Abstract
This is a retrospective observational study including all COVID-19 patients admitted at our Institute throughout three successive pandemic waves, from January 2021 to June 2023. The main in-hospital outcomes (clinical progression [CP], defined as admission to Intensive Care Unit [ICU]/death, and death within 28 days) were compared among participants unvaccinated (NV), fully vaccinated (FV), with one (FV&B1) and two (FV&B2) booster doses. Vaccinated participants were stratified into recently and waned FV/FV&B1/FV&B2, depending on the time elapsed from last dose (≤ and >120 days, respectively). There were 4488 participants: 2224 NV, 674 FV, 1207 FV&B1, and 383 FV&B2. Within 28 days, there were 604 ICU admissions, 396 deaths, and 737 CP. After adjusting for the main confounders, the risk of both in-hospital outcomes was reduced in vaccinated individuals, especially in those who received the booster dose (approximately by 36% for FV and >50% for FV&B1 and FV&B2 compared to NV). Similarly, after restricting the analysis to vaccinated participants only, we observed a risk reduction of approximately 40% for FV&B1 and 50% for FV&B2, compared to FV, regardless of the distance since the last dose. Our data confirm the vaccine’s effectiveness in preventing severe COVID-19 and support the efforts to increase the uptake of booster doses, mainly among older and frailer individuals, still at a greater risk of clinical progression.
1. Introduction
Since SARS-CoV-2 emerged as a new respiratory virus responsible for more than 770 million cases of confirmed COVID-19 so far, including about 7 million deaths worldwide [1], many efforts have been made to control its spread. Among them, the development of vaccines against SARS-CoV-2 at an unprecedented rapid pace and the launch of a mass vaccination campaign have been the key strategy to control the spread of the virus and prevent COVID-19-related hospitalizations and deaths. In Italy, the COVID-19 vaccination campaign started on 27 December 2020 with a frailty- and age-stratified roll-out approach [2]. In this first phase, four types of vaccines were progressively available in Italy: BNT162b2, mRNA-1273, ChAdOx1, and Ad26.COV2.S. By September 2021, the administration of the first booster dose of COVID-19 vaccine was approved by the Italian Ministry of Health for those who completed the first vaccination schedule [3]. The administration of the second booster dose was approved in February 2022 initially for immunocompromised subjects and subsequently extended to the entire population [4]. Booster doses consisted of BNT162b2 or mRNA-1273 vaccines.
COVID-19 vaccines demonstrated a high effectiveness in reducing the risk of SARS-CoV-2 infections and the burden of COVID-19 severe disease and mortality in both clinical trials [5,6] and in the real-world setting [7,8]. Nevertheless, symptomatic SARS-CoV-2 infections still occur in fully vaccinated subjects. The risk of developing vaccine breakthrough infections is related to several factors as host determinants (e.g., age, comorbidities, and immunological status), vaccine-related factors (e.g., dose number, and time elapsed since the last dose), and viral characteristics (e.g., viral variant of concern [VoC]) [9]. In particular, there is now clear evidence that vaccine effectiveness weans over time and the administration of a booster dose is highly effective in restoring good protection against symptomatic infection and severe disease [10,11,12,13,14,15,16]. In addition, concerning the viral variant, a lower vaccine effectiveness has been reported for Omicron compared to previous VoCs, probably related to the greater immune-evasion capacity of this variant [9,17].
Although vaccine breakthrough infections are generally milder and carry a lower risk of hospitalization, the risk of severe COVID-19 remains, particularly among groups at a higher risk of severe disease [9,18]. Vaccinated individuals who require hospitalization for severe SARS-CoV-2 infections seemed to have a lower risk of clinical progression and fatal outcomes [18,19,20,21,22,23,24]. However, limited data on the impact of vaccination on hospitalized individuals are available, especially in the setting of Omicron dominance and in fully vaccinated and boosted subjects.
The aim of this study was to compare clinical characteristics and in-hospital outcomes of patients requiring hospitalization for SARS-CoV-2 infection according to vaccination status over a 30-month observation period starting from the date of the introduction of the anti-SARS-CoV-2 vaccine campaign in Italy.
2. Materials and Methods
2.1. Study Design and Population
We conducted a retrospective observational monocenter study including all adult subjects who were admitted to the National Institute for Infectious Diseases (INMI) “Lazzaro Spallanzani”, in Rome, Italy, between 1 January 2021 to 30 June 2023 with a discharge diagnosis of SARS-CoV-2 infection, confirmed by real-time polymerase chain reaction (RT-PCR) on at least one respiratory specimen, and available complete data on anti-SARS-CoV-2 vaccination from the regional vaccination register (Anagrafe Vaccinale Regione Lazio). According to vaccination status at hospital admission, participants were divided into four exposure groups: 1. Not vaccinated (NV), if they did not receive any vaccine dose, or they received only the first dose of a 2-dose vaccine series less than 14 days before the hospital admission; 2. Fully vaccinated (FV), if they completed the vaccine schedule more than 14 days before the hospital admission; 3. Fully vaccinated and one booster dose (FV&B1), if they completed the primary vaccine schedule and received the first booster dose of vaccine at least 14 days before the hospital admission; and 4. Fully vaccinated and two booster doses (FV&B2), if they completed the primary vaccine schedule and received the second booster dose of vaccine at least 14 days before the hospital admission. Subjects who had received partial vaccination (defined as only the first dose of a 2-dose vaccine series or the complete primary vaccine schedule less than 14 days before the hospital admission) were excluded from the analysis. Likewise, individuals who were not resident in the Lazio region and, therefore, had no vaccination data available from the regional vaccine registry were not included in the present study. Finally, we also excluded patients admitted within 90 days from previous hospitalization, assuming they had a prolonged infection. Demographic and clinical data including age, sex, country of birth, number and type of comorbidities (detailed in supplementary methods), characteristics of COVID-19 (date of symptoms onset, presence of pneumonia, and disease severity) were extracted from hospital discharge records and COVID-19 notification forms. Laboratory and virologic data were taken from hospital laboratory electronic database. Moreover, information about previous SARS-CoV-2 infections were obtained through RT-PCR tests collected by Lazio regional integrated surveillance system platform, COVID-19 notification forms, and results of SARS-CoV-2 serology (particularly Immunoglobulin G [IgG] anti-receptor-binding domain [RBD] of the spike (S) protein) performed at hospital admission for unvaccinated patients. Finally, pandemic waves were defined based on the predominant viral variant in circulation, according to the COVID-19 national surveillance data produced by the Italian National Institute of Health [25] and referring to previously published data from our hospital [26]. Follow-up accrued from the date of hospital admission (study baseline) to the achievement of the defined clinical outcomes (clinical progression or in-hospital death) or to day 28 of hospitalization or to hospital discharge, whichever came first.
2.2. Statistical Analysis
Descriptive characteristics at hospital admission were provided using median values and interquartile ranges (IQR) for continuous variables, and frequencies and percentages for categorical variables, were reported by vaccination status (NV, FV, FV&B1, and FV&B2) and were compared by the four groups using Chi-square test (Fisher’s exact test when applicable) for categorical variables and Kruskal–Wallis test for continuous variables. Similarly, comparison of main clinical and virological outcomes was assessed according to vaccination status. Predictive factors of clinical progression and in-hospital death within 28 days from hospital admission were assessed using a multivariable logistic regression calculating odds ratios (MLR-OR) and their 95% confidence intervals (95% CI), both in total population and after restricting the analysis only to vaccinated population (FV, FV&B1, and FV&B2). To include all potential explanatory variables each factor available at baseline was considered as a covariate in the MLR models. Age was assessed both in its continuous form and categorized in four groups (18–39, 40–59, 60–79, and ≥80); the form included in the logistic models was that which minimized the Bayesian information criterion (BIC). Multicollinearity between predictors was assessed using variance inflation factors (VIF) to exclude the presence of highly correlated variables that merit further investigation (it is generally accepted that a variable with VIF exceeding 10 indicates concerning collinearity) [27,28]. Clinical progression was defined as in-hospital death for any cause or admission to intensive care unit (ICU) within 28 days from hospital admission. In addition, the distribution of the four exposure groups in the study population, only represented by patients hospitalized for COVID-19, was described in comparison with the levels of vaccination coverage observed in the regional population, for each trimester of the study period, both overall and by age group. The data on vaccinations coverage in the Lazio Region were extracted from the open data repository [29]. A two-tailed p-value less than 0.05 indicated conventional statistical significance. All statistical analyses were performed using STATA release 17 (StataCorp. 2021. StataCorp LLC, College Station, TX, USA), while the forestploter R package, version 1.1.2, was utilized for the visual representation of MLR-ORs and their 95% CIs [https://CRAN.R-project.org/package=forestploter, accessed on 16 August 2024].
3. Results
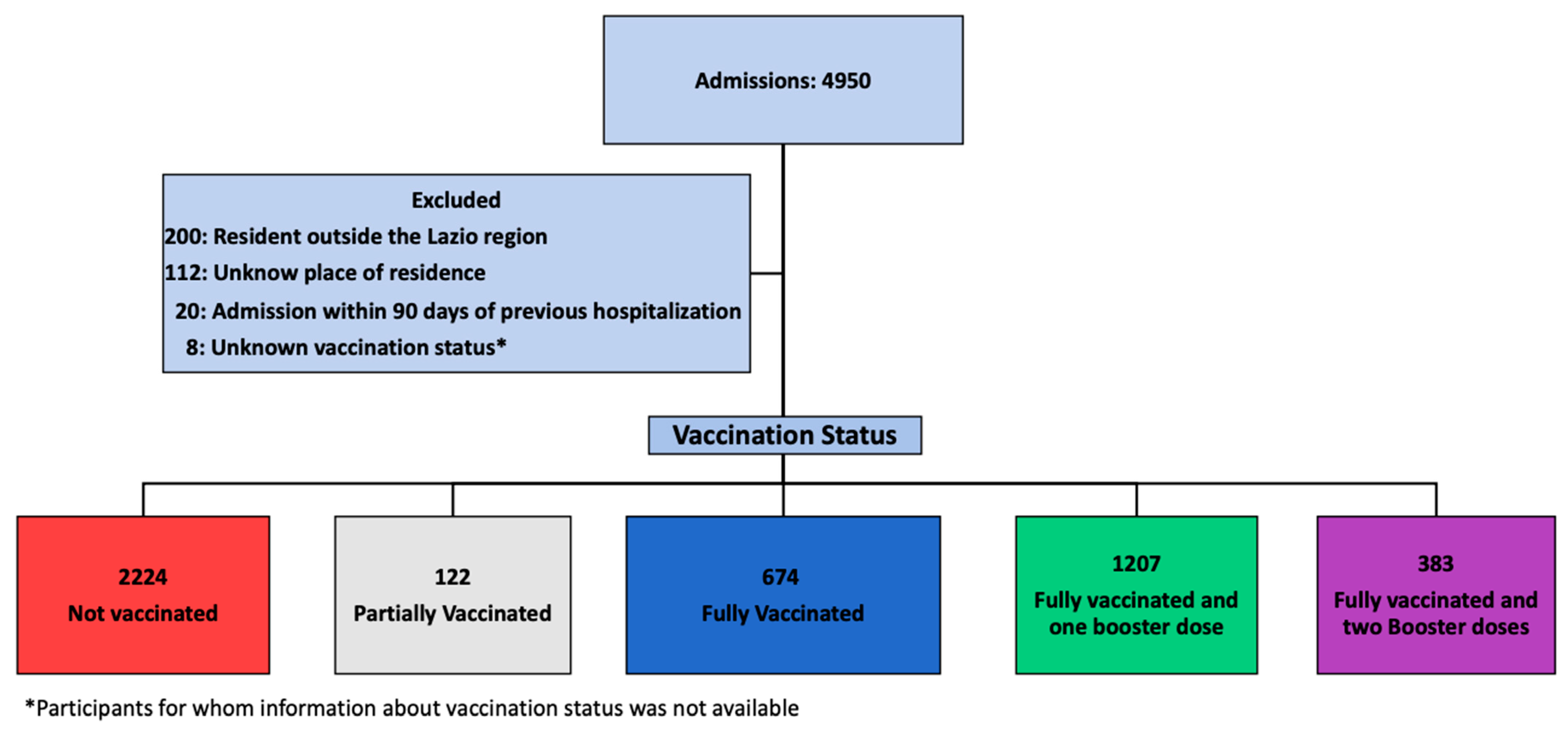
Over an observation period of 30 months, 4950 patients with a diagnosis of COVID-19 were admitted at our Institute. Of these, 4488 were included in the study: 2224 (49.6%) NV, 674 (15.0%) FV, 1207 (26.9%) FV&B1, and 383 (8.5%) FV&B2. The details of the patients’ selection are depicted in Figure 1.
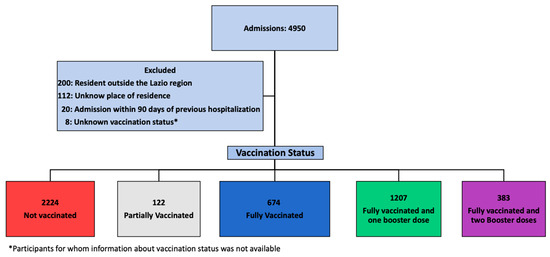
Figure 1.
Study flow chart.
3.1. Patients’ Characteristics at Hospital Admission
The main characteristics at hospital admission according to vaccination status are shown in Table 1.

Table 1.
Study participants’ characteristics at hospital admission according to the vaccination status (n = 4488).
Briefly, the study population consisted mostly of men (59.4%), of Italian origin (88.4%), with a median age of 68 (IQR 54–80) years, and with at least one concomitant disease (71.1%). At hospital admission, which occurred within a median time of 4 days (IQR 2–8) from the symptoms’ onset, 89.9% of patients had evidence of pneumonia. The four exposure groups differed for most of the main baseline characteristics. In particular, vaccinated subjects were more likely to be older (FV&B2 82 [IQR 74–87] years, FV&B1 76 [IQR 64–84] years, FV 68 [IQR 55–79] years, and NV 59 [IQR 48–72] years, p < 0.001) and Italian (FV&B2 98.4% vs. FV&B1 93.6% vs. FV 89.3%, and NV 83.6%, p < 0.001), to be admitted after a shorter median time from symptoms’ onset (FV&B2 2 [IQR 1–4] days, FV&B1 2 [IQR 1–5] days, FV 4 [IQR 2–7] days, and NV 6 [IQR 3–9] days, p < 0.001), and to have multiple comorbidities (patients with more than one comorbidity: FV&B2 53.9 vs. FV&B1 42.0% vs. FV 40.1%, and NV 25.2%, p < 0.001), as compared to NV ones. In all the exposure groups, cardiovascular disease was the most common concomitant illness (FV&B2 69.5% vs. FV&B1 57.2% vs. FV 53.6%, and NV 43.8%, p < 0.001), followed by chronic respiratory disease (FV&B2 31.9% vs. FV&B1 26.6% vs. FV 21.7%, and NV 15.5%, p < 0.001) and diabetes (FV&B2 24.8% vs. FV&B1 16.6% vs. FV 20.0%, and NV 12.6%, p < 0.001). Almost all reported comorbidities, except metabolic disease (including obesity and dyslipidemia), were more frequent among vaccinated patients than not vaccinated ones. At the time of hospital admission, NV patients compared to the other exposure groups were more likely to have lung involvement at the CT scan (NV 95.2% vs. FV 89.0% vs. FV&B1 80.9% vs. FV&B2 88.5%, p < 0.001). Among vaccinated patients, mRNA vaccine BNT162b2 was the most frequent type of vaccine administered for the first dose in the FV, FV&B1, and FV&B2 groups (66.3%, 78.3%, and 81.7%, respectively, p < 0.001). Of note, a total of 441 (19.5%) subjects (16.8% FV, 18.2% FV&B1, and 28.2% FV&B2) received their last dose within 120 days from hospital admission.
3.2. Vaccination Coverage over Time in the Study Population and in the Lazio Region
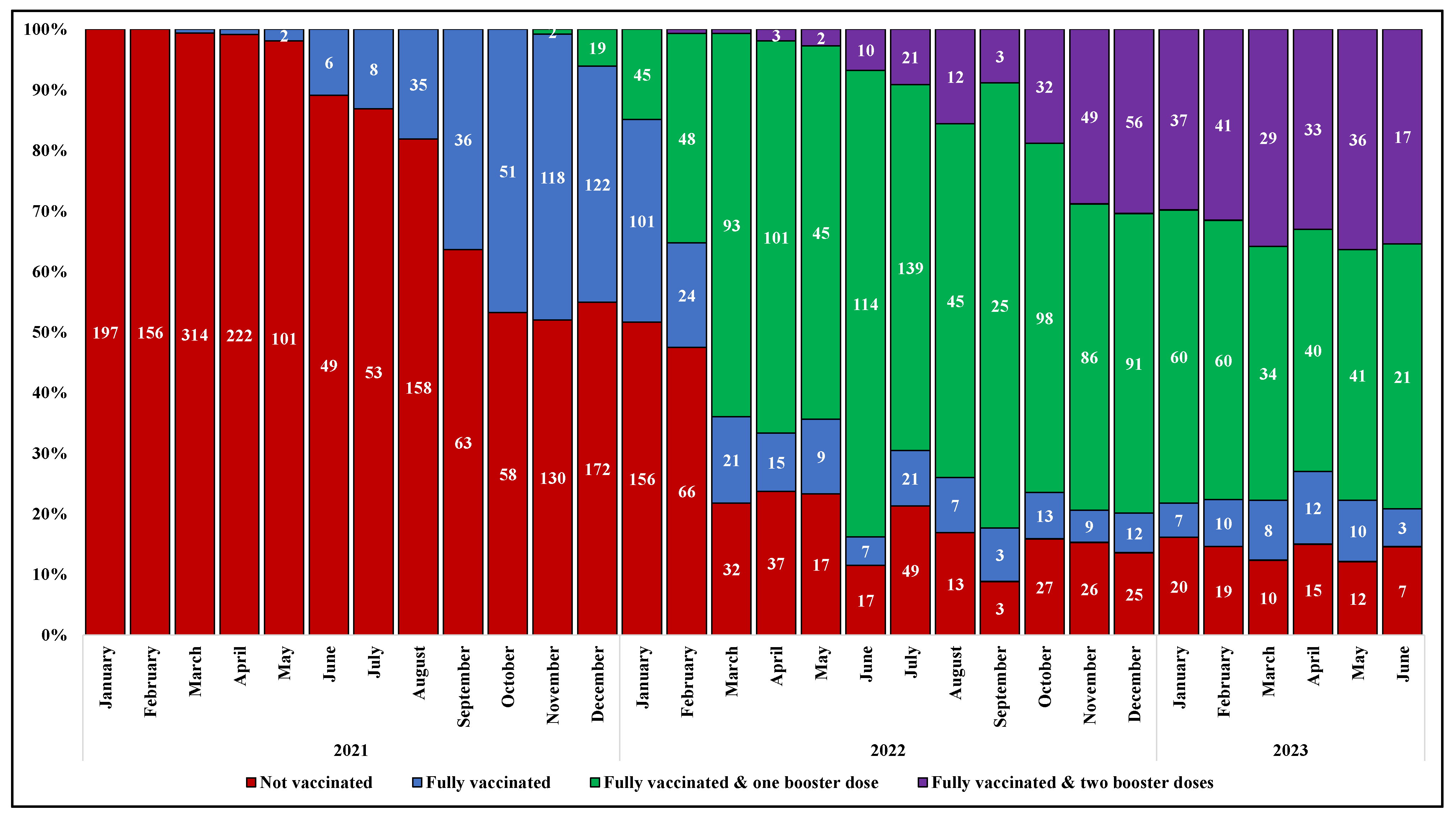
The prevalence of subjects who received a full primary cycle of anti-SARS-CoV-2 vaccination before hospital admission increased gradually over time, from none of the 197 cases hospitalized in January 2021 to 47% of those (n = 250) hospitalized in November 2021, two months after the approval of a booster dose in Italy. The proportion of subjects who received the additional dose of vaccine after the primary cycle (FV&B1) progressively raised, ranging from around 1% of the patients admitted for COVID-19 in November 2021 (n = 250) to 77% in June 2022 (n = 148); similarly, an increasing proportion of individuals who received a second booster dose was observed from February to December 2022 (from less than 1% [1/139] to 30% [56/184]). Thereafter, the proportions remained roughly stable (Figure 2).
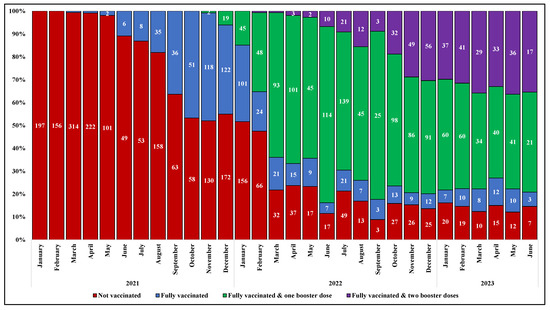
Figure 2.
Prevalence of vaccination among study population by month and year of hospital admission.
The temporal change in anti-SARS-CoV-2 vaccination coverage described in our study population over the observation period is consistent with the regional data. Of note, the proportion of NV was higher in our population than in the general population across all age strata. Similarly, the proportion of FV&B2 was higher among our study population compared to the regional data in the strata from 20 to 69 years (Figure S1, Supplementary Materials).
3.3. Main Clinical and Virological Outcomes
Over a median follow-up of 14 days (IQR 9–22), 396 subjects (8.8%) died, 604 (13.5%) were admitted to ICU, and 737 (16.4%) experienced clinical progression within 28 days from hospital admission. Looking at the comparisons among crude rates, we did not observe any significant differences in terms of 28-day in-hospital death by vaccination status (NV 8.3% vs. FV 9.2% vs. FV&B1 9.5% vs. FV&B2 9.1%, p = 0.627). Conversely, a higher proportion of NV participants compared to vaccinated ones was admitted to the ICU (NV 16.4% vs. FV 11.7% vs. FV&B1 10.1% vs. FV&B2 10.2%, p < 0.001) and experienced clinical progression (NV 18.2% vs. FV 15.6% vs. FV&B1 14.3% vs. FV&B2 14.4%, p = 0.012) within 28 days from hospitalization. The median length of hospital stay and ICU stay did not significantly differ among the exposure groups (Table 2). Concerning virological outcomes, a higher proportion of individuals with persistent viral shedding within day 28 of hospitalization was observed among those who received booster doses (NV 83.2% vs. FV 82.4% vs. FV&B1 89.2% vs. FV&B2 92.2%, p < 0.001) (Table 2).

Table 2.
Main clinical outcomes by vaccination status.
3.4. Predictors of 28-Day in-Hospital Death and Clinical Progression
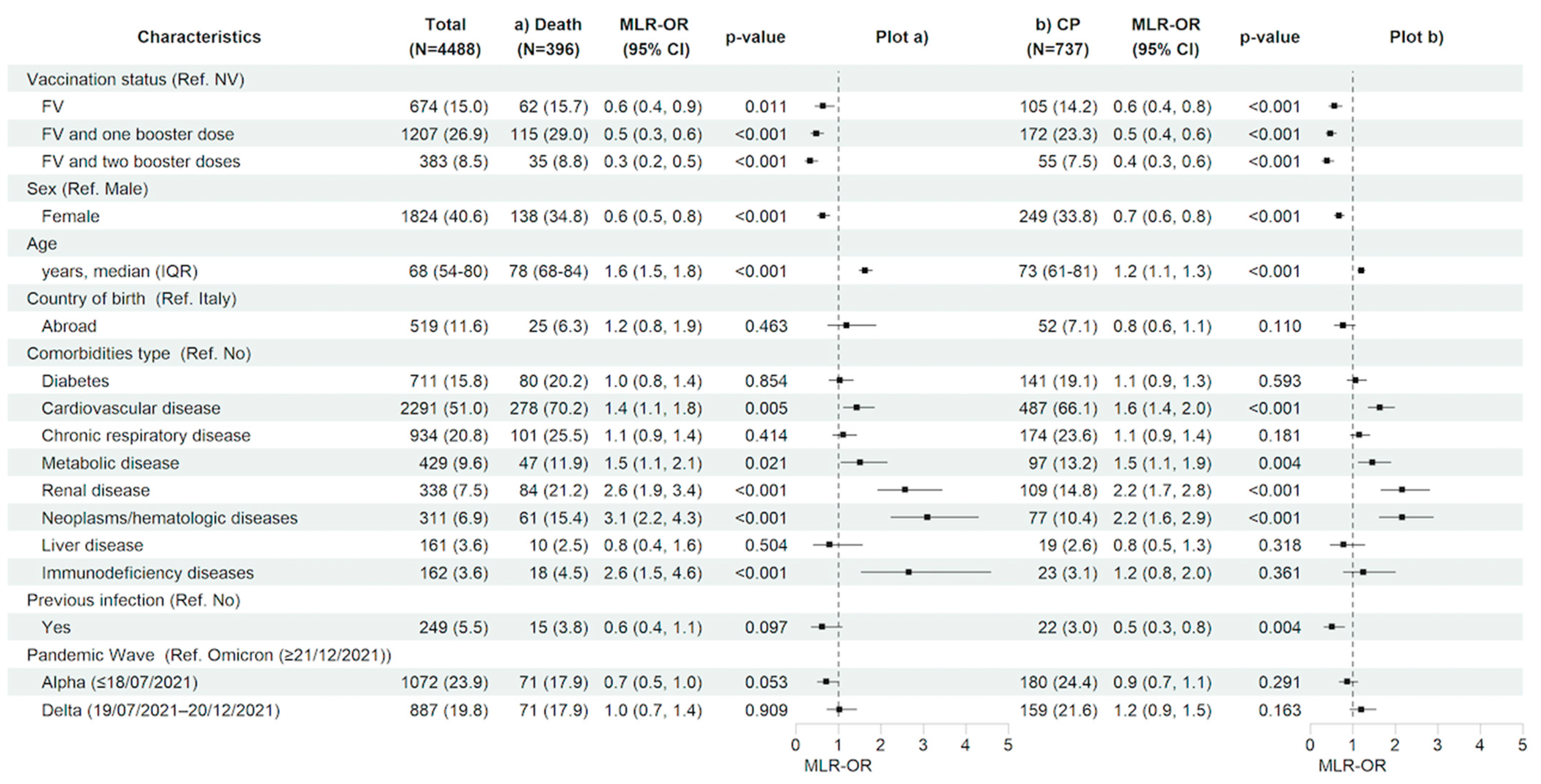
Looking to the predictive factors for the main in-hospital outcomes, after adjusting for the main confounders listed in the methods section, having received a complete primary cycle of anti-SARS-CoV-2 vaccination compared to being not vaccinated was associated with a 36% (MLR-OR FV vs. NV 0.64, 95% CI 0.45–0.90, p = 0.011) and 43% (MLR-OR FV vs. NV 0.57, 95% CI 0.44–0.75, p < 0.001) reduction in the risk of 28-day in-hospital death and clinical progression, respectively. Similarly, having received booster doses versus not being vaccinated reduced the risk for both outcomes by more than 50% (MLR-OR FV&B1 vs. NV 0.48, 95% CI 0.35–0.65, p < 0.001, and MLR-OR FV&B2 vs. NV 0.33, 95% CI 0.21–0.51, p < 0.001 for in-hospital death; MLR-OR FV&B1 vs. NV 0.47, 95% CI 0.37–0.61, p < 0.001, and MLR-OR FV&B2 vs. NV 0.39, 95% CI 0.27–0.55, p < 0.001 for clinical progression). The female gender was also associated to a lower risk of both 28-day in-hospital death (MLR-OR 0.63, 95% CI 0.50–0.80, p < 0.001) and clinical progression (MLR-OR 0.67, 95% CI 0.56–0.80, p < 0.001). On the contrary, older age (MLR-OR per 10 years older, for in-hospital death, 1.63, 95% CI 1.48–1.80, p < 0.001 and, for clinical progression, 1.20, 95% CI 1.12–1.28, p < 0.001) and certain underlying comorbidities such as cardiovascular disease (MLR-OR, for in-hospital death, 1.43, 95% CI 1.12–1.84, p = 0.005 and, for clinical progression, 1.64, 95% CI 1.36–1.98, p < 0.001), metabolic disease (MLR-OR, for in-hospital death, 1.51, 95% CI 1.06–2.14, p = 0.021 and, for clinical progression, 1.46, 95% CI 1.13–1.89, p = 0.004), renal diseases (MLR-OR, for in-hospital death, 2.57, 95% CI 1.92–3.44, p < 0.001 and, for clinical progression, 2.16, 95% CI 1.66–2.80, p < 0.001), and neoplasms/hematologic diseases (MLR-OR, for in-hospital death, 3.09, 95% CI 2.23–4.29, p < 0.001 and, for clinical progression, 2.16, 95% CI 1.62–2.89, p < 0.001) showed a significant higher risk for both in-hospital outcomes; having an immunodeficiency status was associated with a higher risk only for in-hospital death (MLR-OR 2.65, 95% CI 1.53–4.58, p < 0.001). Finally, no association between pandemic waves and clinical outcomes for both in-hospital outcomes was observed (Figure 3a,b and Table S1a,b).
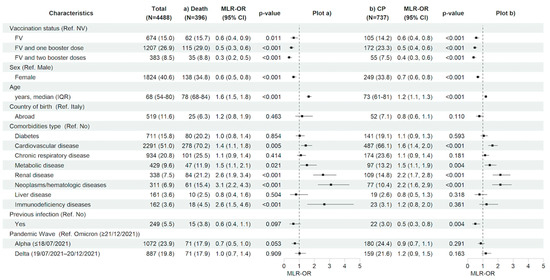
Figure 3.
Characteristics of the participants and multivariable logistic regression results with forest plot representation for (a) in-hospital death and (b) clinical progression within 28 days from hospital admission, in the entire study population (n = 4488). For age, MLR-OR is for 10-year increase. Abbreviations: N, number of participants; MLR, multivariable logistic regression; OR, odds ratio; CP, clinical progression; CI, confidence interval; Ref, reference category; NV, not vaccinated; FV, fully vaccinated; IQR, interquartile range.
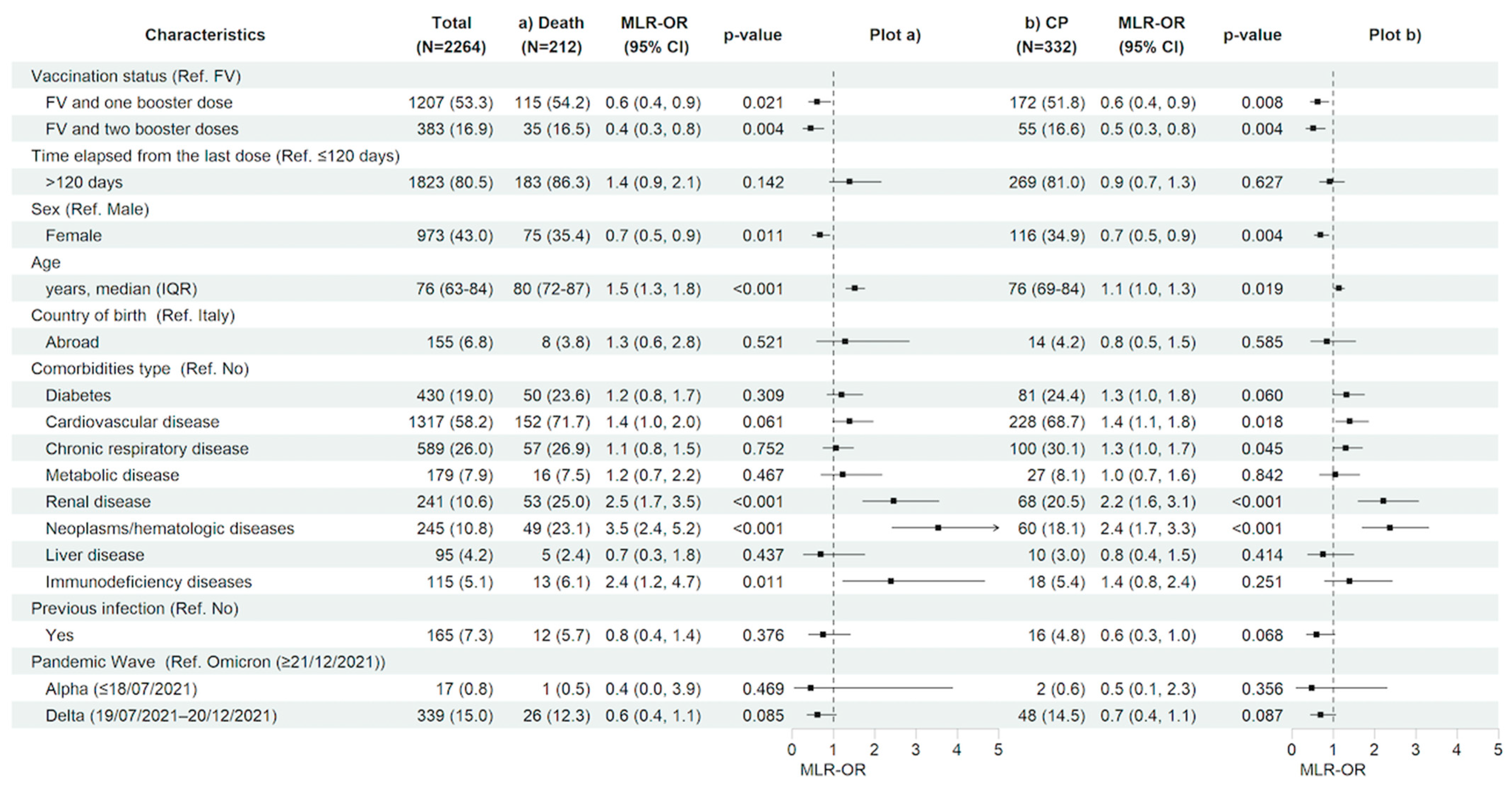
After restricting the analysis to the vaccinated population having received an immunization booster, compared with having completed only the first vaccination cycle, regardless of the time since the last vaccine dose, significantly decreased the risk of 28-day clinical progression and mortality (MLR-OR 0.63 for FV&B1, 95% CI 0.45–0.89, p = 0.008 and MLR-OR 0.52 for FV&B2, 95% CI 0.34–0.81, p = 0.004 vs. MLR-OR 0.61 for FV&B1, 95% CI 0.40–0.93, p = 0.021 and MLR-OR 0.45 for FV&B2, 95% CI 0.26–0.77, p = 0.004, respectively). In contrast, the time elapsed from the last dose did not show a significant effect on the risk of 28-day clinical progression and mortality. Similarly to the total population, in vaccinated subjects, older age (MLR-OR per 10 years increase for clinical progression 1.14, 95% CI 1.02–1.27, p = 0.019 and MLR-OR per 10 years increase for in-hospital death 1.52, 95% CI 1.30–1.76, p < 0.001) and having hematologic/neoplastic diseases (MLR-OR for clinical progression 2.37, 95% CI 1.70–3.31, p < 0.001 and MLR-OR for in-hospital death 3.53, 95% CI 2.42–5.17, p < 0.001), and renal impairment (MLR-OR for clinical progression 2.22, 95% CI 1.60–3.07, p < 0.001 and MLR-OR for in-hospital death 2.46, 95% CI 1.71–3.54, p < 0.001) were associated with a higher risk of both 28-day clinical outcomes. In addition, having an immunodeficiency status increased the risk of in-hospital mortality (MLR-OR for in-hospital death 2.37, 95% CI 1.22–4.63, p = 0.011). Finally, the female gender was confirmed to be associated with a reduced risk of clinical progression and in-hospital death also in the vaccinated population (MLR-OR 0.69, 95% CI 0.54–0.89, p = 0.004 vs. MLR-OR 0.67, 95% CI 0.49–0.91, p = 0.011, respectively) (Figure 4a,b and Table S2a,b).
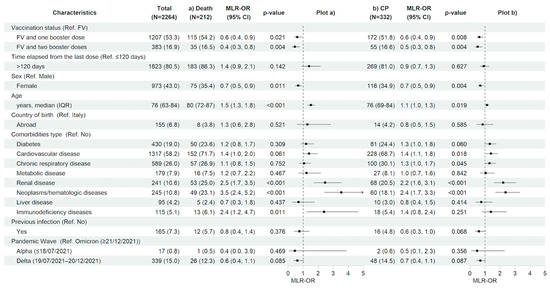
Figure 4.
Characteristics of the participants and multivariable logistic regression results with forest plot representation for (a) in-hospital death and (b) clinical progression within 28 days from hospital admission, restricted to vaccinated study population (n = 2726). For age, MLR-OR is for 10-year increase. Abbreviations: N, number of participants; MLR, multivariable logistic regression; OR, odds ratio; CP, clinical progression; CI, confidence interval; Ref, reference category; FV, fully vaccinated; IQR, interquartile range.
4. Discussion
In this retrospective observational study, including 4488 subjects who were hospitalized at INMI “Lazzaro Spallanzani” between January 2021 and June 2023, the efficacy of the anti-SARS-CoV-2 vaccination, and particularly of booster doses, in reducing the risk of severe COVID-19 was confirmed over a considerable observation period including several pandemic waves.
Indeed, in our analysis, unvaccinated participants were more prone to developing critical disease as shown by the lower rate of ICU admission and clinical progression (in-hospital death and ICU admission within 28 days from hospital admission) among vaccinated individuals. Importantly, this result was more evident for boosted participants, underlying the importance of promoting access to additional doses mainly for frailer subjects. Conversely, when compared crude rates, we did not observe any difference by vaccination status in terms of in-hospital mortality for any causes, however lower (nearly 9% in the overall study population) compared to other reports in similar settings [19,23], and length of hospital and ICU stay, probably because other unidentified confounders may play a causative role and several complications may characterize the hospital stay of a population of old subjects underlying several medical conditions. Nevertheless, our adjusted analysis showed an increasing protection from in-hospital death and clinical progression as the number of vaccine doses received increases. In particular, we observed a risk reduction of more than 50% for boosted individuals compared to unvaccinated ones and, after restricting the analysis to vaccinated participants, we demonstrated a similar risk reduction for those who have received additional doses after the full primary cycle. This result is in line with the existing literature which demonstrates the association of vaccination status and additional doses with in-hospital death or ICU admission, among individuals hospitalized for COVID-19 [18,19,20,21,22,23,24]. Moreover, this study underlines the accuracy and robustness of some previous molecular studies indicating the efficacy of booster-induced memory B cells against Omicron and its variants at the atomic, cellular, and animal levels [30,31,32].
As expected, older participants and those with concomitant medical conditions demonstrated a heightened vulnerability to clinical progression and mortality, regardless of the number of doses received. Interestingly, among comorbidities, the greatest risk of developing critical COVID-19 and, in particular, in-hospital death was observed in the case of underlying renal disease or any immunocompromised status, due to an active treatment and/or immunocompromising medical condition such as immunodeficiency diseases and solid or hematologic neoplasms. The association was even stronger after considering only our vaccinated population. Increased clinical risk for older people has been already demonstrated [11,33] as a consequence of underlying frailty, comorbidity, and immune senescence. Similarly, patients with a chronic renal disease, in particular, those who are under dialysis or kidney transplant recipients, have been known to be at an increased risk of severe infection with SARS-CoV-2, and to have an impaired response to standard vaccination [34]. Finally, immunocompromised subjects are less likely to mount an adequate immune response to vaccination and more likely to develop severe COVID-19 and may have persistent clinical complications and prolonged SARS-CoV-2 positivity in respiratory samples [35]. Indeed, we observed a prolonged viral shedding among individuals vaccinated with booster doses, which is not surprising considering that they consist mainly of elderly, frail, and immunocompromised individuals who are less likely to shed the virus from respiratory samples. Of note, we did not find any association between pandemic waves and in-hospital clinical outcomes, underlying the importance of vaccination for specific categories of patients even with the more recent and less pathogenic SARS-CoV-2 variant [26,36].
In the present study, the time elapsing from the last dose was not associated with the risk of developing critical illness or mortality. Our result is apparently in contrast with the evidence arising from a recent report comparing the effectiveness of several booster doses with that of full primary vaccination received from April 2022 to July 2023 [15]. In fact, this study confirmed the importance of booster doses in restoring individual protection against COVID-19-related hospitalization and death and demonstrated the rapid decline in vaccine efficacy after 12 weeks, especially among individuals aged ≥ 80 years. However, our study was conducted over a larger observation period, covering a range of viral variants, and in a different setting, including only hospitalized patients of different ages, half of whom were not vaccinated. Indeed, other analyses from the US during 2022–2023 showed a similar waning of COVID-19 vaccine effectiveness against hospitalization, but also demonstrated a more sustained effectiveness against critical illness, with protection lasting well over 1 year after the most recent dose. Furthermore, other studies [37,38] found a waning of protection against COVID-19 in elderly patients after six months following the second vaccine dose, while, in the current analysis, we evaluated a distance of 120 days from the last dose. It is, therefore, clear that, particularly after hospitalization, a number of factors in addition to vaccination status should be considered when assessing key clinical outcomes, such as ICU admission or mortality, especially in a more medically fragile population, and further studies are needed to identify the real association between the waning of immunity after vaccination and the declining protection from severe COVID-19.
Finally, given the extensive observation period of our study, which spans from the beginning of the anti-SARS-CoV-2 vaccination campaign in Italy to June 2023, when the second booster dose had been available for over a year, we observed an increasing vaccination coverage, which reflects the regional data on vaccination uptake. Interestingly, by the end of 2022, the proportion of subjects who had received a second booster remained almost stable at a low level both in the study population and at the regional level, particularly among the youngest, indicating that, despite public initiatives promoting the administration of booster doses, vaccine hesitancy regarding additional doses persists, especially for those individuals who have a self-perception of being at a low risk of severe disease [39]. As expected, the proportion of unvaccinated patients was persistently higher in the study population, confirming the effectiveness of vaccination against hospitalization for COVID-19. In addition, it is worth noting that, the proportions of study participants with a second booster dose, compared with regional data, were similar or even higher, reflecting a high vaccination coverage mainly among those more vulnerable due to concomitant diseases and being at high risk of being hospitalized, regardless of age.
To our knowledge, this is the largest study ever conducted in the target population of individuals hospitalized for COVID-19 covering an observation period with a considerable length when several VoCs were circulating, allowing the inclusion of Omicron variants, associated to a less severe presentation of the disease [26,36], and boosted individuals. However, several limitations of our analysis need to be mentioned. First, the observational nature of the study is prone to residual and potential unmeasured confounding bias. Second, the monocentric design of the study, on the one hand, limits the generalizability of our findings, but, on the other hand, ensures homogeneity in background care, disease management, and resource availability, even in the absence of information on therapeutic approaches had during the hospitalization and any previous early treatment received. Finally, there is a lack of SARS-CoV-2 sequencing data to have a more detailed and precise definition of pandemic periods.
5. Conclusions
In conclusion, the present study confirms the effectiveness of complete anti-SARS-CoV-2 vaccination and, in particular, booster doses in reducing the risk of severe clinical progression and in-hospital death among individuals hospitalized for COVID-19. Indeed, unvaccinated participants were more prone to develop critical disease. In addition, our findings indicate a range of demographic and clinical factors, such as older age and the presence of certain concomitant medical conditions, associated with an increased clinical risk of severe COVID-19 outcomes despite booster vaccination. These data support the efforts to promote SARS-CoV-2 vaccination and increase the uptake of booster doses in order to prevent COVID-19-associated severe outcomes, particularly in older and frail individuals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12091018/s1, Classification of comorbidities; Figure S1: Anti-SARS-CoV-2 vaccination coverage in Lazio Region (red) and in the study population (black), including only individuals hospitalized for COVID-19, represented for each trimester of the study period (January 2021–June 2023), both overall and by age group; Table S1: Univariable logistic regression analysis for (a) in-hospital death and (b) clinical progression within 28 days from hospital admission, in the entire study population (n = 4488); Table S2: Univariable logistic regression analysis for a) in-hospital death and b) clinical progression within 28 days from hospital admission, restricted to vaccinated study participants (n = 2726).
Author Contributions
Conceptualization and methodology: A.M., I.M., A.N., E.G. and A.A. (Andrea Antinori). Data curation: A.N., C.C., A.A. (Alessandro Agresta) and A.D. Formal analysis: A.N. Funding acquisition: E.G. and A.A. (Andrea Antinori). Investigation: A.M., I.M., C.P., V.M., A.C., A.Z., S.A.M., L.L., M.G.B., G.M., P.G., F.M. and F.V. Supervision: P.G., F.M., F.V., E.G. and A.A. (Andrea Antinori). Writing—original draft: A.M. and I.M. Writing—review and editing: A.N., C.C., C.P., V.M., A.A. (Alessandro Agresta), A.C., A.Z., S.A.M., L.L., M.G.B., G.M., A.D., P.G., F.M., F.V., E.G. and A.A. (Andrea Antinori). All authors have read and agreed to the published version of the manuscript.
Funding
The study was performed in the framework of the SARS-CoV-2 surveillance and response program implemented by the Lazio Region Health Authority. This study was supported by funds to the National Institute for Infectious Diseases “Lazzaro Spallanzani”, IRCCS, Rome (Italy), from Italian Ministry of Health (Programme CCM 2020; Ricerca Corrente—Linea 1 on emerging and re-emerging infections).
Institutional Review Board Statement
The study was approved by the Ethical Committee of the National Institute for Infectious Diseases “Lazzaro Spallanzani” in Rome, Italy, as the National Review Board for the COVID-19 pandemic in Italy (ethic approval number 164/2020). All procedures contributing to the work described comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge all collaborators, the members of the National Institute for Infectious Diseases ReCOVeRI study group, all the nurses and the clinical and laboratory staff, and all the study participants.
Conflicts of Interest
A.M. received travel fees from ViiV Healthcare. V.M. received personal payments/honoraria from AstraZeneca, Pfizer, and GSK, and support for attending meetings and/or travel by AstraZeneca. E.G. received personal payments from Gilead Sciences and institutional grants from Gilead Sciences and Mylan. A.A. (Andrea Antinori) has served as a paid consultant to AstraZeneca, Bavarian Nordic, Gilead Sciences, GSK, Janssen-Cilag, Merck, Moderna, Pfizer, and ViiV Healthcare and received research funding through the National Institute for Infectious Diseases Lazzaro Spallanzani IRCCS from AstraZeneca, Gilead Sciences, and ViiV Healthcare. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 10 July 2024).
- COVID-19 National Vaccination Strategic Plan. Available online: https://www.epicentro.iss.it/vaccini/covid-19-piano-vaccinazione (accessed on 10 July 2024).
- Italian Ministry of Health. Circolare Ministeriale del. 14 September 2021. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=82776&parte=1%20&serie=null (accessed on 10 July 2024).
- Italian Ministry of Health. Circolare Ministeriale del. 20 February 2022. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&codLeg=85813&parte=1%20&serie=null (accessed on 10 July 2024).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111, Corrected in Lancet 2021, 397, 98. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829, Corrected in Lancet 2021, 398, 212. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, Ι.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol. Open. 2022, 14, 100180. [Google Scholar] [CrossRef]
- Solante, R.; Alvarez-Moreno, C.; Burhan, E.; Chariyalertsak, S.; Chiu, N.C.; Chuenkitmongkol, S.; Dung, D.V.; Hwang, K.P.; Ortiz Ibarra, J.; Kiertiburanakul, S.; et al. Expert review of global real-world data on COVID-19 vaccine booster effectiveness and safety during the omicron-dominant phase of the pandemic. Expert. Rev. Vaccines. 2023, 22, 1–16. [Google Scholar] [CrossRef]
- Agrawal, U.; Bedston, S.; McCowan, C.; Oke, J.; Patterson, L.; Robertson, C.; Akbari, A.; Azcoaga-Lorenzo, A.; Bradley, D.T.; Fagbamigbe, A.F.; et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: Pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wale. Lancet 2022, 400, 1305–1320, Corrected in Lancet 2024, 403, 1140. [Google Scholar] [CrossRef]
- Hansen, C.H.; Moustsen-Helms, I.R.; Rasmussen, M.; Søborg, B.; Ullum, H.; Valentiner-Branth, P. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: A national cohort study. Lancet Infect. Dis. 2024, 24, e73–e74. [Google Scholar] [CrossRef]
- van Werkhoven, C.H.; Valk, A.W.; Smagge, B.; de Melker, H.E.; Knol, M.J.; Hahné, S.J.; van den Hof, S.; de Gier, B. Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, The Netherlands, 9 October to 5 December 2023. Eurosurveillance 2024, 29, 2300703. [Google Scholar] [CrossRef] [PubMed]
- DeCuir, J.; Payne, A.B.; Self, W.H.; Rowley, E.A.K.; Dascomb, K.; DeSilva, M.B.; Irving, S.A.; Grannis, S.J.; Ong, T.C.; Klein, N.P.; et al. Interim Effectiveness of Updated 2023–2024 (Monovalent XBB.1.5) COVID-19 Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalization Among Immunocompetent Adults Aged ≥18 Years—VISION and IVY Networks, September 2023-January 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 180–188. [Google Scholar]
- Luxembourg, M. Interim Analysis of COVID-19 Vaccine Effectiveness Against Hospitalisation Due to COVID-19 and Death Using Electronic Health Records in Eight European Countries: First Update; ECDC: Stockholm, UK, 2024. [Google Scholar]
- Yeh, Y.P.; Lin, T.Y.; Yao, Y.C.; Hsu, C.Y.; Yen, A.M.F.; Chen, S.L.S.; Chen, T.H.H. New insights into three trajectories of omicron-related all-cause death reduced by COVID-19 booster vaccination. J. Infect. Public Health 2024, 17, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.Y.; et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell 2022, 185, 1539–1548.e5. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- d’Arminio Monforte, A.; Tavelli, A.; De Benedittis, S.; Bai, F.; Tincati, C.; Gazzola, L.; Viganò, O.; Allegrini, M.; Mondatore, D.; Tesoro, D.; et al. Real World Estimate of Vaccination Protection in Individuals Hospitalized for COVID-19. Vaccines 2022, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Kristofferson, A.B.; Salamanca, B.V.; Seppälä, E.; Golestani, K.; Kvåle, R.; Watle, S.V.; Buanes, E.A. Length of hospital stay and risk of intensive care admission and in-hospital death among COVID-19 patients in Norway: A register-based cohort study comparing patients fully vaccinated with an mRNA vaccine to unvaccinated patients. Clin. Microbiol. Infect. 2022, 28, 871–878. [Google Scholar] [CrossRef]
- Mielke, N.; Johnson, S.; Bahl, A. Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. Lancet Reg. Health Am. 2022, 8, 100227. [Google Scholar] [CrossRef]
- Ruiz-Giardin, J.M.; Rivilla, M.; Mesa, N.; Morales, A.; Rivas, L.; Izquierdo, A.; Escribá, A.; San Martín, J.V.; Bernal-Bello, D.; Madroñal, E.; et al. Comparative Study of Vaccinated and Unvaccinated Hospitalised Patients: A Retrospective Population Study of 500 Hospitalised Patients with SARS-CoV-2 Infection in a Spanish Population of 220,000 Inhabitants. Viruses 2022, 14, 2284. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Huang, C.; Yang, H.; Jiang, C.; Yu, X.; Zhao, R.; Hong, J.; Zhang, Y.; Wang, Y.; et al. Booster vaccines dose reduced mortality in hospitalized COVID-19 patients requiring oxygen supplementation: Evidence from the Beijing Omicron outbreak. Hum. Vaccin. Immunother. 2024, 20, 2361500. [Google Scholar] [CrossRef]
- Gholinataj Jelodar, M.; Mirzaei, S.; Saghafi, F.; Rafieian, S.; Rezaei, S.; Saatchi, A.; Dehghani Avare, Z.; Dehghan Niri, M. Impact of vaccination status on clinical outcomes of hospitalized COVID-19 patients. BMC Infect. Dis. 2024, 24, 254. [Google Scholar] [CrossRef]
- Italian National Institute of Health (Istituto Superiore di Sanità, ISS). Monitoraggio Delle Varianti del Virus SARS-CoV-2 di Interesse in Sanità Pubblica in Italia. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-monitoraggio-varianti-rapporti-periodici (accessed on 10 July 2024).
- Mondi, A.; Mastrorosa, I.; Piselli, P.; Cimaglia, C.; Matusali, G.; Carletti, F.; Giannico, G.; Milozzi, E.; Biliotti, E.; Di Bari, S.; et al. Evolution of SARS-CoV-2 variants of concern over a period of Delta and Omicron cocirculation, among patients hospitalized for COVID-19 in an Italian reference hospital: Impact on clinical outcomes. J. Med. Virol. 2023, 95, e28831. [Google Scholar] [CrossRef]
- Vittinghoff, E.; Glidden, D.V.; Shiboski, S.C.; McCulloch, C.E. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models; Springer Publishing Co.: New York, NY, USA, 2005. [Google Scholar]
- Chatterjee, S.; Simonoff, J.S. Handbook of Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Struttura Commissariale per l’Emergenza COVID-19. Open Data on COVID-19 Vaccination in Italy. Available online: https://github.com/italia/covid19-opendata-vaccini (accessed on 10 July 2024).
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jia, Z.; Bao, L.; Wang, L.; Cao, L.; Chi, H.; Hu, Y.; Li, Q.; Zhou, Y.; Jiang, Y.; et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature 2022, 603, 919–925. [Google Scholar] [CrossRef]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Ben Tanfous, T.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Patel, K.; Patton, M.E.; Reingold, A.; Kawasaki, B.; Meek, J.; Openo, K.; Ryan, P.A.; Falkowski, A.; Bye, E.; et al. COVID-19-Associated Hospitalizations Among U.S. Adults Aged ≥ 65 Years—COVID-NET, 13 States, January-August 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1089–1094. [Google Scholar] [CrossRef]
- Taheri, S. Efficacy and safety of booster vaccination against SARS-CoV-2 in dialysis and renal transplant patients: Systematic review and meta-analysis. Int. Urol. Nephrol. 2023, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: Insights from the observational population-based INFORM study. Lancet Reg. Health Eur. 2023, 35, 100747. [Google Scholar] [CrossRef]
- Hedberg, P.; Parczewski, M.; Serwin, K.; Marchetti, G.; Bai, F.; Jensen, B.E.O.; Pereira, J.P.; Drobniewski, F.; Reschreiter, H.; Naumovas, D.; et al. In-hospital mortality during the wild-type, alpha, delta, and omicron SARS-CoV-2 waves: A multinational cohort study in the EuCARE project. Lancet Reg. Health Eur. 2024, 38, 100855. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Ayyalasomayajula, S.; Dhawan, A.; Karattuthodi, M.S.; Thorakkattil, S.A.; Abdulsalim, S.; Elnaem, M.H.; Sridhar, S.; Unnikrishnan, M.K. A Systematic Review on Sociodemographic, Financial and Psychological Factors Associated with COVID-19 Vaccine Booster Hesitancy among Adult Population. Vaccines 2023, 11, 623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).