Establishment of a Reference Material in Quality Control for Use in Infectivity and Identity Assays of Recombinant COVID-19 Vaccine, in Accordance with International Standards Organization Guidance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Batch of RCV to Be Tested as RM for Infectivity and Identity Assays
2.2. Chimpanzee Adenovirus AZD1222 Virus Titration
2.3. Identity of the Chimpanzee Adenovirus AZD1222 Virus by Real-Time PCR
2.4. Determination of Homogeneity
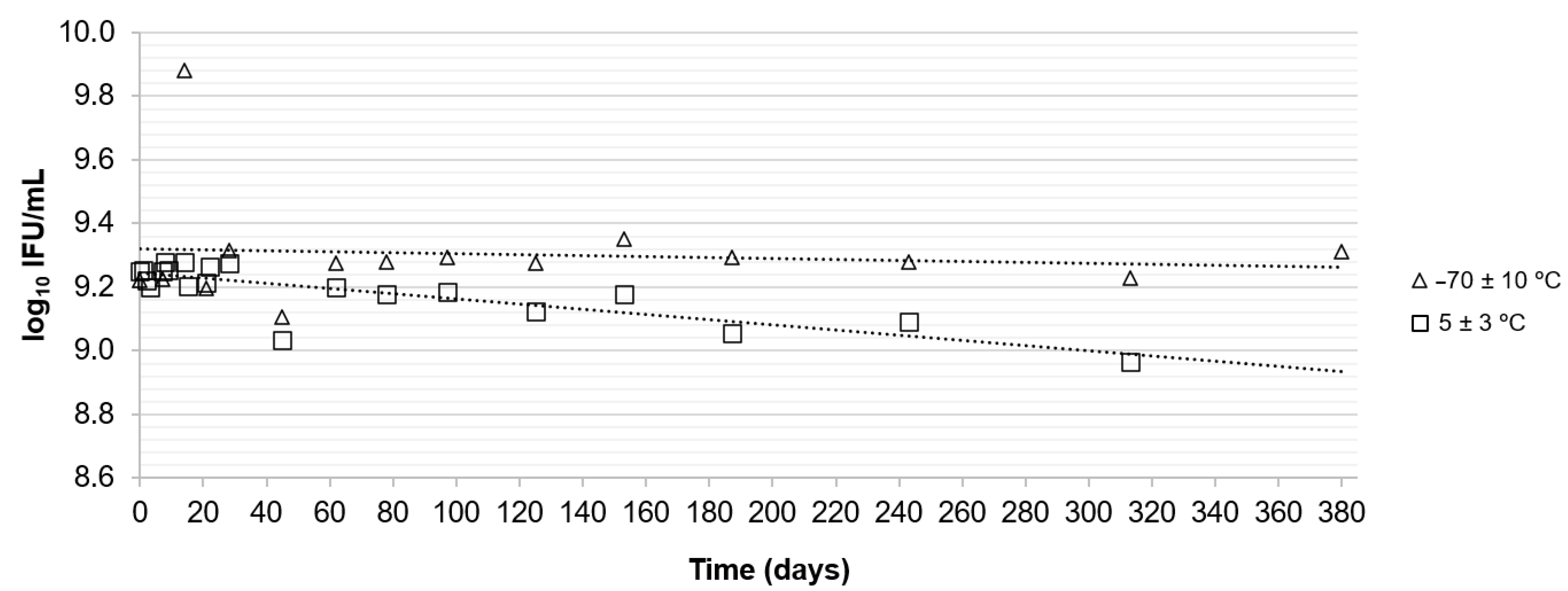
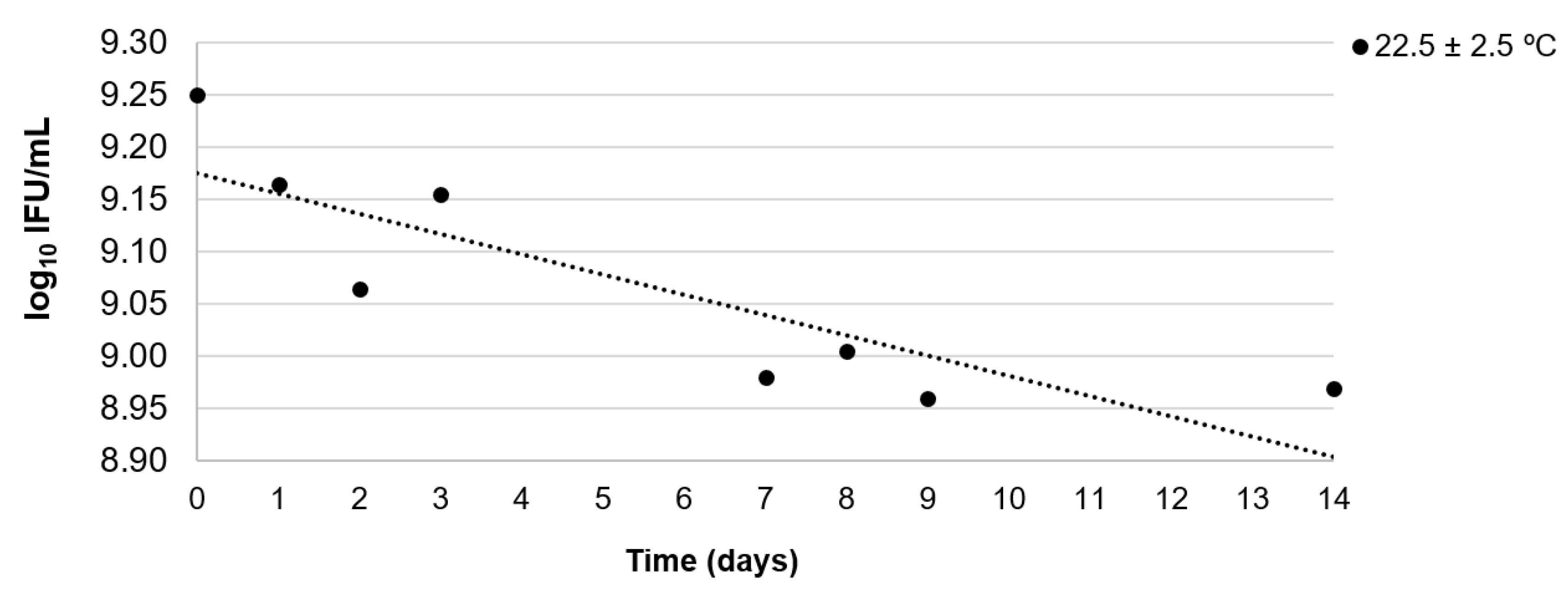
2.5. Determination of Stability
2.6. Certified Value Metrological Traceability
3. Results
3.1. Homogeneity Assessment
3.2. Stability Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, M.C.; Kim, S.; Barberia, L.; Ribeiro, A.F.; Gurzenda, S.; Ribeiro, K.B.; Abbott, E.; Blossom, J.; Rache, B.; Singer, B.H. Spatiotemporal pattern of COVID-19 spread in Brazil. Science 2021, 372, 821–826. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Brazil. Statistics. The Current COVID-19 Situation. Available online: https://www.who.int/countries/bra (accessed on 19 January 2024).
- Sreepadmanabh, M.; Sahu, A.K.; Chande, A. COVID-19: Advances in diagnostic tools, treatment strategies, and vaccine development. J. Biosci. 2020, 45, 148. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.M.A.S.; Maranhão, A.G.K.; Teixeira, A.M.; Fantinato, F.F.S.; Domingues, R.A.S. The Brazilian National Immunization Program: 46 years of achievements and challenges. Cad. Saude Publica 2020, 36, e00222919. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Massuda, A.; Almeida, G.; Menezes-Filho, N.A.; Andrade, M.V.; de Souza Noronha, K.V.M.; Rocha, R.; Macinko, J.; Hone, T.; Tasca, R.; et al. Brazil’s unified health system: The first 30 years and prospects for the future. Lancet 2019, 394, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Dantas, S.I.; Amaral, L.F.G.; da Costa, J.C.S. Technological order and technology transfer of the vaccine for COVID-19 in Brazil: A case study of the model used by AstraZeneca/Oxford and Fiocruz. Vigil. Sanit. Debate 2022, 10, 57–68. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Explainer. COVID-19 Vaccine ChAdOx1-S [Recombinant]—Developed by Oxford University and Astra Zeneca. 2021. Available online: https://www.who.int/publications/m/item/chadox1-s-recombinant-covid-19-vaccine (accessed on 19 January 2024).
- World Health Organization. WHO good manufacturing practices for biological products. In WHO Team: Health Product Policy and Standards, Technical Standards and Specifications; WHO Technical Report Series, No. 999, Annex 2; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- ISO Guide 30:2017; Reference Materials—Selected Terms and Definitions. 3rd ed. International Standards Organization: Geneva, Switzerland, 2017; 8p.
- Ajorio, A.C.F.B.; Rhodes, V.P.; Rodrigues, A.P.; Diniz, V.A.; Mattoso, J.M.V.; da Silva, I.B.; D’aiuto, D.; Brandão, M.L.L. Evaluation of hydrogen peroxide efficacy against AZD1222 chimpanzee adenovirus strain in the recombinant COVID-19 vaccine for application in cleaning validation in a pharmaceutical manufacturing industry. Lett. Appl. Microbiol. 2022, 74, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ellison, S.L.R.; Wood, R. The International Harmonized Protocol for the Proficiency Testing of Analytical Chemistry Laboratories. Pure Appl. Chem. 2006, 78, 145–196. [Google Scholar] [CrossRef]
- Ajorio, A.C.F.B.; Rhodes, V.P.; Rodrigues, A.P.; Diniz, V.A.; da Conceição, G.M.S.; Forsythe, S.J.; da Silva, I.B.; Brandão, M.L.L. Establishment of certified reference material for the infectivity assay in yellow fever vaccine quality control, in accordance with International Standards Organization guidance. J. Pharm. Biomed. Anal. 2023, 230, 115395. [Google Scholar] [CrossRef] [PubMed]
- ISO Guide 35:2017; Reference Materials—Guidance for Characterization and Assessment of Homogeneity and Stability. 4th ed. International Standards Organization: Geneva, Switzerland, 2017; 105p.
- WHO/IVB/11.03; Manual for the Establishment of the National and Other Secondary Standard for Vaccines. World Health Organization: Geneva, Switzerland, 2011.
- Ferguson, M.; Heath, A. Collaborative study to assess the suitability of a candidate International Standard for yellow fever vaccine. Biologicals 2004, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- ISO 17034:2016; General Requirements for the Competence of Reference Material Producers. 1st ed. International Standards Organization: Geneva, Switzerland, 2016; 24p.
- Singh, K.A.; Rai, R.; Nair, S.S. Review on development of assigned value microbiological reference materials used in food testing. Food Microbiol. 2022, 102, 103904. [Google Scholar] [CrossRef] [PubMed]
- Abdelmassih, M.; Planchon, V.; Anceau, C.; Mahillon, J. Development and validation of stable reference materials for food microbiology using Bacillus cereus and Clostridium perfringens spores. J. Appl. Microbiol. 2011, 110, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Kempster, S.L.; Dougall, T.; Morris, C.; Gonzalez-Escobar, G.; Almond, N.; Anderson, R. Utility of reference materials for Zika Virus nucleic acid testing. Biologicals 2019, 61, 55–60. [Google Scholar] [CrossRef]
- Penaud-Budloo, M.; Broucque, F.; Harrouet, K.; Bouzelha, M.; Saleun, S.; Douthe, S.; D’costa, S.; Ogram, S.; Adjali, O.; Blouin, V.; et al. Stability of the adeno-associated virus 8 reference standard material. Gene Ther. 2019, 26, 211–215. [Google Scholar] [CrossRef]
- Ayuso, E.; Blouin, V.; Lock, M.; McGorray, S.; Leon, X.; Alvira, M.R.; Auricchio, A.; Bucher, S.; Chtarto, A.; Clark, K.R.; et al. Manufacturing and characterization of a recombinant adeno-associated virus type 8 reference standard material. Hum. Gene Ther. 2014, 25, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Perraut, R.; Girault, G.; Moreau, J.P. Stability-related studies on 17D yellow fever vaccine. Microbes Infect. 2000, 2, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Rosas, C.O.; Rodrigues, J.M.; de La Cruz, M.H.C.; Lopes, S.M.R.; Souto, A.S.S.; Brandão, M.L.L.; Capasso, I.R.V.d.F. Microbiological reference material (bacterial and fungal domains): Definition, production rules, use and need for establishment in Brazil. Braz. J. Food Technol. 2019, 22, e2017208. [Google Scholar] [CrossRef]
| Vial | Infectivity Assay (log10 IFU 1/mL) | Identity Assay (Ct 2) | ||||
|---|---|---|---|---|---|---|
| Flank | Spike | |||||
| Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | |
| 1 | 9.31 | 9.31 | 22.99 | 21.52 | 20.70 | 20.42 |
| 2 | 9.27 | 9.26 | 23.40 | 20.87 | 21.93 | 20.11 |
| 3 | 9.24 | 9.22 | 22.60 | 21.25 | 21.65 | 20.78 |
| 4 | 9.24 | 9.29 | 21.66 | 23.07 | 20.95 | 21.36 |
| 5 | 9.22 | 9.25 | 22.87 | 22.16 | 23.76 | 20.51 |
| 6 | 9.30 | 9.20 | 24.70 | 22.39 | 25.19 | 20.55 |
| 7 | 9.30 | 9.25 | 23.70 | 22.19 | 22.76 | 19.65 |
| 8 | 9.28 | 9.31 | 22.31 | 21.35 | 20.39 | 20.63 |
| 9 | 9.21 | 9.22 | 22.20 | 22.19 | 20.97 | 20.20 |
| 10 | 9.24 | 9.25 | 24.91 | 20.97 | 19.66 | 19.94 |
| 11 | 9.30 | 9.33 | 19.62 | 22.11 | 20.10 | 20.99 |
| 12 | 9.25 | 9.26 | 19.67 | 21.38 | 19.71 | 20.51 |
| 13 | 9.26 | 9.18 | 19.49 | 21.36 | 20.02 | 18.71 |
| 14 | 9.19 | 9.20 | 19.73 | 20.51 | 19.53 | 19.65 |
| 15 | 9.26 | 9.29 | 20.60 | 21.64 | 20.44 | 20.04 |
| 16 | 9.26 | 9.23 | 20.04 | 22.01 | 20.38 | 21.11 |
| 17 | 9.20 | 9.24 | 19.89 | 21.66 | 19.80 | 20.58 |
| 18 | 9.19 | 9.21 | 21.17 | 22.07 | 21.07 | 20.14 |
| 19 | 9.25 | 9.26 | 20.92 | 23.86 | 20.59 | 19.49 |
| 20 | 9.21 | 9.27 | 21.89 | 21.88 | 21.13 | 19.02 |
| Time (Days) | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| −70 ± 10 | 5 ± 3 | 22.5 ± 2.5 | |||||||
| Infectivity 1 | Flank 2 | Spike 2 | Infectivity | Flank | Spike | Infectivity | Flank | Spike | |
| 0 | 9.23 | 21.26 | 20.57 | 9.25 | 20.92 | 19.83 | 9.25 | 21.37 | 20.45 |
| 1 | NR 3 | NR | NR | 9.24 | 21.69 | 20.68 | 9.16 | 21.56 | 20.60 |
| 2 | NR | NR | NR | 9.22 | 20.56 | 20.10 | 9.06 | 20.44 | 20.25 |
| 3 | NR | NR | NR | 9.19 | 20.68 | 20.28 | 9.12 | 20.10 | 19.72 |
| 7 | 9.23 | 20.56 | 19.43 | 9.25 | 20.53 | 19.62 | 8.98 | 20.48 | 19.98 |
| 8 | NR | NR | NR | 9.28 | 20.75 | 20.07 | 9.00 | 20.48 | 19.42 |
| 9 | NR | NR | NR | 9.25 | 20.67 | 19.90 | 8.96 | 20.43 | 19.56 |
| 14 | NR | 19.73 | 18.70 | 9.28 | 19.87 | 19.38 | 8.97 | 19.99 | 19.19 |
| 21 | 9.20 | 21.38 | 20.32 | 9.21 | 21.36 | 20.54 | NR | NR | NR |
| 28 | 9.32 | 20.78 | 19.77 | 9.27 | 20.67 | 19.91 | NR | NR | NR |
| 45 | 9.11 | 20.04 | 18.87 | 9.03 | 20.13 | 19.32 | NR | NR | NR |
| 62 | 9.28 | 20.64 | 19.49 | 9.20 | 20.69 | 19.99 | NR | NR | NR |
| 153 | 9.35 | 21.81 | 20.66 | 9.18 | 21.06 | 20.13 | NR | NR | NR |
| 243 | 9.28 | 17.99 | 17.87 | 9.09 | 18.39 | 18.00 | NR | NR | NR |
| 313 | 9.23 | 20.95 | 19.08 | 8.96 | 21.04 | 20.25 | NR | NR | NR |
| 380 | 9.31 | 21.50 | 20.45 | NR | NR | NR | NR | NR | NR |
| Storage Temperature | Type of Study | Period (Days) | log10 IFU 1/mL | 95% Confidence Interval | Result | ||
|---|---|---|---|---|---|---|---|
| Slope Per Day | Standard Error Per Day | Lower | Higher | ||||
| −70 ± 10 °C | Long-term | 380 | −0.00016 | 0.00040 | −0.0010 | 0.00069 | Sufficiently stable |
| 5 ± 3 °C | Long-term | 313 | −0.00081 | 0.00013 | −0.0011 | −0.00053 | Insufficiently stable |
| 97 | −0.00094 | 0.00049 | −0.0020 | 0.00011 | Sufficiently stable | ||
| 22.5 ± 2.5 °C | Short-term | 14 | −0.018 | 0.005 | −0.030 | −0.007 | Insufficiently stable |
| 3 | −0.048 | 0.026 | −0.16 | 0.064 | Sufficiently stable | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajorio, A.C.F.B.; Chagas, M.G.; Rhodes, V.P.; Rodrigues, A.P.; Gonçalves, N.P.; Godinho, R.M.d.C.; Forsythe, S.J.; da Costa, L.V.; Brandão, M.L.L. Establishment of a Reference Material in Quality Control for Use in Infectivity and Identity Assays of Recombinant COVID-19 Vaccine, in Accordance with International Standards Organization Guidance. Vaccines 2024, 12, 967. https://doi.org/10.3390/vaccines12090967
Ajorio ACFB, Chagas MG, Rhodes VP, Rodrigues AP, Gonçalves NP, Godinho RMdC, Forsythe SJ, da Costa LV, Brandão MLL. Establishment of a Reference Material in Quality Control for Use in Infectivity and Identity Assays of Recombinant COVID-19 Vaccine, in Accordance with International Standards Organization Guidance. Vaccines. 2024; 12(9):967. https://doi.org/10.3390/vaccines12090967
Chicago/Turabian StyleAjorio, Ana Carolina Ferreira Ballestê, Michel Gomes Chagas, Vinicius Pessanha Rhodes, Anderson Peclat Rodrigues, Natália Pedra Gonçalves, Rodrigo Maciel da Costa Godinho, Stephen James Forsythe, Luciana Veloso da Costa, and Marcelo Luiz Lima Brandão. 2024. "Establishment of a Reference Material in Quality Control for Use in Infectivity and Identity Assays of Recombinant COVID-19 Vaccine, in Accordance with International Standards Organization Guidance" Vaccines 12, no. 9: 967. https://doi.org/10.3390/vaccines12090967







