Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Construction of Recombinant Transfer Plasmids
2.3. Expression and Identification of Recombinant Baculovirus
2.4. Expression and Identification of Recombinant Protein
2.5. Optimization of Protein Expression Conditions and Protein Purification
2.6. Characterization by Transmission Electron Microscopy
2.7. Animals and Animal Welfare
2.8. Rabbits’ Immunization and Challenge
2.9. Detection of Specific Antibody Titers by ELISA
2.10. Detection of Neutralizing Antibody Titers
2.11. Lymphocyte Proliferation Assay
2.12. Serum Cytokine Levels Were Detected by ELISA
2.13. Viral Load Detection by qPCR
2.14. Data Analysis
3. Results
3.1. Construction and Identification of the Recombinant Ferritin Nanoparticles
3.2. Expression and Purification of the Recombinant Protein
3.3. Ferritin Nanoparticles Were Identified by TEM
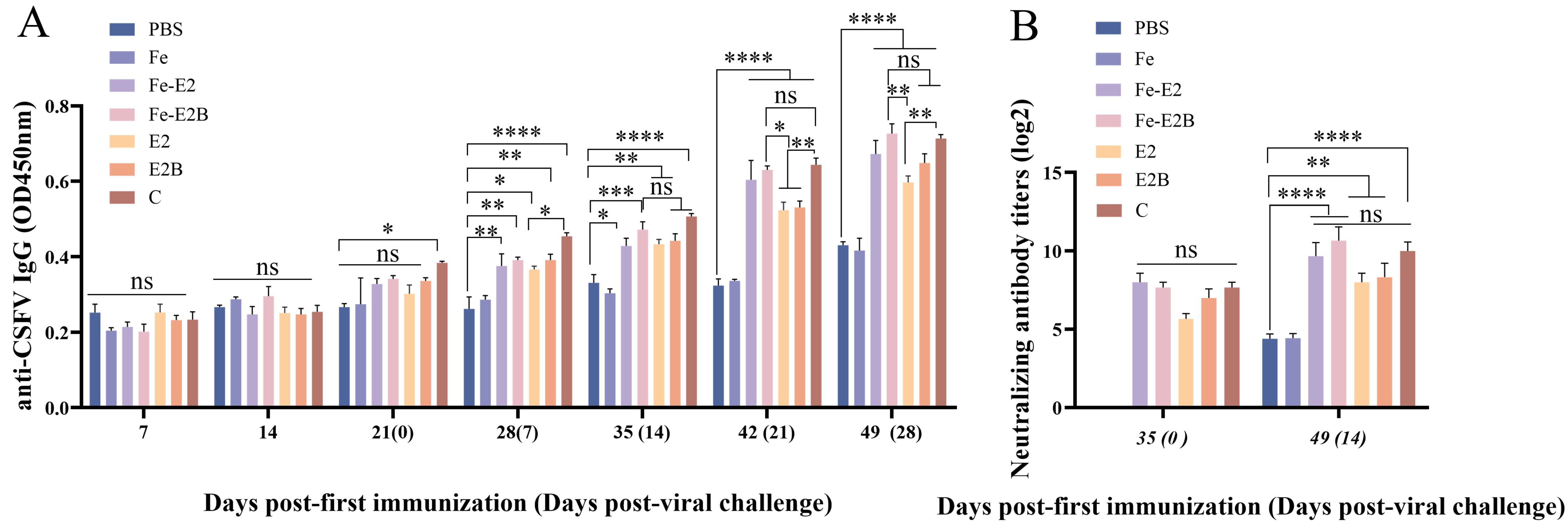
3.4. Detection of Antibody Titers in the Immunized Rabbits
3.5. Detection of the Proliferation of Lymphocytes and the Levels of Cytokines in Rabbits
3.6. Rectal Temperature Changes in Rabbits after Challenge
3.7. Viral Load Was Measured by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatima, M.; Luo, Y.; Zhang, L.; Wang, P.Y.; Song, H.; Fu, Y.; Li, Y.; Sun, Y.; Li, S.; Bao, Y.J.; et al. Genotyping and Molecular Characterization of Classical Swine Fever Virus Isolated in China during 2016–2018. Viruses 2021, 13, 664. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Guo, Z.; Ding, N.Z.; He, C.Q. Studying classical swine fever virus: Making the best of a bad virus. Virus Res. 2015, 197, 35–47. [Google Scholar] [PubMed]
- Becher, P.; Ramirez, R.A.; Orlich, M.; Rosales, S.C.; König, M.; Schweizer, M.; Stalder, H.; Schirrmeier, H.; Thiel, H.J. Genetic and antigenic characterization of novel pestivirus genotypes: Implications for classification. Virology 2003, 311, 96–104. [Google Scholar]
- Lohse, L.; Nielsen, J.; Uttenthal, S. Early pathogenesis of classical swine fever virus (CSFV) strains in Danish pigs. Vet. Microbiol. 2012, 159, 327–336. [Google Scholar] [PubMed]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever-An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- Coronado, L.; Perera, C.L.; Rios, L.; Frías, M.T.; Pérez, L.J. A Critical Review about Different Vaccines against Classical Swine Fever Virus and Their Repercussions in Endemic Regions. Vaccines 2021, 9, 154. [Google Scholar] [CrossRef]
- Van Oirschot, J.T. Vaccinology of classical swine fever: From lab to field. Vet. Microbiol. 2003, 96, 367–384. [Google Scholar]
- Wei, Q.; Liu, Y.; Zhang, G. Research Progress and Challenges in Vaccine Development against Classical Swine Fever Virus. Viruses 2021, 13, 445. [Google Scholar] [CrossRef]
- Postel, A.; Austermann-Busch, S.; Petrov, A.; Moennig, V.; Becher, P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound. Emerg. Dis 2018, 1, 248–261. [Google Scholar]
- Li, S.; Wang, J.; Yang, Q.; Muhammad, N.A.; Yu, S.; Qiu, H.J. Complex Virus–Host Interactions Involved in the Regulation of Classical Swine Fever Virus Replication: A Minireview. Viruses 2017, 9, 171. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.Y.; Tian, D.Y.; Han, Q.Y.; Zhang, X.; Li, N.; Qiu, H.J. A novel alphavirus replicon-vectored vaccine delivered by adenovirus induces sterile immunity against classical swine fever. Vaccine 2011, 29, 8364–8372. [Google Scholar] [PubMed]
- Li, N.; Qiu, H.J.; Zhao, J.J.; Li, Y.; Wang, M.J.; Lu, B.W.; Han, C.G.; Hou, Q.; Wang, Z.H.; Gao, H.; et al. A Semliki Forest virus replicon vectored DNA vaccine expressing the E2 glycoprotein of classical swine fever virus protects pigs from lethal challenge. Vaccine 2007, 25, 2907–2912. [Google Scholar]
- Lin, G.J.; Deng, M.C.; Chen, Z.W.; Liu, T.Y.; Wu, C.W.; Cheng, C.Y.; Chien, M.S.; Huang, C. Yeast expressed classical swine fever E2 subunit vaccine candidate provides complete protection against lethal challenge infection and prevents horizontal virus transmission. Vaccine 2012, 30, 2336–2341. [Google Scholar] [PubMed]
- Dewulf, J.; Laevens, H.; Koenen, F.; Mintiens, K.; de Kruif, A. An E2 sub-unit marker vaccine does not prevent horizontal or vertical transmission of classical swine fever virus. Vaccine 2001, 20, 86–91. [Google Scholar] [PubMed]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar]
- Bijker, M.S.; Melief, C.J.; Offringa, R.; van der Burg, S.H. Design and development of synthetic peptide vaccines: Past, present and future. Expert Rev. Vaccines 2007, 6, 591–603. [Google Scholar] [PubMed]
- Zhu, Y.; Shi, Z.; Drew, T.W.; Wang, Q.; Qiu, H.; Guo, H.; Tu, C. Antigenic differentiation of classical swine fever viruses in China by monoclonal antibodies. Virus Res. 2009, 142, 169–174. [Google Scholar]
- Zhou, B.; Liu, K.; Jiang, Y.; Wei, J.C.; Chen, P.Y. Multiple linear B-cell epitopes of classical swine fever virus glycoprotein E2 expressed in E.coli as multiple epitope vaccine induces a protective immune response. Virol. J. 2011, 8, 378. [Google Scholar]
- Chang, C.Y.; Huang, C.C.; Lin, Y.J.; Deng, M.C.; Tsai, C.H.; Chang, W.M.; Wang, F.I. Identification of antigen-specific residues on E2 glycoprotein of classical swine fever virus. Virus Res. 2010, 152, 65–72. [Google Scholar]
- Harwansh, R.K.; Deshmukh, R.; Barkat, M.A.; Rahman, M.A. Bioinspired Polymeric-based Core-shell Smart Nano-systems. Pharm. Nanotechnol. 2019, 7, 181–205. [Google Scholar]
- Deshayes, S.; Gref, R. Synthetic and bioinspired cage nanoparticles for drug delivery. Nanomedicine 2014, 9, 1545–1564. [Google Scholar]
- Jelinkova, P.; Mazumdar, A.; Sur, V.P.; Kociova, S.; Dolezelikova, K.; Jimenez, A.M.; Koudelkova, Z.; Mishra, P.K.; Smerkova, K.; Heger, Z.; et al. Nanoparticle-drug conjugates treating bacterial infections. J. Control. Release 2019, 307, 166–185. [Google Scholar] [PubMed]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Edit. 2019, 58, 670–680. [Google Scholar]
- Heng, W.T.; Yew, J.S.; Poh, C.L. Nanovaccines against Viral Infectious Diseases. Pharmaceutics 2022, 14, 2554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar]
- Kalathiya, U.; Padariya, M.; Fahraeus, R.; Chakraborti, S.; Hupp, T.R. Multivalent Display of SARS-CoV-2 Spike (RBD Domain) of COVID-19 to Nanomaterial, Protein Ferritin Nanocages. Biomolecules 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.F.; Sun, C.; Zhuang, Z.; Yuan, R.Y.; Zheng, Q.; Li, J.P.; Zhou, P.P.; Chen, X.C.; Liu, Z.; Zhang, X.; et al. Rapid Development of SARS-CoV-2 Spike Protein Receptor-Binding Domain Self-Assembled Nanoparticle Vaccine Candidates. ACS Nano 2021, 15, 2738–2752. [Google Scholar]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.; Rowntree, L.C.; et al. Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar]
- Liu, T.; Li, M.; Tian, Y.; Dong, Y.; Liu, N.; Wang, Z.; Zhang, H.; Zheng, A.; Cui, C. Immunogenicity and safety of a self-assembling ZIKV nanoparticle vaccine in mice. Int. J. Pharm. 2024, 660, 124320. [Google Scholar]
- Li, Q.; Zhang, J.; Deng, Q.; Liao, C.; Qian, J.; Chen, Z.; Lu, J. A Divalent Chikungunya and Zika Nanovaccine with Thermostable Self-Assembly Multivalent Scaffold LS-SUMO. Adv. Healthc. Mater. 2024, 13, e2303619. [Google Scholar]
- Wu, F.; Qin, M.; Wang, H.; Sun, X. Nanovaccines to combat virus-related diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1857. [Google Scholar]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 2021, 268, 120597. [Google Scholar]
- Harrison, P.M.; Hoy, T.G.; Macara, I.G.; Hoare, R.J. Ferritin iron uptake and release. Structure-function relationships. Biochemistry 1974, 143, 445–451. [Google Scholar]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar]
- Lee, L.A.; Wang, Q. Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine 2006, 2, 137–149. [Google Scholar] [PubMed]
- Parker, D.C. T cell-dependent B cell activation. Annu. Rev. Immunol. 1993, 11, 331–360. [Google Scholar]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines 2021, 6, 70. [Google Scholar]
- Sokolenko, S.; George, S.; Wagner, A.; Tuladhar, A.; Andrich, J.M.; Aucoin, M.G. Co-expression vs. co-infection using bacu-lovirus expression vectors in insect cell culture: Benefits and drawbacks. Biotechnol. Adv. 2012, 30, 766–781. [Google Scholar]
- Xia, H.; Wahlberg, N.; Qiu, H.J.; Widén, F.; Belák, S.; Liu, L. Lack of phylogenetic evidence that the Shimen strain is the parental strain of the lapinized Chinese strain (C-strain) vaccine against classical swine fever. Arch. Virol. 2011, 56, 1041–1044. [Google Scholar]
- Moennig, V. Introduction to classical swine fever: Virus, disease and control policy. Vet. Microbiol. 2000, 73, 93–102. [Google Scholar]
- Li, C.; Li, Y.; Shen, L.; Huang, J.; Sun, Y.; Luo, Y.; Zhao, B.; Wang, C.; Yuan, J.; Qiu, H.J. The role of noncoding regions of classical swine fever virus C-strain in its adaptation to the rabbit. Virus Res. 2014, 183, 117–122. [Google Scholar] [PubMed]
- Cao, Z.; Zhang, H.; Yang, Q.; Zhang, H.; Fan, G. Establishment of a method for evaluation of the efficacy of a classical swine fever virus subunit vaccine in rabbits. Am. J. Vet. Res. 2020, 81, 521–526. [Google Scholar]
- Wei, Q.; Bai, Y.; Song, Y.; Liu, Y.; Yu, W.; Sun, Y.; Wang, L.; Deng, R.; Xing, G.; Zhang, G. Generation and immunogenicity analysis of recombinant classical swine fever virus glycoprotein E2 and E(rns) expressed in baculovirus expression system. Virol. J. 2021, 18, 44. [Google Scholar] [PubMed]
- Cao, T.; Wang, Z.; Li, X.; Zhang, S.; Paudyal, N.; Zhang, X.; Li, X.; Fang, W. E2 and E(rns) of classical swine fever virus C-strain play central roles in its adaptation to rabbits. Virus Genes 2019, 55, 238–242. [Google Scholar] [PubMed]
- Zhang, H.; Li, X.; Peng, G.; Tang, C.; Zhu, S.; Qian, S.; Xu, J.; Qian, P. Glycoprotein E2 of classical swine fever virus expressed by baculovirus induces the protective immune responses in rabbits. Vaccine 2014, 32, 6607–6613. [Google Scholar] [PubMed]
- Chen, J.Y.; Wu, C.M.; Chia, M.Y.; Huang, C.; Chien, M.S. A prospective CSFV-PCV2 bivalent vaccine effectively protects against classical swine fever virus and porcine circovirus type 2 dual challenge and prevents horizontal transmission. Vet. Res. 2023, 54, 57. [Google Scholar] [PubMed]
- Dar, P.; Kalaivanan, R.; Sied, N.; Mamo, B.; Kishore, S.; Suryanarayana, V.V.; Kondabattula, G. Montanide ISA™ 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle. Vaccine 2013, 31, 3327–3332. [Google Scholar]
- Postel, A.; Nishi, T.; Kameyama, K.I.; Meyer, D.; Suckstorff, O.; Fukai, K.; Beche, P. Reemergence of Classical Swine Fever, Japan, 2018. Emerg. Infect. Dis. 2019, 25, 1228–1231. [Google Scholar]
- Je, S.H.; Kwon, T.; Yoo, S.J.; Lee, D.U.; Lee, S.; Richt, J.A.; Lyoo, Y.S. Classical Swine Fever Outbreak after Modified Live LOM Strain Vaccination in Naive Pigs, South Korea. Emerg. Infect. Dis. 2018, 24, 798–800. [Google Scholar]
- Xu, H.; Wang, Y.; Han, G.; Fang, W.; He, F. Identification of E2 with improved secretion and immunogenicity against CSFV in piglets. BMC Microbiol. 2020, 20, 26. [Google Scholar]
- Sánchez, O.; Barrera, M.; Farnós, O.; Parra, N.C.; Salgado, E.R.; Saavedra, P.A.; Meza, C.D.; Meza, C.I.; Cortez-San Martín, M.; Toledo, J.R. Effectiveness of the E2-classical swine fever virus recombinant vaccine produced and formulated within whey from genetically transformed goats. Clin. Vaccine Immunol. 2014, 21, 1628–1634. [Google Scholar] [PubMed]
- Huang, Y.L.; Deng, M.C.; Wang, F.I.; Huang, C.C.; Chang, C.Y. The challenges of classical swine fever control: Modified live and E2 subunit vaccines. Virus Res. 2014, 179, 1–11. [Google Scholar] [PubMed]
- Blome, S.; Moß, C.; Reimann, I.; König, P.; Beer, M. Classical swine fever vaccines-State-of-the-art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [PubMed]
- Zinkernagel, R.M. On natural and artificial vaccinations. Annu. Rev. Immunol. 2003, 21, 515–546. [Google Scholar]
- Zhao, Z.; Chen, X.; Chen, Y.; Li, H.; Fang, K.; Chen, H.; Li, X.; Qian, P. A Self-Assembling Ferritin Nanoplatform for Designing Classical Swine Fever Vaccine: Elicitation of Potent Neutralizing Antibody. Vaccines 2021, 9, 45. [Google Scholar] [CrossRef]
- Suzuki, D.; Furukawa, K.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Effects of perioperative immunonutrition on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy. Surgery 2010, 148, 573–581. [Google Scholar]
- Tarradas, J.; Argilaguet, J.M.; Rosell, R.; Nofrarías, M.; Crisci, E.; Córdoba, L.; Pérez-Martín, E.; Díaz, I.; Rodríguez, F.; Domingo, M.; et al. Interferon-gamma induction correlates with protection by DNA vaccine expressing E2 glycoprotein against classical swine fever virus infection in domestic pigs. Vet. Microbiol. 2010, 142, 51–58. [Google Scholar] [PubMed]
- Palatnik-de-Sousa, C.B.; Soares, I.S.; Rosa, D.S. Editorial: Epitope Discovery and Synthetic Vaccine Design. Front. Immunol. 2018, 9, 826. [Google Scholar]
- Zhang, H.; Wen, W.; Zhao, Z.; Wang, J.; Chen, H.; Qian, P.; Li, X. Enhanced protective immunity to CSFV E2 subunit vaccine by using IFN-γ as immunoadjuvant in weaning piglets. Vaccine 2018, 36, 7353–7360. [Google Scholar] [PubMed]
- Peng, W.P.; Hou, Q.; Xia, Z.H.; Chen, D.; Li, N.; Sun, Y.; Qiu, H.J. Identification of a conserved linear B-cell epitope at the N-terminus of the E2 glycoprotein of Classical swine fever virus by phage-displayed random peptide library. Virus Res. 2008, 135, 267–272. [Google Scholar] [PubMed]
- Liu, S.; Tu, C.; Wang, C.; Yu, X.; Wu, J.; Guo, S.; Shao, M.; Gong, Q.; Zhu, Q.; Kong, X. The protective immune response induced by B cell epitope of classical swine fever virus glycoprotein E2. J. Virol. Methods 2006, 134, 125–129. [Google Scholar] [PubMed]




| Groups | Numbers of Rabbits | Vaccination | Type and Composition | Immune Dose | Immunization Pathway | Number of Injection | Challenge |
|---|---|---|---|---|---|---|---|
| 1 | 5 | PBS | PBS | 1 mL (PBS) | IM | 2 (21 dpi, Booster immunization) | 100 RID50 C-strain (35 dpi, IV, 1dose) |
| 2 | 5 | Fe | W/O/W ISA 201 VG | 1 mL (40 µg, purified) | |||
| 3 | 5 | Fe-E2 | W/O/W ISA 201 VG | 1 mL (40 µg, purified) | |||
| 4 | 5 | Fe-E2B | W/O/W ISA 201 VG | 1 mL (40 µg, purified) | |||
| 5 | 5 | E2 | W/O/W ISA 201 VG | 1 mL (40 µg, purified) | |||
| 6 | 5 | E2B | W/O/W ISA 201 VG | 1 mL (40 µg, purified) | |||
| 7 | 5 | C-strain vaccine | C-strain attenuated vaccine (commercial vaccine) | 1 dose (commercial vaccine) |
| Groups | Number | C-Strain Viral Load (copies/μL) | ||
|---|---|---|---|---|
| Spleen | Lymph Nodes | Peripheral Blood | ||
| PBS | PBS-1 | 2.37 × 103 | 3.64 × 103 | 0.98 × 103 |
| PBS-3 | 3.45 × 103 | 1.98 × 103 | 2.23 × 103 | |
| PBS-5 | 4.32 × 103 | 3.12 × 103 | 2.36 × 103 | |
| Fe | Fe-7 | 5.26 × 103 | 3.29 × 103 | 3.12 × 103 |
| Fe-9 | 5.23 × 103 | 2.17 × 103 | 2.98 × 103 | |
| Fe-10 | 2.13 × 103 | 4.23 × 103 | 3.01 × 103 | |
| E2 | E2-12 | - | - | - |
| E2-14 | - | - | - | |
| E2-15 | - | - | - | |
| E2B | E2B-17 | - | - | - |
| E2B-18 | - | - | - | |
| E2B-20 | - | - | - | |
| Fe-E2 | Fe-E2-22 | - | - | - |
| Fe-E2-24 | - | - | - | |
| Fe-E2-25 | - | - | - | |
| Fe-E2B | Fe-E2B-26 | - | - | - |
| Fe-E2B-27 | - | - | - | |
| Fe-E2B-29 | - | - | - | |
| C | C-32 | - | - | - |
| C-34 | - | - | - | |
| C-35 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Yuan, Z.; Ji, J.; Ruan, Y.; Li, X.; Wang, L.; Zeng, W.; Wu, K.; Hu, W.; Yi, L.; et al. Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever. Vaccines 2024, 12, 948. https://doi.org/10.3390/vaccines12080948
Song Y, Yuan Z, Ji J, Ruan Y, Li X, Wang L, Zeng W, Wu K, Hu W, Yi L, et al. Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever. Vaccines. 2024; 12(8):948. https://doi.org/10.3390/vaccines12080948
Chicago/Turabian StyleSong, Yiwan, Zhongmao Yuan, Junzhi Ji, Yang Ruan, Xiaowen Li, Lianxiang Wang, Weijun Zeng, Keke Wu, Wenshuo Hu, Lin Yi, and et al. 2024. "Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever" Vaccines 12, no. 8: 948. https://doi.org/10.3390/vaccines12080948
APA StyleSong, Y., Yuan, Z., Ji, J., Ruan, Y., Li, X., Wang, L., Zeng, W., Wu, K., Hu, W., Yi, L., Ding, H., Zhao, M., Fan, S., Li, Z., & Chen, J. (2024). Development of a Ferritin-Based Nanoparticle Vaccine against Classical Swine Fever. Vaccines, 12(8), 948. https://doi.org/10.3390/vaccines12080948