The Improvement of Adaptive Immune Responses towards COVID-19 Following Diphtheria–Tetanus–Pertussis and SARS-CoV-2 Vaccinations in Indonesian Children: Exploring the Roles of Heterologous Immunity
Abstract
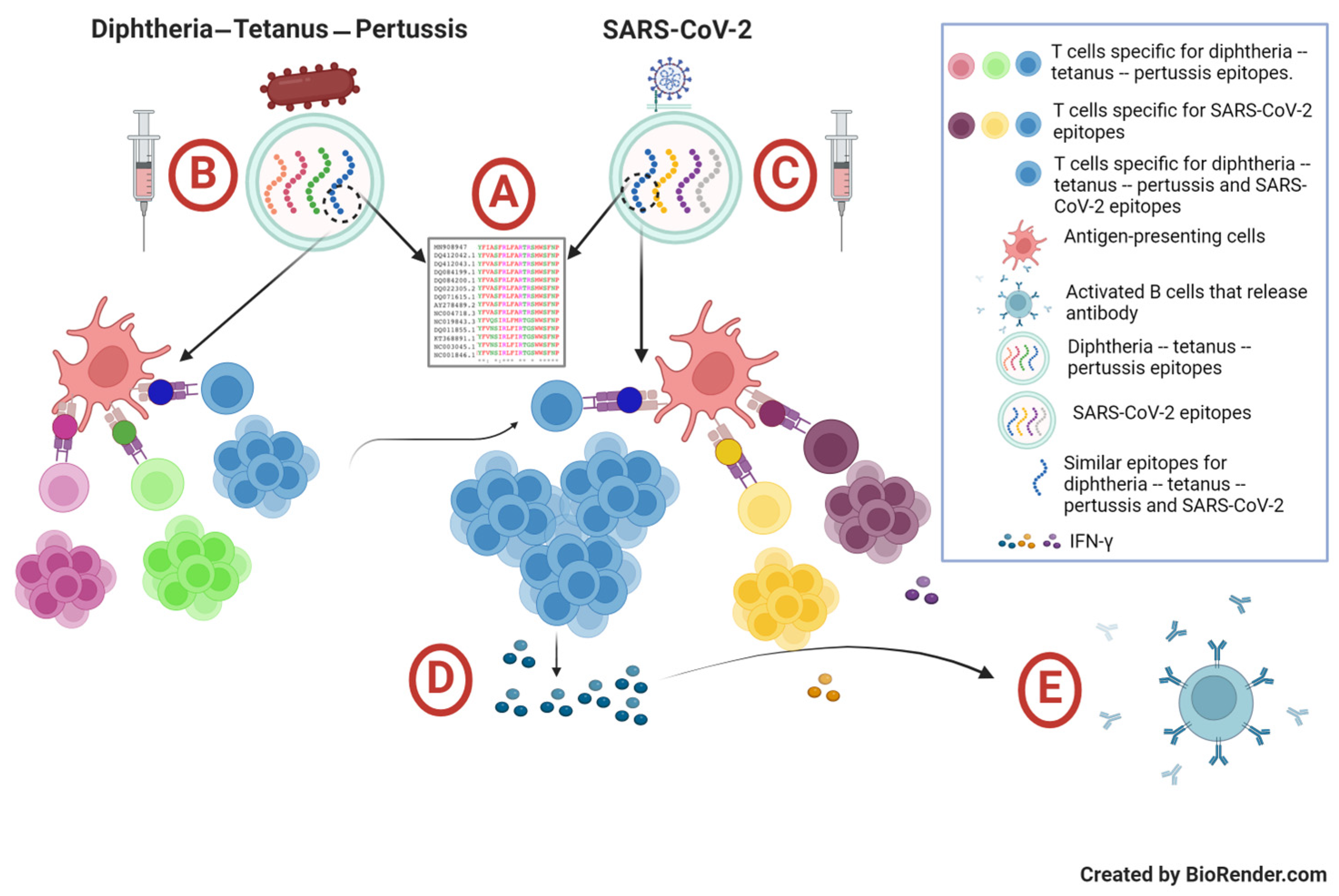
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Humoral Immune Response Assays
2.3. Cellular Immune Response Assay
2.4. Statistical Analysis
3. Results
3.1. Characteristic of Study Participants
3.2. Humoral Immune Response Following DTP and/or COVID-19 Vaccination
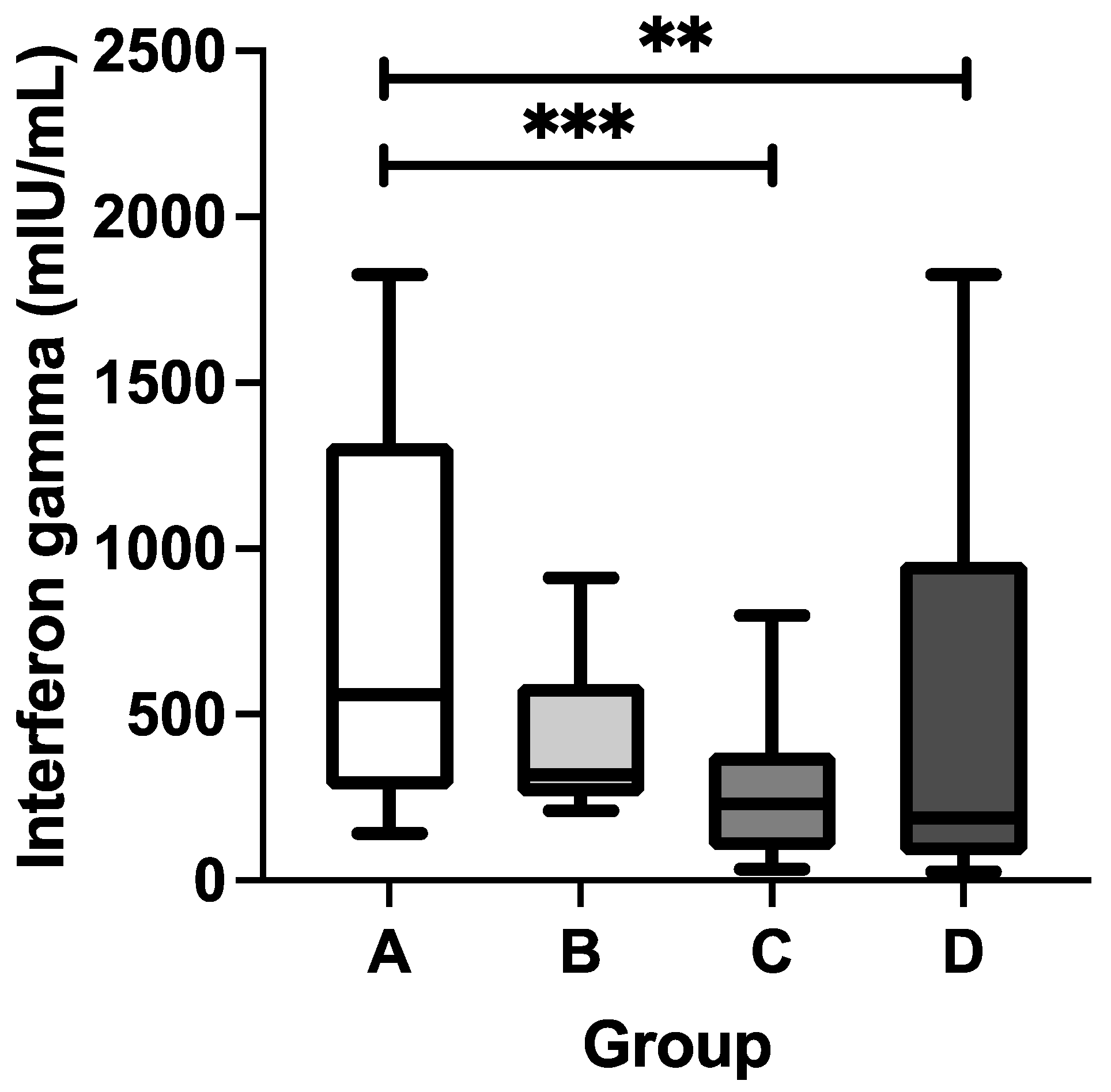
3.3. Cellular Immune Response Following DTP and COVID-19 Vaccinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization WHO. COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 22 June 2024).
- Barouch, D.H. COVID-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Shoorekchali, J.M.; Faraji, M.R.; Seyyedkolaee, M.A.; Pagán, J.A.; Wilson, F.A. COVID-19 Vaccine Inequality: A Global Perspective. J. Glob. Health 2022, 12, 10–13. [Google Scholar] [CrossRef]
- Gozzi, N.; Chinazzi, M.; Dean, N.E.; Longini, I.M.; Halloran, M.E.; Perra, N.; Vespignani, A. Estimating the Impact of COVID-19 Vaccine Inequities: A Modeling Study. Nat. Commun. 2023, 14, 3272. [Google Scholar] [CrossRef]
- Steinman, J.B.; Lum, F.M.; Ho, P.P.K.; Kaminski, N.; Steinman, L. Reduced Development of COVID-19 in Children Reveals Molecular Checkpoints Gating Pathogenesis Illuminating Potential Therapeutics. Proc. Natl. Acad. Sci. USA 2020, 117, 24620–24626. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A Review of the Proposed Mechanisms Underlying the Age-Related Difference in Severity of SARS-CoV-2 Infections. Arch. Dis. Child. 2021, 106, 429–439. [Google Scholar] [CrossRef]
- Axfors, C.; Pezzullo, A.M.; Contopoulos-Ioannidis, D.G.; Apostolatos, A.; Ioannidis, J.P.A. Differential COVID-19 Infection Rates in Children, Adults, and Elderly: Systematic review and Meta-Analysis of 38 Pre-Vaccination National Seroprevalence Studies. J. Glob. Health 2023, 13, 06004. [Google Scholar] [CrossRef]
- Powell, A.A.; Dowell, A.C.; Moss, P.; Ladhani, S.N. Current State of COVID-19 in Children: 4 Years On. J. Infect. 2024, 88, 106134. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Jones, C.E.; Faust, S.N. Vaccination against COVID-19—Risks and Benefits in Children. Eur. J. Pediatr. 2024, 183, 1107–1112. [Google Scholar] [CrossRef]
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe Acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020, 174, 882–889. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Child. Adolesc. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem Inflammatory Syndrome in Children Related to COVID-19: A Systematic Review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Pudjiadi, A.H.; Putri, N.D.; Sjakti, H.A.; Yanuarso, P.B.; Gunardi, H.; Roeslani, R.D.; Pasaribu, A.D.; Nurmalia, L.D.; Sambo, C.M.; Ugrasena, I.D.G.; et al. Pediatric COVID-19: Report From Indonesian Pediatric Society Data Registry. Front. Pediatr. 2021, 9, 716898. [Google Scholar] [CrossRef] [PubMed]
- Nachega, J.B.; Sam-Agudu, N.A.; MacHekano, R.N.; Rabie, H.; Van Der Zalm, M.M.; Redfern, A.; Dramowski, A.; O’Connell, N.; Pipo, M.T.; Tshilanda, M.B.; et al. Assessment of Clinical Outcomes among Children and Adolescents Hospitalized with COVID-19 in 6 Sub-Saharan African Countries. JAMA Pediatr. 2022, 176, E216436. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Izquierdo-Condoy, J.S.; Fernandez-Naranjo, R.; Vasconez, J.; Dávila Rosero, M.G.; Revelo-Bastidas, D.; Herrería-Quiñonez, D.; Rubio-Neira, M. The Deadly Impact of COVID-19 among Children from Latin America: The Case of Ecuador. Front. Pediatr. 2023, 11, 1060311. [Google Scholar] [CrossRef]
- Martínez-Valdez, L.; Richardson, V.L.; Bautista-Márquez, A.; Camacho Franco, M.A.; Cruz Cruz, V.; Hernández Ávila, M. Three Years of COVID-19 in Children That Attend the Mexican Social Security Institute’s 1350 Child Day-Care Centers, 2020–2023. Front. Pediatr. 2023, 11, 1292629. [Google Scholar] [CrossRef]
- Kulkarni, D.; Ismail, N.F.; Zhu, F.; Wang, X.; del Carmen Morales, G.; Srivastava, A.; Allen, K.E.; Spinardi, J.; Rahman, A.E.; Kyaw, M.H.; et al. Epidemiology and Clinical Features of SARS-CoV-2 Infection in Children and Adolescents in the Pre-Omicron Era: A Global Systematic Review and Meta-Analysis. J. Glob. Health 2024, 14, 05003. [Google Scholar] [CrossRef]
- Yonker, L.M.; Boucau, J.; Regan, J.; Choudhary, M.C.; Burns, M.D.; Young, N.; Farkas, E.J.; Davis, J.P.; Moschovis, P.P.; Bernard Kinane, T.; et al. Virologic Features of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children. J. Infect. Dis. 2021, 224, 1821–1829. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, P.; Liang, Y.; Du, B.; Li, L.; Yu, Z.; Wang, H.; Wang, Q.; Zhang, X.; Zhang, W. A Systematic Review of Current Status and Challenges of Vaccinating Children against SARS-CoV-2. J. Infect. Public Health 2022, 15, 1212–1224. [Google Scholar] [CrossRef]
- Gao, P.; Kang, L.-Y.; Liu, J.; Liu, M. Immunogenicity, Efectiveness, and Safety of COVID-19 Vaccines among Children and Adolescents Aged 2–18 Years: An Updated Systematic Review and Meta-analysis. World J. Pediatr. 2023, 19, 1041–1054. [Google Scholar] [CrossRef]
- Leung, D.; Rosa Duque, J.S.; Yip, K.M.; So, H.K.; Wong, W.H.S.; Lau, Y.L. Effectiveness of BNT162b2 and CoronaVac in Children and Adolescents against SARS-CoV-2 Infection during Omicron BA.2 Wave in Hong Kong. Commun. Med. 2023, 3, 3. [Google Scholar] [CrossRef]
- Ladhani, S.N. COVID-19 Vaccination for Children Aged 5–11 Years. Lancet 2022, 400, 74–76. [Google Scholar] [CrossRef]
- Piechotta, V.; Siemens, W.; Thielemann, I.; Toews, M.; Koch, J.; Vygen-Bonnet, S.; Kothari, K.; Grummich, K.; Braun, C.; Kapp, P.; et al. Safety and Effectiveness of Vaccines against COVID-19 in Children Aged 5–11 Years: A Systematic Review and Meta-Analysis. Lancet Child Adolesc. Health 2023, 7, 379–391. [Google Scholar] [CrossRef]
- da Fonseca Lima, E.J.; Leite, R.D. COVID-19 Vaccination in Children: A Public Health Priority. J. Pediatr. (Rio. J.) 2023, 99, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Copland, E.; Patone, M.; Saatci, D.; Handunnetthi, L.; Hirst, J.; Hunt, D.P.J.; Mills, N.L.; Moss, P.; Sheikh, A.; Coupland, C.A.C.; et al. Safety Outcomes Following COVID-19 Vaccination and Infection in 5.1 Million Children in England. Nat. Commun. 2024, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (CoronaVac) in Healthy Children and Adolescents: A Double-Blind, Randomised, Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef]
- Zhang, D.S.; Bao, X.P.; Zhu, J.J.; Zheng, W.J.; Sun, L.X. Safety of an Inactivated COVID-19 Vaccine (CoronaVac) in Children Aged 7-14 Years in Taizhou, China. Diagn. Microbiol. Infect. Dis. 2024, 109, 116253. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.O.; Eun, B.W. COVID-19 in Immunocompromised Children and Adolescents. Clin. Exp. Pediatr. 2023, 66, 182–189. [Google Scholar] [CrossRef]
- Poparn, H.; Srichumpuang, C.; Sosothikul, D.; Jantarabenjakul, W.; Lauhasurayotin, S.; Techavichit, P.; Chiengthong, K.; Poovorapan, Y. Immune Response after 2 Doses of BNT162b2 MRNA COVID-19 Vaccinations in Children and Adolescents with Cancer and Hematologic Diseases. Asian Pac. J. Cancer Prev. 2022, 23, 2049–2055. [Google Scholar] [CrossRef]
- Leung, D.; Mu, X.; Duque, J.S.R.; Cheng, S.M.S.; Wang, M.; Zhang, W.; Zhang, Y.; Tam, I.Y.S.; Lee, T.S.S.; Lam, J.H.Y.; et al. Safety and Immunogenicity of 3 Doses of BNT162b2 and CoronaVac in Children and Adults with Inborn Errors of Immunity. Front. Immunol. 2022, 13, 982155. [Google Scholar] [CrossRef]
- Cho, K.; Park, S.; Kim, E.Y.; Koyanagi, A.; Jacob, L.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Radua, J.; Elena, D.; et al. Immunogenicity of COVID-19 Vaccines in Patients with Diverse Health Conditions: A Comprehensive Systematic Review. J. Med. Virol. 2022, 94, 4144–4155. [Google Scholar] [CrossRef]
- Schmidt, K.L.J.; Dautzenberg, N.M.M.; Hoogerbrugge, P.M.; Lindemans, C.A.; Nierkens, S.; Smits, G.; Van Binnendijk, R.S.; Bont, L.J.; Tissing, W.J.E. Immune Response Following BNT162b2 MRNA COVID-19 Vaccination in Pediatric Cancer Patients. Cancers 2023, 15, 2562. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Funaki, T.; Yamada, M.; Mikami, M.; Miyake, K.; Ueno, S.; Tao, C.; Myojin, S.; Aiba, H.; Matsui, T.; et al. Safety of and Antibody Response to the BNT162b2 COVID-19 Vaccine in Adolescents and Young Adults with Underlying Disease. J. Infect. Chemother. 2023, 29, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zendt, M.; Bustos Carrillo, F.A.; Kelly, S.; Saturday, T.; DeGrange, M.; Ginigeme, A.; Wu, L.; Callier, V.; Ortega-Villa, A.; Faust, M.; et al. Characterization of the Antispike IgG Immune Response to COVID-19 Vaccines in People with a Wide Variety of Immunodeficiencies. Sci. Adv. 2023, 9, eadh3150. [Google Scholar] [CrossRef] [PubMed]
- Hartantri, Y.; Debora, J.; Widyatmoko, L.; Giwangkancana, G.; Suryadinata, H.; Susandi, E.; Hutajulu, E.; Hakiman, A.P.A.; Pusparini, Y.; Alisjahbana, B. Clinical and Treatment Factors Associated with the Mortality of COVID-19 Patients Admitted to a Referral Hospital in Indonesia. Lancet Reg. Health 2023, 11, 100167. [Google Scholar] [CrossRef]
- Santi, T.; Hegar, B.; Munasir, Z.; Prayitno, A.; Werdhani, R.A.; Bandar, I.N.S.; Jo, J.; Uswa, R.; Widia, R.; Vandenplas, Y. Factors Associated with Parental Intention to Vaccinate Their Preschool Children against COVID-19: A Cross-Sectional Survey in Urban Area of Jakarta, Indonesia. Clin. Exp. Vaccine Res. 2023, 12, 240–248. [Google Scholar] [CrossRef]
- Ministry of Health Republic of Indonesia Vaksinasi COVID-19 Nasional. Available online: https://vaksin.kemkes.go.id/#/vaccines (accessed on 24 August 2024).
- Widianto, S. Indonesia to Start Vaccinating Children Aged 6–11 against COVID-19. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/indonesia-start-vaccinating-children-aged-6-11-against-covid-19-2021-12-13/ (accessed on 22 June 2024).
- Suhenda, D. COVID-19 Vaccine Shortages Reported across Country. Available online: https://www.thejakartapost.com/indonesia/2022/10/21/covid-19-vaccine-shortages-reported-across-country.html (accessed on 22 June 2024).
- Messina, N.L.; Zimmermann, P.; Curtis, N. The Impact of Vaccines on Heterologous Adaptive Immunity. Clin. Microbiol. Infect. 2019, 25, 1484–1493. [Google Scholar] [CrossRef]
- Reche, P.A. Potential Cross-Reactive Immunity to SARS-CoV-2 from Common Human Pathogens and Vaccines. Front. Immunol. 2020, 11, 586984. [Google Scholar] [CrossRef]
- Monereo-Sánchez, J.; Luykx, J.J.; Pinzón-Espinosa, J.; Richard, G.; Motazedi, E.; Westlye, L.T.; Andreassen, O.A.; van der Meer, D. Diphtheria And Tetanus Vaccination History Is Associated with Lower Odds of COVID-19 Hospitalization. Front. Immunol. 2021, 12, 749264. [Google Scholar] [CrossRef]
- Nicholson, L.; Adkins, E.; Karyanti, M.R.; Ong-Lim, A.; Shenoy, B.; Huoi, C.; Vargas-Zambrano, J.C. What Is the True Burden of Diphtheria, Tetanus, Pertussis and Poliovirus in Children Aged 3–18 Years in Asia? A Systematic Literature Review. Int. J. Infect. Dis. 2022, 117, 116–129. [Google Scholar] [CrossRef]
- Yosephine, P. Vaccine Preventable Diseases, Current Status, Outbreak and Mitigation. In Proceeding Book Childhood Immunization Update 2023; Hadinegoro; Gunardi, S.R.H., Handryastuti, H., Kaswandhani, S., Raihan, N., Eds.; Badan Penerbit Ikatan Dokter Anak Indonesia: Jakarta, Indonesia, 2023. [Google Scholar]
- World Health Organization Diphtheria Vaccine: WHO Position Paper, August 2017. Wkly. Epidemiol. Rec. 2017, 92, 417–436.
- Zasada, A.A.; Rastawicki, W.; Śmietańska, K.; Rokosz, N.; Jagielski, M. Comparison of Seven Commercial Enzyme-Linked Immunosorbent Assays for the Detection of Anti-Diphtheria Toxin Antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Sanjaya, A.; Pinontoan, R.; Aruan, M.; Wahyuni, R.M.; Viktaria, V. Assessment on Anti-SARS-CoV-2 Receptor-Binding Domain Antibodies among CoronaVac-Vaccinated Indonesian Adults. Clin. Exp. Vaccine Res. 2022, 11, 116. [Google Scholar] [CrossRef]
- Saad Albichr, I.; Mzougui, S.; Devresse, A.; Georgery, H.; Goffin, E.; Kanaan, N.; Yombi, J.C.; Belkhir, L.; De Greef, J.; Scohy, A.; et al. Evaluation of a Commercial Interferon-γ Release Assay for the Detection of SARS-CoV-2 T-Cell Response after Vaccination. Heliyon 2023, 9, e17186. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, E.B. Identifying and Evaluating the Metabolic Syndrome in Children and Adolescents. Ethn. Dis. 2007, 17, S4-1-6. [Google Scholar] [PubMed]
- Tan, A.T.; Lim, J.M.E.; Le Bert, N.; Kunasegaran, K.; Chia, A.; Qui, M.D.C.; Tan, N.; Chia, W.N.; de Alwis, R.; Ying, D.; et al. Rapid Measurement of SARS-CoV-2 Spike T Cells in whole Blood from Vaccinated and Naturally Infected Individuals. J. Clin. Investig. 2021, 131, e152379. [Google Scholar] [CrossRef]
- Indonesian Pediatric Society Proceeding Book Childhood Immunization Update 2023; IDAI: Jakarta, Indonesia, 2023.
- Harapan, H.; Anwar, S.; Dimiati, H.; Hayati, Z.; Mudatsir, M. Diphtheria Outbreak in Indonesia, 2017: An Outbreak of an Ancient and Vaccine-Preventable Disease in the Third Millennium. Clin. Epidemiol. Glob. Health 2019, 7, 261–262. [Google Scholar] [CrossRef]
- Itiakorit, H.; Sathyamoorthi, A.; O’Brien, B.E.; Nguyen, D. COVID-19 Impact on Disparity in Childhood Immunization in Low- and Middle-Income Countries Through the Lens of Historical Pandemics. Curr. Trop. Med. Rep. 2022, 9, 225–233. [Google Scholar] [CrossRef]
- Arguni, E.; Karyanti, M.R.; Satari, H.I.; Hadinegoro, S.R. Diphtheria Outbreak in Jakarta and Tangerang, Indonesia: Epidemiological and Clinical Predictor Factors for Death. PLoS ONE 2021, 16, e0246301. [Google Scholar] [CrossRef]
- Sunarno, S.; Sofiah, S.N.; Amalia, N.; Hartoyo, Y.; Rizki, A.; Puspandari, N.; Saraswati, R.D.; Febriyana, D.; Febrianti, T.; Susanti, I.; et al. Laboratory and Epidemiology Data of Pertussis Cases and Close Contacts: A 5-Year Case-Based Surveillance of Pertussis in Indonesia, 2016–2020. PLoS ONE 2022, 17, e0266033. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and Safety of a Third Dose of CoronaVac, and Immune Persistence of a Two-Dose Schedule, in Healthy Adults: Interim Results from Two Single-Centre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Clinical Trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Lim, J.M.E.; Hang, S.K.; Hariharaputran, S.; Chia, A.; Tan, N.; Lee, E.S.; Chng, E.; Lim, P.L.; Young, B.E.; Lye, D.C.; et al. A Comparative Characterization of SARS-CoV-2-Specific T Cells Induced by MRNA or Inactive Virus COVID-19 Vaccines. Cell Rep. Med. 2022, 3, 100793. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ying, L.; Wang, J.; Xia, J.; Zhang, Y.; Mao, H.; Zhang, R.; Zang, R.; Le, Z.; Shu, Q.; et al. Non-Spike and Spike-Specific Memory T Cell Responses after the Third Dose of Inactivated COVID-19 Vaccine. Front. Immunol. 2023, 14, 1139620. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N.; Tan, A.T. SARS-CoV-2-Specific T Cells in the Changing Landscape of the COVID-19 Pandemic. Immunity 2022, 55, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Kang, A.Y.H.; Tay, C.J.X.; Li, H.E.; Elyana, N.; Tan, C.W.; Yap, W.C.; Lim, J.M.E.; Le Bert, N.; Chan, K.R.; et al. Correlates of Protection against Symptomatic SARS-CoV-2 in Vaccinated Children. Nat. Med. 2024, 30, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Santi, T.; Sungono, V.; Kamarga, L.; De Samakto, B.; Hidayat, F.; Hidayat, F.K.; Satolom, M.; Permana, A.; Yusuf, I.; Suriapranata, I.M.; et al. Heterologous Prime-Boost with the MRNA-1273 Vaccine among CoronaVac-Vaccinated Healthcare Workers in Indonesia. Clin. Exp. Vaccine Res. 2022, 11, 209–216. [Google Scholar] [CrossRef]
- Barin, B.; Kasap, U.; Selçuk, F.; Volkan, E.; Uluçkan, Ö. Comparison of SARS-CoV-2 Anti-Spike Receptor Binding Domain IgG Antibody Responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 Vaccines, and a Single Booster Dose: A Prospective, Longitudinal Population-Based Study. Lancet Microbe 2022, 3, e274–e283. [Google Scholar] [CrossRef]
- Clemens, S.A.C.; Weckx, L.; Clemens, R.; Mendes, A.V.A.; Souza, A.R.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Pinto, M.I.M.; Gonzalez, I.G.S.; et al. Heterologous versus Homologous COVID-19 Booster Vaccination in Previous Recipients of Two Doses of CoronaVac COVID-19 Vaccine in Brazil (RHH-001): A Phase 4, Non-Inferiority, Single Blind, Randomised Study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Fadlyana, E.; Setiabudi, D.; Kartasasmita, C.B.; Putri, N.D.; Rezeki Hadinegoro, S.; Mulholland, K.; Sofiatin, Y.; Suryadinata, H.; Hartantri, Y.; Sukandar, H.; et al. Immunogenicity and Safety in Healthy Adults of Full Dose versus Half Doses of COVID-19 Vaccine (ChAdOx1-S or BNT162b2) or Full-Dose CoronaVac Administered as a Booster Dose after Priming with CoronaVac: A Randomised, Observer-Masked, Controlled Trial in I. Lancet Infect. Dis. 2023, 23, 545–555. [Google Scholar] [CrossRef]
- Sauré, D.; O’Ryan, M.; Torres, J.P.; Zuñiga, M.; Soto-Rifo, R.; Valiente-Echeverría, F.; Gaete-Argel, A.; Neira, I.; Saavedra, V.; Acevedo, M.L.; et al. COVID-19 Lateral Flow IgG Seropositivity and Serum Neutralising Antibody Responses after Primary and Booster Vaccinations in Chile: A Cross-Sectional Study. Lancet Microbe 2023, 4, e149–e158. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic Review of COVID-19 in Children Shows Milder Cases and a Better Prognosis than Adults. Acta Paediatr. Int. J. Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Mysore, V.; Cullere, X.; Settles, M.L.; Ji, X.; Kattan, M.W.; Desjardins, M.; Durbin-Johnson, B.; Gilboa, T.; Baden, L.R.; Walt, D.R.; et al. Protective Heterologous T Cell Immunity in COVID-19 Induced by the Trivalent MMR and Tdap Vaccine Antigens. Med 2021, 2, 1050–1071.e7. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory Analysis of Immunization Records Highlights Decreased SARS-CoV-2 Rates in Individuals with Recent Non-COVID-19 Vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef]
- Haddad-Boubaker, S.; Othman, H.; Touati, R.; Ayouni, K.; Lakhal, M.; Ben Mustapha, I.; Ghedira, K.; Kharrat, M.; Triki, H. In Silico Comparative Study of SARS-CoV-2 Proteins and Antigenic Proteins in BCG, OPV, MMR and Other Vaccines: Evidence of a Possible Putative Protective Effect. BMC Bioinform. 2021, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Balz, K.; Trassl, L.; Härtel, V.; Nelson, P.P.; Skevaki, C. Virus-Induced T Cell-Mediated Heterologous Immunity and Vaccine Development. Front. Immunol. 2020, 11, 513. [Google Scholar] [CrossRef]
- Gil, A.; Kenney, L.L.; Mishra, R.; Watkin, L.B.; Aslan, N.; Selin, L.K. Vaccination and Heterologous Immunity: Educating the Immune System. Trans. R. Soc. Trop. Med. Hyg. 2014, 109, 62–69. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Chang, J.; Cheong, R.M.Y.; Yellapragada, A.; Wong, W.Y.; Ting, Y.T.; Monk, J.A.; Gan, P.Y.; Holdsworth, S.R.; et al. Heterologous Immunity Between SARS-CoV-2 and Pathogenic Bacteria. Front. Immunol. 2022, 13, 821595. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ashok, G.; Debroy, R.; Ramaiah, S.; Livingstone, P.; Anbarasu, A. Impact of the COVID-19 Pandemic on Routine Vaccine Landscape: A Global Perspective. Hum. Vaccines Immunother. 2023, 19, 2199656. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Acevedo, J.; Pizarro, A.; Vergara, V.; Soto-Marchant, M.; Gilabert, R.; Flores, J.C.; et al. Effectiveness of CoronaVac in Children 3–5 Years of Age during the SARS-CoV-2 Omicron Outbreak in Chile. Nat. Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Flores, J.C.; Zubizarreta, J.R.; González, C.; Pizarro, A.; Ortuño-Borroto, D.; Acevedo, J.; Leo, K.; Paredes, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Children and Adolescents: A Large-Scale Observational Study. Lancet Reg. Health 2023, 21, 100487. [Google Scholar] [CrossRef]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Chia, W.N.; Tham, C.Y.L.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.A.; Shankar, N.; et al. Highly Functional Virus-Specific Cellular Immune Response in Asymptomatic SARS-CoV-2 Infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef] [PubMed]
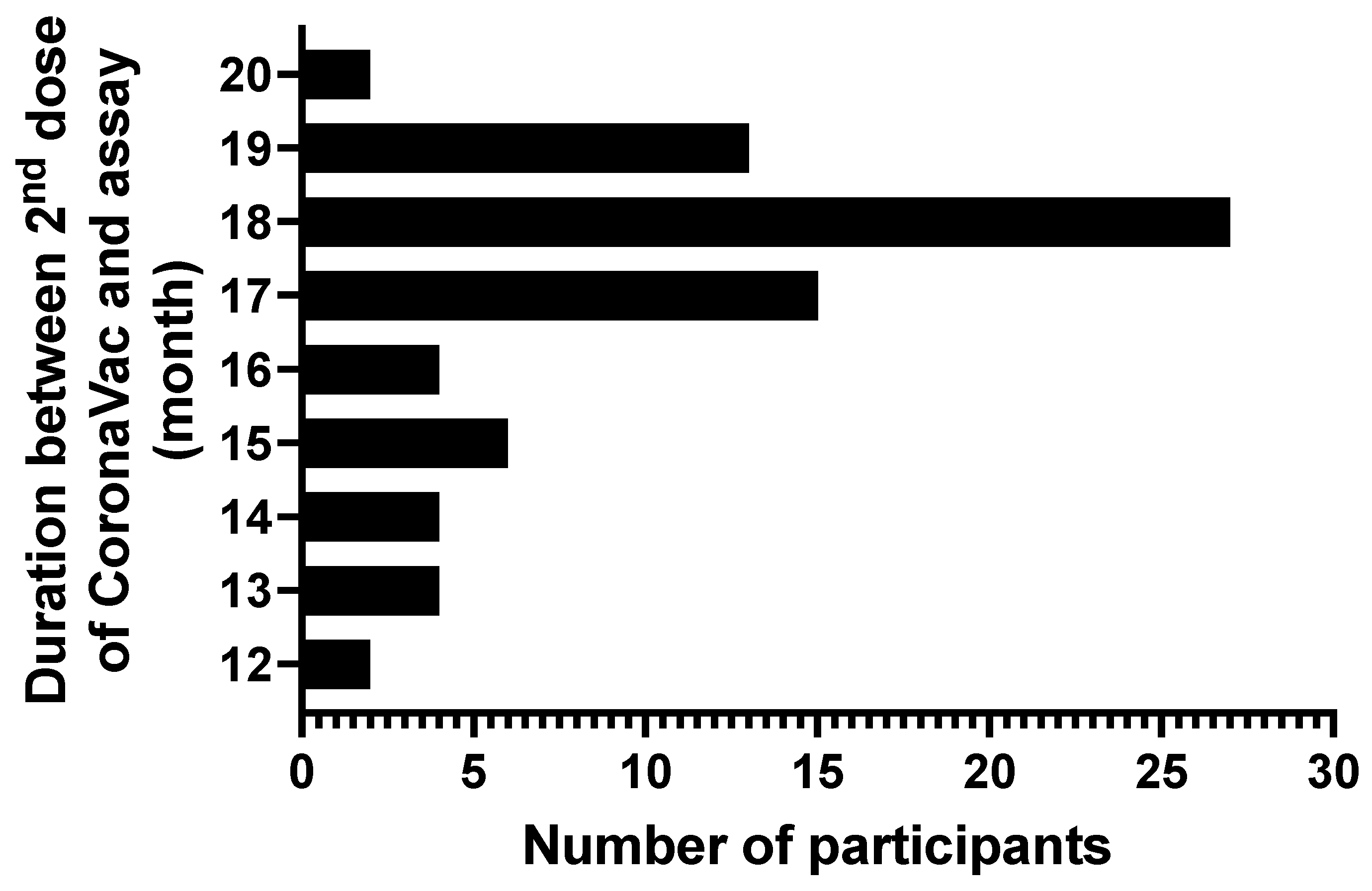
| Variable | Value |
|---|---|
| Age, months [median (minimum–maximum)] | 92 (81–103) |
| BMI, kg/m2 [median (minimum–maximum)] | 14.8 (12.3–19.7) |
| Sex [n (%)] | |
| Male | 60 (39) |
| Female | 94 (61) |
| Parental occupation [n (%)] | |
| Working | 71 (46.1) |
| Not working | 83 (53.9) |
| Parental income [n (%)] | |
| Equal or above the minimum wage | 24 (15.6) |
| Below the minimum wage | 130 (84.4) |
| History of acute respiratory infection in last 6 months [n (%)] | |
| <3 times | 106 (68.8) |
| ≥3 times | 48 (31.2) |
| History of COVID-19 disease in other family members [n (%)] | |
| No | 73 (47.4) |
| Yes | 81 (52.6) |
| Classification based on vaccination statuses [n (%)] | |
| Group A (COVID-19 yes/DTP yes) | 39 (25.3) |
| Group B (COVID-19 yes/DTP no) | 38 (24.7) |
| Group C (COVID-19 no/DTP yes) | 38 (24.7) |
| Group D (COVID-19 no/DTP no) | 39 (25.3) |
| DTP Vaccination Status | n | Anti-SARS-CoV-2 S-RBD (U/mL) Median (Min–Max) | p-Value |
|---|---|---|---|
| Yes | 77 | 1182 (0.3–22,269) | 0.026 |
| No | 77 | 612.5 (0.3–14,589) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, T.; Jo, J.; Harahap, A.R.; Werdhani, R.A.; Hadinegoro, S.R.S.; SahBandar, I.N.; Prayitno, A.; Munasir, Z.; Vandenplas, Y.; Hegar, B. The Improvement of Adaptive Immune Responses towards COVID-19 Following Diphtheria–Tetanus–Pertussis and SARS-CoV-2 Vaccinations in Indonesian Children: Exploring the Roles of Heterologous Immunity. Vaccines 2024, 12, 1032. https://doi.org/10.3390/vaccines12091032
Santi T, Jo J, Harahap AR, Werdhani RA, Hadinegoro SRS, SahBandar IN, Prayitno A, Munasir Z, Vandenplas Y, Hegar B. The Improvement of Adaptive Immune Responses towards COVID-19 Following Diphtheria–Tetanus–Pertussis and SARS-CoV-2 Vaccinations in Indonesian Children: Exploring the Roles of Heterologous Immunity. Vaccines. 2024; 12(9):1032. https://doi.org/10.3390/vaccines12091032
Chicago/Turabian StyleSanti, Theresia, Juandy Jo, Alida Roswita Harahap, Retno Asti Werdhani, Sri Rezeki S. Hadinegoro, Ivo Novita SahBandar, Ari Prayitno, Zakiudin Munasir, Yvan Vandenplas, and Badriul Hegar. 2024. "The Improvement of Adaptive Immune Responses towards COVID-19 Following Diphtheria–Tetanus–Pertussis and SARS-CoV-2 Vaccinations in Indonesian Children: Exploring the Roles of Heterologous Immunity" Vaccines 12, no. 9: 1032. https://doi.org/10.3390/vaccines12091032








