Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development
Abstract
1. Introduction
2. Methods
3. The Basis of Cross-Reactivity and Cross-Protection
4. Challenges of Assessing Cross-Reactivity and Cross-Protection of Pneumococcal Vaccines
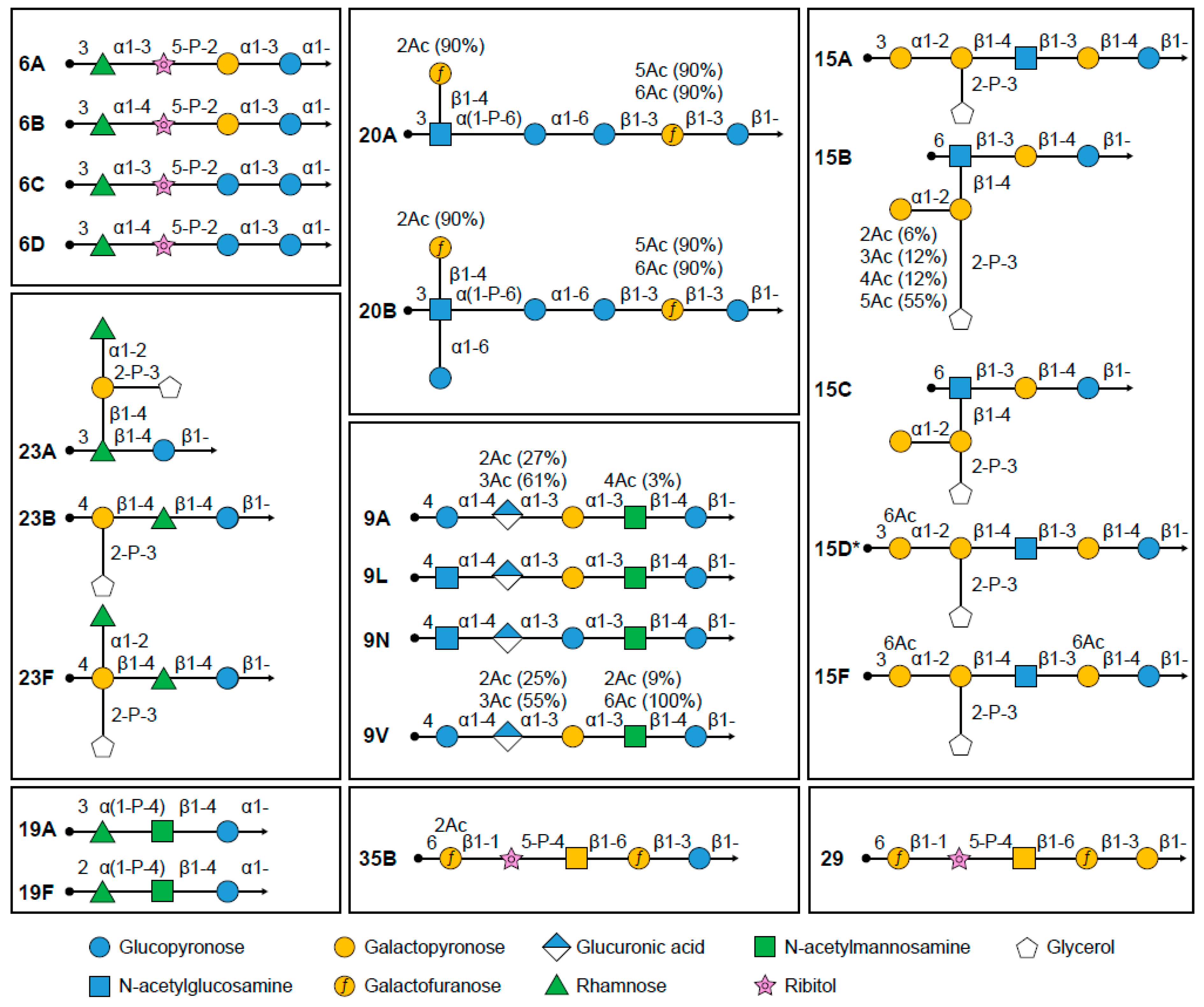
4.1. Serotype Classification
4.2. Assessment of Immune Response
5. From Past to Present: The Role of Cross-Reactivity and Cross-Protection in Pneumococcal Vaccine Design
6. Lessons Learned from PCV Clinical and Real-World Evidence
6.1. PCV7
6.2. PCV10
6.3. PCV13
6.4. PCV15 and PCV20
6.5. PCV21
6.6. Investigational Vaccines
7. What Is Known about Cross-Reactivity and Cross-Protection from Pneumococcal Vaccines against Other Serotypes?
7.1. Serotypes 9A/9N/9L/9V
7.2. Serotypes 15A/15B/15C/15D/15F
7.3. Serotypes 20A/20B
7.4. Serotypes 23A/23B/23F
7.5. Emerging Serotypes
8. Expert Analysis and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. The Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th Edition. Chapter 17: Pneumococcal Disease. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html (accessed on 12 June 2023).
- Overturf, G.D. American Academy of Pediatrics. Committee on Infectious Diseases. Technical Report: Prevention of Pneumococcal Infections, including the Use of Pneumococcal Conjugate and Polysaccharide Vaccines and Antibiotic Prophylaxis. Pediatrics 2000, 106, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.F.; Fadilah, F.; Maladan, Y.; Sarassari, R.; Safari, D. A narrative review of genomic characteristics, serotype, immunogenicity, and vaccine development of Streptococcus pneumoniae capsular polysaccharide. Clin. Exp. Vaccine Res. 2024, 13, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M. Pneumococcal serotypes and their clinical relevance. Thorax 1998, 53, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Yu, X.; Sidhu, M.; Nahm, M.H.; Fernsten, P.; Jansen, K.U. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to. Vaccine 2011, 29, 7207–7211. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.L.; Childs, L.; Ouattara, M.; Akhter, F.; Britton, A.; Pilishvili, T.; Kobayashi, M. Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults. Pathogens 2023, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Lagousi, T.; Basdeki, P.; Routsias, J.; Spoulou, V. Novel Protein-Based Pneumococcal Vaccines: Assessing the Use of Distinct Protein Fragments Instead of Full-Length Proteins as Vaccine Antigens. Vaccines 2019, 7, 9. [Google Scholar] [CrossRef]
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum. Vaccines Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef]
- Pfizer. Pneumococcal 7-Valent Conjugate Vaccine (Diphtheria CRM197 Protein) Prevnar®. Available online: http://labeling.pfizer.com/showlabeling.aspx?id=134 (accessed on 16 August 2023).
- United States Food and Drug Administration. PREVNAR 13 (Pneumococcal 13-Valent Conjugate Vaccine [Diphtheria CRM197 Protein]) Prescribing Information. Available online: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM574852.pdf (accessed on 8 April 2023).
- Pfizer. Prevnar 13 Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/medicine/22689#DOCREVISION (accessed on 29 September 2023).
- Merck Sharp & Dohme LLC. VAXNEUVANCE™ (Pneumococcal 15-Valent Conjugate Vaccine) Prescribing Information. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1158fa93-ef41-4a29-8252-9251f94c53c8 (accessed on 1 June 2023).
- Food and Drug Administration. PREVNAR 20 (Pneumococcal 20-Valent Conjugate Vaccine) Prescribing Information. Available online: https://www.fda.gov/media/149987/download (accessed on 20 September 2023).
- Food and Drug Administration. CAPVAXIVE™ (Pneumococcal 21-Valent Conjugate Vaccine) Injection, for Intramuscular Use, Prescribing Information. Available online: https://www.fda.gov/media/179426/download?attachment (accessed on 10 July 2023).
- Serum Institute of India Pvt Ltd. PNEUMOSIL Package Insert. Available online: https://extranet.who.int/prequal/vaccines/p/pneumosilr-0 (accessed on 10 July 2023).
- European Medicines Agency. SYNFLORIX Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/synflorix-epar-product-information_en.pdf (accessed on 15 September 2023).
- Alderson, M.R.; Sethna, V.; Newhouse, L.C.; Lamola, S.; Dhere, R. Development strategy and lessons learned for a 10-valent pneumococcal conjugate vaccine (PNEUMOSIL(R)). Hum. Vaccines Immunother. 2021, 17, 2670–2677. [Google Scholar] [CrossRef]
- World Health Organization. Prequalified Vaccines. Available online: https://extranet.who.int/prequal/vaccines/prequalified-vaccines (accessed on 23 July 2023).
- Izurieta, P.; Bahety, P.; Adegbola, R.; Clarke, C.; Hoet, B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: Assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev. Vaccines 2018, 17, 479–493. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 23 October 2023).
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network. Available online: www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2020.pdf (accessed on 22 February 2023).
- Wasserman, M.D.; Perdrizet, J.; Grant, L.; Hayford, K.; Singh, S.; Saharia, P.; Horn, E.K.; Farkouh, R.A. Clinical and economic burden of pneumococcal disease due to serotypes contained in current and investigational pneumococcal conjugate vaccines in children under five years of age. Infect. Dis. Ther. 2021, 10, 2701–2720. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Dawood, H.N.; Ikram, D.; Al-Jabban, A. Pneumococcal Disease: Global Disease Prevention Strategies with a Focus on the Challenges in Iraq. Int. J. Gen. Med. 2023, 16, 2095–2110. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, I.; Hanage, W.P.; Lipsitch, M.; Shea, K.M.; Stevenson, A.; Finkelstein, J.; Huang, S.S.; Lee, G.M.; Kleinman, K.; Pelton, S.I. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 2010, 29, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.M.; Calix, J.J.; Yu, J.; Geno, K.A.; Cutter, G.R.; Nahm, M.H. Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation. J. Infect. Dis. 2014, 210, 1155–1165. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. Chapter 11: Pneumococcal. Available online: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html (accessed on 5 August 2023).
- Lochen, A.; Croucher, N.J.; Anderson, R.M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 2020, 10, 18977. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper—February 2019. Wkly. Epidemiol. Rec. 2019, 94, 85–104. [Google Scholar]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Lynfield, R.; Reingold, A.; Cieslak, P.R.; Pilishvili, T.; Jackson, D.; et al. Decline in Invasive Pneumococcal Disease after the Introduction of Protein–Polysaccharide Conjugate Vaccine. N. Engl. J. Med. 2003, 348, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Klugman, K.P. Historical changes in pneumococcal serogroup distribution: Implications for the era of pneumococcal conjugate vaccines. Clin. Infect. Dis. 2002, 35, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Calix, J.J.; Brady, A.M.; Du, V.Y.; Saad, J.S.; Nahm, M.H. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J. Clin. Microbiol. 2014, 52, 758–765. [Google Scholar] [CrossRef]
- Ganaie, F.A.; Saad, J.S.; Lo, S.W.; McGee, L.; van Tonder, A.J.; Hawkins, P.A.; Calix, J.J.; Bentley, S.D.; Nahm, M.H. Novel pneumococcal capsule type 33E results from the inactivation of glycosyltransferase WciE in vaccine type 33F. J. Biol. Chem. 2023, 299, 105085. [Google Scholar] [CrossRef] [PubMed]
- Vojtek, I.; Buchy, P.; Doherty, T.M.; Hoet, B. Would immunization be the same without cross-reactivity? Vaccine 2019, 37, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.A. Chapter 4: Specificity and Cross-Reactivity. In Immunology and Evolution of Infectious Disease; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Chadwick, S.J. Chapter 2—Principles of Allergy Management. In Managing the Allergic Patient; Krouse, J.H., Derebery, M.J., Chadwick, S.J., Eds.; W.B. Saunders: Edinburgh, UK, 2008; pp. 19–72. [Google Scholar]
- Miyaji, E.N.; Ferreira, D.M.; Lopes, A.P.; Brandileone, M.C.; Dias, W.O.; Leite, L.C. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against. Infect. Immun. 2002, 70, 5086–5090. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Wohlbold, T.J.; Zheng, N.Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429.e10. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Baldovin, T.; Pellegrini, M.; Angiolelli, G.; Majori, S.; Floreani, A.; Busana, M.C.; Bertoncello, C.; Trivello, R. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin. Dev. Immunol. 2010, 2010, 517198. [Google Scholar] [CrossRef] [PubMed]
- De Vincenzo, R.; Ricci, C.; Conte, C.; Scambia, G. HPV vaccine cross-protection: Highlights on additional clinical benefit. Gynecol. Oncol. 2013, 130, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Wulferink, M.; Dierkes, S.; Gleichmann, E. Cross-sensitization to haptens: Formation of common haptenic metabolites, T cell recognition of cryptic peptides, and true T cell cross-reactivity. Eur. J. Immunol. 2002, 32, 1338–1348. [Google Scholar] [CrossRef]
- Walters, C.S.; Wigzell, H. Heteroclitic cells in anti-hapten systems: Some studies at the cellular and serum level. Cell Immunol. 1972, 5, 570–578. [Google Scholar] [CrossRef]
- Nahm, M.H.; Yu, J.; Vlach, J.; Bar-Peled, M. A Common Food Glycan, Pectin, Shares an Antigen with Streptococcus pneumoniae Capsule. mSphere 2020, 5, e00074-20. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Khan, R.; Ferreira, D.M.; Mitsi, E.; German, E.; Rorvik, G.H.; Berild, D.; Schenck, K.; Kwon, K.; Petersen, F. Antibodies Reactive to Commensal. Front. Immunol. 2018, 9, 747. [Google Scholar] [CrossRef]
- Park, I.H.; Pritchard, D.G.; Cartee, R.; Brandao, A.; Brandileone, M.C.; Nahm, M.H. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 2007, 45, 1225–1233. [Google Scholar] [CrossRef]
- Grant, L.R.; O’Brien, S.E.; Burbidge, P.; Haston, M.; Zancolli, M.; Cowell, L.; Johnson, M.; Weatherholtz, R.C.; Reid, R.; Santosham, M.; et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS ONE 2013, 8, e74906. [Google Scholar] [CrossRef] [PubMed]
- Eskola, J.; Kilpi, T.; Palmu, A.; Jokinen, J.; Haapakoski, J.; Herva, E.; Takala, A.; Kayhty, H.; Karma, P.; Kohberger, R.; et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 2001, 344, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kilpi, T.; Ahman, H.; Jokinen, J.; Lankinen, K.S.; Palmu, A.; Savolainen, H.; Gronholm, M.; Leinonen, M.; Hovi, T.; Eskola, J.; et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: Randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 2003, 37, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Parameswar, A.R.; Demchenko, A.V.; Nahm, M.H. Identification of a simple chemical structure associated with protective human antibodies against multiple pneumococcal serogroups. Infect. Immun. 2009, 77, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; He, J.; Kaufhold, R.M.; McGuinness, D.; McHugh, P.; Nawrocki, D.; Xie, J.; Skinner, J.M. Evaluation of cross-protection between. Vaccine 2023, 41, 1774–1777. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Balloch, A.; Russell, F.M.; Mulholland, E.K.; Tang, M.L. Antibodies to serotype 9V exhibit novel serogroup cross-reactivity following infant pneumococcal immunization. Vaccine 2010, 28, 3793–3800. [Google Scholar] [CrossRef] [PubMed]
- Laufer, A.S.; Thomas, J.C.; Figueira, M.; Gent, J.F.; Pelton, S.I.; Pettigrew, M.M. Capacity of serotype 19A and 15B/C. Vaccine 2010, 28, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Andam, C.P.; Mitchell, P.K.; Callendrello, A.; Chang, Q.; Corander, J.; Chaguza, C.; McGee, L.; Beall, B.W.; Hanage, W.P. Genomic Epidemiology of Penicillin-Nonsusceptible Pneumococci with Nonvaccine Serotypes Causing Invasive Disease in the United States. J. Clin. Microbiol. 2017, 55, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Kapatai, G.; Sheppard, C.L.; Al-Shahib, A.; Litt, D.J.; Underwood, A.P.; Harrison, T.G.; Fry, N.K. Whole genome sequencing of Streptococcus pneumoniae: Development, evaluation and verification of targets for serogroup and serotype prediction using an automated pipeline. PeerJ 2016, 4, e2477. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cui, Y.; Huang, X.; Lei, S.; Zhou, W.; Tong, W.; Chen, W.; Shen, M.; Wu, K.; Jiang, Y. Molecular Characterization Based on Whole-Genome Sequencing of Streptococcus pneumoniae in Children Living in Southwest China during 2017–2019. Front. Cell. Infect. Microbiol. 2021, 11, 726740. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Morita, M.; Lee, K.I.; Ohnishi, M. Whole-Genome Sequence Analysis of Streptococcus pneumoniae Strains That Cause Hospital-Acquired Pneumonia Infections. J. Clin. Microbiol. 2018, 56, e01822-17. [Google Scholar] [CrossRef]
- Lee, H.; Nahm, M.H.; Burton, R.; Kim, K.H. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin. Vaccine Immunol. 2009, 16, 376–381. [Google Scholar] [CrossRef]
- Wernette, C.M.; Frasch, C.E.; Madore, D.; Carlone, G.; Goldblatt, D.; Plikaytis, B.; Benjamin, W.; Quataert, S.A.; Hildreth, S.; Sikkema, D.J.; et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 2003, 10, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Henckaerts, I.; Durant, N.; De Grave, D.; Schuerman, L.; Poolman, J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 2007, 25, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Truelove, S.; Zhu, H.; Lessler, J.; Riley, S.; Read, J.M.; Wang, S.; Kwok, K.O.; Guan, Y.; Jiang, C.Q.; Cummings, D.A. A comparison of hemagglutination inhibition and neutralization assays for characterizing immunity to seasonal influenza A. Influenza Other Respir. Viruses 2016, 10, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kim, H.W.; Lee, J.H.; Lee, S.; Kim, K.H. Validation of a Multiplexed Opsonophagocytic Assay for 11 Additional Pneumococcal Serotypes and Its Application to Functional Antibody Evaluation Induced by Pneumococcal Polysaccharide Vaccine. J. Korean Med. Sci. 2018, 33, e340. [Google Scholar] [CrossRef]
- Burton, R.L.; Nahm, M.H. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 2006, 13, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Kuttel, M.M.; Ravenscroft, N.; Thompson, A.; Prasad, A.K.; Gangolli, S.; Tan, C.; Cooper, D.; Watson, W.; Liberator, P.; et al. Streptococcus pneumoniae serotype 15B polysaccharide conjugate elicits a cross-functional immune response against serotype 15C but not 15A. Vaccine 2022, 40, 4872–4880. [Google Scholar] [CrossRef]
- Shi, Y.; Nolan, K.M.; Burton, R.L.; Shekar, T.; Murphy, R.D.; Banniettis, N.; Musey, L.; Buchwald, U.K. The 15-valent pneumococcal conjugate vaccine V114 induces cross-reactive antibodies against pneumococcal serotype 6C. Hum. Vaccines Immunother. 2023, 19, 2235238. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.H.; Gurtman, A.; Hurley, D.C.; Block, S.L.; Schwartz, R.H.; Patterson, S.; Jansen, K.U.; Love, J.; Gruber, W.C.; Emini, E.A.; et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics 2010, 126, e493–e505. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Hoet, B.; Schuerman, L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr. 2010, 10, 4. [Google Scholar] [CrossRef]
- Vesikari, T.; Wysocki, J.; Chevallier, B.; Karvonen, A.; Czajka, H.; Arsene, J.P.; Lommel, P.; Dieussaert, I.; Schuerman, L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 2009, 28, S66–S76. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, J.; Tejedor, J.C.; Grunert, D.; Konior, R.; Garcia-Sicilia, J.; Knuf, M.; Bernard, L.; Dieussaert, I.; Schuerman, L. Immunogenicity of the 10-valent pneumococcal non-typeable. Pediatr. Infect. Dis. J. 2009, 28, S77–S88. [Google Scholar] [CrossRef]
- Cremers, A.J.; Mobegi, F.M.; de Jonge, M.I.; van Hijum, S.A.; Meis, J.F.; Hermans, P.W.; Ferwerda, G.; Bentley, S.D.; Zomer, A.L. The post-vaccine microevolution of invasive. Sci. Rep. 2015, 5, 14952. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Anderson, R.; Feldman, C. The remarkable history of pneumococcal vaccination: An ongoing challenge. Pneumonia 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Austrian, R.; Lee, C.J.; Rastogi, S.C.; Schiffman, G.; Henrichsen, J.; Makela, P.H.; Broome, C.V.; Facklam, R.R.; Tiesjema, R.H.; et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 1983, 148, 1136–1159. [Google Scholar] [CrossRef] [PubMed]
- Merck Sharp & Dohme LLC Rahway NJ USA. Pneumovax 23 Prescribing Information. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb362a20-6d91-4ae8-bebb-9ee8b2591814 (accessed on 16 August 2023).
- Kim, H.W.; Lee, S.; Kim, K.H. Serotype 6B from a pneumococcal polysaccharide vaccine induces cross-functional antibody responses in adults to serotypes 6A, 6C, and 6D. Medicine 2016, 95, e4854. [Google Scholar] [CrossRef] [PubMed]
- Nuorti, J.P.; Whitney, C.G.; Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–18. [Google Scholar] [PubMed]
- Food and Drug Administration. Pneumococcal 7-Valent Conjugate Vaccine (Diphtheria CRM197 Protein) Prevnar®. Available online: https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm137038.pdf (accessed on 20 September 2023).
- GlaxoSmithKline. Synflorix™. Pneumococcal Polysaccharide and Non-Typeable. Available online: https://gskpro.com/content/dam/global/hcpportal/en_BD/PI/Synflorix_GDS15_IPI_15_Clean_1_03_2019_1_03_2019.pdf (accessed on 15 September 2023).
- Lecrenier, N.; Marijam, A.; Olbrecht, J.; Soumahoro, L.; Nieto Guevara, J.; Mungall, B. Ten years of experience with the pneumococcal non-typeable. Expert Rev. Vaccines 2020, 19, 247–265. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. PREVENAR 13 Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/prevenar-13-epar-product-information_en.pdf (accessed on 26 May 2023).
- Food and Drug Administration. VAXNEUVANCE™ (Pneumococcal 15-Valent Conjugate Vaccine) Prescribing Information. Available online: https://www.fda.gov/media/150819/download (accessed on 7 April 2024).
- European Medical Agency. Vaxneuvance Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/vaxneuvance-epar-product-information_en.pdf (accessed on 20 September 2023).
- Kobayashi, M.; Farrar, J.L.; Gierke, R.; Britton, A.; Childs, L.; Leidner, A.J.; Campos-Outcalt, D.; Morgan, R.L.; Long, S.S.; Talbot, H.K.; et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: Updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 109–117. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Prevnar 20 Package Insert. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20 (accessed on 8 April 2024).
- Pfizer. Prevnar 20 Summary of Product Characteristics. Available online: www.ema.europa.eu/en/documents/product-information/apexxnar-epar-product-information_en.pdf (accessed on 8 April 2023).
- Center for Disease Control and Prevention (CDC). V116: An Investigational Adult Specific Pneumococcal Conjugate Vaccine; Key Results from the Phase 3 Clinical Development Program. In Proceedings of the ACIP Meeting, Atlanta, GA, USA, 28–29 February 2024. [Google Scholar]
- Yu, X.; Gray, B.; Chang, S.; Ward, J.I.; Edwards, K.M.; Nahm, M.H. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 1999, 180, 1569–1576. [Google Scholar] [CrossRef]
- Platt, H.L.; Bruno, C.; Buntinx, E.; Pelayo, E.; Garcia-Huidobro, D.; Barranco-Santana, E.A.; Sjoberg, F.; Song, J.Y.; Grijalva, C.G.; Orenstein, W.A.; et al. Safety, tolerability, and immunogenicity of an adult pneumococcal conjugate vaccine, V116 (STRIDE-3): A randomised, double-blind, active comparator controlled, international phase 3 trial. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, N.; Kline, M.J.; Center, K.J.; Thompson, A.; Baugher, G.; Drozd, J.; Scully, I.L.; Giardina, P.; Trammel, J.; Lei, L.; et al. 1728. Immune Responses to Cross-Reactive Serotypes 6C and 15C after 20-Valent Pneumococcal Conjugate Vaccine in Infants. Open Forum Infect. Dis. 2023, 10, ofad500.1560. [Google Scholar]
- Andrews, N.; Kent, A.; Amin-Chowdhury, Z.; Sheppard, C.; Fry, N.; Ramsay, M.; Ladhani, S.N. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: The indirect cohort design, 2006–2018. Vaccine 2019, 37, 4491–4498. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Pondo, T.; Xing, W.; McGee, L.; Farley, M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.H.; Lynfield, R.; et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions-United States. Clin. Infect. Dis. 2020, 70, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kokko, H.; Auranen, K.; Toropainen, M.; Nuorti, J.P.; Nohynek, H.; Siira, L.; Palmu, A.A. Effectiveness of 10-valent pneumococcal conjugate vaccine estimated with three parallel study designs among vaccine-eligible children in Finland. Vaccine 2020, 38, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Hanquet, G.; Lernout, T.; Vergison, A.; Verhaegen, J.; Kissling, E.; Tuerlinckx, D.; Malfroot, A.; Swennen, B.; Sabbe, M.; Belgian IPD Scientific Committee. Impact of conjugate 7-valent vaccination in Belgium: Addressing methodological challenges. Vaccine 2011, 29, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Frasch, C.; Nurkka, A.; Käyhty, H.; Biemans, R.; Schuerman, L. Impact of the conjugation method on the immunogenicity of. Clin. Vaccine Immunol. 2011, 18, 327–336. [Google Scholar] [CrossRef]
- Whitney, C.G.; Pilishvili, T.; Farley, M.M.; Schaffner, W.; Craig, A.S.; Lynfield, R.; Nyquist, A.C.; Gershman, K.A.; Vazquez, M.; Bennett, N.M.; et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet 2006, 368, 1495–1502. [Google Scholar] [CrossRef]
- Park, I.H.; Moore, M.R.; Treanor, J.J.; Pelton, S.I.; Pilishvili, T.; Beall, B.; Shelly, M.A.; Mahon, B.E.; Nahm, M.H.; Active Bacterial Core Surveillance Team. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J. Infect. Dis. 2008, 198, 1818–1822. [Google Scholar] [CrossRef]
- Harboe, Z.B.; Valentiner-Branth, P.; Benfield, T.L.; Christensen, J.J.; Andersen, P.H.; Howitz, M.; Krogfelt, K.A.; Lambertsen, L.; Konradsen, H.B. Early effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish Childhood Immunization Programme. Vaccine 2010, 28, 2642–2647. [Google Scholar] [CrossRef]
- Lepoutre, A.; Varon, E.; Georges, S.; Gutmann, L.; Lévy-Bruhl, D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001–2006. Eurosurveillance 2008, 13, 18962. [Google Scholar] [CrossRef]
- Rückinger, S.; van der Linden, M.; Reinert, R.R.; von Kries, R.; Burckhardt, F.; Siedler, A. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine 2009, 27, 4136–4141. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, G.D.; de Greeff, S.C.; Jansen, A.G.; de Melker, H.E.; Schouls, L.M.; Hak, E.; Spanjaard, L.; Sanders, E.A.; van der Ende, A. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg. Infect. Dis. 2010, 16, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Bratcher, P.E.; Kim, K.H.; Kang, J.H.; Hong, J.Y.; Nahm, M.H. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical and serological characterization. Microbiology 2010, 156, 555–560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, I.H.; Park, S.; Hollingshead, S.K.; Nahm, M.H. Genetic basis for the new pneumococcal serotype, 6C. Infect. Immun. 2007, 75, 4482–4489. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cha, J.H.; Nahm, M.H.; Burton, R.L.; Kim, K.H. The 7-valent pneumococcal conjugate vaccine elicits cross-functional opsonophagocytic killing responses to Streptococcus pneumoniae serotype 6D in children. BMC Infect. Dis. 2013, 13, 474. [Google Scholar] [CrossRef]
- Palmu, A.A.; Kaijalainen, T.; Jokinen, J.; Kilpi, T.M. Efficacy of the 7-Valent Pneumococcal Conjugate Vaccine Against Acute Otitis Media Caused by Serotype 6C Pneumococcus. Pediatr. Infect. Dis. J. 2015, 34, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Nahm, M.H.; Lin, J.; Finkelstein, J.A.; Pelton, S.I. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J. Infect. Dis. 2009, 199, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R.; Good, C.E.; Bajaksouzian, S.; Windau, A.R. Emergence of Streptococcus pneumoniae serotypes 19A, 6C, and 22F and serogroup 15 in Cleveland, Ohio, in relation to introduction of the protein-conjugated pneumococcal vaccine. Clin. Infect. Dis. 2008, 47, 1388–1395. [Google Scholar] [CrossRef][Green Version]
- Prymula, R.; Peeters, P.; Chrobok, V.; Kriz, P.; Novakova, E.; Kaliskova, E.; Kohl, I.; Lommel, P.; Poolman, J.; Prieels, J.P.; et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both. Lancet 2006, 367, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, P.; Nieto Guevara, J. Exploring the evidence behind the comparable impact of the pneumococcal conjugate vaccines PHiD-CV and PCV13 on overall pneumococcal disease. Hum. Vaccines Immunother. 2022, 18, 1872341. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.M.; Verani, J.R.; Montenegro Renoiner, E.I.; de Cunto Brandileone, M.C.; Flannery, B.; de Oliveira, L.H.; Santos, J.B.; de Moraes, J.C.; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: A matched case-control study. Lancet Respir. Med. 2014, 2, 464–471. [Google Scholar] [CrossRef]
- Verani, J.R.; Domingues, C.M.; de Moraes, J.C.; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Indirect cohort analysis of 10-valent pneumococcal conjugate vaccine effectiveness against vaccine-type and vaccine-related invasive pneumococcal disease. Vaccine 2015, 33, 6145–6148. [Google Scholar] [CrossRef] [PubMed]
- Deceuninck, G.; De Serres, G.; Boulianne, N.; Lefebvre, B.; De Wals, P. Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine 2015, 33, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Peckeu, L.; van der Ende, A.; de Melker, H.E.; Sanders, E.A.M.; Knol, M.J. Impact and effectiveness of the 10-valent pneumococcal conjugate vaccine on invasive pneumococcal disease among children under 5 years of age in the Netherlands. Vaccine 2021, 39, 431–437. [Google Scholar] [CrossRef]
- Hausdorff, W.P.; Hoet, B.; Adegbola, R.A. Predicting the impact of new pneumococcal conjugate vaccines: Serotype composition is not enough. Expert Rev. Vaccines 2015, 14, 413–428. [Google Scholar] [CrossRef]
- Jokinen, J.; Rinta-Kokko, H.; Siira, L.; Palmu, A.A.; Virtanen, M.J.; Nohynek, H.; Virolainen-Julkunen, A.; Toropainen, M.; Nuorti, J.P. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children--A population-based study. PLoS ONE 2015, 10, e0120290. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.; Ciruela, P.; Hernandez, S.; Garcia-Garcia, J.J.; Soldevila, N.; Izquierdo, C.; Moraga-Llop, F.; Diaz, A.; de Sevilla, M.F.; Gonzalez-Peris, S.; et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7-59 months. A matched case-control study. PLoS ONE 2017, 12, e0183191. [Google Scholar] [CrossRef]
- Desmet, S.; Theeten, H.; Laenen, L.; Cuypers, L.; Maes, P.; Bossuyt, W.; Van Heirstraeten, L.; Peetermans, W.E.; Lagrou, K. Characterization of emerging serotype 19A pneumococcal strains in invasive disease and carriage, Belgium. Emerg. Infect. Dis. 2022, 28, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.; Van Heirstraeten, L.; Willen, L.; Desmet, S.; Wouters, I.; Vermeulen, H.; Lammens, C.; Goossens, H.; Van Damme, P.; Verhaegen, J.; et al. Serotype 19A and 6C Account for One-Third of Pneumococcal Carriage Among Belgian Day-Care Children Four Years After a Shift to a Lower-Valent PCV. J. Pediatr. Infect. Dis. Soc. 2023, 12, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Anglemyer, A.; McNeill, A.; DuBray, K.; Sonder, G.J.B.; Walls, T. Invasive pneumococcal disease: Concerning trends in serotype 19A notifications in New Zealand. Clin. Infect. Dis. 2022, 74, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Isturiz, R.; Sings, H.L.; Hilton, B.; Arguedas, A.; Reinert, R.R.; Jodar, L. Streptococcus pneumoniae serotype 19A: Worldwide epidemiology. Expert Rev. Vaccines 2017, 16, 1007–1027. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.; Chiu, C.; Quinn, H.; Menzies, R.; Gilmour, R.; McIntyre, P. Effectiveness of 7- and 13-Valent Pneumococcal Conjugate Vaccines in a Schedule Without a Booster Dose: A 10-Year Observational Study. Clin. Infect. Dis. 2018, 67, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Levy, C.; Bingen, E.; Koskas, M.; Nave, I.; Varon, E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr. Infect. Dis. J. 2012, 31, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Porat, N.; Benisty, R.; Givon-Lavi, N.; Trefler, R.; Dagan, R. The impact of pneumococcal conjugate vaccines on carriage of and disease caused by Streptococcus pneumoniae serotypes 6C and 6D in southern Israel. Vaccine 2016, 34, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.; Menzies, R.; Chiu, C.; Toms, C.; Blyth, C.C.; Krause, V.; McIntyre, P. Long-term impact of a “3 + 0” schedule for 7- and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in Australia, 2002–2014. Clin. Infect. Dis. 2017, 64, 175–183. [Google Scholar] [CrossRef]
- Ono, T.; Watanabe, M.; Hashimoto, K.; Kume, Y.; Chishiki, M.; Okabe, H.; Sato, M.; Norito, S.; Chang, B.; Hosoya, M. Serotypes and Antibiotic Resistance of Streptococcus pneumoniae before and after the Introduction of the 13-Valent Pneumococcal Conjugate Vaccine for Adults and Children in a Rural Area in Japan. Pathogens 2023, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Savulescu, C.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.; Rinta-Kokko, H.; Levy, C.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022, 40, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.R.; Hanquet, G.; Sepúlveda-Pachón, I.T.; Theilacker, C.; Baay, M.; Slack, M.P.E.; Jodar, L.; Gessner, B.D. Effects of PCV10 and PCV13 on pneumococcal serotype 6C disease, carriage, and antimicrobial resistance. Vaccine 2024, 42, 2983–2993. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Study to Evaluate the Safety, Tolerability, and Immunogenicity of V116 in Pneumococcal Vaccine-Experienced Adults (V116-006, STRIDE-6). Available online: https://clinicaltrials.gov/ct2/show/NCT05420961?term=V116&draw=2&rank=3 (accessed on 5 December 2023).
- Clinicaltrials.gov. Safety and Immunogenicity of V116 in Adults Living with Human Immunodeficiency Virus (HIV) (V116-007) (STRIDE-7). Available online: https://clinicaltrials.gov/ct2/show/NCT05393037?term=V116&draw=2&rank=1 (accessed on 4 December 2023).
- Clinicaltrials.gov. Safety and Immunogenicity of V116 in Adults with Increased Risk for Pneumococcal Disease (V116-008) (STRIDE-8). Available online: https://clinicaltrials.gov/study/NCT05696080 (accessed on 4 December 2023).
- Scott, P.; Haranaka, M.; Yang, Y.-C.; Choi, J.; Stacey, H.; Dionne, M.; Greenberg, D.; Grijalva, C.G.; Orenstein, W.; Fernsler, D.; et al. A phase 3 clinical study to evaluate the safety and immunogenicity of V116, an investigational adult-specific pneumococcal conjugate vaccine, in pneumococcal vaccine-experienced adults 50 years of age or older (STRIDE-6). In Proceedings of the International Society of Pneumonia and Pneumococcal Diseases (ISPPD), Cape Town, South Africa, 17–20 March 2024. [Google Scholar]
- Li, S.; Liang, H.; Zhao, S.; Yang, X.; Guo, Z. Recent progress in pneumococcal protein vaccines. Front. Immunol. 2023, 14, 1278346. [Google Scholar] [CrossRef]
- Henrichsen, J. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 1995, 33, 2759–2762. [Google Scholar] [CrossRef]
- Pichichero, M.; Malley, R.; Kaur, R.; Zagursky, R.; Anderson, P. Acute otitis media pneumococcal disease burden and nasopharyngeal colonization in children due to serotypes included and not included in current and new pneumococcal conjugate vaccines. Expert Rev. Vaccines 2023, 22, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.; Moiane, B.; Gertz, R.E., Jr.; Chochua, S.; Snippes Vagnone, P.M.; Lynfield, R.; Sigauque, B.; Carvalho, M.D.G.; Beall, B. New Pneumococcal Serotype 15D. J. Clin. Microbiol. 2021, 59, e00329-21. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.E.; Lindberg, B.; Lindquist, U.; Ljungberg, J. Structural studies of the capsular polysaccharide from. Carbohydr. Res. 1987, 162, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gertz, R.E., Jr.; McEllistrem, M.C.; Boxrud, D.J.; Li, Z.; Sakota, V.; Thompson, T.A.; Facklam, R.R.; Besser, J.M.; Harrison, L.H.; Whitney, C.G.; et al. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J. Clin. Microbiol. 2003, 41, 4194–4216. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.L.; Shenoy, A.T.; Orihuela, C.J.; Nahm, M.H. The pneumococcal serotype 15C capsule is partially O-acetylated and allows for limited evasion of 23-valent pneumococcal polysaccharide vaccine-elicited anti-serotype 15B antibodies. Clin. Vaccine Immunol. 2017, 24, e00099-17. [Google Scholar] [CrossRef] [PubMed]
- Rajam, G.; Carlone, G.M.; Romero-Steiner, S. Functional antibodies to the O-acetylated pneumococcal serotype 15B capsular polysaccharide have low cross-reactivities with serotype 15C. Clin. Vaccine Immunol. 2007, 14, 1223–1227. [Google Scholar] [CrossRef]
- Curry, S.; Kaufhold, R.M.; Monslow, M.A.; Zhang, Y.; McGuinness, D.; Kim, E.; Nawrocki, D.K.; McHugh, P.M.; Briggs, M.L.; Smith, W.J.; et al. Preclinical evaluation of an investigational 21-valent pneumococcal conjugate vaccine, V116, in adult-rhesus monkey, rabbit, and mouse models. Vaccine 2023, 41, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.L.; Nahm, M.H. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin. Vaccine Immunol. 2012, 19, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Calix, J.J.; Porambo, R.J.; Brady, A.M.; Larson, T.R.; Yother, J.; Abeygunwardana, C.; Nahm, M.H. Biochemical, genetic, and serological characterization of two capsule subtypes among. J. Biol. Chem. 2012, 287, 27885–27894. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Wassil, J.; Sisti, M.; Fairman, J.; Davis, M.; Fierro, C.; Bennett, S.; Johnson, D.; Migone, T.S.; Nguyen, K.; Sauer, P.; et al. Evaluating the safety, tolerability, and immunogenicity of a 24-valent pneumococcal conjugate vaccine (VAX-24) in healthy adults aged 18 to 64 years: A phase 1/2, double-masked, dose-finding, active-controlled, randomised clinical trial. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, N.; Omar, A.; Hlozek, J.; Edmonds-Smith, C.; Follador, R.; Serventi, F.; Lipowsky, G.; Kuttel, M.M.; Cescutti, P.; Faridmoayer, A. Genetic and structural elucidation of capsular polysaccharides from. Carbohydr. Res. 2017, 450, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Azarian, T.; Grant, L.R.; Arnold, B.J.; Hammitt, L.L.; Reid, R.; Santosham, M.; Weatherholtz, R.; Goklish, N.; Thompson, C.M.; Bentley, S.D.; et al. The impact of serotype-specific vaccination on phylodynamic parameters of Streptococcus pneumoniae and the pneumococcal pan-genome. PLoS Pathog. 2018, 14, e1006966. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). 1998–2021 Serotype Data for Invasive Pneumococcal Disease Cases by Age Group from Active Bacterial Core Surveillance. Available online: https://data.cdc.gov/Public-Health-Surveillance/1998-2021-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data (accessed on 11 July 2023).
- World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Pneumococcal Conjugate Vaccines; WHO TRS N°977, Annex 3. Available online: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-standardization/pneumococcus/trs_977_annex_3.pdf?sfvrsn=344f81e_3&download=true (accessed on 1 October 2023).
| Vaccine Name | Marketed Name | Approval Year | Age Indication | Serotypes Included in Vaccine | Adjuvant | Carrier Protein | References |
|---|---|---|---|---|---|---|---|
| Pneumococcal conjugate vaccines (PCVs) | |||||||
| PCV7 | Prevnar® Prevenar® | 2000 | ≥2 months | 4, 6B, 9V, 14, 18C, 19F, and 23F | Aluminum phosphate | CRM197 | [73,74] |
| PCV10 | Synflorix™ | 2009 | ≥6 weeks–5 years | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F | Aluminum phosphate | Protein D, except for 18 C (tetanus toxoid) and 19F (diphtheria toxoid) | [16,75,76] |
| PCV10 | Pneumosil® | 2019 | ≥6 weeks–2 years | 1, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, and 23F | Aluminum phosphate | CRM197 | [15,17] |
| PCV13 | Prevnar 13© Prevenar 13® | 2010 | ≥6 weeks | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, and 23F | Aluminum phosphate | CRM197 | [73,77] |
| PCV15 (V114) | Vaxneuvance™ | 2021 2022 | ≥18 years ≥6 weeks | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F | Aluminum phosphate | CRM197 | [78,79,80] |
| PCV20 | Apexxnar® Prevnar 20© Prevenar 20® | 2021 2023 | ≥18 years ≥6 weeks | 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F | Aluminum phosphate | CRM197 | [81,82] |
| PCV21 (V116) | Capvaxive™ | 2024 | ≥18 years | 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B | N/A | CRM197 | [14,83] |
| Pneumococcal polysaccharide vaccine (PPSV) | |||||||
| PPSV23 | Pneumovax 23® | 1983 | ≥50 years ≥2 years with increased risk of PD | 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F | N/A | N/A | [71] |
| Study | PCV | Study Design | Region | Serotype(s) of Interest * | Evidence | Limitations Reported |
|---|---|---|---|---|---|---|
| Cross-reactivity | ||||||
| Platt H et al., 2024 [85] | PCV21 | Phase III (n = 2663 adults) | Multi- national (11 countries) | 6C, 15B |
|
|
| Tamimi N et al., 2023 [86] | PCV20 | RCT sub-analysis (n = 397 infants) | Multi- national | 6C, 15C |
|
|
| Shi Y et al., 2023 [63] | PCV15 | Post-hoc analysis (n = 150 infants, n = 250 adults) | – | 6C |
|
|
| Cooper D, et al. 2011 [5] | PCV13 | RCT sub-analysis (n = 52 infants) | Germany | 6C, 7A |
|
|
| Lee H et al., 2009 [56] | PCV7 | Observational (n = 31 infants) | Republic of Korea | 6A, 19A |
|
|
| Cross-protection | ||||||
| Andrews N et al., 2019 [87] | PCV13 | Indirect cohort (n = 3421 children) | England | 6C |
|
|
| Ahmed S et al., 2020 [88] | PCV13 | Surveillance (n = 7640 adults > 19 years of age) | United States | 6C |
|
|
| Rinta-Kokko H et al., 2020 [89] | PCV10 | Full and indirect cohort and case control study (n = 150 children 6–102 months of age) | Finland | 19A |
|
|
| Hanquet G et al., 2011 [90] | PCV7 | Surveillance (n = 1144 children < 5 years of age) | Belgium | 6A |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feemster, K.; Hausdorff, W.P.; Banniettis, N.; Platt, H.; Velentgas, P.; Esteves-Jaramillo, A.; Burton, R.L.; Nahm, M.H.; Buchwald, U.K. Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development. Vaccines 2024, 12, 974. https://doi.org/10.3390/vaccines12090974
Feemster K, Hausdorff WP, Banniettis N, Platt H, Velentgas P, Esteves-Jaramillo A, Burton RL, Nahm MH, Buchwald UK. Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development. Vaccines. 2024; 12(9):974. https://doi.org/10.3390/vaccines12090974
Chicago/Turabian StyleFeemster, Kristen, William P. Hausdorff, Natalie Banniettis, Heather Platt, Priscilla Velentgas, Alejandra Esteves-Jaramillo, Robert L. Burton, Moon H. Nahm, and Ulrike K. Buchwald. 2024. "Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development" Vaccines 12, no. 9: 974. https://doi.org/10.3390/vaccines12090974
APA StyleFeemster, K., Hausdorff, W. P., Banniettis, N., Platt, H., Velentgas, P., Esteves-Jaramillo, A., Burton, R. L., Nahm, M. H., & Buchwald, U. K. (2024). Implications of Cross-Reactivity and Cross-Protection for Pneumococcal Vaccine Development. Vaccines, 12(9), 974. https://doi.org/10.3390/vaccines12090974






