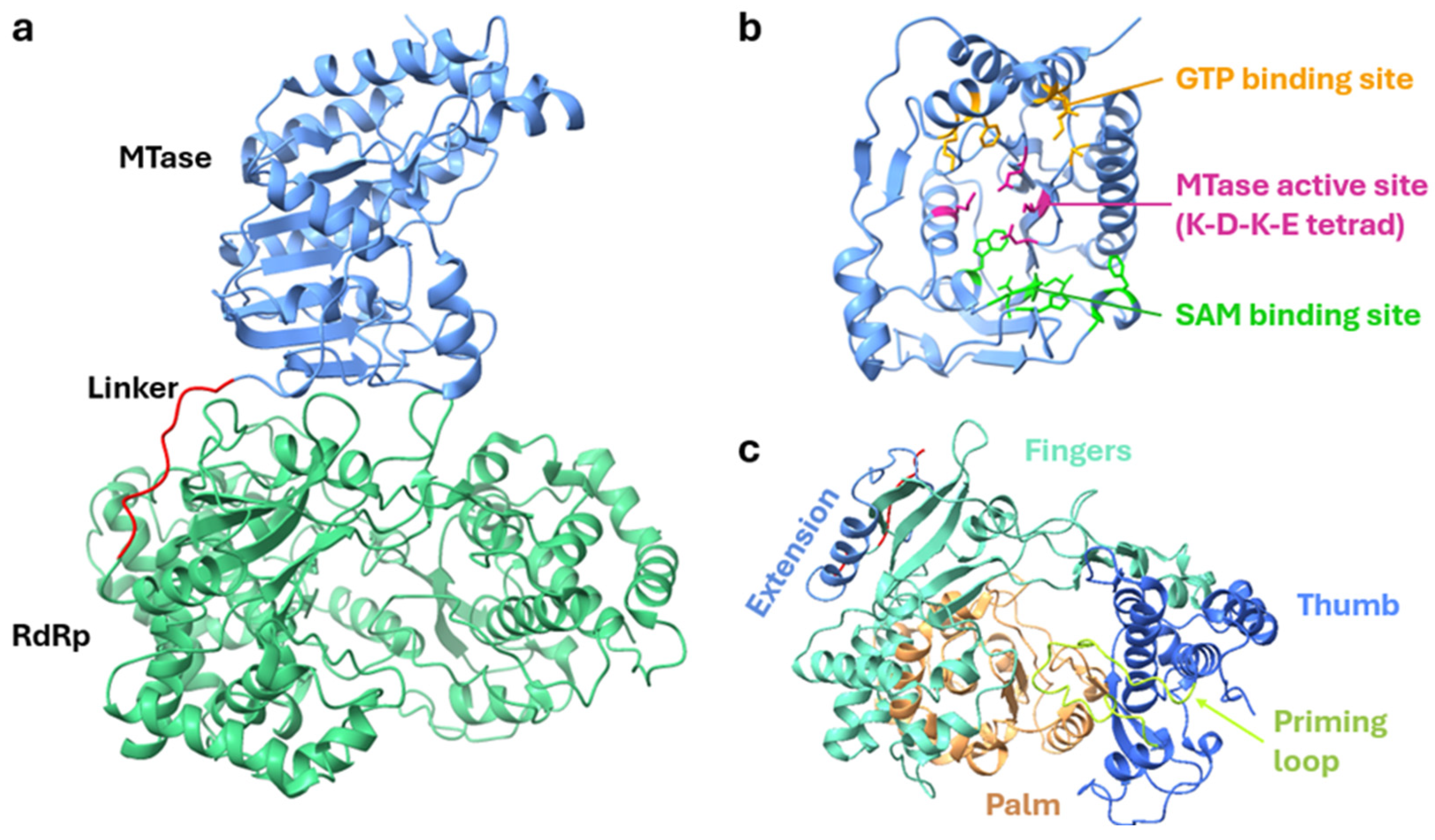
3.1. Structure of NS5 Protein and Its Role in Viral Replication
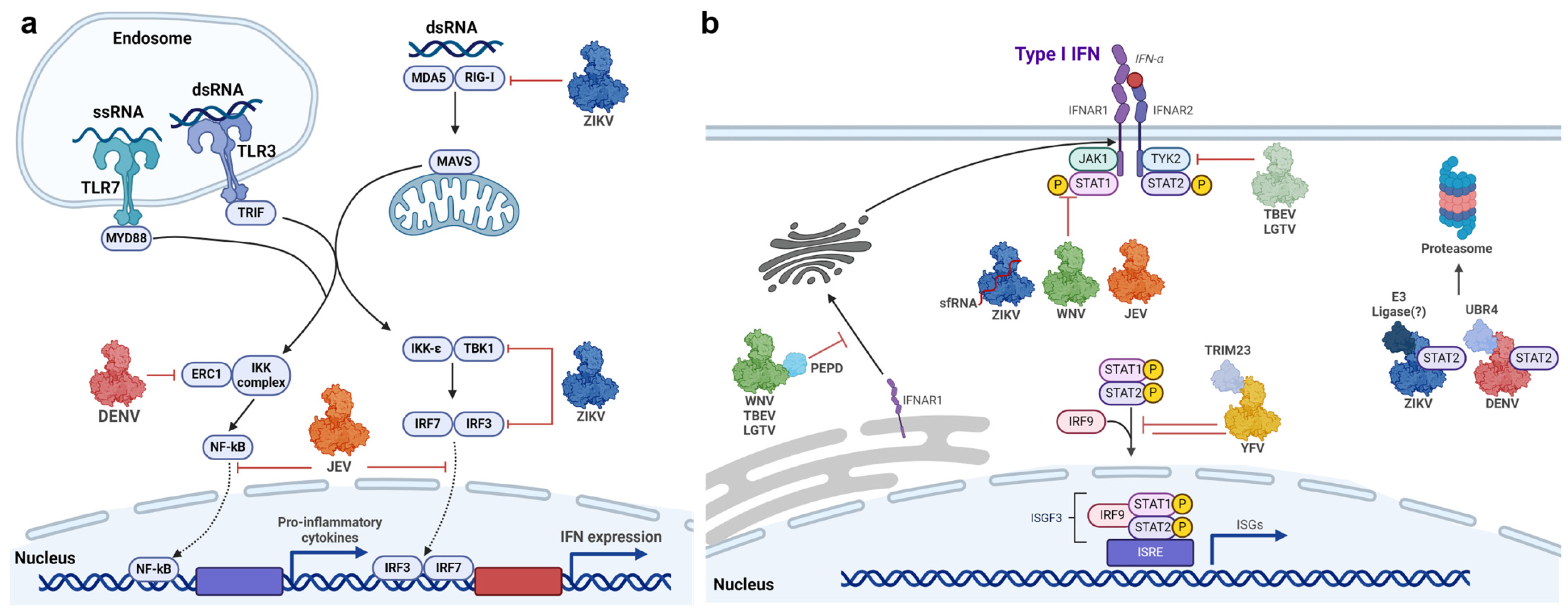
3.2. Flavivirus NS5-Mediated Inhibition of Interferon Signalling
3.3. Nuclear Localization of NS5: A Potential Mechanism for Disruption
4. The Flavivirus NS5 as a Target for Development of Novel Vaccines and Therapeutics
4.1. Targeting the NS5 for Design of Live Attenuated Vaccine Candidates
4.2. The NS5 Elicit Potent T-Cell Immune Responses
4.3. Targeting the NS5 for Proteasomal Degradation as a Novel Strategy for Therapeutics
4.4. The NS5 Is a Prime Target for Development of Small Molecule Antiviral Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, A.; Díaz, Y.; López-Vergès, S. Interaction of Flavivirus with Their Mosquito Vectors and Their Impact on the Human Health in the Americas. Biochem. Biophys. Res. Commun. 2017, 492, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P. Tick-Borne Encephalitis: A Review of Epidemiology, Clinical Characteristics, and Management. World J. Clin. Cases WJCC 2015, 3, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Pustijanac, E.; Buršić, M.; Talapko, J.; Škrlec, I.; Meštrović, T.; Lišnjić, D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms 2023, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.G.; Costa, M.d.C.N.; de Oliveira, W.K.; Nunes, M.L.; Rodrigues, L.C. The Epidemic of Zika Virus–Related Microcephaly in Brazil: Detection, Control, Etiology, and Future Scenarios. Am. J. Public Health 2016, 106, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-Year Historical Review of West Nile Virus since Its Initial Emergence in North America: Has West Nile Virus Become a Neglected Tropical Disease? PLoS Neglected Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, S.L.; Lau, C.L.; Leder, K. The Evolving Japanese Encephalitis Situation in Australia and Implications for Travel Medicine. J. Travel Med. 2023, 30, taad029. [Google Scholar] [CrossRef]
- Farooq, Z.; Sjödin, H.; Semenza, J.C.; Tozan, Y.; Sewe, M.O.; Wallin, J.; Rocklöv, J. European Projections of West Nile Virus Transmission under Climate Change Scenarios. One Health 2023, 16, 100509. [Google Scholar] [CrossRef]
- Xu, Z.; Bambrick, H.; Frentiu, F.D.; Devine, G.; Yakob, L.; Williams, G.; Hu, W. Projecting the Future of Dengue under Climate Change Scenarios: Progress, Uncertainties and Research Needs. PLoS Neglected Trop. Dis. 2020, 14, e0008118. [Google Scholar] [CrossRef]
- Samrat, S.K.; Xu, J.; Li, Z.; Zhou, J.; Li, H. Antiviral Agents against Flavivirus Protease: Prospect and Future Direction. Pathogens 2022, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Bisaillon, M.; Geiss, B.J. Organization of the Flavivirus RNA Replicase Complex. Wiley RNA 2017, 8, e1437. [Google Scholar] [CrossRef]
- Ng, W.C.; Soto-Acosta, R.; Bradrick, S.S.; Garcia-Blanco, M.A.; Ooi, E.E. The 5′ and 3′ Untranslated Regions of the Flaviviral Genome. Viruses 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Roby, J.; Slonchak, A.; Khromykh, A. Functional Non-Coding RNAs Derived from the Flavivirus 3′ Untranslated Region. Virus Res. 2015, 206, 53–61. [Google Scholar] [CrossRef]
- Slonchak, A.; Parry, R.; Pullinger, B.; Sng, J.D.J.; Wang, X.; Buck, T.F.; Torres, F.J.; Harrison, J.J.; Colmant, A.M.G.; Hobson-Peters, J.; et al. Structural Analysis of 3′UTRs in Insect Flaviviruses Reveals Novel Determinants of sfRNA Biogenesis and Provides New Insights into Flavivirus Evolution. Nat. Commun. 2022, 13, 1279. [Google Scholar] [CrossRef]
- Khromykh, A.A.; Meka, H.; Guyatt, K.J.; Westaway, E.G. Essential Role of Cyclization Sequences in Flavivirus RNA Replication. J. Virol. 2001, 75, 6719–6728. [Google Scholar] [CrossRef] [PubMed]
- Bujalowski, P.J.; Bujalowski, W.; Choi, K.H. Identification of the Viral RNA Promoter Stem Loop A (SLA)-Binding Site on Zika Virus Polymerase NS5. Sci. Rep. 2020, 10, 13306. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chan, K.W.K.; Tan, M.J.A.; Flory, C.; Luo, D.; Lescar, J.; Forwood, J.K.; Vasudevan, S.G. A Conserved Arginine in NS5 Binds Genomic 3′ Stem–Loop RNA for Primer-Independent Initiation of Flavivirus RNA Replication. RNA 2022, 28, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Freier, M.; Schmidt, T.; Rostowski, K.; Zwoch, J.; Lilie, H.; Behrens, S.-E.; Friedrich, S. An RNA Thermometer Activity of the West Nile Virus Genomic 3′-Terminal Stem-Loop Element Modulates Viral Replication Efficiency during Host Switching. Viruses 2020, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, G.P.; Funk, A.; Kondratieva, N.; Leung, J.; Torres, S.; van der Aa, L.; Liu, W.J.; Palmenberg, A.C.; Shi, P.-Y.; Hall, R.A.; et al. A Highly Structured, Nuclease-Resistant, Noncoding RNA Produced by Flaviviruses Is Required for Pathogenicity. Cell Host Microbe 2008, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, A.T.; Pham, O.H.; Kenaston, M.W.; Beesabathuni, N.S.; Shah, P.S. Let’s Get Physical: Flavivirus-Host Protein–Protein Interactions in Replication and Pathogenesis. Front. Microbiol. 2022, 13, 847588. [Google Scholar] [CrossRef] [PubMed]
- Berzal-Herranz, A.; Berzal-Herranz, B.; Ramos-Lorente, S.E.; Romero-López, C. The Genomic 3′ UTR of Flaviviruses Is a Translation Initiation Enhancer. Int. J. Mol. Sci. 2022, 23, 8604. [Google Scholar] [CrossRef] [PubMed]
- Barrows, N.J.; Campos, R.K.; Liao, K.-C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.-C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Roby, J.A.; Hall, R.A.; Setoh, Y.X.; Khromykh, A.A. Post-Translational Regulation and Modifications of Flavivirus Structural Proteins. J. Gen. Virol. 2015, 96, 1551–1569. [Google Scholar] [CrossRef]
- Slonchak, A.; Khromykh, A.A. Subgenomic Flaviviral RNAs: What Do We Know after the First Decade of Research. Antivir. Res. 2018, 159, 13–25. [Google Scholar] [CrossRef]
- Slonchak, A.; Wang, X.; Aguado, J.; Sng, J.D.J.; Chaggar, H.; Freney, M.E.; Yan, K.; Torres, F.J.; Amarilla, A.A.; Balea, R.; et al. Zika Virus Noncoding RNA Cooperates with the Viral Protein NS5 to Inhibit STAT1 Phosphorylation and Facilitate Viral Pathogenesis. Sci. Adv. 2022, 8, eadd8095. [Google Scholar] [CrossRef] [PubMed]
- Schuessler, A.; Funk, A.; Lazear, H.M.; Cooper, D.A.; Torres, S.; Daffis, S.; Jha, B.K.; Kumagai, Y.; Takeuchi, O.; Hertzog, P.; et al. West Nile Virus Noncoding Subgenomic RNA Contributes to Viral Evasion of the Type I Interferon-Mediated Antiviral Response. J. Virol. 2012, 86, 5708–5718. [Google Scholar] [CrossRef]
- Slonchak, A.; Chaggar, H.; Aguado, J.; Wolvetang, E.; Khromykh, A.A. Noncoding RNA of Zika Virus Affects Interplay between Wnt-Signaling and Pro-Apoptotic Pathways in the Developing Brain Tissue. Viruses 2023, 15, 1062. [Google Scholar] [CrossRef] [PubMed]
- Doets, K.; Pijlman, G.P. Subgenomic Flavivirus RNA as Key Target for Live-Attenuated Vaccine Development. J. Virol. 2024, 98, e00100-23. [Google Scholar] [CrossRef]
- Mazeaud, C.; Freppel, W.; Chatel-Chaix, L. The Multiples Fates of the Flavivirus RNA Genome During Pathogenesis. Front. Genet. 2018, 9, 595. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Zhang, X.-C.; Jia, R.-Y. Toll-Like Receptors and RIG-I-Like Receptors Play Important Roles in Resisting Flavivirus. J. Immunol. Res. 2018, 2018, 6106582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like Receptor 3 (TLR3) Regulation Mechanisms and Roles in Antiviral Innate Immune Responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Oshiumi, H.; Seya, T. Antiviral Responses Induced by the TLR3 Pathway. Rev. Med. Virol. 2011, 21, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, R.; Mundt, F.; Gilthorpe, J.D.; Wölfel, S.; Gekara, N.O.; Kröger, A.; Överby, A.K. Fast Type I Interferon Response Protects Astrocytes from Flavivirus Infection and Virus-Induced Cytopathic Effects. J. Neuroinflamm. 2016, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.D.; Roehrig, J.T. Monoclonal Antibodies That Bind to Domain III of Dengue Virus E Glycoprotein Are the Most Efficient Blockers of Virus Adsorption to Vero Cells. J. Virol. 2001, 75, 7769–7773. [Google Scholar] [CrossRef] [PubMed]
- Gollins, S.W.; Porterfield, J.S. A New Mechanism for the Neutralization of Enveloped Viruses by Antiviral Antibody. Nature 1986, 321, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Haslwanter, D.; Blaas, D.; Heinz, F.X.; Stiasny, K. A Novel Mechanism of Antibody-Mediated Enhancement of Flavivirus Infection. PLoS Pathog. 2017, 13, e1006643. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Katzelnick, L.C.; Russell, P.K.; Markoff, L.; Aguiar, M.; Dans, L.R.; Dans, A.L. Ethics of a Partially Effective Dengue Vaccine: Lessons from the Philippines. Vaccine 2020, 38, 5572–5576. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, D.T.; Fernando, S.; Gomes, L.; Jeewandara, C.; Ginneliya, A.; Samarasekara, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; Malavige, G.N. Quantification of Dengue Virus Specific T Cell Responses and Correlation with Viral Load and Clinical Disease Severity in Acute Dengue Infection. PLoS Neglected Trop. Dis. 2018, 12, e0006540. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.H.; Alves, R.P.d.S.; Bizerra, R.; Pereira, S.A.; Pereira, L.R.; Fabris, D.L.N.; Santos, R.A.; Romano, C.M.; Ferreira, L.C.d.S. Antibodies Are Not Required to a Protective Immune Response against Dengue Virus Elicited in a Mouse Encephalitis Model. Virology 2016, 487, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; López, C.; Blanco, A.; Lazo, L.; Martín, J.; Valdés, I.; Romero, Y.; Figueroa, Y.; Guillén, G.; Hermida, L. The Cellular Immune Response Plays an Important Role in Protecting Against Dengue Virus in the Mouse Encephalitis Model. Viral Immunol. 2009, 22, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Tang, W.W.; Eddy, W.E.; King, K.; Sanchez, M.C.; Shresta, S. CD8+ T Cells Can Mediate Short-Term Protection against Heterotypic Dengue Virus Reinfection in Mice. J. Virol. 2015, 89, 6494–6505. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The Human CD8+ T Cell Responses Induced by a Live Attenuated Tetravalent Dengue Vaccine Are Directed against Highly Conserved Epitopes. J. Virol. 2014, 89, 120–128. [Google Scholar] [CrossRef]
- Mladinich, K.M.; Piaskowski, S.M.; Rudersdorf, R.; Eernisse, C.M.; Weisgrau, K.L.; Martins, M.A.; Furlott, J.R.; Partidos, C.D.; Brewoo, J.N.; Osorio, J.E.; et al. Dengue Virus-Specific CD4+ and CD8+ T Lymphocytes Target NS1, NS3 and NS5 in Infected Indian Rhesus Macaques. Immunogenetics 2012, 64, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; George, S.L.; Stinchcomb, D.T.; Osorio, J.E.; Partidos, C.D. CD8+ T-Cell Responses in Flavivirus-Naive Individuals Following Immunization with a Live-Attenuated Tetravalent Dengue Vaccine Candidate. J. Infect. Dis. 2015, 212, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Young, P.R. The Flavivirus NS1 Protein: Molecular and Structural Biology, Immunology, Role in Pathogenesis and Application as a Diagnostic Biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef] [PubMed]
- Barnard, T.R.; Abram, Q.H.; Lin, Q.F.; Wang, A.B.; Sagan, S.M. Molecular Determinants of Flavivirus Virion Assembly. Trends Biochem. Sci. 2021, 46, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Loh, Y.R.; Phoo, W.W.; Hung, A.W.; Kang, C.; Luo, D. Crystal Structure of Unlinked NS2B-NS3 Protease from Zika Virus. Science 2016, 354, 1597–1600. [Google Scholar] [CrossRef]
- Luo, D.; Xu, T.; Watson, R.P.; Scherer-Becker, D.; Sampath, A.; Jahnke, W.; Yeong, S.S.; Wang, C.H.; Lim, S.P.; Strongin, A.; et al. Insights into RNA Unwinding and ATP Hydrolysis by the Flavivirus NS3 Protein. EMBO J. 2008, 27, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Klaitong, P.; Smith, D.R. Roles of Non-Structural Protein 4A in Flavivirus Infection. Viruses 2021, 13, 2077. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C. Dengue Virus NS4B Protein as a Target for Developing Antivirals. Front. Cell. Infect. Microbiol. 2022, 12, 959727. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.-S.; Lee, S.-A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Klema, V.J.; Padmanabhan, R.; Choi, K.H. Flaviviral Replication Complex: Coordination between RNA Synthesis and 5′-RNA Capping. Viruses 2015, 7, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Godói, I.P.; Lemos, L.L.P.; de Araújo, V.E.; Bonoto, B.C.; Godman, B.; Júnior, A.A.G. CYD-TDV Dengue Vaccine: Systematic Review and Meta-Analysis of Efficacy, Immunogenicity and Safety. J. Comp. Eff. Res. 2017, 6, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Bhatnagar, S.; Kumari, K.; Mittal, N.; Sukhralia, S.; At, S.G.; Dhanaraj, P.; Lal, R. Highly Conserved Epitopes of DENV Structural and Non-Structural Proteins: Candidates for Universal Vaccine Targets. Gene 2019, 695, 18–25. [Google Scholar] [CrossRef]
- Xu, X.; Vaughan, K.; Weiskopf, D.; Grifoni, A.; Diamond, M.S.; Sette, A.; Peters, B. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Quadeer, A.A.; Barton, J.P.; McKay, M.R. Cross-Serotypically Conserved Epitope Recommendations for a Universal T Cell-Based Dengue Vaccine. PLoS Neglected Trop. Dis. 2020, 14, e0008676. [Google Scholar] [CrossRef]
- Khan, A.M.; Miotto, O.; Nascimento, E.J.M.; Srinivasan, K.N.; Heiny, A.T.; Zhang, G.L.; Marques, E.T.; Tan, T.W.; Brusic, V.; Salmon, J.; et al. Conservation and Variability of Dengue Virus Proteins: Implications for Vaccine Design. PLoS Neglected Trop. Dis. 2008, 2, e272. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chuang, Y.-C.; Lin, Y.-S.; Liu, H.-S.; Liu, C.-C.; Perng, G.-C.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Neglected Trop. Dis. 2016, 10, e0004828. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular Leakage in Severe Dengue Virus Infections: A Potential Role for the Nonstructural Viral Protein NS1 and Complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika Virus Evades Interferon-Mediated Antiviral Response through the Co-Operation of Multiple Nonstructural Proteins in Vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Choi, J.Y.; Uyangaa, E.; Kim, S.B.; Kim, J.H.; Kim, B.S.; Kim, K.; Eo, S.K. Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways. PLoS Pathog. 2014, 10, e1004319. [Google Scholar] [CrossRef] [PubMed]
- Melian, E.B.; Hinzman, E.; Nagasaki, T.; Firth, A.E.; Wills, N.M.; Nouwens, A.S.; Blitvich, B.J.; Leung, J.; Funk, A.; Atkins, J.F.; et al. NS1′ of Flaviviruses in the Japanese Encephalitis Virus Serogroup Is a Product of Ribosomal Frameshifting and Plays a Role in Viral Neuroinvasiveness. J. Virol. 2010, 84, 1641–1647. [Google Scholar] [CrossRef]
- Young, L.B.; Melian, E.B.; Khromykh, A.A. NS1′ Colocalizes with NS1 and Can Substitute for NS1 in West Nile Virus Replication. J. Virol. 2013, 87, 9384–9390. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.Y.; Pijlman, G.P.; Kondratieva, N.; Hyde, J.; Mackenzie, J.M.; Khromykh, A.A. Role of Nonstructural Protein NS2A in Flavivirus Assembly. J. Virol. 2008, 82, 4731–4741. [Google Scholar] [CrossRef]
- Liu, W.J.; Chen, H.B.; Wang, X.J.; Huang, H.; Khromykh, A.A. Analysis of Adaptive Mutations in Kunjin Virus Replicon RNA Reveals a Novel Role for the Flavivirus Nonstructural Protein NS2A in Inhibition of Beta Interferon Promoter-Driven Transcription. J. Virol. 2004, 78, 12225–12235. [Google Scholar] [CrossRef] [PubMed]
- Bessaud, M.; Pastorino, B.A.; Peyrefitte, C.N.; Rolland, D.; Grandadam, M.; Tolou, H.J. Functional Characterization of the NS2B/NS3 Protease Complex from Seven Viruses Belonging to Different Groups inside the Genus Flavivirus. Virus Res. 2006, 120, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. Nuclear Localization of Non-Structural Protein 3 (NS3) during Dengue Virus Infection. Arch. Virol. 2021, 166, 1439–1446. [Google Scholar] [CrossRef]
- Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.H.; Vasudevan, S.G.; Hommel, U. Structural Basis for the Activation of Flaviviral NS3 Proteases from Dengue and West Nile Virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373. [Google Scholar] [CrossRef]
- Uchil, P.D.; Satchidanandam, V. Architecture of the Flaviviral Replication Complex. Protease, Nuclease, and Detergents Reveal Encasement within Double-Layered Membrane Compartments. J. Biol. Chem. 2003, 278, 24388–24398. [Google Scholar] [CrossRef]
- Muñoz-Jordán, J.L.; Laurent-Rolle, M.; Ashour, J.; Martínez-Sobrido, L.; Ashok, M.; Lipkin, W.I.; García-Sastre, A. Inhibition of Alpha/Beta Interferon Signaling by the NS4B Protein of Flaviviruses. J. Virol. 2005, 79, 8004–8013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Shi, P.-Y. Flavivirus NS4B Protein: Structure, Function, and Antiviral Discovery. Antivir. Res. 2022, 207, 105423. [Google Scholar] [CrossRef] [PubMed]
- Issur, M.; Geiss, B.J.; Bougie, I.; Picard-Jean, F.; Despins, S.; Mayette, J.; Hobdey, S.E.; Bisaillon, M. The Flavivirus NS5 Protein Is a True RNA Guanylyltransferase That Catalyzes a Two-Step Reaction to Form the RNA Cap Structure. RNA 2009, 15, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thurmond, S.; Zhou, K.; Sánchez-Aparicio, M.T.; Fang, J.; Lu, J.; Gao, L.; Ren, W.; Cui, Y.; Veit, E.C.; et al. Structural Basis for STAT2 Suppression by Flavivirus NS5. Nat. Struct. Mol. Biol. 2020, 27, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Selisko, B.; Wang, C.; Harris, E.; Canard, B. Regulation of Flavivirus RNA Synthesis and Replication. Curr. Opin. Virol. 2014, 9, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Slastnikova, T.A.; Ulasov, A.V.; Rosenkranz, A.A.; Sobolev, A.S. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol. 2018, 9, 1208. [Google Scholar] [CrossRef]
- Lopez-Denman, A.J.; Russo, A.; Wagstaff, K.M.; White, P.A.; Jans, D.A.; Mackenzie, J.M. Nucleocytoplasmic Shuttling of the West Nile Virus RNA-Dependent RNA Polymerase NS5 Is Critical to Infection. Cell. Microbiol. 2018, 20, e12848. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High Circulating Levels of the Dengue Virus Nonstructural Protein NS1 Early in Dengue Illness Correlate with the Development of Dengue Hemorrhagic Fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue Virus NS1 Protein Activates Cells via Toll-like Receptor 4 and Disrupts Endothelial Cell Monolayer Integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue Virus NS1 Triggers Endothelial Permeability and Vascular Leak That Is Prevented by NS1 Vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Chuang, Y.-C.; Liu, C.-C.; Ho, T.-S.; Lin, Y.-S.; Anderson, R.; Yeh, T.-M. Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection. Sci. Rep. 2017, 7, 6975. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-W.; Lu, Y.-T.; Huang, C.-H.; Lin, C.-F.; Anderson, R.; Liu, H.-S.; Yeh, T.-M.; Yen, Y.-T.; Wu-Hsieh, B.A.; Lin, Y.-S. Protection against Dengue Virus Infection in Mice by Administration of Antibodies against Modified Nonstructural Protein 1. PLoS ONE 2014, 9, e92495. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Lin, C.-F.; Wan, S.-W.; Wei, L.-S.; Chen, M.-C.; Yeh, T.-M.; Liu, H.-S.; Anderson, R.; Lin, Y.-S. Anti–Dengue Virus Nonstructural Protein 1 Antibodies Cause NO-Mediated Endothelial Cell Apoptosis via Ceramide-Regulated Glycogen Synthase Kinase-3β and NF-κB Activation. J. Immunol. 2013, 191, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Wan, S.-W.; Chen, M.-C.; Lin, S.-C.; Cheng, C.-C.; Chiu, S.-C.; Hsiao, Y.-L.; Lei, H.-Y.; Liu, H.-S.; Yeh, T.-M.; et al. Liver Injury Caused by Antibodies against Dengue Virus Nonstructural Protein 1 in a Murine Model. Mod. Pathol. 2008, 88, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Lin, J.; Lin, Y.-S.; Wang, S.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1–Induced Antibodies Cross-React with Human Plasminogen and Enhance Its Activation. J. Immunol. 2016, 196, 1218–1226. [Google Scholar] [CrossRef]
- Chen, H.-R.; Lai, Y.-C.; Yeh, T.-M. Dengue Virus Non-Structural Protein 1: A Pathogenic Factor, Therapeutic Target, and Vaccine Candidate. J. Biomed. Sci. 2018, 25, 58. [Google Scholar] [CrossRef]
- Carpio, K.L.; Barrett, A.D.T. Flavivirus NS1 and Its Potential in Vaccine Development. Vaccines 2021, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, N.J.; Afonso, M.Q.L.; Pedersolli, N.G.; de Oliveira, L.C.; Andrade, D.S.; Bleicher, L. Sequence, Structure and Function Relationships in Flaviviruses as Assessed by Evolutive Aspects of Its Conserved Non-Structural Protein Domains. Biochem. Biophys. Res. Commun. 2017, 492, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fink, K.; Züst, R.; Lim, S.P.; Qin, C.-F.; Shi, P.-Y. Flavivirus RNA Methylation. J. Gen. Virol. 2014, 95, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhong, Y.; Peng, C.; Gong, P. Crystal Structures of Flavivirus NS5 Guanylyltransferase Reveal a GMP-Arginine Adduct. J. Virol. 2022, 96, e00418-22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yi, G.; Du, F.; Chuang, Y.-C.; Vaughan, R.C.; Sankaran, B.; Kao, C.C.; Li, P. Structure and Function of the Zika Virus Full-Length NS5 Protein. Nat. Commun. 2017, 8, 14762. [Google Scholar] [CrossRef]
- Coloma, J.; Jain, R.; Rajashankar, K.R.; García-Sastre, A.; Aggarwal, A.K. Structures of NS5 Methyltransferase from Zika Virus. Cell Rep. 2016, 16, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Soh, T.S.; Chan, K.W.K.; Fung, S.S.Y.; Swaminathan, K.; Lim, S.P.; Shi, P.-Y.; Huber, T.; Lescar, J.; Luo, D.; et al. Flexibility of NS5 Methyltransferase-Polymerase Linker Region Is Essential for Dengue Virus Replication. J. Virol. 2015, 89, 10717–10721. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.S.; Lima, G.M.A.; Oliveira, K.I.Z.; Torres, N.U.; Maluf, F.V.; Guido, R.V.C.; Oliva, G. Crystal Structure of Zika Virus NS5 RNA-Dependent RNA Polymerase. Nat. Commun. 2017, 8, 14764. [Google Scholar] [CrossRef] [PubMed]
- Selisko, B.; Potisopon, S.; Agred, R.; Priet, S.; Varlet, I.; Thillier, Y.; Sallamand, C.; Debart, F.; Vasseur, J.-J.; Canard, B. Molecular Basis for Nucleotide Conservation at the Ends of the Dengue Virus Genome. PLoS Pathog. 2012, 8, e1002912. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tan, X.-F.; Thurmond, S.; Zhang, Z.-M.; Lin, A.; Hai, R.; Song, J. The Structure of Zika Virus NS5 Reveals a Conserved Domain Conformation. Nat. Commun. 2017, 8, 14763. [Google Scholar] [CrossRef]
- Zhao, Y.; Soh, T.S.; Zheng, J.; Chan, K.W.K.; Phoo, W.W.; Lee, C.C.; Tay, M.Y.F.; Swaminathan, K.; Cornvik, T.C.; Lim, S.P.; et al. A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication. PLoS Pathog. 2015, 11, e1004682. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Gong, P. Crystal Structure of the Full-Length Japanese Encephalitis Virus NS5 Reveals a Conserved Methyltransferase-Polymerase Interface. PLoS Pathog. 2013, 9, e1003549. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Cyr, M.; Longenecker, K.; Tripathi, R.; Sun, C.; Kempf, D.J. Crystal Structure of Full-Length Zika Virus NS5 Protein Reveals a Conformation Similar to Japanese Encephalitis Virus NS5. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Dubankova, A.; Boura, E. Structure of the Yellow Fever NS5 Protein Reveals Conserved Drug Targets Shared among Flaviviruses. Antivir. Res. 2019, 169, 104536. [Google Scholar] [CrossRef] [PubMed]
- El Sahili, A.; Soh, T.S.; Schiltz, J.; Gharbi-Ayachi, A.; Seh, C.C.; Shi, P.-Y.; Lim, S.P.; Lescar, J. NS5 from Dengue Virus Serotype 2 Can Adopt a Conformation Analogous to That of Its Zika Virus and Japanese Encephalitis Virus Homologues. J. Virol. 2019, 94, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, H.-Q.; Zhang, Q.-Y.; Lu, G.; Zhang, B.; Gong, P. A Conformation-Based Intra-Molecular Initiation Factor Identified in the Flavivirus RNA-Dependent RNA Polymerase. PLoS Pathog. 2020, 16, e1008484. [Google Scholar] [CrossRef]
- Biswal, M.; Yao, W.; Lu, J.; Chen, J.; Morrison, J.; Hai, R.; Song, J. A Conformational Selection Mechanism of Flavivirus NS5 for Species-Specific STAT2 Inhibition. Commun. Biol. 2024, 7, 76. [Google Scholar] [CrossRef]
- Klema, V.J.; Ye, M.; Hindupur, A.; Teramoto, T.; Gottipati, K.; Padmanabhan, R.; Choi, K.H. Dengue Virus Nonstructural Protein 5 (NS5) Assembles into a Dimer with a Unique Methyltransferase and Polymerase Interface. PLoS Pathog. 2016, 12, e1005451. [Google Scholar] [CrossRef]
- Saw, W.G.; Pan, A.; Manimekalai, M.S.S.; Grüber, G. Structural Features of Zika Virus Non-Structural Proteins 3 and -5 and Its Individual Domains in Solution as Well as Insights into NS3 Inhibition. Antivir. Res. 2017, 141, 73–90. [Google Scholar] [CrossRef]
- Ferrero, D.S.; Ruiz-Arroyo, V.M.; Soler, N.; Usón, I.; Guarné, A.; Verdaguer, N. Supramolecular Arrangement of the Full-Length Zika Virus NS5. PLoS Pathog. 2019, 15, e1007656. [Google Scholar] [CrossRef]
- Saw, W.; Chan, K.W.; Vasudevan, S.G.; Grüber, G. Zika Virus Nonstructural Protein 5 Residue R681 Is Critical for Dimer Formation and Enzymatic Activity. FEBS Lett. 2019, 593, 1272–1291. [Google Scholar] [CrossRef]
- Zoladek, J.; Nisole, S. Mosquito-Borne Flaviviruses and Type I Interferon: Catch Me If You Can! Front. Microbiol. 2023, 14, 1257024. [Google Scholar] [CrossRef] [PubMed]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.-Y.; García-Sastre, A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Bacardit, J.; Bachmair, A.; Holdsworth, M.J. The Eukaryotic N-End Rule Pathway: Conserved Mechanisms and Diverse Functions. Trends Cell Biol. 2014, 24, 603–611. [Google Scholar] [CrossRef]
- Morrison, J.; Laurent-Rolle, M.; Maestre, A.M.; Rajsbaum, R.; Pisanelli, G.; Simon, V.; Mulder, L.C.F.; Fernandez-Sesma, A.; García-Sastre, A. Dengue Virus Co-opts UBR4 to Degrade STAT2 and Antagonize Type I Interferon Signaling. PLoS Pathog. 2013, 9, e1003265. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M. The Many Faces of the Flavivirus NS5 Protein in Antagonism of Type I Interferon Signaling. J. Virol. 2017, 91, e01970-16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bühler, S.; Selisko, B.; Davidson, A.; Mulder, K.; Canard, B.; Miller, S.; Bartenschlager, R. Nuclear Localization of Dengue Virus Nonstructural Protein 5 Does Not Strictly Correlate with Efficient Viral RNA Replication and Inhibition of Type I Interferon Signaling. J. Virol. 2013, 87, 4545–4557. [Google Scholar] [CrossRef]
- Su, C.-I.; Tseng, C.-H.; Yu, C.-Y.; Lai, M.M.C. SUMO Modification Stabilizes Dengue Virus Nonstructural Protein 5 To Support Virus Replication. J. Virol. 2016, 90, 4308–4319. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, M.M.G.L.; Navarro, G.C.; Pallares, H.M.; Paletta, A.; De Maio, F.; Iglesias, N.G.; Gebhard, L.; Rouco, S.O.; Ojeda, D.S.; de Borba, L.; et al. Dengue Virus NS5 Degrades ERC1 during Infection to Antagonize NF-kB Activation. Proc. Natl. Acad. Sci. USA 2023, 120, e2220005120. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Morrison, J.; Rajsbaum, R.; Macleod, J.M.L.; Pisanelli, G.; Pham, A.; Ayllon, J.; Miorin, L.; Martínez-Romero, C.; Tenoever, B.R.; et al. The Interferon Signaling Antagonist Function of Yellow Fever Virus NS5 Protein Is Activated by Type I Interferon. Cell Host Microbe 2014, 16, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Chang, B.-L.; Yu, H.-P.; Liao, C.-L.; Lin, Y.-L. Blocking of Interferon-Induced Jak-Stat Signaling by Japanese Encephalitis Virus NS5 through a Protein Tyrosine Phosphatase-Mediated Mechanism. J. Virol. 2006, 80, 5908–5918. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, S.; Lai, C.; Lu, K.; Chiu, M.; Hsieh, T.; Wan, L.; Lin, C. Proteomic Analysis for Type I Interferon Antagonism of Japanese Encephalitis Virus NS5 Protein. Proteomics 2013, 13, 3442–3456. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, Z.; Li, Y.; Zhao, Z.; He, W.; Zohaib, A.; Song, Y.; Deng, C.; Zhang, B.; Chen, H.; et al. Japanese Encephalitis Virus NS5 Inhibits Type I Interferon (IFN) Production by Blocking the Nuclear Translocation of IFN Regulatory Factor 3 and NF-κB. J. Virol. 2017, 91, e00039-17. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, W.; Wang, Y.; Chen, K.; Xiao, F.; Hu, D.; Hui, L.; Liu, W.; Feng, Y.; Li, G.; et al. NS5 Conservative Site Is Required for Zika Virus to Restrict the RIG-I Signaling. Front. Immunol. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, S.; He, J.; Guest, J.D.; Ma, Z.; Yang, L.; Pierce, B.G.; Tang, Q.; Zhang, Y.-J. Zika Virus NS5 Protein Antagonizes Type I Interferon Production via Blocking TBK1 Activation. Virology 2019, 527, 180–187. [Google Scholar] [CrossRef]
- Lundberg, R.; Melén, K.; Westenius, V.; Jiang, M.; Österlund, P.; Khan, H.; Vapalahti, O.; Julkunen, I.; Kakkola, L. Zika Virus Non-Structural Protein NS5 Inhibits the RIG-I Pathway and Interferon Lambda 1 Promoter Activation by Targeting IKK Epsilon. Viruses 2019, 11, 1024. [Google Scholar] [CrossRef]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.G.; Vasconcelos, P.F.C.; et al. An Evolutionary NS1 Mutation Enhances Zika Virus Evasion of Host Interferon Induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Roby, J.A.; Esser-Nobis, K.; Dewey-Verstelle, E.C.; Fairgrieve, M.R.; Schwerk, J.; Lu, A.Y.; Soveg, F.W.; Hemann, E.A.; Hatfield, L.D.; Keller, B.C.; et al. Flavivirus Nonstructural Protein NS5 Dysregulates HSP90 to Broadly Inhibit JAK/STAT Signaling. Cells 2020, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Tomasi, T.B. The Heat Shock Protein 90-CDC37 Chaperone Complex Is Required for Signaling by Types I and II Interferons. J. Biol. Chem. 2006, 281, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika Virus Inhibits Type-I Interferon Production and Downstream Signaling. EMBO Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.A.; Zaheer, T.; Paracha, R.Z.; Ali, A. Structural Analysis and Insight into Zika Virus NS5 Mediated Interferon Inhibition. Infect. Genet. Evol. 2017, 51, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.Y.G.; Sng, J.D.J.; Setoh, Y.X.; Khromykh, A.A. Residue K28 of Zika Virus NS5 Protein Is Implicated in Virus Replication and Antagonism of STAT2. Microorganisms 2024, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chu, H.; Wen, L.; Yuan, S.; Chik, K.K.-H.; Yuen, T.T.-T.; Yip, C.C.-Y.; Wang, D.; Zhou, J.; Yin, F.; et al. Targeting SUMO Modification of the Non-Structural Protein 5 of Zika Virus as a Host-Targeting Antiviral Strategy. Int. J. Mol. Sci. 2019, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-E.; Ahn, J.-H. Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus. PLoS Pathog. 2015, 11, e1004785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tao, M.; Han, W.; Fan, Z.; Imran, M.; Cao, S.; Ye, J. Nuclear Localization of Zika Virus NS5 Contributes to Suppression of Type I Interferon Production and Response. J. Gen. Virol. 2019, 102, 001376. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Yuen, K.-S.; Chan, J.F.-W.; Chan, C.-P.; Wang, P.-H.; Cai, J.-P.; Zhang, S.; Liang, M.; Kok, K.-H.; Yuen, K.-Y.; et al. Selective Activation of Type II Interferon Signaling by Zika Virus NS5 Protein. J. Virol. 2017, 91, e00163-17. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, J.; Dias Junior, A.G.; Rigby, R.E.; Donald, C.L.; Mayer, A.; Sezgin, E.; Song, C.; Jin, B.; Hublitz, P.; Eggeling, C.; et al. Infection with a Brazilian Isolate of Zika Virus Generates RIG-I Stimulatory RNA and the Viral NS5 Protein Blocks Type I IFN Induction and Signaling. Eur. J. Immunol. 2018, 48, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Crown, R.A.; Sohn, J.A.; Seeger, C. West Nile Virus Infection Induces Depletion of IFNAR1 Protein Levels. Viral Immunol. 2011, 24, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Lubick, K.J.; Robertson, S.J.; McNally, K.L.; Freedman, B.A.; Rasmussen, A.L.; Taylor, R.T.; Walts, A.D.; Tsuruda, S.; Sakai, M.; Ishizuka, M.; et al. Flavivirus Antagonism of Type I Interferon Signaling Reveals Prolidase as a Regulator of IFNAR1 Surface Expression. Cell Host Microbe 2015, 18, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-T.; Hayashi, J.; Seeger, C. West Nile Virus Inhibits the Signal Transduction Pathway of Alpha Interferon. J. Virol. 2005, 79, 1343–1350. [Google Scholar] [CrossRef]
- Laurent-Rolle, M.; Boer, E.F.; Lubick, K.J.; Wolfinbarger, J.B.; Carmody, A.B.; Rockx, B.; Liu, W.; Ashour, J.; Shupert, W.L.; Holbrook, M.R.; et al. The NS5 Protein of the Virulent West Nile Virus NY99 Strain Is a Potent Antagonist of Type I Interferon-Mediated JAK-STAT Signaling. J. Virol. 2010, 84, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M.; Morris, K.L.; Shannon, J.G.; Robertson, S.J.; Mitzel, D.N.; Park, G.S.; Boer, E.; Wolfinbarger, J.B.; Bloom, M.E. Inhibition of Interferon-Stimulated JAK-STAT Signaling by a Tick-Borne Flavivirus and Identification of NS5 as an Interferon Antagonist. J. Virol. 2005, 79, 12828–12839. [Google Scholar] [CrossRef] [PubMed]
- Park, G.S.; Morris, K.L.; Hallett, R.G.; Bloom, M.E.; Best, S.M. Identification of Residues Critical for the Interferon Antagonist Function of Langat Virus NS5 Reveals a Role for the RNA-Dependent RNA Polymerase Domain. J. Virol. 2007, 81, 6936–6946. [Google Scholar] [CrossRef] [PubMed]
- Werme, K.; Wigerius, M.; Johansson, M. Tick-Borne Encephalitis Virus NS5 Associates with Membrane Protein Scribble and Impairs Interferon-Stimulated JAK-STAT Signalling. Cell. Microbiol. 2008, 10, 696–712. [Google Scholar] [CrossRef]
- Lopez-Denman, A.J.; Mackenzie, J.M. The IMPORTance of the Nucleus during Flavivirus Replication. Viruses 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Saade, M.; Ferrero, D.S.; Blanco-Ameijeiras, J.; Gonzalez-Gobartt, E.; Flores-Mendez, M.; Ruiz-Arroyo, V.M.; Martínez-Sáez, E.; Cajal, S.R.Y.; Akizu, N.; Verdaguer, N.; et al. Multimerization of Zika Virus-NS5 Causes Ciliopathy and Forces Premature Neurogenesis. Cell Stem Cell 2020, 27, 920–936.e8. [Google Scholar] [CrossRef] [PubMed]
- Pryor, M.J.; Rawlinson, S.M.; Butcher, R.E.; Barton, C.L.; Waterhouse, T.A.; Vasudevan, S.G.; Bardin, P.G.; Wright, P.J.; Jans, D.A.; Davidson, A.D. Nuclear Localization of Dengue Virus Nonstructural Protein 5 Through Its Importin α/β–Recognized Nuclear Localization Sequences Is Integral to Viral Infection. Traffic 2007, 8, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, H.; Sung, P.-Y.; Chiu, H.-C.; Yousuf, A.; Bird, J.; Lim, S.P.; Davidson, A.D. Serotype-Specific Differences in Dengue Virus Non-Structural Protein 5 Nuclear Localization. J. Biol. Chem. 2013, 288, 22621–22635. [Google Scholar] [CrossRef]
- Tay, M.Y.F.; Smith, K.; Ng, I.H.W.; Chan, K.W.K.; Zhao, Y.; Ooi, E.E.; Lescar, J.; Luo, D.; Jans, D.A.; Forwood, J.K.; et al. The C-Terminal 18 Amino Acid Region of Dengue Virus NS5 Regulates its Subcellular Localization and Contains a Conserved Arginine Residue Essential for Infectious Virus Production. PLoS Pathog. 2016, 12, e1005886. [Google Scholar] [CrossRef]
- Cheng, C.X.; Tan, M.J.A.; Chan, K.W.K.; Choy, M.M.J.; Roman, N.; Arnold, D.D.R.; Bifani, A.M.; Zu Kong, S.Y.; Bist, P.; Nath, B.K.; et al. Serotype-Specific Regulation of Dengue Virus NS5 Protein Subcellular Localization. ACS Infect. Dis. 2024, 10, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Luo, G. Zika Virus NS5 Nuclear Accumulation Is Protective of Protein Degradation and Is Required for Viral RNA Replication. Virology 2020, 541, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.N.; Smith, K.; Forwood, J.K.; Jans, D.A. Nuclear Import Inhibitor N-(4-Hydroxyphenyl) Retinamide Targets Zika Virus (ZIKV) Nonstructural Protein 5 to Inhibit ZIKV Infection. Biochem. Biophys. Res. Commun. 2017, 493, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M.; et al. A Nuclear Transport Inhibitor That Modulates the Unfolded Protein Response and Provides In Vivo Protection Against Lethal Dengue Virus Infection. J. Infect. Dis. 2014, 210, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Malone, W.; Perloff, M.; Buranelli, F.; Campa, T.; Dossena, G.; Magni, A.; Pizzichetta, M.; Andreoli, C.; Del Vecchio, M.; et al. Tolerability of the Synthetic Retinoid Fenretinide® (HPR). Eur. J. Cancer Clin. Oncol. 1989, 25, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.J.; Kang, M.H.; Villablanca, J.G.; Janeba, J.; Groshen, S.; Matthay, K.K.; Sondel, P.M.; Maris, J.M.; Jackson, H.A.; Goodarzian, F.; et al. Phase I Trial of Fenretinide Delivered Orally in a Novel Organized Lipid Complex in Patients with Relapsed/Refractory Neuroblastoma: A Report from the New Approaches to Neuroblastoma Therapy (NANT) Consortium. Pediatr. Blood Cancer 2013, 60, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, J.G.; London, W.B.; Naranjo, A.; McGrady, P.; Ames, M.M.; Reid, J.M.; McGovern, R.M.; Buhrow, S.A.; Jackson, H.; Stranzinger, E.; et al. Phase II Study of Oral Capsular 4-Hydroxyphenylretinamide (4-HPR/Fenretinide) in Pediatric Patients with Refractory or Recurrent Neuroblastoma: A Report from the Children’s Oncology Group. Clin. Cancer Res. 2011, 17, 6858–6866. [Google Scholar] [CrossRef] [PubMed]
- López-Denman, A.J.; Tuipulotu, D.E.; Ross, J.B.; Trenerry, A.M.; White, P.A.; Mackenzie, J.M. Nuclear Localisation of West Nile Virus NS5 Protein Modulates Host Gene Expression. Virology 2021, 559, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, S.M.; Pryor, M.J.; Wright, P.J.; Jans, D.A. CRM1-Mediated Nuclear Export of Dengue Virus RNA Polymerase NS5 Modulates Interleukin-8 Induction and Virus Production. J. Biol. Chem. 2009, 284, 15589–15597. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.A.; Risso, G.; Iglesias, N.G.; Shah, P.; Pozzi, B.; Gebhard, L.G.; Mammi, P.; Mancini, E.; Yanovsky, M.J.; Andino, R.; et al. The Dengue Virus NS5 Protein Intrudes in the Cellular Spliceosome and Modulates Splicing. PLoS Pathog. 2016, 12, e1005841. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cruz-Cosme, R.; Armstrong, N.; Obwolo, L.A.; Wen, F.; Hu, W.; Luo, M.-H.; Tang, Q. Molecular Cloning and Characterization of the Genes Encoding the Proteins of Zika Virus. Gene 2017, 628, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kovanich, D.; Saisawang, C.; Sittipaisankul, P.; Ramphan, S.; Kalpongnukul, N.; Somparn, P.; Pisitkun, T.; Smith, D.R. Analysis of the Zika and Japanese Encephalitis Virus NS5 Interactomes. J. Proteome Res. 2019, 18, 3203–3218. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.H.W.; Chan, K.W.-K.; Tan, M.J.A.; Gwee, C.P.; Smith, K.M.; Jeffress, S.J.; Saw, W.-G.; Swarbrick, C.M.D.; Watanabe, S.; Jans, D.A.; et al. Zika Virus NS5 Forms Supramolecular Nuclear Bodies That Sequester Importin-α and Modulate the Host Immune and Pro-Inflammatory Response in Neuronal Cells. ACS Infect. Dis. 2019, 5, 932–948. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Liu, S.; Lu, R.; Jiang, H.; Wang, N.; Luo, M.; Guo, L.; Xiao, J.; Bu, L.; et al. The RNA Polymerase of Cytoplasmically Replicating Zika Virus Binds with Chromatin DNA in Nuclei and Regulates Host Gene Transcription. Proc. Natl. Acad. Sci. USA 2022, 119, e2205013119. [Google Scholar] [CrossRef] [PubMed]
- Kesari, A.S.; Heintz, V.J.; Poudyal, S.; Miller, A.S.; Kuhn, R.J.; LaCount, D.J. Zika Virus NS5 Localizes at Centrosomes during Cell Division. Virology 2020, 541, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Ryman, K.D.; Barrett, A.D.; A Campbell, G. Mutation in NS5 Protein Attenuates Mouse Neurovirulence of Yellow Fever 17D Vaccine Virus. J. Gen. Virol. 1998, 79, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Lee, J.J.; Blaney, J.E.; Murphy, B.R.; Whitehead, S.S. Paired Charge-to-Alanine Mutagenesis of Dengue Virus Type 4 NS5 Generates Mutants with Temperature-Sensitive, Host Range, and Mouse Attenuation Phenotypes. J. Virol. 2002, 76, 525–531. [Google Scholar] [CrossRef] [PubMed]
- A Hanley, K.; Manlucu, L.R.; Manipon, G.G.; Hanson, C.T.; Whitehead, S.S.; Murphy, B.R.; Blaney, J.E. Introduction of Mutations into the Non-Structural Genes or 3′ Untranslated Region of an Attenuated Dengue Virus Type 4 Vaccine Candidate Further Decreases Replication in Rhesus Monkeys While Retaining Protective Immunity. Vaccine 2004, 22, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.H.; Durbin, A.P.; Marron, J.A.; Wanionek, K.A.; Thumar, B.; Pierro, D.J.; Schmidt, A.C.; Blaney, J.E.; Murphy, B.R.; Whitehead, S.S. Phase I Clinical Evaluation of rDEN4Δ30-200,201: A Live Attenuated Dengue 4 Vaccine Candidate Designed for Decreased Hepatotoxicity. Am. J. Trop. Med. Hyg. 2008, 79, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.E., Jr.; Durbin, A.P.; Murphy, B.R.; Whitehead, S.S. Development of a Live Attenuated Dengue Virus Vaccine Using Reverse Genetics. Viral Immunol. 2006, 19, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.E.; Speicher, J.; Hanson, C.T.; Sathe, N.S.; Whitehead, S.S.; Murphy, B.R.; Pletnev, A.G. Evaluation of St. Louis Encephalitis Virus/Dengue Virus Type 4 Antigenic Chimeric Viruses in Mice and Rhesus Monkeys. Vaccine 2008, 26, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, A.A.; Chanock, R.M.; Murphy, B.R.; Pletnev, A.G. Comparison of Live and Inactivated Tick-Borne Encephalitis Virus Vaccines for Safety, Immunogenicity and Efficacy in Rhesus Monkeys. Vaccine 2006, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.R.; Rumyantsev, A.A.; Maximova, O.A.; Speicher, J.M.; Heiss, B.; Murphy, B.R.; Pletnev, A.G. The Neurovirulence and Neuroinvasiveness of Chimeric Tick-Borne Encephalitis/Dengue Virus Can Be Attenuated by Introducing Defined Mutations into the Envelope and NS5 Protein Genes and the 3′ Non-Coding Region of the Genome. Virology 2010, 405, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.-Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2′-O Methylation of the Viral mRNA Cap Evades Host Restriction by IFIT Family Members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Züst, R.; Dong, H.; Li, X.-F.; Chang, D.C.; Zhang, B.; Balakrishnan, T.; Toh, Y.-X.; Jiang, T.; Li, S.-H.; Deng, Y.-Q.; et al. Rational Design of a Live Attenuated Dengue Vaccine: 2′-O-Methyltransferase Mutants Are Highly Attenuated and Immunogenic in Mice and Macaques. PLoS Pathog. 2013, 9, e1003521. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Dong, H.; Li, X.-F.; Xie, X.; Zhao, H.; Deng, Y.-Q.; Wang, X.-Y.; Ye, Q.; Zhu, S.-Y.; Wang, H.-J.; et al. Rational Design of a Flavivirus Vaccine by Abolishing Viral RNA 2′-O Methylation. J. Virol. 2013, 87, 5812–5819. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Katoh, H.; Kayama, H.; Saiga, H.; Okuyama, M.; Okamoto, T.; Umemoto, E.; Matsuura, Y.; Yamamoto, M.; Takeda, K. Ifit1 Inhibits Japanese Encephalitis Virus Replication through Binding to 5′ Capped 2′-O Unmethylated RNA. J. Virol. 2013, 87, 9997–10003. [Google Scholar] [CrossRef]
- Taylor, R.T.; Best, S.M. Assessing Ubiquitination of Viral Proteins: Lessons from Flavivirus NS5. Methods 2011, 55, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Ploegh, H.L. Antigen Presentation and the Ubiquitin-Proteasome System in Host–Pathogen Interactions. In Advances in Immunology; Academic Press: Cambridge, MA, USA, 2006; Volume 92, pp. 225–305. [Google Scholar]
- Rivino, L.; Kumaran, E.A.P.; Jovanovic, V.; Nadua, K.; Teo, E.W.; Pang, S.W.; Teo, G.H.; Gan, V.C.H.; Lye, D.C.; Leo, Y.S.; et al. Differential Targeting of Viral Components by CD4+ versus CD8+T Lymphocytes in Dengue Virus Infection. J. Virol. 2013, 87, 2693–2706. [Google Scholar] [CrossRef]
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silvera, C.G.T.; Costa, P.R.; et al. Human CD8+ T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated with Distinct Patterns of Protein Targets. J. Infect. Dis. 2015, 212, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.F.; Magnani, D.M.; Ricciardi, M.; Shin, Y.C.; Domingues, A.; Bailey, V.K.; Gonzalez-Nieto, L.; Rakasz, E.G.; Watkins, D.I.; Desrosiers, R.C. Use of a Recombinant Gamma-2 Herpesvirus Vaccine Vector against Dengue Virus in Rhesus Monkeys. J. Virol. 2017, 91, e00525-17. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Cantaert, T.; Colas, C.; Prot, M.; Casadémont, I.; Levillayer, L.; Thalmensi, J.; Langlade-Demoyen, P.; Gerke, C.; Bahl, K.; et al. A Modified mRNA Vaccine Targeting Immunodominant NS Epitopes Protects Against Dengue Virus Infection in HLA Class I Transgenic Mice. Front. Immunol. 2019, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.P.d.S.; Pereira, L.R.; Fabris, D.L.N.; Salvador, F.S.; Santos, R.A.; Zanotto, P.M.d.A.; Romano, C.M.; Amorim, J.H.; Ferreira, L.C.d.S. Production of a Recombinant Dengue Virus 2 NS5 Protein and Potential Use as a Vaccine Antigen. Clin. Vaccine Immunol. 2016, 23, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Schouest, B.; Grifoni, A.; Pham, J.; Mateus, J.; Sydney, J.; Brien, J.D.; De Silva, A.D.; Balmaseda, A.; Harris, E.; Sette, A.; et al. Pre-existing T Cell Memory against Zika Virus. J. Virol. 2021, 95, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika Virus Epitopes Reveals Immunodominant and Protective Roles for Dengue Virus Cross-Reactive CD8+ T Cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.d.S.; Vidal, P.O.; Amorim, J.H. In Silico Design of a Zika Virus Non-Structural Protein 5 Aiming Vaccine Protection against Zika and Dengue in Different Human Populations. J. Biomed. Sci. 2017, 24, 88. [Google Scholar] [CrossRef]
- Li, Z.; Bai, H.; Xi, X.; Tian, W.; Zhang, J.Z.; Zhou, D.; Si, L. PROTAC Vaccine: A New Way to Live Attenuated Vaccines. Clin. Transl. Med. 2022, 12, e1081. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A Live-Attenuated Zika Virus Vaccine Candidate Induces Sterilizing Immunity in Mouse Models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Arriaga, J.; Gromowski, G.D.; Tsetsarkin, K.A.; Firestone, C.-Y.; Castro-Jiménez, T.; Pletnev, A.G.; Cedillo-Barrón, L.; Whitehead, S.S. Decreased Accumulation of Subgenomic RNA in Human Cells Infected with Vaccine Candidate DEN4Δ30 Increases Viral Susceptibility to Type I Interferon. Vaccine 2018, 36, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M. Structural Chemistry of Human RNA Methyltransferases. ACS Chem. Biol. 2016, 11, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Brecher, M.; Chen, H.; Li, Z.; Banavali, N.K.; Jones, S.A.; Zhang, J.; Kramer, L.D.; Li, H. Identification and Characterization of Novel Broad-Spectrum Inhibitors of the Flavivirus Methyltransferase. ACS Infect. Dis. 2015, 1, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Samrat, S.K.; Bashir, Q.; Huang, Y.; Trieshmann, C.W.; Tharappel, A.M.; Zhang, R.; Chen, K.; Zheng, Y.G.; Li, Z.; Li, H. Broad-Spectrum Small-Molecule Inhibitors Targeting the SAM-Binding Site of Flavivirus NS5 Methyltransferase. ACS Infect. Dis. 2023, 9, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Stephen, P.; Baz, M.; Boivin, G.; Lin, S.-X. Structural Insight into NS5 of Zika Virus Leading to the Discovery of MTase Inhibitors. J. Am. Chem. Soc. 2016, 138, 16212–16215. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Butler, K.V.; Coloma, J.; Jin, J.; Aggarwal, A.K. Development of a S-Adenosylmethionine Analog That Intrudes the RNA-Cap Binding Site of Zika Methyltransferase. Sci. Rep. 2017, 7, 1632. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Sonntag, L.S.; Noble, C.; Nilar, S.H.; Ng, R.H.; Zou, G.; Monaghan, P.; Chung, K.Y.; Dong, H.; Liu, B.; et al. Small Molecule Inhibitors That Selectively Block Dengue Virus Methyltransferase. J. Biol. Chem. 2011, 286, 6233–6240. [Google Scholar] [CrossRef] [PubMed]
- Stahla-Beek, H.J.; April, D.G.; Saeedi, B.J.; Hannah, A.M.; Keenan, S.M.; Geiss, B.J. Identification of a Novel Antiviral Inhibitor of the Flavivirus Guanylyltransferase Enzyme. J. Virol. 2012, 86, 8730–8739. [Google Scholar] [CrossRef] [PubMed]
- Thames, J.E.; Waters, C.D.; Valle, C.; Bassetto, M.; Aouadi, W.; Martin, B.; Selisko, B.; Falat, A.; Coutard, B.; Brancale, A.; et al. Synthesis and Biological Evaluation of Novel Flexible Nucleoside Analogues That Inhibit Flavivirus Replication in Vitro. Bioorg. Med. Chem. 2020, 28, 115713. [Google Scholar] [CrossRef] [PubMed]
- Coutard, B.; Barral, K.; Lichière, J.; Selisko, B.; Martin, B.; Aouadi, W.; Lombardia, M.O.; Debart, F.; Vasseur, J.-J.; Guillemot, J.C.; et al. Zika Virus Methyltransferase: Structure and Functions for Drug Design Perspectives. J. Virol. 2017, 91, e02202-16. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, F.; Trist, I.; Coutard, B.; Decroly, E.; Querat, G.; Brancale, A.; Barral, K. Discovery of Novel Dengue Virus NS5 Methyltransferase Non-Nucleoside Inhibitors by Fragment-Based Drug Design. Eur. J. Med. Chem. 2017, 125, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mishra, S.; Shreya; Maurya, S.K. Recent Advances in the Discovery of Potent RNA-Dependent RNA-Polymerase (RdRp) Inhibitors Targeting Viruses. RSC Med. Chem. 2021, 12, 306–320. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, T.; Savini, C.; Seyedkazemi, S. Sofosbuvir, a Significant Paradigm Change in HCV Treatment. J. Clin. Transl. Hepatol. 2015, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Picarazzi, F.; Vicenti, I.; Saladini, F.; Zazzi, M.; Mori, M. Targeting the RdRp of Emerging RNA Viruses: The Structure-Based Drug Design Challenge. Molecules 2020, 25, 5695. [Google Scholar] [CrossRef]
- Zandi, K.; Bassit, L.; Amblard, F.; Cox, B.D.; Hassandarvish, P.; Moghaddam, E.; Yueh, A.; Rodrigues, G.O.L.; Passos, I.; Costa, V.V.; et al. Nucleoside Analogs with Selective Antiviral Activity against Dengue Fever and Japanese Encephalitis Viruses. Antimicrob. Agents Chemother. 2019, 63, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Eyer, L.; Zouharová, D.; Širmarová, J.; Fojtíková, M.; Štefánik, M.; Haviernik, J.; Nencka, R.; de Clercq, E.; Růžek, D. Antiviral Activity of the Adenosine Analogue BCX4430 against West Nile Virus and Tick-Borne Flaviviruses. Antivir. Res. 2017, 142, 63–67. [Google Scholar] [CrossRef]
- Hercík, K.; Kozak, J.; Šála, M.; Dejmek, M.; Hřebabecký, H.; Zborníková, E.; Smola, M.; Ruzek, D.; Nencka, R.; Boura, E. Adenosine Triphosphate Analogs Can Efficiently Inhibit the Zika Virus RNA-Dependent RNA Polymerase. Antivir. Res. 2017, 137, 131–133. [Google Scholar] [CrossRef]
- Konkolova, E.; Dejmek, M.; Hřebabecký, H.; Šála, M.; Böserle, J.; Nencka, R.; Boura, E. Remdesivir Triphosphate Can Efficiently Inhibit the RNA-Dependent RNA Polymerase from Various Flaviviruses. Antivir. Res. 2020, 182, 104899. [Google Scholar] [CrossRef]
- de Freitas, C.S.; Higa, L.M.; Sacramento, C.Q.; Ferreira, A.C.; Reis, P.A.; Delvecchio, R.; Monteiro, F.L.; Barbosa-Lima, G.; Westgarth, H.J.; Vieira, Y.R.; et al. Yellow Fever Virus Is Susceptible to Sofosbuvir Both in Vitro and in Vivo. PLoS Neglected Trop. Dis. 2019, 13, e0007072. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-T.; Colby-Germinario, S.P.; Hassounah, S.A.; Fogarty, C.; Osman, N.; Palanisamy, N.; Han, Y.; Oliveira, M.; Quan, Y.; Wainberg, M.A. Evaluation of Sofosbuvir (β-D-2′-Deoxy-2′-α-Fluoro-2′-β-C-Methyluridine) as an Inhibitor of Dengue Virus Replication. Sci. Rep. 2017, 7, 6345. [Google Scholar] [CrossRef]
- Dragoni, F.; Boccuto, A.; Picarazzi, F.; Giannini, A.; Giammarino, F.; Saladini, F.; Mori, M.; Mastrangelo, E.; Zazzi, M.; Vicenti, I. Evaluation of Sofosbuvir Activity and Resistance Profile against West Nile Virus in Vitro. Antivir. Res. 2020, 175, 104708. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, C.Q.; de Melo, G.R.; de Freitas, C.S.; Rocha, N.; Hoelz, L.V.B.; Miranda, M.; Fintelman-Rodrigues, N.; Marttorelli, A.; Ferreira, A.C.; Barbosa-Lima, G.; et al. The Clinically Approved Antiviral Drug Sofosbuvir Inhibits Zika Virus Replication. Sci. Rep. 2017, 7, 40920. [Google Scholar] [CrossRef] [PubMed]
- Niyomrattanakit, P.; Chen, Y.-L.; Dong, H.; Yin, Z.; Qing, M.; Glickman, J.F.; Lin, K.; Mueller, D.; Voshol, H.; Lim, J.Y.H.; et al. Inhibition of Dengue Virus Polymerase by Blocking of the RNA Tunnel. J. Virol. 2010, 84, 5678–5686. [Google Scholar] [CrossRef]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting Flavivirus RNA Dependent RNA Polymerase through a Pyridobenzothiazole Inhibitor. Antivir. Res. 2016, 134, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.Y.; Lescar, J.; Arora, R.; Benson, T.; Nilar, S.; et al. Potent Allosteric Dengue Virus NS5 Polymerase Inhibitors: Mechanism of Action and Resistance Profiling. PLoS Pathog. 2016, 12, e1005737. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Song, W.; Si, S.; Han, Y.; Jiang, J. Identification and Characterization of Zika Virus NS5 RNA-Dependent RNA Polymerase Inhibitors. Int. J. Antimicrob. Agents 2019, 54, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Celegato, M.; Sturlese, M.; Costa, V.V.; Trevisan, M.; Dias, A.S.L.; Passos, I.B.S.; Queiroz-Junior, C.M.; Messa, L.; Favaro, A.; Moro, S.; et al. Small-Molecule Inhibitor of Flaviviral NS3-NS5 Interaction with Broad-Spectrum Activity and Efficacy In Vivo. mBio 2023, 14, e03097-22. [Google Scholar] [CrossRef] [PubMed]
- Cannalire, R.; Chan, K.W.K.; Burali, M.S.; Gwee, C.P.; Wang, S.; Astolfi, A.; Massari, S.; Sabatini, S.; Tabarrini, O.; Mastrangelo, E.; et al. Pyridobenzothiazolones Exert Potent Anti-Dengue Activity by Hampering Multiple Functions of NS5 Polymerase. ACS Med. Chem. Lett. 2020, 11, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Franco, L.; Gushi, L.T.; Luiz, W.B.; Amorim, J.H. Seeking Flavivirus Cross-Protective Immunity. Front. Immunol. 2019, 10, 2260. [Google Scholar] [CrossRef]

| Flavivirus | Vaccine Candidate | Manufacturer | Vaccine Technology | Current Progress |
|---|---|---|---|---|
| DENV | Dengvaxia (CYD_TDV) | Sanofi Pasteur | Live chimeric virus | Licensed |
| Qdenga (TAK-003) | Takeda | Live chimeric virus | Licensed | |
| YFV | YF-17DD | Bio-Manguinhos/Fiocruz | Live attenuated virus | Licensed |
| YF-VAX/Stamaril | Sanofi Pasteur | Live attenuated virus | Licensed | |
| YFV-17D-213 | Federal State Unitary Enterprise of Chumakov Institute | Live attenuated virus | Licensed | |
| 17D-204 | Institut Pasteur Dakar (Senegal) | Live attenuated virus | Licensed | |
| JEV | IXIARO/JESPECT/JEEV (JE-VC) | Valneva | Inactivated whole virus | Licensed |
| IMOJEV (JE-CV) | Acambis/Sanofi Pastuer | Live attenuated virus | Licensed | |
| SA 14-14-2 | BBIL, CDIBP, Chengdu Institute of Biological Product | Live attenuated virus | Licensed | |
| JEVAC | Liaoning Cheng Da Biotechnology Co., Ltd. | Inactivated whole virus | Licensed | |
| JEBIK®V | Biken | Inactivated whole virus | Licensed | |
| ENCEVAC | KM-Biologics, Kaketsuken; Boryung | Inactivated whole virus | Licensed | |
| JEVAX | VABIOTECH | Live attenuated virus | Licensed | |
| JEV-GCC | Green Cross Corp | Inactivated whole virus | Licensed | |
| JENVAC | Bharat Biotech | Inactivated whole virus | Licensed | |
| TBEV | TBE-Moscow | Chumakov Institute of Poliomyelitis and Viral Encephalitides | Inactivated whole virus | Licensed |
| EnceVir | Microgen | Inactivated whole virus | Licensed | |
| FSME-Immun/Tico Vac | Baxter, Pfizer | Inactivated whole virus | Licensed | |
| Tick-E-Vac | Chumakov FSC R&D IBP RAS | Inactivated whole virus | Licensed | |
| SenTaiBao | Changchun Institute of Biological Products | Inactivated whole virus | Licensed | |
| Encepur | Bavarian Nordic | Inactivated whole virus | Licensed | |
| WNV | WN/DEN4-3′Δ30 | NIAID | Live chimeric virus | In Trials |
| HydroVax-001 | Najit Technologies | Inactivated whole virus | In Trials | |
| WN-80E | Hawaii Biotech | Protein subunit | In Trials | |
| VRC-WNVDNA020-00-VP | NIAID, NIH, DVBD, CDC | Genomic (DNA) | In Trials | |
| VRC-WNVDNA017-00-VP | NIAID, NIH, DVBD, CDC | Genomic (DNA) | In Trials | |
| ChimeriVax-WN02 | Sanofi Pasteur | Live chimeric virus | In Trials | |
| DENV | TetraVax-DV—TV-003 | NIAID | Live attenuated virus | In Trials |
| TetraVax-DV—TV-005 | NIAID | Live attenuated virus | In Trials | |
| TetraVax-DV—V181 | Merck & Co., Instituto Butantan, and Medigen Vaccine Biologics | Protein subunit | In Trials | |
| TDEN | WRAIR and GSK | Live attenuated virus | In Trials | |
| TDEN-F17 | WRAIR and GSK | Live attenuated virus | In Trials | |
| TDEN-F19 | WRAIR and GSK | Live attenuated virus | In Trials | |
| TVDV | WRAIR | Genomic (DNA) | In Trials | |
| CYD-1,2,3,4/VDV-2 | Sanofi | Live chimeric virus | In Trials | |
| Dengusiil | Serum Institute of India Pvt. Ltd. | Live attenuated virus | In Trials | |
| DSV4 | International Centre for Genetic Engineering and Biotechnology | Protein subunit | In Trials | |
| E80-mRNA | CAS laboratory of Molecular Virology and Immunology, Institute Pasteur of Shanghai | mRNA | In Trials | |
| TDENV-PIV | WRAIR and GSK | Inactivated whole virus | In Trials | |
| TDENV-LAV | WRAIR and GSK | Live attenuated virus | In Trials | |
| PepGNP-Dengue | Emergex Vaccines | Nanoparticle antigen delivery | In Trials | |
| rDEN1∆30 | NIAID | Live attenuated virus | In Trials | |
| rDEN2/4Δ30 | NIAID | Live chimeric virus | In Trials | |
| rDEN2/4Δ30(ME) | NIAID | Live chimeric virus | In Trials | |
| rDEN3∆30 | NIAID | Live attenuated virus | In Trials | |
| rDEN3/4Δ30(ME) | NIAID | Live chimeric virus | In Trials | |
| rDEN3Δ30/31-7164 | NIAID | Live attenuated virus | In Trials | |
| rDEN3-3′D4Δ30 | NIAID | Live chimeric virus | In Trials | |
| rDEN4Δ30 | NIAID | Live attenuated virus | In Trials | |
| rDEN4Δ30-200,201 | NIAID | Live attenuated virus | In Trials | |
| rDEN4Δ30-4995 | NIAID | Live attenuated virus | In Trials | |
| ZIKV | ZPIV | NIAID/WRAIR/BIDMC | Inactivated whole virus | In Trials |
| rZIKV/D4Δ30-713 | NIAID | Live chimeric virus | In Trials | |
| mRNA-1893 | Moderna | mRNA | In Trials | |
| MV-ZIKA | Themis Bioscience GmbH | Live chimeric virus | In Trials | |
| MV-ZIKA-RSP | Themis Bioscience GmbH | Viral vector-based | In Trials | |
| VRC-ZKADNA085-00-VP | NIAID/VRC | Genomic (DNA) | In Trials | |
| VRC-ZKADNA090-00-VP | NIAID/VRC | Genomic (DNA) | In Trials | |
| VLA1601 | Valneva | Inactivated whole virus | In Trials | |
| ChAdOx1 Zika | University of Oxford | Viral vector-based | In Trials | |
| BBV121 | Bharat Biotech | Inactivated whole virus | In Trials | |
| GLS-5700 | Inovio Pharmaceutical | Genomic (DNA) | In Trials | |
| PIZV (TAK-426) | Takeda Pharmaceuticals | Inactivated whole virus | In Trials | |
| ChimeriVax-Zika (CYZ) | Sanofi | Live chimeric virus | In Trials | |
| YFV | XRX-001 | Xcellerex | Inactivated whole virus | In Trials |
| SII YFV | Serum Institute of India Pvt. Ltd. | Live attenuated virus | In Trials | |
| vYF | Sanofi Pasteur | Live attenuated virus | In Trials | |
| JEV | JECEVAX | VABIOTECH | Inactivated whole virus | In Trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, J.Z.H.; De Hayr, L.; Khromykh, A.A.; Slonchak, A. The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics. Vaccines 2024, 12, 865. https://doi.org/10.3390/vaccines12080865
Goh JZH, De Hayr L, Khromykh AA, Slonchak A. The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics. Vaccines. 2024; 12(8):865. https://doi.org/10.3390/vaccines12080865
Chicago/Turabian StyleGoh, Jarvis Z. H., Lachlan De Hayr, Alexander A. Khromykh, and Andrii Slonchak. 2024. "The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics" Vaccines 12, no. 8: 865. https://doi.org/10.3390/vaccines12080865
APA StyleGoh, J. Z. H., De Hayr, L., Khromykh, A. A., & Slonchak, A. (2024). The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics. Vaccines, 12(8), 865. https://doi.org/10.3390/vaccines12080865






