Strengthening of Vaccine-Preventable Disease (VPD) Surveillance to Enhance National Health Capacity and Security: Perspective from India
Abstract
:1. Introduction
2. Methods
2.1. VPD Surveillance Structure
- 1.
- Reporting units send regular weekly reports, including nil reports. As of January 2024, there are 12,022 reporting units in the country.
- 2.
2.2. Capacity Building of the Reporting Network
2.3. Response to Reported VPD Cases
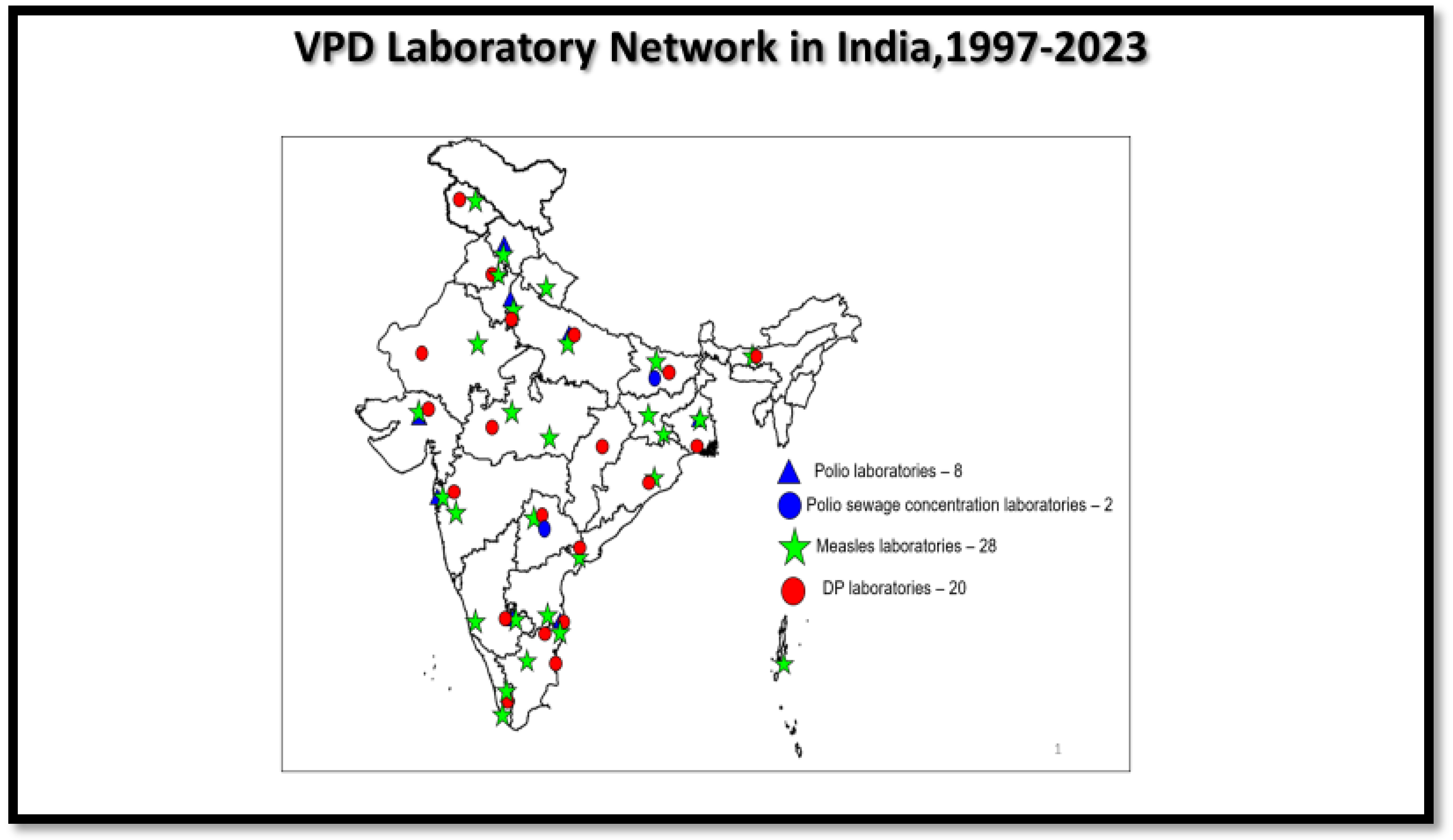
2.4. Laboratory Network for VPD Surveillance
2.4.1. Polio Laboratory Network
2.4.2. MR Laboratory Network
2.4.3. DP Laboratory Network
3. Results
- Identification and sensitization of potential informers (faith healers, temple sites, Indian System of Medicine [ISM] practitioners, and others): There has been an expansion of the reporting network to increase VPD surveillance reporting. From 41,800 reporting sites in 2015, the network has expanded to more than 51,000 reporting sites in the country.
- ∙ Capacity building of health functionaries: Between January 2020 and December 2023, more than 22,000 workshops on surveillance have been conducted, with more than 400,000 health personnel trained and oriented on VPDs through these cascaded training sessions across India. In 2023, approximately 5500 workshops were conducted at health facilities.
- Active case searches (ACSs) at the reporting sites: Between January 2020 and December 2023, more than 500,000 ACS visits have been made by government officers (District Immunization Officers/District Surveillance Officers/Surveillance Nodal Medical Officers) and WHO-NPSN Surveillance Medical Officers. In 2023 alone, more than 125,000 of these ACS visits were conducted at health facilities.
- Expansion of VPD laboratory network across the country: The expansion of surveillance has resulted in the expansion of both virology and bacteriology laboratories in the country. There is a strong component of quality assurance and quality control in these laboratories.
- Program review: Annually, two to three state-level VPD surveillance reviews are conducted in the country. These reviews have integrated the UIP reviews as well. Further, there is a close tracking of the progress of the immunization and surveillance program in the country through task force meetings, which are conducted periodically, usually monthly.
- Efficient data analysis and feedback mechanism: At the time of the initiation of AFP surveillance in 1997, data collation and analysis were mainly conducted using an Excel-based system. From 2005 to June 2023, the data management had been conducted through an offline epi-info based system called the Surveillance Information Management system (SIMS). From July 2023, the VPD surveillance data are being managed through a real time web-based tool called the ‘vaccine-preventable disease surveillance information management system (VSIMS)’.
3.1. AFP Surveillance
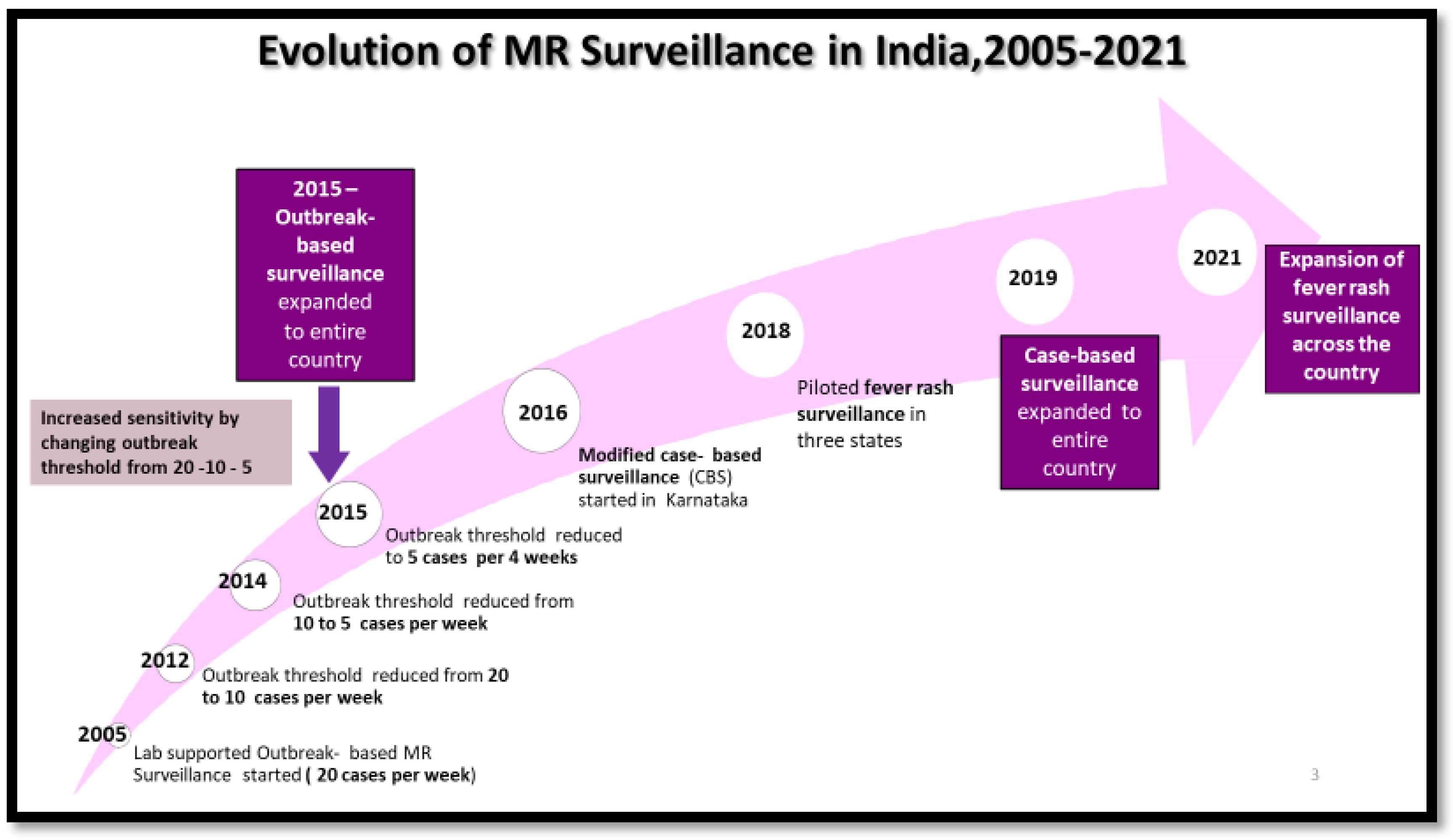
3.2. Measles and Rubella Surveillance
3.3. Diphtheria, Pertussis, and Neonatal Tetanus Surveillance
3.4. VPD Laboratory Network
4. Discussion
4.1. Best Practices towards Health Capacity Enhancement
4.1.1. Capacity Building of Health Personnel
4.1.2. Ensuring Quality of Surveillance System at Par with the Global Standards
4.1.3. Surveillance-Guided Program Planning and Implementation
4.1.4. Strengthening of VPD Surveillance and Routine Immunization (RI) Program [19]
- High-risk areas identified through VPD surveillance were linked to RI session sites to improve immunization coverage;
- Advocacy and accountability framework through state, district/city, and block task forces for the polio program were utilized for other VPD surveillance and RI program reviews;
- VPD surveillance field reviews integrated the UIP field review to identify the gaps in the surveillance and routine immunization program, thereby strengthening VPD surveillance and RI;
- The identification and enlistment of areas vulnerable to MR outbreaks and carrying out the risk reprioritization of outbreak areas into high-risk areas for risk mitigation measures are crucial to prevent the outbreaks. This has resulted in the strengthening of the immunization and health system [20].
4.1.5. Epidemic Response Team (ERT) at State/District/Block Levels
4.1.6. VPD Surveillance System Backed by an Effective Network of Diagnostic Laboratories
4.1.7. Leveraging VPD Surveillance System for COVID-19 Response and Other Public Health Priorities
4.2. Best Practices towards Health Security Enhancement
4.2.1. Governance Mechanism and Intersectoral Coordination
4.2.2. Cross-Border Cooperation
4.2.3. Data Management System
4.2.4. Support in Public Health Emergencies of National and International Concern
5. Strengths and Limitations of the Surveillance System
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Vaccine Preventable Diseases Surveillance Standards. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/surveillance/surveillance-for-vpds/vpd-surveillance-standards (accessed on 8 April 2024).
- Ministry of Health and Family Welfare; Government of India. A Big Leap Towards Measles & Rubella Elimination. Intensified Mission Indradhanush 5.0, Operational Guidelines; Government of India: New Delhi, India, 2023. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. National Immunization Schedule. Available online: https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/report/National_%20Immunization_Schedule.pdf (accessed on 8 April 2024).
- Child Health Division; Department of Family Welfare Ministry of Health & Family Welfare New Delhi. Field Guide. Surveillance of Acute Flaccid Paralysis, 3rd ed.; Ministry of Health & Family Welfare: New Delhi, India, 2005. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Measles and Rubella Surveillance Field Guide 2020; Government of India: New Delhi, India, 2020. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Surveillance of Diphtheria, Pertussis and Neonatal Tetanus, Field Guide, India, 1st ed.; Government of India: New Delhi, India, 2020. [Google Scholar]
- Blanchard, J.; Washington, R.; Becker, M.; Vasanthakumar, N.; Madangopal, K.; Sarwal, R.; Becker, M. Vision 2035: Public Health Surveillance in India. A White Paper; NITI Aayog/Government of India: New Delhi, India, 2020. [Google Scholar]
- Brown, L.; LaFond, A.; Macintyre, K. Measuring Capacity Building; Carolina Population Center/University of North Carolina: Chapel Hill, NC, USA, 2001. [Google Scholar]
- Beaglehole, R.; Dal Poz, M.R. Public health workforce: Challenges and policy issues. Hum. Resour. Health 2003, 1, 4. [Google Scholar] [CrossRef]
- World Health Organization. Health Security. Available online: https://www.who.int/health-topics/health-security#tab=tab_1 (accessed on 8 April 2024).
- World Health Organization. International Health Regulations. 2005. Available online: https://www.who.int/publications/i/item/9789241580410 (accessed on 8 April 2024).
- Sharma, D.; Sangal, L.; Vijay, N.; Nalavade, U.; Krishnasamy, K.; Pawar, S.; Kaur, H.; Narayan, J.; Rane, S.; Narkar, M. Expansion of the measles and rubella laboratory network, India. Bull. World Health Organ. 2022, 100, 247–255. [Google Scholar] [CrossRef]
- World Health Organization. Health. Acute Flaccid Paralysis Surveillance Bulletin; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- World Health Organization. Health. Measles Rubella Surveillance Bulletin; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- World Health Organization. Health. Diphtheria, Pertussis and Neonatal Tetanus Surveillance Bulletin; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Polio Eradication Initiative, Bihar. Kosi Operational Plan Bihar. Standard Operating Procedures; Government of India: New Delhi, India, 2009. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Introduction of Measles Rubella Vaccine (Campaign and Routine Immunization. National Operational Guidelines; Government of India: New Delhi, India, 2017. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Strengthening Td 10 and Td 16 Vaccine Implementation, Operational Guidelines and Strategic Plan; Government of India: New Delhi, India, 2019. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Immunization Handbook for Medical Officers, 2nd ed.; Government of India: New Delhi, India, 2017. [Google Scholar]
- Ministry of Health and Family Welfare; Government of India. Roadmap to Measles & Rubella Elimination in India by 2023; Government of India: New Delhi, India, 2022. [Google Scholar]
- Mulders, M.N.; Serhan, F.; Goodson, J.L.; Icenogle, J.; Johnson, B.W.; Rota, P.A. Expansion of Surveillance for Vaccine-preventable Diseases: Building on the Global Polio Laboratory Network and the Global Measles and Rubella Laboratory Network Platforms. J. Infect. Dis. 2017, 216 (Suppl. 1), S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Manor, Y.; Handsher, R.; Halmut, T.; Neuman, M.; Bobrov, A.; Rudich, H.; Vansover, A.; Shulman, L.; Kew, O.; Mendelson, E. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian authority. J. Clin. Microbiol. 1999, 37, 1670–1675. [Google Scholar] [CrossRef]
- Pöyry, T.; Stenvik, M.; Hovi, T. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl. Environ. Microbiol. 1998, 30, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Avoort, H.G.V.D.; Reimerink, J.H.; Ras, A.; Mulders, M.N.; Van Loon, A.M. Isolation of epidemic poliovirus from sewage during the 1992–3 type 3 outbreak in The Netherlands. Epidemiol. Infect. 1995, 114, 481–491. [Google Scholar] [CrossRef]
- Deshpande, J.M.; Shetty, S.J.; Siddiqui, Z.A. Environmental surveillance system to track wild poliovirus transmission. Appl. Environ. Microbiol. 2003, 69, 2919–2927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Centre for Disease Control; Government of India. Standard Operating Procedures (SOPs) for Investigation of a Suspected COVID-19 Case Using Case Investigation Form (CIF); Government of India: New Delhi, India, 2020. [Google Scholar]
- Kaur, H.; Mukhopadhyay, L.; Gupta, N.; Aggarwal, N.; Sangal, L.; Potdar, V.; Inbanathan, F.Y.; Narayan, J.; Gupta, S.; Rana, S.; et al. External quality assessment of COVID-19 real time reverse transcription PCR laboratories in India. PLoS ONE 2022, 17, e0263736. [Google Scholar] [CrossRef]
- Directorate of Health and Family Welfare Services; Government of Karnataka. Operational Manual Kyasanur Forest Disease; Government of Karnataka: Karnataka, India, 2020. [Google Scholar]

| Age | Vaccines Given |
|---|---|
| Birth | BCG, OPV 0, Hepatitis B birth dose |
| 6 weeks | OPV 1, Pentavalent 1 *, Rotavirus 1, fractional IPV 1, PCV 1 |
| 10 weeks | OPV 2, Pentavalent 2, Rotavirus 2 |
| 14 weeks | OPV 3, Pentavalent 3, Rotavirus 3, fractional IPV 2, PCV 2 |
| 9–12 months | MR 1, JE 1 **, PCV booster, fIPV 3 |
| 16–24 months | MR 2, JE 2 **, DPT booster 1, OPV booster |
| 5–6 years | DPT booster 2 |
| 10 years | Td |
| 16 years | Td |
| Pregnant mother | Td 1, Td 2, Td booster *** |
| 2021 | 2022 | 2023 | ||
|---|---|---|---|---|
| Fever–rash cases | 33,379 | 112,226 | 154,372 | |
| Age group | <5 years | 50% | 53% | 48% |
| 5–10 years | 20% | 24% | 26% | |
| >10 years | 30% | 23% | 26% | |
| Sex | Male | 56% | 55% | 55% |
| Female | 44% | 45% | 45% | |
| Setting | Rural | 71% | 64% | 69% |
| Urban | 29% | 36% | 31% |
| Year | 1997–2023 | 2005–2023 | 2006–2023 | 2014–2023 | 2019–2023 |
|---|---|---|---|---|---|
| Polio (AFP) | 1,721,408 | ||||
| Polio (ES) | 17,100 | ||||
| MR (Serology) | 309,200 | ||||
| MR (Molecular) | 161,364 | ||||
| Diphtheria (TS) | 16,985 | ||||
| Pertussis (NPS and Serology) | 23,256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Murugan, R.; Donkatti, S.; Sharma, D.; Kaundal, N.; Avagyan, T.; Kumar, P.; Bahl, S.; Khanal, S.; Bura, V. Strengthening of Vaccine-Preventable Disease (VPD) Surveillance to Enhance National Health Capacity and Security: Perspective from India. Vaccines 2024, 12, 941. https://doi.org/10.3390/vaccines12080941
Kumar A, Murugan R, Donkatti S, Sharma D, Kaundal N, Avagyan T, Kumar P, Bahl S, Khanal S, Bura V. Strengthening of Vaccine-Preventable Disease (VPD) Surveillance to Enhance National Health Capacity and Security: Perspective from India. Vaccines. 2024; 12(8):941. https://doi.org/10.3390/vaccines12080941
Chicago/Turabian StyleKumar, Arun, Ratnesh Murugan, Satishchandra Donkatti, Deepa Sharma, Nirmal Kaundal, Tigran Avagyan, Pawan Kumar, Sunil Bahl, Sudhir Khanal, and Vinod Bura. 2024. "Strengthening of Vaccine-Preventable Disease (VPD) Surveillance to Enhance National Health Capacity and Security: Perspective from India" Vaccines 12, no. 8: 941. https://doi.org/10.3390/vaccines12080941





