Rotavirus A Infection Prevalence and Spatio-Temporal Genotype Shift among Under-Five Children in Amhara National Regional State, Ethiopia: A Multi-Center Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Sociodemographic and Clinical Data Collection
2.3. Nutritional Assessment and Clinical Severity Scorning of Diarrhea
2.4. Sample Collection, Transport, and Storage
2.5. RNA Extraction and RVA Detection
2.6. RNA Shipment for Genotyping and Sequencing
2.7. VP7 (G) and VP4 (P) Based Genotyping PCR
2.8. Gel Electrophoresis and Sequencing
2.9. Genotyping and Phylogenetic Analysis
2.10. VP7 and VP4 Protein Modeling
2.11. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics
3.2. Rotavirus A Prevalence
3.3. Genotypic Distribution of RVAs
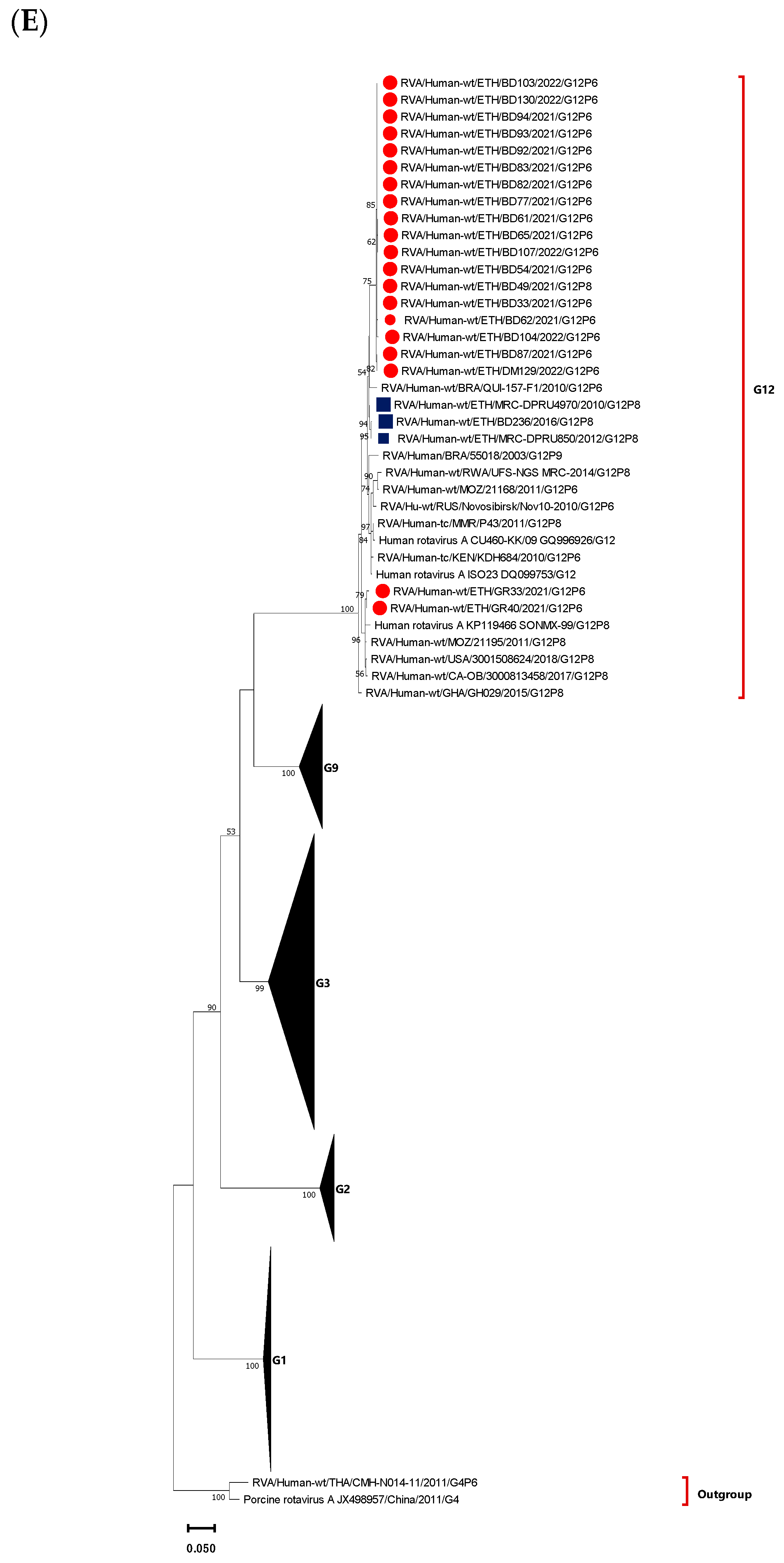
3.4. Phylogenetic Analysis of VP7 Gene of the Circulating RVA Strains
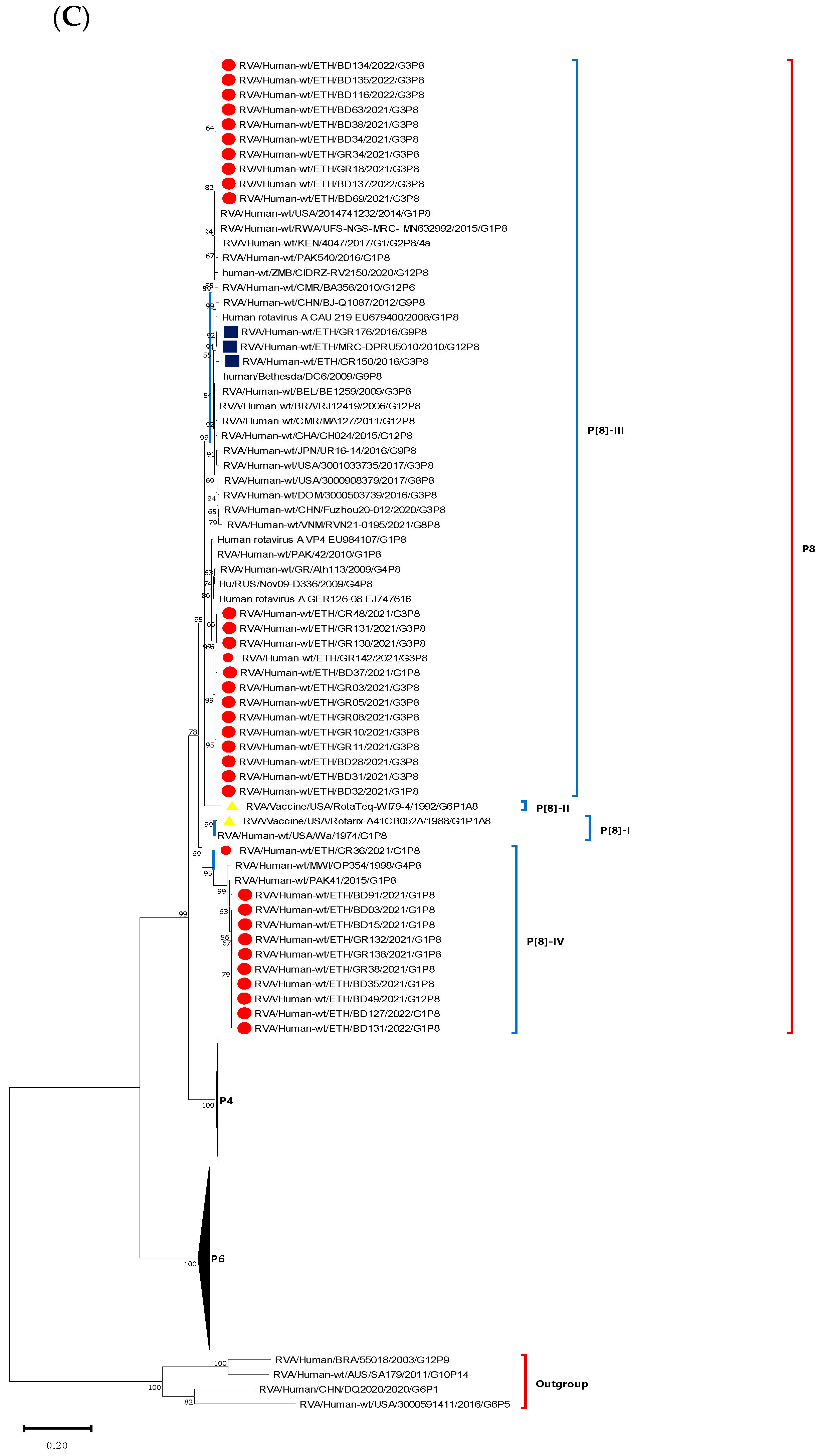
3.5. Phylogenetic Analysis of VP4 Gene of the Circulating RVA Strains
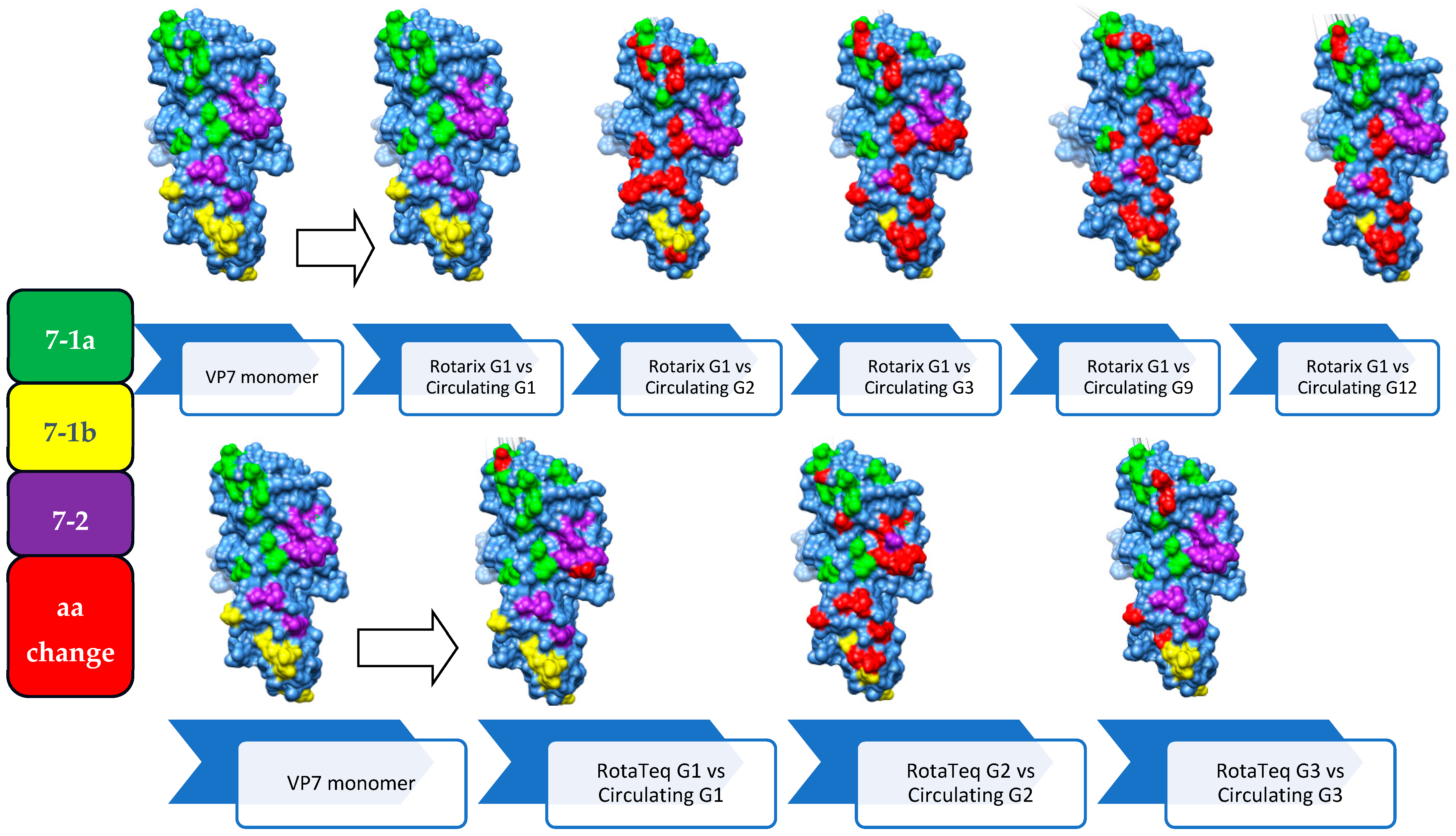
3.6. Comparison of the VP7 Antigenic Epitopes with Vaccine Strains
3.7. Comparison of VP4 Antigenic Epitopes with Vaccine Strains
4. Discussion
5. Conclusions and Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea among Children Younger than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Burton, A.; Lanata, C.; Boschi-Pinto, C.; Shibuya, K.; Steele, D.; Birmingham, M.; Glass, R.I. Global mortality associated with rotavirus disease among children in 2004. J. Infect. Dis. 2009, 200 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Bilcke, J.; Van Damme, P.; Van Ranst, M.; Hens, N.; Aerts, M.; Beutels, P. Estimating the incidence of symptomatic rotavirus infections: A systematic review and meta-analysis. PLoS ONE 2009, 4, e6060. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R. ICTV virus taxonomy profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Greenberg, H.B. Rotaviruses. In Fields Virology, 6th ed.; Howley, P.M., Knipe, D.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1347–1401. [Google Scholar]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef]
- Mihalov-Kovács, E.; Gellért, Á.; Marton, S.; Farkas, S.L.; Fehér, E.; Oldal, M.; Jakab, F.; Martella, V.; Bányai, K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infect. Dis. 2015, 21, 660. [Google Scholar] [CrossRef]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Martella, V.; Buonavoglia, C.; O’Shea, H. Identification of a G2-like porcine rotavirus bearing a novel VP4 type, P[32]. Vet. Res. 2010, 41, 73. [Google Scholar] [CrossRef] [PubMed]
- Esona, M.D.; Mijatovic-Rustempasic, S.; Conrardy, C.; Tong, S.; Kuzmin, I.V.; Agwanda, B.; Breiman, R.F.; Banyai, K.; Niezgoda, M.; Rupprecht, C.E. Reassortant group A rotavirus from straw-colored fruit bat (Eidolon helvum). Emerg. Infect. Dis. 2010, 16, 1844. [Google Scholar] [CrossRef]
- Abe, M.; Ito, N.; Masatani, T.; Nakagawa, K.; Yamaoka, S.; Kanamaru, Y.; Suzuki, H.; Shibano, K.-i.; Arashi, Y.; Sugiyama, M. Whole genome characterization of new bovine rotavirus G21P[29] and G24P[33] strains provides evidence for interspecies transmission. J. Gen. Virol. 2011, 92, 952–960. [Google Scholar] [CrossRef]
- Hayashi, M.; Murakami, T.; Kuroda, Y.; Takai, H.; Ide, H.; Awang, A.; Suzuki, T.; Miyazaki, A.; Nagai, M.; Tsunemitsu, H. Reinfection of adult cattle with rotavirus B during repeated outbreaks of epidemic diarrhea. Can. J. Vet. Res. = Rev. Can. Rech. Vet. 2016, 80, 189–196. [Google Scholar]
- Santos, N.; Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005, 15, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Khamrin, P.; Maneekarn, N.; Peerakome, S.; Chan-it, W.; Yagyu, F.; Okitsu, S.; Ushijima, H. Novel porcine rotavirus of genotype P[27] shares new phylogenetic lineage with G2 porcine rotavirus strain. Virology 2007, 361, 243–252. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Bányai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gómara, M.; Kirkwood, C.D.; Martella, V. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Mwenda, J.M.; Ntoto, K.M.; Abebe, A.; Enweronu-Laryea, C.; Amina, I.; Mchomvu, J.; Kisakye, A.; Mpabalwani, E.M.; Pazvakavambwa, I.; Armah, G.E. Burden and epidemiology of rotavirus diarrhea in selected African countries: Preliminary results from the African Rotavirus Surveillance Network. J. Infect. Dis. 2010, 202 (Suppl. S1), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- WHO. Meeting of the Strategic Advisory Group of Experts on immunization, October 2009—Conclusions and recommendations. Wkly. Epidemiol. Rec. = Relev. Épidémiol. Hebd. 2009, 84, 517–532. [Google Scholar]
- FMoH. Ethiopian National Expanded Programme on Immunization: Comprhencive Munlti-Year Plan 2016–2020 EPI; Federal Ministry of Health of Ethiopia: Addis Ababa, Ethiopia, 2015; p. 28.
- Wondimu, A.; Cao, Q.; Wilschut, J.C.; Postma, M.J. Factors associated with the uptake of newly introduced childhood vaccinations in Ethiopia: The cases of rotavirus and pneumococcal conjugate vaccines. BMC Public Health 2019, 19, 1656. [Google Scholar] [CrossRef] [PubMed]
- Atalell, K.A.; Liyew, A.M.; Alene, K.A. Spatial distribution of rotavirus immunization coverage in Ethiopia: A geospatial analysis using the Bayesian approach. BMC Infect. Dis. 2022, 22, 830. [Google Scholar] [CrossRef]
- Muhe, L.; Fredrikzon, B.; Habte, D. Clinical profile of rotavirus enteritis in Ethiopian children. Ethiop. Med. J. 1986, 24, 1–6. [Google Scholar]
- Abebe, A.; Abebe, S.; Giday, M.; Taffesse, B. Rotavirus infection in under-five children in Yekatit 12 Hospital. Ethiop. J. Health Dev. (EJHD) 1995, 9, 71–75. [Google Scholar]
- Stintzing, G.; Bäck, E.; Tufvesson, B.; Johnsson, T.; Wadström, T.; Habte, D. Seasonal fluctuations in the occurrence of enterotoxigenic bacteria and rotavirus in paediatric diarrhoea in Addis Ababa. Bull. World Health Organ. 1981, 59, 67. [Google Scholar] [PubMed]
- Bizuneh, T.; Abebe, A.; Lema, E. Rotavirus infection in under-five children in Jimma Hospital, Southwest Ethiopia. Ethiop. J. Health Dev. (EJHD) 2004, 18, 1–66. [Google Scholar] [CrossRef]
- Gelaw, A.; Pietsch, C.; Liebert, U.G. Molecular epidemiology of rotaviruses in Northwest Ethiopia after national vaccine introduction. Infect. Genet. Evol. 2018, 65, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Group, W.M.G.R.S.; de Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar]
- Lewis, K.D.; Dallas, M.J.; Victor, J.C.; Ciarlet, M.; Mast, T.C.; Ji, M.; Armah, G.; Zaman, K.; Ferraro, A.; Neuzil, K.M. Comparison of two clinical severity scoring systems in two multi-center, developing country rotavirus vaccine trials in Africa and Asia. Vaccine 2012, 30, A159–A166. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, T.; Vesikari, T. Rotavirus disease in Finnish children: Use of numerical scores for clinical severity of diarrhoeal episodes. Scand. J. Infect. Dis. 1990, 22, 259–267. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.-Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Gault, E.; Chikhi-Brachet, R.; Delon, S.; Schnepf, N.; Albiges, L.; Grimprel, E.; Girardet, J.-P.; Begue, P.; Garbarg-Chenon, A. Distribution of human rotavirus G types circulating in Paris, France, during the 1997–1998 epidemic: High prevalence of type G4. J. Clin. Microbiol. 1999, 37, 2373–2375. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Glass, R.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.; Bhan, M. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Maes, P.; Matthijnssens, J.; Rahman, M.; Van Ranst, M. RotaC: A web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.; Getahun, M.; Mapaseka, S.L.; Beyene, B.; Assefa, E.; Teshome, B.; Tefera, M.; Kebede, F.; Habtamu, A.; Haile-Mariam, T. Impact of rotavirus vaccine introduction and genotypic characteristics of rotavirus strains in children less than 5 years of age with gastroenteritis in Ethiopia: 2011–2016. Vaccine 2018, 36, 7043–7047. [Google Scholar] [CrossRef]
- Yassin, M.A.; Kirby, A.; Mengistu, A.A.; Arbide, I.; Dove, W.; Beyer, M.; Cunliffe, N.A.; Cuevas, L.E. Unusual norovirus and rotavirus genotypes in Ethiopia. Paediatr. Int. Child Health 2012, 32, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Assis, A.S.F.; Valle, D.A.; Antunes, G.R.; Tibiriça, S.H.C.; De Assis, R.M.S.; Leite, J.P.G.; De Carvalho, I.P.; da Rosa e Silva, M.L. Rotavirus epidemiology before and after vaccine introduction. J. Pediatr. 2013, 89, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Hallowell, B.D.; Parashar, U.D.; Curns, A.; DeGroote, N.P.; Tate, J.E. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination—United States, 2000–2018. Morb. Mortal. Wkly. Rep. 2019, 68, 539. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Rahman, M.; Heylen, E.; De Coster, S.; De Vos, S.; Arijs, I.; Novo, L.; Verstappen, N.; Van Ranst, M.; Matthijnssens, J. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010, 28, 7507–7513. [Google Scholar] [CrossRef]
- Saso, A.; Kampmann, B. (Eds.) Vaccine responses in newborns. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Lewnard, J.A.; Lopman, B.A.; Parashar, U.D.; Bar-Zeev, N.; Samuel, P.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Kang, G.; Pitzer, V.E. Naturally acquired immunity against rotavirus infection and gastroenteritis in children: Paired reanalyses of birth cohort studies. J. Infect. Dis. 2017, 216, 317–326. [Google Scholar] [CrossRef]
- Ojobor, C.; Olovo, C.; Onah, L.; Ike, A. Prevalence and associated factors to rotavirus infection in children less than 5 years in Enugu State, Nigeria. Virusdisease 2020, 31, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Junaid, S.A.; Umeh, C.; Olabode, A.O.; Banda, J.M. Incidence of rotavirus infection in children with gastroenteritis attending Jos university teaching hospital, Nigeria. Virol. J. 2011, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.B.; Fialho, A.M.; Maranhão, A.G.; Malta, F.C.; Andrade, J.d.S.R.d.; Assis, R.M.S.d.; Mouta, S.d.S.e.; Miagostovich, M.P.; Leite, J.P.G.; Machado Fumian, T. Rotavirus A in Brazil: Molecular epidemiology and surveillance during 2018–2019. Pathogens 2020, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Pitzer, V.E.; Alonso, W.J.; Vera, D.; Lopman, B.; Tate, J.; Viboud, C.; Parashar, U.D. Global seasonality of rotavirus disease. Pediatr. Infect. Dis. J. 2013, 32, e134–e147. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Castro, K.; Ávila, S.; Gutierrez, M.; Naumova, E.N.; Ulloa-Gutierrez, R.; Mora-Guevara, A. Seasonality of rotavirus hospitalizations at Costa Rica’s National Children’s Hospital in 2010–2015. Int. J. Environ. Res. Public Health 2019, 16, 2321. [Google Scholar] [CrossRef]
- Levy, K.; Hubbard, A.E.; Eisenberg, J.N. Seasonality of rotavirus disease in the tropics: A systematic review and meta-analysis. Int. J. Epidemiol. 2009, 38, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Staat, M.A.; Azimi, P.H.; Berke, T.; Roberts, N.; Bernstein, D.I.; Ward, R.L.; Pickering, L.K.; Matson, D.O. Clinical presentations of rotavirus infection among hospitalized children. Pediatr. Infect. Dis. J. 2002, 21, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Intusoma, U.; Sornsrivichai, V.; Jiraphongsa, C.; Varavithaya, W. Epidemiology, clinical presentations and burden of rotavirus diarrhea in children under five seen at Ramathibodi Hospital, Thailand. J. Med. Assoc. Thail. 2008, 91, 1350–1355. [Google Scholar]
- Rerksuppaphol, S.; Rerksuppaphol, L. Prevalence and clinical manifestations of rotavirus diarrhea in children of rural area of Thailand. Int. J. Collab. Res. Intern. Med. Public Health 2011, 3, 695–702. [Google Scholar]
- Ferdous, F.; Das, S.K.; Ahmed, S.; Farzana, F.D.; Latham, J.R.; Chisti, M.J.; Ud-Din, A.I.; Azmi, I.J.; Talukder, K.A.; Faruque, A.S. Severity of diarrhea and malnutrition among under five-year-old children in rural Bangladesh. Am. J. Trop. Med. Hyg. 2013, 89, 223. [Google Scholar] [CrossRef]
- Samani, E.F.Z.E.; Willett, W.C.; Ware, J.H. Association of malnutrition and diarrhea in children aged under five years: A prospective follow-up study in a rural Sudanese community. Am. J. Epidemiol. 1988, 128, 93–105. [Google Scholar] [CrossRef]
- Damtie, D.; Melku, M.; Tessema, B.; Vlasova, A.N. Prevalence and genetic diversity of rotaviruses among under-five children in Ethiopia: A systematic review and meta-analysis. Viruses 2020, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Roczo-Farkas, S.; Kirkwood, C.D.; Cowley, D.; Barnes, G.L.; Bishop, R.F.; Bogdanovic-Sakran, N.; Boniface, K.; Donato, C.M.; Bines, J.E. The impact of rotavirus vaccines on genotype diversity: A comprehensive analysis of 2 decades of Australian surveillance data. J. Infect. Dis. 2018, 218, 546–554. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Arcila-Quiceno, V.; Trejos-Suarez, J.; Ruiz-Saenz, J. Prevalence and molecular typing of rotavirus in children with acute diarrhoea in Northeastern Colombia. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e34. [Google Scholar] [CrossRef]
- Abebe, A.; Teka, T.; Kassa, T.; Seheri, M.; Beyene, B.; Teshome, B.; Kebede, F.; Habtamu, A.; Maake, L.; Kassahun, A.; et al. Hospital-based surveillance for rotavirus gastroenteritis in children younger than 5 years of age in Ethiopia: 2007–2012. Pediatr. Infect. Dis. J. 2014, 33 (Suppl. S1), S28–S33. [Google Scholar] [CrossRef]
- Esona, M.D.; Armah, G.E.; Duncan Steele, A. Rotavirus VP4 and VP7 genotypes circulating in Cameroon: Identification of unusual types. J. Infect. Dis. 2010, 202 (Suppl. S1), S205–S211. [Google Scholar] [CrossRef]
- Kiulia, N.M.; Nyaga, M.M.; Seheri, M.L.; Wolfaardt, M.; Van Zyl, W.B.; Esona, M.D.; Irimu, G.; Inoti, M.; Gatinu, B.W.; Njenga, P.K. Rotavirus G and P types circulating in the eastern region of Kenya: Predominance of G9 and emergence of G12 genotypes. Pediatr. Infect. Dis. J. 2014, 33, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, G.; Li, C.; Niu, P.; Li, X.; Gao, Q.; Guo, K.; Zhang, R.; Wang, J.; Ma, X. Rotavirus Infection and Genotyping in Yantai, Shandong Province, 2017–2019. Trop. Med. Infect. Dis. 2023, 8, 101. [Google Scholar] [CrossRef]
- João, E.D.; Munlela, B.; Chissaque, A.; Chilaúle, J.; Langa, J.; Augusto, O.; Boene, S.S.; Anapakala, E.; Sambo, J.; Guimarães, E. Molecular epidemiology of rotavirus a strains pre-and post-vaccine (Rotarix®) introduction in Mozambique, 2012–2019: Emergence of genotypes G3P[4] and G3P[8]. Pathogens 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Lartey, B.L.; Damanka, S.; Dennis, F.E.; Enweronu-Laryea, C.C.; Addo-Yobo, E.; Ansong, D.; Kwarteng-Owusu, S.; Sagoe, K.W.; Mwenda, J.M.; Diamenu, S.K. Rotavirus strain distribution in Ghana pre-and post-rotavirus vaccine introduction. Vaccine 2018, 36, 7238–7242. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Delogu, R.; Fiore, L.; Ruggeri, F.M.; Group, R.-I.S. Genetic variability of VP7, VP4, VP6 and NSP4 genes of common human G1P[8] rotavirus strains circulating in Italy between 2010 and 2014. Virus Res. 2016, 220, 117–128. [Google Scholar] [CrossRef]
- Mao, T.; Wang, M.; Wang, J.; Ma, Y.; Liu, X.; Wang, M.; Sun, X.; Li, L.; Li, H.; Zhang, Q. Phylogenetic analysis of the viral proteins VP4/VP7 of circulating human rotavirus strains in China from 2016 to 2019 and comparison of their antigenic epitopes with those of vaccine strains. Front. Cell. Infect. Microbiol. 2022, 12, 927490. [Google Scholar] [CrossRef]
- Mathew, S.; Al Khatib, H.A.; Al Ibrahim, M.; Al Ansari, K.; Smatti, M.K.; Nasrallah, G.K.; Ibrahim, E.; Al Thani, A.A.; Zaraket, H.; Yassine, H.M. Vaccine evaluation and genotype characterization in children infected with rotavirus in Qatar. Pediatr. Res. 2023, 94, 477–485. [Google Scholar] [CrossRef]
- Zeller, M.; Patton, J.T.; Heylen, E.; De Coster, S.; Ciarlet, M.; Van Ranst, M.; Matthijnssens, J. Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in Rotarix and RotaTeq. J. Clin. Microbiol. 2012, 50, 966–976. [Google Scholar] [CrossRef]
- Manouana, G.P.; Niendorf, S.; Tomazatos, A.; Ngwese, M.M.; Maloum, M.N.; Moure, P.A.N.; Matsougou, G.B.; Ategbo, S.; Rossatanga, E.G.; Bock, C.T. Molecular surveillance and genetic divergence of rotavirus A antigenic epitopes in Gabonese children with acute gastroenteritis. EBioMedicine 2021, 73, 103648. [Google Scholar] [CrossRef]
- Mwape, I.; Laban, N.M.; Chibesa, K.; Moono, A.; Silwamba, S.; Malisheni, M.M.; Chisenga, C.; Chauwa, A.; Simusika, P.; Phiri, M. Characterization of Rotavirus Strains Responsible for Breakthrough Diarrheal Diseases among Zambian Children Using Whole Genome Sequencing. Vaccines 2023, 11, 1759. [Google Scholar] [CrossRef]
- Bonura, F.; Mangiaracina, L.; Filizzolo, C.; Bonura, C.; Martella, V.; Ciarlet, M.; Giammanco, G.M.; De Grazia, S. Impact of vaccination on rotavirus genotype diversity: A nearly two-decade-long epidemiological study before and after rotavirus vaccine introduction in Sicily, Italy. Pathogens 2022, 11, 424. [Google Scholar] [CrossRef]
- Ogden, K.M.; Tan, Y.; Akopov, A.; Stewart, L.S.; McHenry, R.; Fonnesbeck, C.J.; Piya, B.; Carter, M.H.; Fedorova, N.B.; Halpin, R.A. Multiple introductions and antigenic mismatch with vaccines may contribute to increased predominance of G12P[8] rotaviruses in the United States. J. Virol. 2019, 93, e01476-18. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Pal, D. The interrelationships of side-chain and main-chain conformations in proteins. Prog. Biophys. Mol. Biol. 2001, 76, 1–102. [Google Scholar] [CrossRef] [PubMed]






| Variables | Frequency (%) |
|---|---|
| Age in months (mean ± SD) 26.4 ± 15.2 range 2–59 MO | |
| Sex | |
| Male | 308 (57.4) |
| Female | 229 (42.6) |
| Residence | |
| Urban | 494 (92) |
| Rural | 43 (8.0) |
| Immunization | |
| Yes | 524 (97.6) |
| No | 13 (2.4) |
| Admission status | |
| Yes | 41 (7.6) |
| No | 496 (92.4) |
| Vomiting | |
| Yes | 233 (43.4) |
| No | 304 (56.6) |
| Child’s thirst status | |
| Drink normally | 366 (68.2) |
| Thirsty/drink eagerly | 152 (28.3) |
| Drink poorly/not able to drink | 13 (2.4) |
| N/A | 6 (1.1) |
| Sunken eyes | |
| Yes | 83 (15.5) |
| No | 454 (84.5) |
| Irritable/restless | |
| Yes | 133 (24.8) |
| No | 404 (75.2) |
| Lethargic | |
| Yes | 108 (20.1) |
| No | 429 (79.9) |
| Mental status of the child | |
| Normal | 528 (98.3) |
| Altered | 9 (1.7) |
| Fever | |
| Yes | 182 (33.9) |
| No | 355 (66.1) |
| Dehydration status | |
| No dehydration | 496 (92.2) |
| Some dehydration | 40 (7.4) |
| Severe dehydration | 1 (0.2) |
| Given IV fluid | |
| Yes | 57 (10.6) |
| No | 480 (89.4) |
| Vesikari clinical severity | |
| Mild | 198 (36.9) |
| Moderate | 257 (47.9) |
| Severe | 82 (15.2) |
| Variables | RVA | ||
|---|---|---|---|
| Positive (%) | Negative (%) | p-Value | |
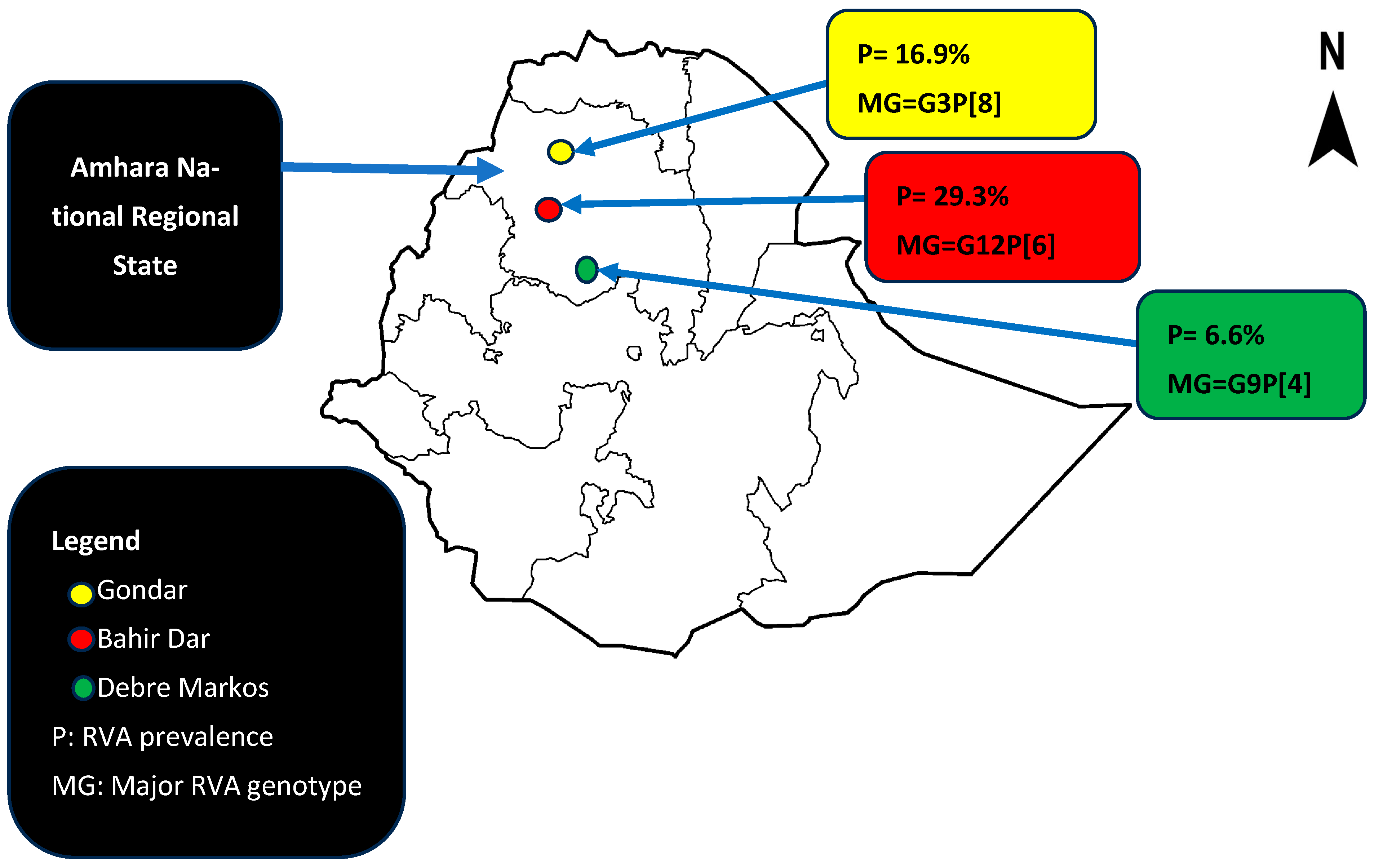
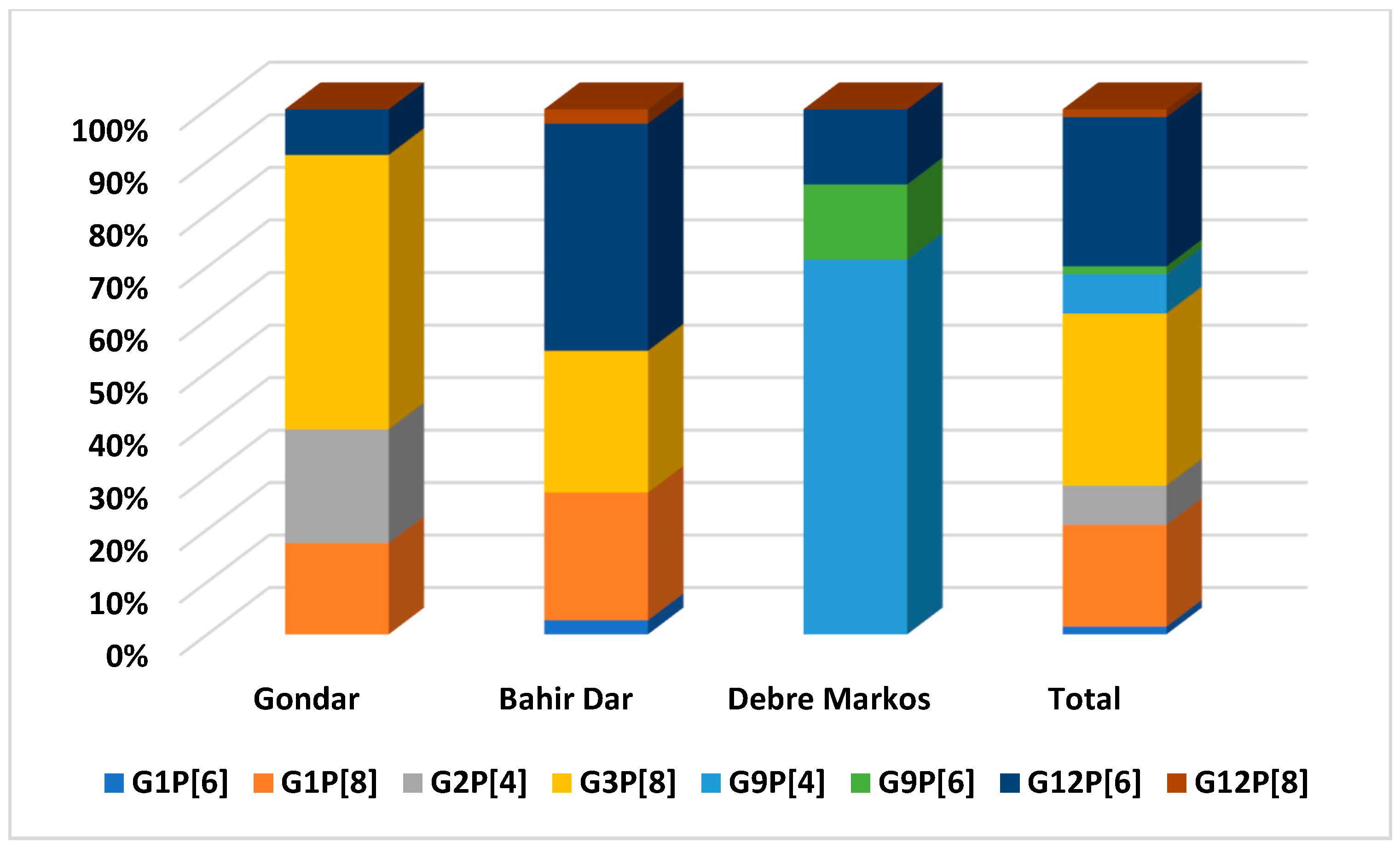
| Study locations | |||
| Gondar | 44 (16.9) | 217 (83.1) | 0.001 |
| Bahir Dar | 41 (29.3) | 99 (70.7) | |
| Debre Markos | 9 (6.6) | 127 (93.4) | |
| Age (in months) | |||
| 0–11 | 17 (19.8) | 69 (80.2) | 0.04 |
| 12–23 | 46 (23.8) | 147 (76.2) | |
| 24–59 | 31 (12.0) | 227 (88.0) | |
| Vomiting | |||
| Yes | 54 (23.2) | 179 (76.8) | 0.002 |
| No | 30 (13.2) | 263 (86.8) | |
| Sunken eyes | |||
| Yes | 22 (26.5) | 61 (73.5) | 0.019 |
| No | 72 (15.9) | 382 (84.1) | |
| IV fluid given | |||
| Yes | 16 (28.1) | 41 (71.9) | 0.026 |
| No | 78 (16.2) | 402 (83.8) | |
| Vesikari clinical severity | |||
| Mild | 23 (11.6) | 175 (88.4) | 0.006 |
| Moderate | 49 (19.1) | 208 (80.9) | |
| Severe | 22 (26.8) | 60 (73.2) | |
| Stunting (HFA) | |||
| Normal | 63 (16.8) | 313 (83.2) | 0.329 |
| Moderately stunted | 14 (15.9) | 74 (84.1) | |
| Severely stunted | 15 (24.2) | 47 (75.8) | |
| Weight (WFA) | |||
| Normal | 75 (16.8) | 372 (83.2) | 0.005 |
| Moderately underweight | 10 (15.4) | 55 (84.6) | |
| Severely underweight | 7 (50) | 7 (50) | |
| Wasting (WFH) | |||
| Normal | 75 (16.6) | 376 (83.4) | 0.007 |
| Moderately wasted | 8 (14.8) | 46 (85.2) | |
| Severely wasted | 9 (42.9) | 12 (57.1) | |
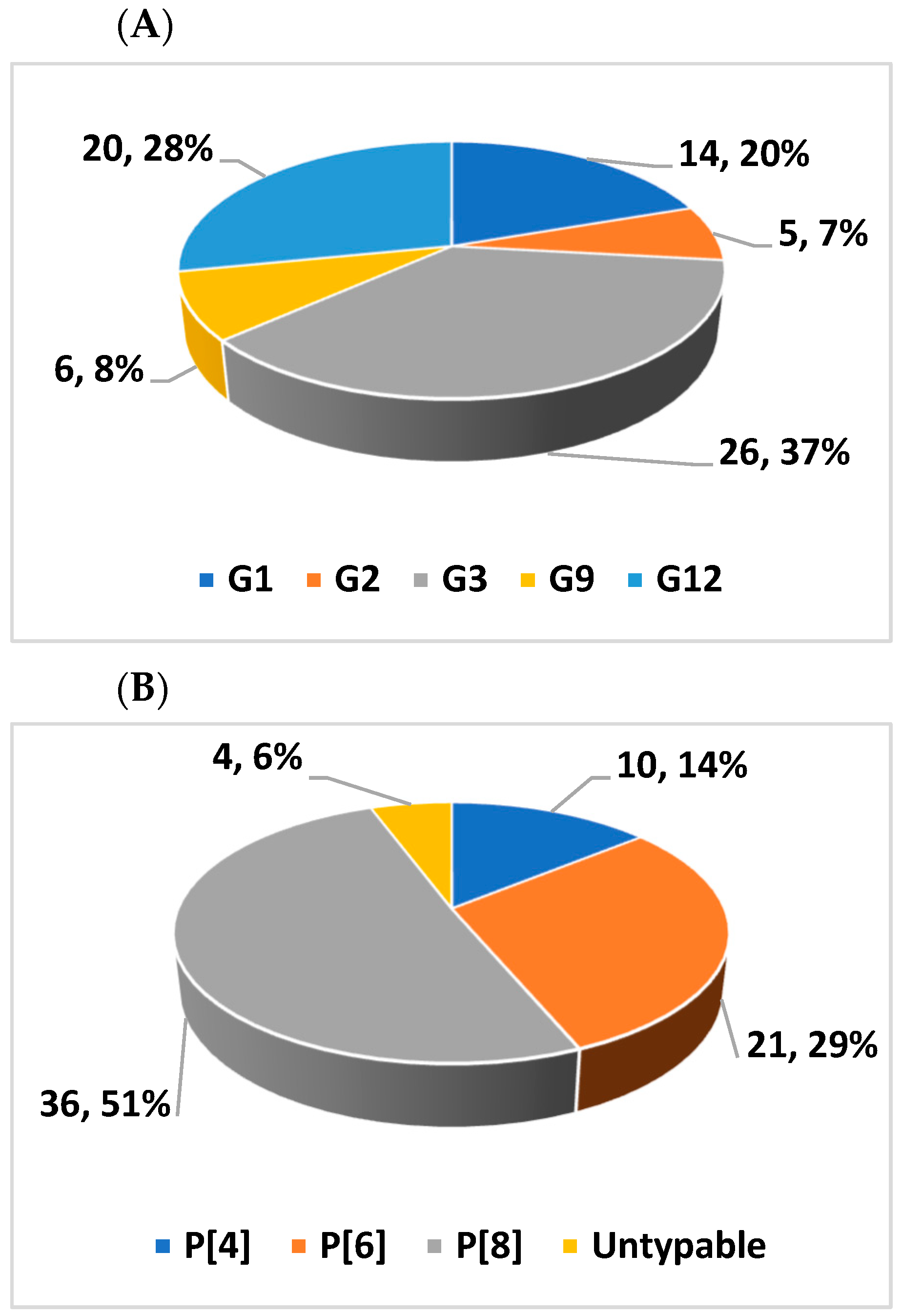
| G/P Combinations | Isolates (n = 67) | Proportion (%) |
|---|---|---|
| G1P[6] | 1 | 1.5 |
| G1P[8] | 13 | 19.4 |
| G2P[4] | 5 | 7.5 |
| G3P[8] | 22 | 32.8 |
| G9P[4] | 5 | 7.5 |
| G9P[6] | 1 | 1.5 |
| G12P[6] | 19 | 28.4 |
| G12P[8] | 1 | 3 |
| Total | 67 | 100 |
| Strain (No. of Sequences) | Lineage | Epitope 7-1a | Epitope 7-1b | Epitope 7-2 | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 87 | 91 | 94 | 96 | 97 | 98 | 99 | 100 | 104 | 123 | 125 | 129 | 130 | 291 | 201 | 211 | 212 | 213 | 238 | 242 | 143 | 145 | 146 | 147 | 148 | 190 | 217 | 221 | 264 | ||
| Rotarix/A41CB052A/G1P[8] | II | T | T | N | G | E | W | K | D | Q | S | V | V | D | K | Q | N | V | D | N | T | K | D | Q | N | L | S | M | N | G |
| RotaTeq/W179-9/G1P7[5] | III | T | T | N | G | D | W | K | D | Q | S | V | V | D | K | Q | N | V | D | N | T | K | D | Q | S | L | S | M | N | G |
| GR36/2021/G1P8 (12) | II | T | T | N | G | E | W | K | D | Q | S | V | V | D | K | Q | N | V | D | N | T | K | D | Q | N | L | S | M | N | G |
| BD44/2021/G1P6 (1) | II | T | T | N | G | E | W | K | D | Q | S | V | V | D | K | Q | N | V | D | N | T | K | D | Q | N | L | S | M | N | G |
| RotaTeq/SC2-9/G2P7[5] | II | A | N | S | D | E | W | E | N | Q | D | T | M | N | K | Q | D | T | M | N | K | Q | D | V | S | N | S | R | D | N |
| GR13/2021/G2P4 (5) | IV | T | N | S | N | E | W | E | N | Q | D | T | M | N | K | Q | D | V | D | N | N | R | D | N | T | S | D | I | S | G |
| RotaTeq/wi78-8/G3P7[5] | II | T | T | N | N | S | W | K | D | Q | D | A | V | D | K | Q | D | A | N | K | D | K | D | A | T | L | S | E | A | G |
| GR03/2021/G3P8 (11) | I | T | N | N | N | S | W | K | D | Q | D | A | V | D | K | Q | D | T | N | N | N | K | D | A | T | L | S | E | A | G |
| GR10/2021/G3P8 (1) | I | T | N | D | N | S | W | K | D | Q | D | A | V | D | K | Q | D | T | N | N | N | K | D | A | T | L | S | E | A | G |
| BD34/2021/G3P8 (12) | I | T | T | N | N | S | W | K | D | Q | D | A | V | D | K | Q | D | T | N | N | N | K | D | A | T | L | S | E | D | G |
| DM01/2021/G9P4 (5) | I | T | T | G | T | E | W | K | D | Q | D | A | I | D | K | Q | N | T | A | D | N | K | D | S | T | L | S | E | S | G |
| DM31/2021/G9P6 (1) | I | T | T | G | T | E | W | K | D | Q | D | A | I | D | K | Q | N | T | A | D | N | K | D | S | T | L | S | E | S | G |
| RotaTeq/Br-B-9/G4P7[5] | II | S | T | S | T | E | W | K | D | Q | N | L | I | D | K | Q | D | T | A | D | T | R | A | S | G | E | S | T | S | G |
| RotaTeq/WI79-4/G6P1A[8] | - | V | N | A | T | E | W | K | D | Q | D | A | V | E | K | Q | N | P | D | N | A | K | D | S | T | Q | S | T | T | G |
| BD33/2021/G12P6 (18) | S | T | T | P | D | W | T | N | Q | D | S | V | D | K | Q | D | V | T | N | N | Q | Q | N | S | L | S | E | A | G | |
| BD49/2021/G12P8 (1) | S | T | T | P | D | W | T | N | Q | D | S | V | D | K | Q | D | V | T | N | N | Q | Q | N | S | L | S | E | A | G | |
| BD104/2022/G12P6 (1) | S | T | T | P | D | W | T | S | Q | D | S | V | D | E | Q | D | V | T | N | N | Q | Q | N | S | L | S | E | A | G | |
 Different from both Rotarix and RotaTeq.
Different from both Rotarix and RotaTeq.  Different from Rotarix.
Different from Rotarix.  Different from the closest RotaTeq.
Different from the closest RotaTeq.  Sites involved in neutralization escape.
Sites involved in neutralization escape.| Strain (No. of Sequences) | Lineage | Epitope 8-1 | Epitope 8-2 | Epitope 8-3 | Epitope 8-4 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 146 | 148 | 150 | 188 | 190 | 192 | 193 | 194 | 195 | 196 | 180 | 183 | 113 | 114 | 115 | 116 | 125 | 131 | 132 | 133 | 135 | 86 | 87 | 88 | 89 | 90 | ||
| Rotarix/A41CB52A/G1P[8] | I | D | S | Q | E | S | T | N | L | N | N | I | T | A | N | P | V | D | S | S | N | D | N | S | N | T | N | G |
| RotaTeq/WI79-4/G6P1A[8] | II | D | S | Q | E | S | T | N | L | N | D | I | T | A | N | P | V | D | N | R | N | D | D | S | N | T | N | G |
| GR03/2021/G3P8 (12) | III | D | S | Q | D | S | T | N | L | D | G | I | T | A | N | P | V | D | N | N | N | D | D | S | N | T | N | G |
| BD34/2021/G3P8 (9) | III | D | S | Q | D | S | T | N | L | N | G | I | T | A | N | P | V | D | N | N | N | D | D | S | N | T | N | G |
| BD63/2021/G3P8 (1) | III | D | S | Q | D | S | T | N | L | N | G | I | T | A | N | P | V | D | N | R | N | D | D | S | N | T | N | G |
| GR36/2021/G1P8 (1) | IV | D | S | Q | E | S | T | N | L | T | S | I | T | A | N | P | V | D | S | S | N | D | N | S | N | T | N | G |
| GR38/2021/G1P8 (10) | IV | D | S | Q | E | S | T | D | L | T | S | I | T | A | D | P | V | D | S | S | N | D | N | S | N | T | N | G |
| BD49/2021/G12P8 (1) | IV | D | S | Q | E | S | T | D | L | T | S | I | T | A | D | P | V | D | S | S | N | D | N | S | N | T | N | G |
| RotaTeq-WI79-9/G1P7[5] | G | T | I | G | R | I | T | N | Y | A | S | E | N | T | S | E | T | S | S | N | A | D | P | T | G | P | G | |
| BD33/2021/G12P6 (19) | - | D | N | N | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | V | E | N | N | N | P | T | N | Q | Q |
| DM31/2021/G9P6 (1) | - | D | N | N | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | V | E | N | N | N | P | T | N | Q | Q |
| BD44/2021/G1P6 (1) | - | D | N | N | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | V | E | N | N | N | P | T | N | Q | Q |
| GR13/2021/G2P4 (5) | - | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S | D | S | N | T | D | G |
| DM01/2021/G9P4 (5) | - | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | S | D | S | N | T | D | G |
 Different from both Rotarix and RotaTeq.
Different from both Rotarix and RotaTeq.  Different from Rotarix.
Different from Rotarix.  Different from the closest RotaTeq.
Different from the closest RotaTeq.  Sites involved in neutralization escape.
Sites involved in neutralization escape.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damtie, D.; Gelaw, A.; Wondimeneh, Y.; Aleka, Y.; Kick, M.K.; Tigabu, Z.; Sack, U.; Mekuria, Z.H.; Vlasova, A.N.; Tessema, B. Rotavirus A Infection Prevalence and Spatio-Temporal Genotype Shift among Under-Five Children in Amhara National Regional State, Ethiopia: A Multi-Center Cross-Sectional Study. Vaccines 2024, 12, 866. https://doi.org/10.3390/vaccines12080866
Damtie D, Gelaw A, Wondimeneh Y, Aleka Y, Kick MK, Tigabu Z, Sack U, Mekuria ZH, Vlasova AN, Tessema B. Rotavirus A Infection Prevalence and Spatio-Temporal Genotype Shift among Under-Five Children in Amhara National Regional State, Ethiopia: A Multi-Center Cross-Sectional Study. Vaccines. 2024; 12(8):866. https://doi.org/10.3390/vaccines12080866
Chicago/Turabian StyleDamtie, Debasu, Aschalew Gelaw, Yitayih Wondimeneh, Yetemwork Aleka, Maryssa K. Kick, Zemene Tigabu, Ulrich Sack, Zelalem H. Mekuria, Anastasia N. Vlasova, and Belay Tessema. 2024. "Rotavirus A Infection Prevalence and Spatio-Temporal Genotype Shift among Under-Five Children in Amhara National Regional State, Ethiopia: A Multi-Center Cross-Sectional Study" Vaccines 12, no. 8: 866. https://doi.org/10.3390/vaccines12080866
APA StyleDamtie, D., Gelaw, A., Wondimeneh, Y., Aleka, Y., Kick, M. K., Tigabu, Z., Sack, U., Mekuria, Z. H., Vlasova, A. N., & Tessema, B. (2024). Rotavirus A Infection Prevalence and Spatio-Temporal Genotype Shift among Under-Five Children in Amhara National Regional State, Ethiopia: A Multi-Center Cross-Sectional Study. Vaccines, 12(8), 866. https://doi.org/10.3390/vaccines12080866







