Abstract
Ever since the discovery of dendritic cells by Ralph Steinman and Zanvil Cohn in 1973, it is increasingly evident that dendritic cells are integral for adaptive immune responses, and there is an undeniable focus on them for vaccines development. Fungal infections, often thought to be innocuous, are becoming significant threats due to an increased immunocompromised or immune-suppressed population and climate change. Further, the recent COVID-19 pandemic unraveled the wrath of fungal infections and devastating outcomes. Invasive fungal infections cause significant case fatality rates ranging from 20% to 90%. Regrettably, no licensed fungal vaccines exist, and there is an urgent need for preventive and therapeutic purposes. In this review, we discuss the ontogeny, subsets, tissue distribution, and functions of lung dendritic cells. In the latter part, we summarize and discuss the studies on the DC-based vaccines against pulmonary fungal infections. Finally, we highlight some emerging potential avenues that can be incorporated for DC-based vaccines against fungal infections.
1. Introduction
Fungal infections represent a significant global health problem causing approximately 3.8 million annual deaths, mainly in immunocompromised individuals [1]. This mortality rate surpasses that of malaria and is nearly comparable to the deaths caused by HIV/AIDS and tuberculosis [2]. The severe global concern is mostly driven by pulmonary mycoses caused by fungal species such as Aspergillus, Cryptococcus, Coccidioides, Histoplasma, Blastomyces, and Paracoccidioides. The infections caused by these species can be wide-ranging from innocuous, self-clearing to invasive infections leading to severe complications, especially in individuals with compromised immunity, such as those with HIV/AIDS, organ transplant recipients, patients with comorbidities like SARS-CoV2 infection, or patients undergoing chemotherapy, radiotherapy, immunotherapy, or receiving anti-inflammatory agents. According to an estimate, the fungal diseases posed a significant healthcare burden and workforce loss of an estimated 11.5 billion USD [3] and, according to the CDC, it could be as high as 48 billion USD [4]. The current antifungal drug arsenal is limited due to increased drug resistance, severe toxicity, and a high cost [5,6,7]. To overcome these challenges, new strategies for their prevention and treatment are being developed, such as vaccines, immunomodulation, and passive immunotherapy, which can be employed to protect vulnerable patients.
In recent years, the development of dendritic cell (DC)-based vaccines has emerged as a promising frontier in the fight against respiratory fungal infections. Dendritic cells are essential innate immune cells, renowned for their role as professional antigen-presenting cells (APCs). These ‘large stellate cells’ were discovered in 1973 by two scientists named Ralph Steinman and Zanvil Cohn, who named them “dendritic cells” due to their unique structure like multiple extended dendrites or pseudopodia-like cytoplasmic protrusions in their maturation stage [8]. DCs possess the unique abilities of unique abilities in the uptake, processing, and presentation of antigens to T cells, thus initiating and orchestrating a tailored adaptive immune response [9]. DCs also exhibit diversity specialization into distinct subsets, each contributing uniquely to immune function, in different tissues. The major pulmonary DC subsets include conventional DCs (CD103+ cDC1, and CD11b+ cDC2), pDCs, and inflammatory or monocyte-derived DCs (infDCs/moDCs) [10]. cDC1 and cDC2 are involved in the induction of CD8+ and CD4+ T-cell responses, respectively, mainly due to cDC1’s exquisite ability to cross-present exogenous antigens via MHC-I molecules. However, monocyte-derived dendritic cells (moDCs), under the influence of inflammation, can modulate the ongoing T-cell responses [11,12]. Thus, these unique abilities of dendritic cells to induce a robust and specific immune response make them attractive candidates for vaccine targeting or development. Moreover, the advancements in dendritic cell biology and vaccine technology have facilitated the development of novel strategies to optimize their antigen cargo loading, presentation, and functions. Techniques such as extracellular vesicle (EV)-based or nanoparticle-based delivery systems are being explored to enhance the efficacy and durability of dendritic-cell-based vaccines against fungal infections [13,14] by improving antigen uptake, processing, and presentation, and maximizing their ability to elicit robust immune responses.
Despite the promising advancements, various challenges persist in the translational aspect of DC-based vaccines. Challenges include the optimization of vaccine formulations, the standardization of manufacturing, and ensuring safety, stability, and efficacy in diverse groups of patients. Further, the high cost and scalability of these vaccines must be addressed for broad clinical implementation. Moreover, the variability in individual immune responses necessitates further research to identify biomarkers that can predict vaccine efficacy and tailor treatments to individual patients.
In this review, we describe the panoply of pulmonary DC subsets, their origin, development, and their function in anti-fungal immunity in preclinical model systems. We emphasize the strategies involved in DC-based fungal vaccines, highlight the recent DC-focused studies in various pulmonary fungal infection settings, and discuss the prospects of dendritic-cell-based vaccines for the management of respiratory fungal infections and improvement of the patient’s disease outcomes.
2. Pulmonary Dendritic Cells (DCs)
The lung is constantly exposed to particles during each breath and particle deposition in the airways occurs through impaction, sedimentation, interception, diffusion, and electrostatic precipitation largely dictated by the size [15]. Pulmonary DCs are lined along the airways (Figure 1), continuously sampling the environmental antigens, chemicals, and microbes including fungal spores and hyphal fragments as a part of surveillance [16,17]. With apt recognition, the antigens can be transported to the draining mediastinal lymph node or induce a specialized lymphoid structure called induced bronchoalveolar lymphoid tissue (iBALT) [18]. Thus, the airway is an important site and a target for intranasal vaccinations against inhaled fungi, and such vaccine platforms have shown promise in influenza model systems [19]. Here, we will describe the types and the role of pulmonary DCs.
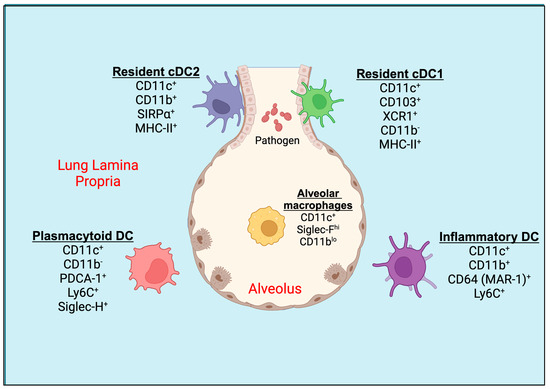
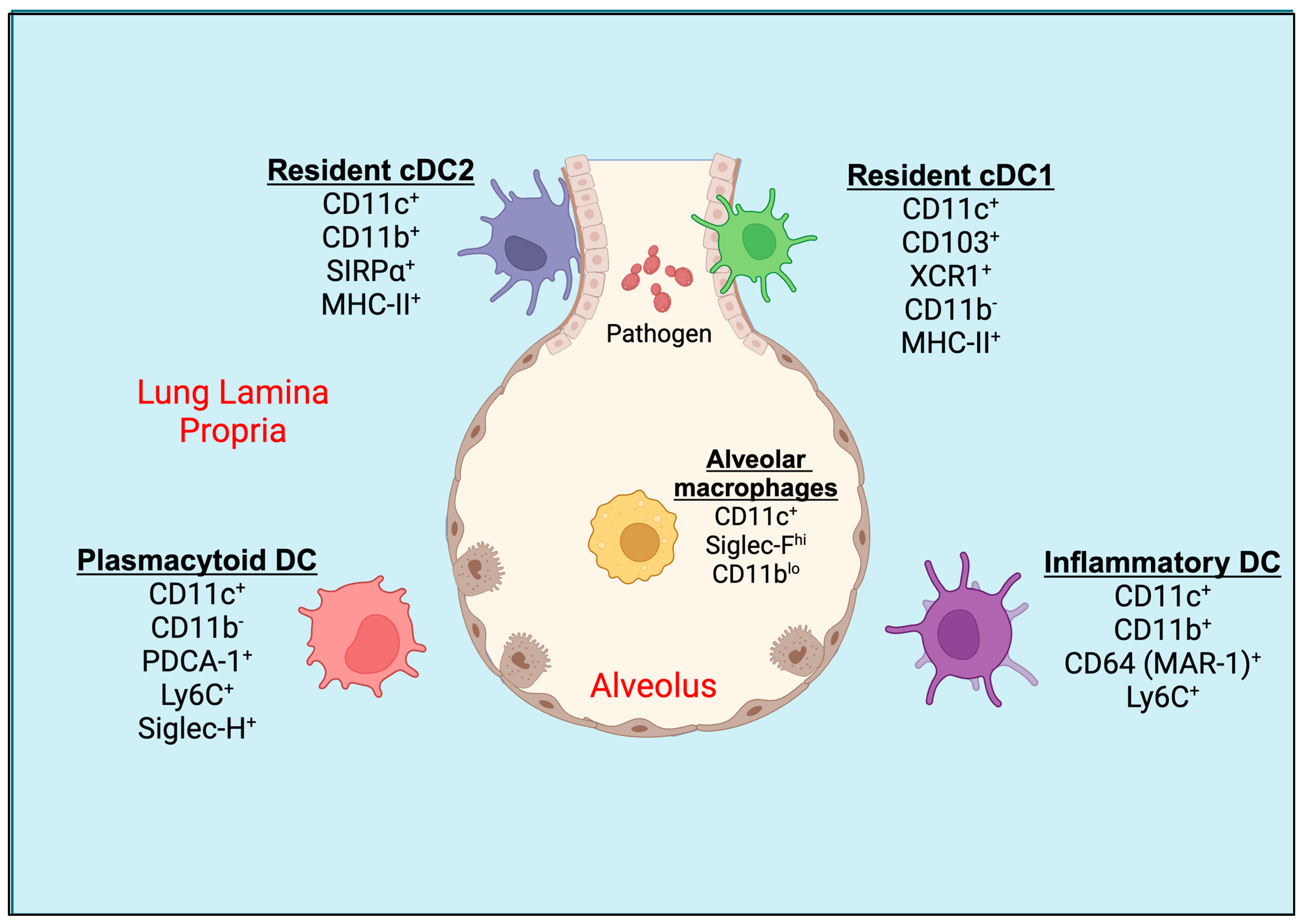
Figure 1.
Pulmonary tissue DC subsets. Mucosal CD103+ resident cDC1s are located in the basolateral space of the epithelium and can extend their dendrites between epithelial cells directly into the lumen of the airway. CD11b+ resident cDC2 and plasmacytoid DCs (pDCs) are located underneath the basement membrane of the lung or in the lung parenchyma. During inflammatory conditions, an activated population of inflammatory DCs expressing CD11b+ and Ly6C+ are seen in the lung tissue. The alveolar macrophages (AMs) are present in alveolar space. DC, dendritic cell; cDC, conventional dendritic cell.
The dendritic cells in the murine lungs are divided into conventional DCs (cDCs) and plasmacytoid DCs (pDCs; Figure 1). The lung cDCs express integrin CD11c at higher levels and are further classified as CD103+ DCs and CD11b+ DCs, also known as cDC1 and cDC2, respectively [20]. During the inflammation in the lungs, the monocytes can differentiate into DCs called monocyte-derived dendritic cells (moDCs) [10]. CD103+ cDC1s express MHC-II, Langerin (CD207), and high levels of the lymphotactin receptor XCR1, but low levels of CD11b [17,21,22]. The cDC1 creates an intricate network within the epithelial layer of the airways and extends elongated cellular projections into the spaces between basal epithelial cells for the sampling of the inhaled particles [17]. cDC1 engulfs soluble as well as apoptotic cell-associated antigens and migrates to the mediastinal lymph nodes during steady-state conditions and inflammation [23]. The cDC1 cells are well-known for their cross-presentation of antigens to cytotoxic T cells (CTLs) [24]. On the other hand, cDC2s express high levels of SIRPα (CD172α, a ligand for CD47), and intermediate levels of CX3CR1, MHC-II, and F4/80, but are mostly negative for CD103, XCR1, or CD207 [20,25]. They reside underneath the lamina propria or basement membrane of the lung [26]. CD11b+ cDC2 are specialized to present exogenous antigens via MHC-II to CD4+ T cells in mediastinal lymph nodes [12]. Both cDC1 and cDC2 migrate to mediastinal lymph nodes under steady-state conditions in the absence of inflammation to induce tolerance to inhaled antigens or particulates or allergens [27].
The airways also consist of pDCs that express PDCA-1 (CD317), Siglec-H, and B220, along with Ly6C, but not CD11b or SIRPα, which are distinct from moDCs [10]. Like cDC2, pDCs reside in the lung parenchyma, the site of gas exchange [16]. Following the exposure of foreign particles to the lungs, such as microbes (bacteria, fungi, parasites, and viruses), TLR ligands, environmental pollutants, or allergens, and the induction of inflammation, another subset of DC, CD11b+ moDC, is increased in the lung parenchyma and conducting airways [10]. The circulating monocytes, upon inflammatory stimuli, leave the blood vessel lumen within minutes to hours, cross the endothelial barrier, migrate towards the site of inflammation, and, with apt signals, differentiate into inflammatory DCs [28]. The surface markers of moDCs often overlap with CD11b+ cDC2 and express CD11b, SIRPα, F4/80, and MHC-II. It has been reported recently that the expression of CD64 (MAR-1 clone), MerTK, and Ly6C on moDCs helps differentiate from cDC2 in the inflamed lungs of mice [29,30,31]. Even though these subsets are more related to macrophages than DCs based on the MafB lineage, their superior ability of antigen presentation under ex vivo conditions makes them more of a DC phenotype [11,32,33,34]. Some studies have found that CD64+ moDCs account for ~25% of CD11c+CD11b+MHC-II+ lung DCs under steady-state conditions [10]. Further, the cytokine and chemokine production profiles of cDC2 and moDCs differ based on the nature of the pathogen [35]. In the murine lungs, alveolar macrophages also express higher levels of CD11c and often contaminate the DC gating population by flow cytometry. However, they can be distinguished from DCs by their higher autofluorescence, expression of higher levels of Siglec-F, and lower levels of CD11b, Ly6C, and MHC-II [36].
3. Origin and Development of Lung DCs
The origin and development of lung DCs are recently being defined [37]. The differentiation of progenitors occurs in the bone marrow and is a continuous process, necessitated by the constant need to replenish mature DCs [38]. The multipotent progenitors derived from hematopoietic stem cells undergo a series of phase transitions producing common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs) for lymphocytes and myeloid cells, respectively [39,40]. CMPs give rise to monocyte-dendritic cell progenitors (MDPs), which lack the potential to develop into granulocytes. The common dendritic cell precursors (CDPs) are identified as the first DC-restricted progenitors [41] and their development relies on the transcription factor FMS-like tyrosine kinase 3 ligand (FLT3L) and expresses ZBTB46 that is not essential for their development [42]. CDPs produce pre-DCs, which migrate to the peripheral organs and undergo local differentiation into mature cDCs within the tissue ambience [38]. The increased expression of interferon regulatory factor 8 (Irf8) is linked to the specification of plasmacytoid DCs (pDCs) and type 1 classical DCs (cDC1s). Here, Zbtb46 expression is crucial for cDC lineages, initially as pre-cDC1s and pre-cDC2s [40]. The transcription factors (TF) Basic Leucine Zipper ATF-Like Transcription Factor 3 (BATF3) and interferon regulatory factor (IRF8) dictate the lineage commitment of cDC1. Conversely, cDC2 development depends on the transcription factor IRF4 [43]. Fate-mapping studies reveal that almost all CD103+ cDC1 are derived from CDPs, whereas only 50% of CD11b+cDCs are accounted to be of CDP origin [44]. The other developmental pathways are still unknown. In contrast to cDCs, the complete development of pDCs occurs in the bone marrow before they migrate to the lungs, despite their closeness to cDC1 [45,46]. Interestingly, the ontogeny of pre-pDCs is from both CDP and CLPs, and, with their nature of high type-I cytokine producers, the debate exists to classify subsets of them as innate lymphocytes rather than DC [47,48]. pDC generation is mainly dependent on transcription factors like interferon regulatory factor 8 (IRF8), inhibitor of differentiation 2 (ID2), transcription factor 4 (TCF4), signal transducer and activator of transcription 3 (STAT3), and E26-transformation-specific (ETS) family member Spi-B [12,49,50]. In contrast to other subsets of dendritic cells, during infection and inflammation, bone-marrow-derived monocytes differentiate into monocyte-derived dendritic cells (MoDCs) or TNF- and iNOS-producing DCs (Tip-DCs). MoDCs, resembling classical monocytes, depend on macrophage-colony stimulating factor (M-CSF) for their development and CCR2 for their recruitment to inflamed lungs [51].
The studies employing bone marrow depletion techniques have elucidated that dendritic cells (DCs) migrating within the lungs undergo turnover every 2–3 days during steady-state conditions [52]. However, other studies suggested that 5–10% of the lung cDC proliferates at any given point [53]. Various factors, such as exposure to microbial antigens, tissue damage, and inflammatory signals, influence the turnover and replenishment rate of DCs in mucosal tissues. Parabiotic mouse experiments conducted in lung tissues have revealed a notable extended turnover period with CD11b+ DC and CD103+ DC exhibiting half-lives of 15 and 30 days, respectively [53]. Thus, the dynamics of different subsets of DCs and their homeostasis may influence the pathogenesis during infection.
4. Functions of DC against Respiratory Fungal Pathogens
While the role of DCs and their subsets are well-characterized during viral infection models, their studies during fungal infections are limited. Incontrovertibly, DCs are the single most common class of professional antigen-presenting cells that interconnect the innate immune system with adaptive immunity to pathogens including fungi [54]. It is conceivable that specific subsets of DC have distinct functions shaping the ongoing inflammation and T-cell responses. Further, the fungal pathogen can exist in different forms—the filamentous hyphal form, spore or conidia form, and single-cell yeast form—posing unique challenges to the dendritic cells for sensing, phagocytosis, killing, and antigen presentation [55,56].
The DCs activate and differentiate the T cells mainly by three signals (Figure 2); (i) antigen presentation, (ii) co-stimulation, and (iii) the production of lineage-defining cytokines. DCs recognize fungi through a diverse range of surface-bound and intracellular pattern recognition receptor (PRR) sensors [57]. The DC subsets cDC1 and cDC2 primarily process and present the fungal antigens via major histocompatibility complex (MHC) class I or MHC-II molecules to naïve CD8+ or CD4+ T cells, respectively. The migratory DCs (migDCs) engulf the antigen/yeast, migrate to the draining lymph nodes (dLN), and present the antigen to CD4+ and CD8+ T cells or transfer the antigens to cDCs [58]. On the other hand, the pDCs mainly produce type-I interferons and generally mediate immunity to viral infections [51]. moDCs mainly are involved during an ongoing inflammation and shape the ongoing adaptive immune responses. Notably, the process of recognition of fungi and the activation of DCs enhances the expression of the epitope-bound MHC molecules, co-stimulatory molecules (CD80/CD86), and polarizing cytokines essential for priming naïve T cells and their differentiation into multiple distinct subsets such as IFN-γ+ Th/Tc1, IL-4/13+ Th/Tc2, and IL-17+ Th/Tc17 [10,43]. Thus, the nature of DC activation determines the type of T-cell response and fungal immunity.
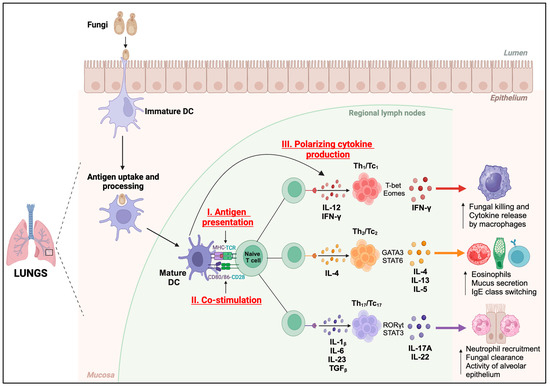
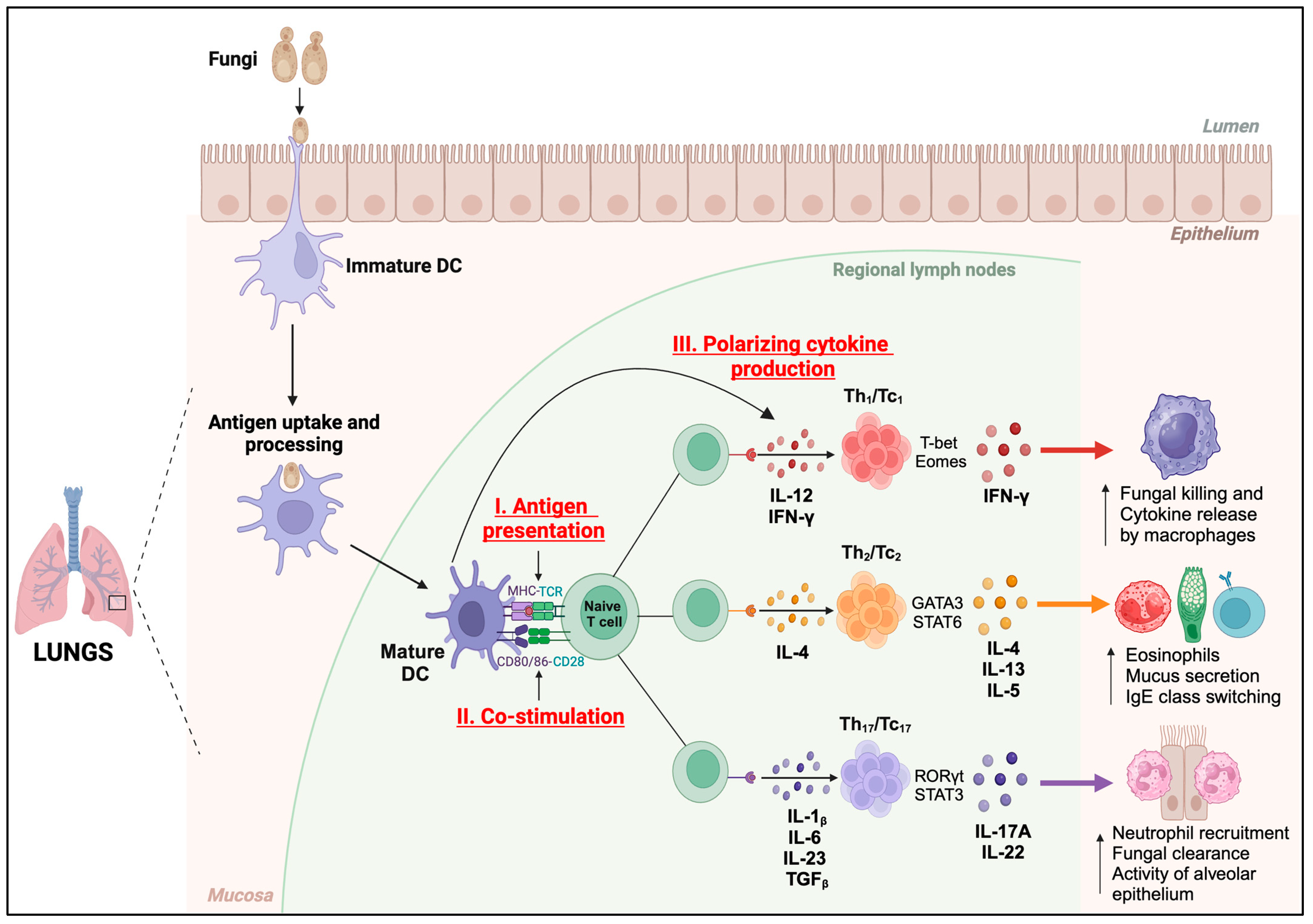
Figure 2.
Functions of DCs. Immature dendritic cells (DCs) perform antigen uptake and processing in the mucosa of the lungs and present the antigenic peptides in MHC-I or MHC-II complexes to cognate TCRs on naïve T cells (Signal I) in regional lymph nodes. Fungal antigen recognition induces the maturation and activation of DCs, resulting in increased expression of co-stimulatory molecules, CD80 and CD86 (Signal II), which bind to CD28 on naïve T cells to initiate their activation. DCs secrete polarizing cytokines (Signal III) which induce the differentiation of T cells into lineage-specific subsets. Th1/Tc1 subset produces IFN-γ which enhances the fungal killing and cytokine release by macrophages. Th2/Tc2 subset secretes IL-4, IL-13, and IL-5, which increases the number of eosinophils, and mucus secretion, and promotes IgE class switching. Th17/Tc17 subset produces IL-17A and IL-22, which increases the neutrophil recruitment, fungal clearance, and alveolar epithelial cell functions.
The functions of different DC subsets may depend on various factors, such as the site of infection/inoculation, the nature or phenotype of the fungal organisms, and the susceptibility of the host. For instance, a study in mice showed that Batf3+ cDC1 induces protective Th1 CD4+ T-cell responses and helps to clear cryptococcal infections [59] by upregulating T-cell recruitment, differentiation, and activation pathways. Contrastingly, conventional type 1 Langerin-expressing DCs (LangDC1) impaired immune responses against Cryptococcus neoformans in mice in the early stages of pulmonary infection [60]. This was associated with the downregulation of type-1 and type-17 cytokine production while enhancing the type-2 responses. In murine histoplasmosis, CD103+ cDCs were the main producers of type-I IFN in the lungs in a TLR7/9-dependent manner, and cause the induction of IFN-γ production by T cells and increased the host survivability [61]. CD11b+ DCs that recognized chitin during pulmonary cryptococcosis induced predominant Th2 cell responses, and their deletion using CD11ccreIRF4fl/fl mice reduced the Th2 pathology [62]. Another murine study showed that CCR2 mediates CD11b+ DC (cDC2) recruitment to the lung during pulmonary cryptococcosis [63], which helps in mounting effective Th1 immune responses [63].
The various DC subsets cooperate with each other in inducing effective T-cell responses. For example, migratory DCs and moDCs shuttled the vaccine antigens to lymph-node resident DCs to induce robust vaccine-induced CD4+ T-cell responses that provided immunity against Blastomyces dermatitidis in mice [64]. Similarly, moDCs and neutrophils produce CXCL9 and CXCL10 through dectin-1, Card9-, and type-I and -III IFN-mediated signaling for the recruitment of pDCs and host defense in the mouse model of aspergillosis [65]. In another study, the FLT3L-dependent cDCs and CCR2-dependent moDCs presented the antigens and primed the T cells during mucosal candidiasis in mice [66]. In murine lung cryptococcal infection, IL-10 signaling reduced the numbers of moDC and the activation of CD11b+ type-2 DCs in an autocrine manner in persistent infection and the ablation of IL-10 promoted the host immunity by these subsets [67].
An important DC subset that increases after inflammation is monocyte-derived dendritic cells (moDCs)/inflammatory DCs. These TNF-expressing moDCs (Tip-DCs) effectively induced CD4+ T-cell responses and IL-17A-dependent airway neutrophilia and fungal killing, but the absence of dectin-1, MyD88, or TNF enhanced the IL-5 and impeded the fungal clearance in persistent aspergillosis in mice [68], suggesting TNF-producing moDC orchestrated neutrophil-dependent airway inflammation and immunity. Similarly, Notch ligands and receptors were upregulated phagocytes and Th1 cells during histoplasmosis, and the inhibition of Notch signaling led to an increased fungal burden in primary infection associated with the reduced differentiation and maturation of moDCs and elevated monocyte-derived alveolar macrophages polarized to M2 [69].
Interestingly, the conidial forms of yeasts can be phagocytosed effectively by DCs leading to a protective Th1 response by the expression of IL-12, but the hyphal or filamentous forms are not and may induce non-protective Th2 cells expressing IL-4 and regulatory cytokine IL-10 [70,71,72]. Human immature DCs seem to be activated upon exposure to Aspergillus germ tubes and expressed proinflammatory cytokines TNF and IL-12 in a dectin-1-dependent manner [73]. Interestingly, the murine moDCs seem to inhibit Th17 responses and promote Th1 responses, likely in a dectin-1-dependent manner, during aspergillosis [74]. The human DCs exposed to Aspergillus α-(1,3)-Glucan polarized naïve T cells to the regulatory type and the PD-L1 pathway facilitated this process while negatively regulating IFN-γ secretion [75]. The sensing of PRR ligands can dictate the outcome of T-cell responses. For example, the live but not the killed Aspergillus activated the dendritic cells mainly due to the differential beta-glucan display and activation of Th1 responses [76,77]. Despite the accumulating studies on the role of DCs during pulmonary fungal infections, distinct DC subsets’ functions directly contributing to immunity or by the activation and polarization of distinct cytokine-producing T cells or modulating other cells are not clear [78]. Further, only a few studies have directly evaluated the specific type of DCs in regulating the immunity to pulmonary fungal infections and we will discuss this where possible under specific DC-based fungal vaccines.
A relatively outlier DC subset, pDC, also has a role in antifungal immunity in a tissue-dependent manner [79]. A study showed that the deletion of pDCs increased the susceptibility of mice to invasive aspergillosis, and in vitro, fungal exposure to human pDCs enhanced the expression of TNF and type-I interferons and direct cytotoxicity [80]. Further, the blood-borne murine pDCs may crosstalk with the neutrophils to enhance NADPH oxidase activity and the Aspergillus conidial killing [65]. Despite the dectin-1 requirement for immunity to aspergillosis, dectin-2-mediated recognition by pDC was essential for TNF/IFN-α release, the formation of neutrophil extracellular traps, and antifungal activity in humans [81]. On the contrary, pDCs mediated the tolerance during lung infection with Paracoccidioides brasiliensis by inducing regulatory T cells [82]. Paracoccidioides infection augmented the number of pDCs’ immunoregulatory enzymes driving regulatory T-cell (Treg) expansion in an indoleamine 2,3-dioxygenase (IDO)-dependent manner [82]. Interestingly, dectin-1 activation in human myeloid DCs reduced the Th2 responses whereas dectin-1 activation in pDCs promoted the Th2 responses apparently due to their disparate surface expression of OX40L and reduced OX40L on myeloid DCs enhanced Th17 cell responses to curdlan treatment [83]. Thus, the pDCs have pattern recognition receptor (PRR)-specific, pathogen-specific anti- or pro-inflammatory immune responses to fungal pathogens.
Another notable function of DCs is “cross-presentation”; DCs process and present extracellular antigens via MHC-I molecules to activate CD8+ T cells [84,85,86]. DC-based cross-presentation is essential for initiating CD8+ T-cell responses against tumors and certain pathogens that do not directly infect antigen-presenting cells or use host translation machinery. The DC cross-presentation enables the immune system to detect and respond to a wider range of antigens than would be possible through direct presentation alone. Despite cDC1 being known to be potent cross-presenting cells, recent studies showed that cDC2, moDCs, and pDCs (in humans) also have a cross-presentation ability [12,54]. Most of these studies deciphered the cross-presentation ability of DC subsets in viral or tumor model systems, and their role during pulmonary fungal infections is scarce. In an in vitro study, the bone-marrow-derived DCs (BMDCs) engulf and cross-present Histoplasma capsulatum antigens either from live or killed yeast to cytotoxic CD8+ T cells or via the uptake of live or killed apoptotic macrophages that harbor Histoplasma spp. [87]. In hematopoietic transplanted patients, the TLR3 on CCR7+ migratory DCs enhances the memory CD8+ T-cell recall responses to A. fumigatus by detecting fungal RNA and promoting cross-presentation via MHC-I [88]. Despite the overwhelming data on cDC1 (CD8+ DC) for cross-presentation, a study showed that splenic cDC2 (CD4+ DC) could cross-present and activate CD8+ T cells using an OVA-expressing strain of yeast Saccharomyces cerevisiae [89]. Although these studies show that resident DCs can acquire and present fungal antigens, further studies are needed to unravel the subset of DCs involved in in vivo cross-presentation.
5. Dendritic-Cell-Based Experimental Fungal Vaccines
The DCs are the most principal innate cells to connect innate and adaptive immune systems mainly by the phagocytosis of fungal pathogens, antigen processing and presentation, co-stimulation, and secretion of polarizing cytokines for the activation, differentiation, and expansion of T cells (Figure 2) [17]. DC-based vaccines have shown promise in cancer immunotherapy and the treatment of infectious diseases [90]. Some of these vaccines have undergone testing in phase I, II, and III clinical trials for various cancers, including myeloma, melanoma, acute myelogenous leukemia (AML), ovarian cancer, and HNSCC [91,92,93,94,95]. Consequently, Sipuleucel-T (Provenge; Dendreon Corp, Seattle, WA, USA) became the inaugural autologous DC-based vaccine approved for treating specific cases of advanced prostate cancer [96]. Thus, the DC-based vaccine approach is gaining significance, and various strategies are being explored as a rational design for vaccines against pulmonary fungal infections, especially in immunocompromised hosts. There is insurmountable evidence that the T cells are instrumental in immunity to fungal infections, including by inducing an apt B cell response, and, not surprisingly, many DC-based vaccines are tailored to elicit T-cell responses.
Recent studies have examined several approaches to target DCs in the development of antifungal vaccines. One of the strategies involves pulsing DCs with fungal antigens through various methods, such as exposure to the fungal proteins, peptides, and cell wall extracts, or introducing fungal nucleic acids (DNA or RNA) [97]. This approach enables the utilization of fungal ligands like β-glucans, zymosans, and mannose, which can enhance effective antigen uptake by DCs and specific PRRs known as C-type lectin receptors (CLRs) signaling. This apt fungal recognition by PRRs activates DCs, allowing them to present antigens to T cells and initiate an adaptive immune response. Additionally, DCs are the cells that express higher levels of co-stimulatory molecules (CD80 and CD86) and cytokines (IL-1β, IL-6, IL-12, IL-23, and IL-4) which help in the priming and subsequent differentiation of T cells into various effector subsets (Th1, Th2, or Th17), and antibody responses following fungal vaccination [43,57].
Another approach involves targeting DCs for directed activation, the induction of inflammation, and the augmenting of T-cell responses. For example, employing nanoparticles that specifically target receptor DC-SIGN or DEC205 on dendritic cells (DCs) where nanoparticles delivered the PRR ligand and antigens initiating robust T-cell responses [98,99]. DCs are efficient in the endocytosis of mannosylated proteins in fungal pathogens via CLRs. For instance, the model antigen OVA upon mannosylation promoted its uptake by DCs through CLR family members DC-SIGN and CD206, leading to enhanced immunogenicity [99]. Similarly, DCs efficiently engulf antigen-loaded β-glucan particles mediated by the dectin-1 pathway [100,101]. This targeted strategy for DCs could potentially facilitate the loading of fungal antigens into DCs, which is essential for triggering effective cellular immune responses against fungal infections. Here, we have discussed the DC-based vaccine strategies that are utilized against a few pulmonary fungal infections, and some examples are listed in Table 1.
5.1. Aspergillus spp.
Aspergillus is a genus of globally ubiquitous filamentous fungi found in various environments including soil and decaying vegetation. Despite the abundance and frequent inhalation of Aspergillus spores (conidia), it rarely causes infection or immune diseases in healthy hosts [102,103]. However, in immunocompromised hosts and patients with pre-existing pulmonary infections, the spores can germinate into invasive hyphae, resulting in a variety of acute to chronic diseases [104,105]. The most common species of Aspergillus associated with invasive infections in humans include A. fumigatus, along with A. flavus, A. niger, and A. terreus, especially in patients receiving chemotherapy, stem cell, and solid organ transplants, and patients experiencing complications with COVID-19. Around 250,000 cases of invasive aspergillosis are reported annually worldwide and are associated with significant mortality rates [74,106]. Moreover, the sensitization to allergens from Aspergillus spp. leads to allergic diseases, and allergic bronchopulmonary aspergillosis contributes to ~5 million cases [106,107]. The current antifungal medications for aspergillosis have limited efficacy and may result in drug resistance. Thus, it is essential to develop therapeutic vaccines to control allergic responses and provide protection against these opportunistic fungi in immunocompromised individuals.
Dendritic-cell-based vaccines have emerged as a promising approach for treating aspergillosis. DC-based vaccines aim to harness the antigen-presenting capabilities of dendritic cells to stimulate a specific immune response to Aspergillus antigens. The experimental vaccines in mice have shown protection given by crude and genetically engineered Aspergillus antigens in a Th1 cell-dependent manner enhanced by the addition of an adjuvant, CpG oligodeoxynucleotides (CpG ODN) [108]. DCs undergo remarkable functional plasticity in response to the conidia and hyphae of A. fumigatus and provide in vivo antifungal immunity upon activation with fungal RNA or live fungi [56,109]. The protection to aspergillosis was mediated by DC-induced Th1 responses in a mouse model of allogeneic hematopoietic transplantation. Similar findings were observed in studies using recombinant Aspergillus proteins (such as Asp f 16) and CpG ODN as adjuvants [56]. Another study used a vaccine strategy involving the transduction of DCs with an adenovirus vector encoding the cDNA of IL-12 and primed with heat-inactivated A. fumigatus (HAF). The adoptive transfer of these genetically modified DCs into syngeneic mice stimulated antigen-specific Th1 responses, reduced the fungal burden, and improved survivability, suggesting the potential use of antigen-pulsed DCs and IL-12 gene therapy to treat invasive aspergillosis [110]. In some of these studies, the specificity of the adaptive immune response elicited by DCs pulsed with Aspergillus antigens may not be specific, which may reduce the effectiveness of these vaccines in providing targeted protection against aspergillosis. Nonetheless, this small clinical trial has shown promising results using DCs pulsed with antigens of A. fumigatus [111]. In this trial, patients who received DC-based therapy demonstrated protection against opportunistic infections following haploidentical transplants, suggesting that DC-based vaccines could be a viable option in preventing infections in high-risk individuals. Despite these positive outcomes, the high cost associated with DC-based vaccine therapies remains a significant barrier to widespread use. Further research is needed to optimize the efficacy and cost-effectiveness of these vaccines for wider implementations in clinical settings.
5.2. Coccidiodes spp.
Coccidiodes spp. especially C. posadasii and C. immitis are highly virulent, dimorphic fungi causing coccidioidomycosis or Valley Fever or San Joaquin Valley fever in the southwestern United States, northern Mexico, and regions of Central and South America [112]. The coccidioidomycosis has re-emerged as a major threat to human health due to climate change and newer geographical spread. In 2019, approximately 20,003 cases were reported to the Centers for Disease Control and Prevention (CDC) in the United States [113]. Patients’ travel history to the endemic areas is the primary cause of the disease reported worldwide [114,115,116]. Dissemination of endospores causes severe pulmonary infections or more widespread diseases affecting organs like the bones, skin, central nervous system, or other organs. The host’s susceptibility to coccidioidomycosis depends on age, racial or genetic predispositions, pregnancy, and immune status [117], and immunocompromised hosts (with AIDS), pregnant women, and patients with dysfunctional T cells are prone to developing severe disseminated forms of Valley Fever [118,119]. Many healthy individuals recover upon exposure to Coccidiodes arthroconidia, but around 5–10% develop active disease [113]. The current therapeutics against coccidioidomycosis are limited, expensive, and ineffective due to relapse or reactivation, and require prolonged use. Hence, there is a need for an effective vaccine or immunotherapy to treat Valley fever.
The earlier study on a DC-based vaccine against Coccidioides spp. in a mouse model utilized the strategy of transfecting the BMDCs with a plasmid DNA encoding for Coccidioides-Ag2/proline-rich antigen (PRA) [120]. A significant number of intranasally administered Coccidioides-Ag2/PRA-cDNA-transfected DCs (Ag2-DC) are retained in the mucosal organs, such as the lungs and gut [112,120], and stimulate the protective IFN-γ responses. A similar intranasal Ag2-DC immunization of highly susceptible BALB/c mice reduced the fungal burden, significantly increased the IFN- γ expression, and alleviated lung pathology. Further, this prophylactic Ag2-DC immunization increased the levels of serum IgG and its isotypes [121]. The indicators of lung injury (protein, lactate, and albumin) were normal, suggesting the DC vaccine itself did not cause any vascular leakage or tissue injury. Further studies showed that the DC vaccine provided IFN-γ-mediated vaccine immunity and protection against coccidioidomycosis in mouse models [122,123]. These DC-immunization strategies showed the feasibility of a therapeutic vaccine.
5.3. Paracoccidioides spp.
Paracoccidioidomycosis (PCM) is a systemic granulomatous infection caused by the Paracoccidioides spp. (P. brasiliensis and P. lutzii) of thermo-dimorphic fungi, which are endemic to Latin America (Mexico to Argentina), mainly affecting workers with significant soil exposures. The high incidence of PCM is reported in Brazil, where around 51% of the confirmed deaths due to systemic mycoses were caused by PCM from 1996 to 2006 [124,125]. Upon the inhalation of fungal propagules, they undergo differentiation into the infective yeast form in the lung, resulting in the development of characteristic granulomatous lesions through the activation of Th1 responses [126]. In immunocompromised individuals, the granulomatous lesions contain abundant viable fungi that have the potential to disseminate to almost all organs and tissues. Th1/Th17 responses provide protection against PCM, while Th2 responses and regulatory T cells (Tregs) lead to a severe form of the disease [127]. Interestingly, PCM vaccines aiming to reduce only the fungal burden are insufficient to lower the morbidity and mortality, and a well-balanced cytokine response remains essential [127,128].
Dendritic cells are shown to be important for initiating the adaptive immune responses for the control of Paracoccidioides spp. infections [126,129,130]. The peptide-based vaccine strategy involves utilizing the P10 peptide, an immunodominant antigenic region of the glycoprotein 43 (gp43) of the fungus P. brasiliensis, containing a sequence of 15 amino-acid epitopes specific for CD4+ T cells. The P10 antigen was used to test the adjuvant-like effects of DC presentation to enhance the resolution of PCM [113]. In experimental mouse models, BMDCs pulsed with the P10 antigen showed both therapeutic and preventive effects against PCM [131]. Upon the subcutaneous immunization of P10-primed DCs and the subsequent challenge with a virulent strain induced a strong Th1 response with an increased production of IFN-γ and IL-12, while reducing the IL-10 and IL-4 levels correlated with a reduction in the number of granulomas, the alleviation of lung damage, and a reduction in the fungal burden, suggesting its potential use as a therapeutic and prophylactic [131]. A study with a similar strategy but using monocyte-differentiated dendritic cells (moDCs) harvested from infected mice were pulsed with P-10 and used as a therapeutic vaccine, giving protection rivaling BMDC-P10-pulsed vaccination [132]. Perhaps, in humans, monocyte-derived DCs could be employed. In vitro experiments also demonstrated that the DC-based approaches activate and upregulate MHC-II, CD80, and CD86 expression on DCs and induce the proliferation of CD4+ and CD8+ T cells.
Considering the apprehension for PCM treatment in immunocompromised patients, the P10-pulsed DC vaccination has been investigated in BALB/c mice injected with dexamethasone to mimic the immunocompromised state. DC-vaccination effectively induces Th1-specific and protective immune responses against P. brasiliensis, the causative agent of a severe form of PCM [133]. Furthermore, a combination of antifungal drugs with a P10-primed DC therapeutic vaccine has a synergistic effect in reducing the fungal burden in immunosuppressed mice with PCM. These studies highlight a range of vaccine candidates for Paracoccidioides, with ongoing efforts to develop a P10-based adjuvant vaccine for clinical trials [127,131,132,133].
5.4. Cryptococcus spp.
Cryptococcosis is a highly virulent invasive fungal infection mainly caused by Cryptococcus spp. (C. neoformans and C. gattii). While C. neoformans infection primarily occurs in immunocompromised individuals, C. gattii can cause infection in apparently healthy individuals. In 1999, C. gattii infection emerged as an infectious disease in Vancouver Island, Canada, and surrounding regions, resulting in deaths among healthy individuals, with a mortality rate of around 8–20% [134,135]. The infection typically occurs through inhalation of the spores, leading to a primary lung infection. While many individuals may remain asymptomatic, the fungus can disseminate from the lungs to other parts of the body, mostly the central nervous system (CNS), causing conditions like meningitis. Patients with compromised T-cell immunity such as HIV/AIDS are highly susceptible to cryptococcosis. In 2020, around 152,000 cryptococcal meningitis cases were reported worldwide, resulting in ~112,000 deaths [136,137], with a case fatality rate of ~74%. Cryptococcosis accounts for 19% of all AIDS-related mortality. The increased global prevalence of cryptococcosis in immunodeficient hosts necessitates the development of new therapeutic avenues, including preventive vaccines.
The whole-cell-based DC-vaccine approach has garnered interest for protection against cryptococcosis. The study utilized the pulsing of BMDCs with the heat-killed acapsular C. gattii (∆cap60) strain of yeast, where the capsule synthesis-related gene cap60 was disrupted using a reverse genetic approach, and pulsed DCs were adoptively transferred intravenously into the mice twice before pulmonary infection with the highly virulent C. gattii strain R265 [138]. This vaccine strategy provided good immunity with a ~8900-fold decrease in the fungal burden, cross-strain protection, a significant reduction in lung pathology, and improved survival rates. The vaccine immunity increased the number of multinucleated giant cells (MGCs) that are involved in phagocytosis and enhanced the production of cytokines such as IL-17A, TNF, and IFN-γ, but reduced protection in mice lacking IFN-γ [138]. Concomitantly, pulsed DCs were required to establish immune memory against cryptococcus infections, called “trained immunity” [139,140] involving epigenetic modifications in the DCs. Several experimental vaccines have elucidated the significance of vaccination leading to protective immunity against cryptococcosis [141,142]. The ex vivo incubation of DCs with C. neoformans or immunizing with H99γ resulted in an increased production of IFN-γ and pro-inflammatory cytokines. Further, the responses showed a memory-like DC response, associated with epigenetic modification, which may aid in providing protective immunity to immunosuppressed hosts [140,143]. A recent study showed that the C. gattii Cap60∆ BMDCs preferentially migrated to the lungs, recruited recipients’ DCs and promoted long-lived tissue-resident memory Th17 cells protective against highly virulent C. gattii. The lung-resident memory Th17 cells activated neutrophils and bolstered granuloma formation, providing immunity to pulmonary cryptococcosis [144]. Overall, these findings suggest that systemic DC vaccines have the potential to induce protective immune memory against cryptococcal infections, particularly in the lungs; however, their effectiveness in humans needs to be studied further.

Table 1.
DC-based fungal vaccines against pulmonary fungal infections.
Table 1.
DC-based fungal vaccines against pulmonary fungal infections.
| Fungi | Vaccine Type | Methodology | Major Outcomes | References |
|---|---|---|---|---|
| Aspergillus spp. | RNA or live fungi/Crude antigen or subunit vaccine | Murine and human DC-pulsed (RNA complexed with DOTAP) | Enhanced DC’s MHC-II and co-stimulatory molecules expression, and increased Th1/Th2 response | [108] |
| Heat-killed fungi/Crude antigen vaccine | Human DC-pulsed | In vivo protective antigen-specific Th1 response (high IFN-γ and IL-10 production) | [109] | |
| Heat-inactivated fungi/viral transduction | Murine DC-pulsed and IL-12 gene therapy | Increased Th1 responses, improved survivability, and reduced fungal burden | [110] | |
| Coccidioides spp. | Ag2/Subunit vaccine (Ag2/PRA-cDNA transfected DC) | Murine transfected DCs | Reduced fungal burden, tissue injury in vaccinated mice, enhanced IgG levels, and increased IFN-γ, IL-4, and IL-17 production | [120,121,122,123] |
| Paracoccidioides spp. | Peptide vaccine (P10) P10 primary DC P10 primary monocyte derived-DC | P10-primed murine DCs | Reduced fungal burden in both immunocompetent and immunosuppressed mice, protection against intratracheal challenge, protective Th1 responses, activation and upregulation of MHC-II, CD80, and CD86 on the DCs, and induction of CD4+ and CD8+ T-cell proliferation. | [131,132,133] |
| Cryptococcus spp. | Heat-killed Cryptococcus gattii mutant ∆cap60 | Murine DC-pulsed | Protection and stimulation of tissue-resident memory Th17 cells in the lungs | [138,144] |
| Live or heat-killed Cryptococcus neoformans mutant | - | Protective Th1-type adaptive immune response Induction of trained immunity of DCs | [140] |
DC: dendritic cell; DOTAP: cationic lipid N-[1-(2,3-dioleoyloxypropyl]-N, N, N,-trimethylammonium methyl sulfate; Ag2/PRA: protein termed antigen 2/proline-rich antigen.
6. Future Perspectives of Fungal DC Vaccines
Despite the several limitations of DC-based vaccines, new emerging techniques of inexpensive and easy culturing of monocyte-derived DCs from PBMCs in humans may bolster therapeutic vaccines in conjunction with antifungals. With traditional DC-based fungal vaccine candidates and strategies, new platforms and techniques are evolving to propel fungal vaccine research. The fungal extracellular vesicles (EVs) have great potential as a fungal vaccine candidate. EVs are lipid bilayer nanoparticles secreted by fungi, that can carry cargo of proteins, enzymes, lipids, nucleic acids, and carbohydrates [14]. For example, EVs from C. neoformans carry immunogenic proteins, like mannoprotein MP88, Vep proteins, and chitin deacetylase Cda family proteins—all of which have been tested as potential vaccine candidates [145]. In recent animal studies, EVs isolated from Paracoccidioides and Candida showed immunogenicity and vaccine immunity [14,146]. Similarly, the mice immunized with EVs derived from Cryptococcus acapsular cap59 mutants secreted antibodies against EV proteins and provided a strong immunity to the wild-type H99 challenge [145]. The possible advantage of an EV-based vaccine is its combination of various natural antigens which induce strong immunity. Further, EVs offer stable structural conditions for protein components, allowing them to circulate in bodily fluids and effectively sensed and taken up by antigen-presenting cells [147]. Some of the challenges with EVs are the heterogeneity of the contents, scaled-up production, and toxicity or immunosuppression of some components. Thus, EV-based antigen delivery may offer a novel approach to DC-based vaccines.
The nanoparticle-mediated targeting of DCs can potentially enhance the fungal vaccine efficacy due to their sub-micron size and specific targeting of DCs [13]. Nanoparticles can be composed of various materials such as fungal cell surface carbohydrates, metallic nanoparticles, and lipid-based particles, and serve as effective carriers for fungal antigens. Targeting dendritic cells (DCs) with nanoparticles allows for the precise delivery of specific antigens combined with an adjuvant and a DC-receptor-targeting (DEC-205) molecule [27]. Several studies depicted the effectiveness of using nanoparticles coated with DEC-205 F(ab’)2 fragment, containing TLRs such as TLR3, TLR7, and TLR8 ligands, and OVA antigen [98]. This combination strategy is designed to effectively target DEC-205+ DC and deliver both the antigens and adjuvants. This technique enhances the immunogenicity, increases the antigen delivery potency, and reduces the overall toxicity. Overall, the nanoparticle-based DC vaccines could be utilized against fungal infections to induce effective immune responses with limited toxicity.
The recent COVID-19 pandemic and the development of mRNA-based vaccines have accelerated the adoption of this technology, providing promise for the development of mRNA-based vaccines against other pathogens. RNA-based vaccines offer the benefits of a straightforward, rapid, versatile, scalable, modifiable, and cost-effective manufacturing process, as only the encoded RNA sequence needs to be modified. Further, mRNA is non-infectious, making it relatively safe to use in immunosuppressed hosts [148]. Additionally, it can be modified to self-replicate within the host, enhancing its ability to stimulate a robust immune response necessary for generating immunological memory [149]. Through the integration of cutting-edge vaccine technologies and the utilization of established mRNA vaccine platforms, one can accelerate the identification of novel and immunogenic fungal vaccine candidates, promising a potential breakthrough in fungal vaccine development in the near future. In the next decade, future research will explore integrating various methods of innovation for the development of vaccines against fungal infections. Strategies like DNA vaccines, subunit vaccines, attenuated vaccines, and advancements in adjuvant optimization should guide us to advance into new phases of innovative effective vaccine development.
7. Conclusions
In this review, we have highlighted the origin, development, subsets, and functions of pulmonary DCs, linking them to the activation of naïve T cells, effector T-cell formation, and immunity against fungal infections. Further, we have discussed in depth the DC-based vaccines used against several respiratory fungal pathogens viz. Aspergillus spp., Cryptococcus spp., Coccidioides spp., and Paracoccidioides spp. The utilization of various DC-based vaccine development strategies in animal models has made immense progress in the fungal field, further embracing a new perspective on the utilization of EV, nanoparticle-based, and mRNA-based vaccines that hold potential for the future of fungal vaccine development. Further elucidating the involvement, stability, longevity, plasticity, and dynamics of DC subsets in antifungal immunity will unveil novel avenues for the development of effective fungal vaccines.
Author Contributions
Conceptualization, S.G.N.; software, N.A.K.; writing—original draft preparation, N.A.K.; writing—review and editing, S.G.N.; supervision, S.G.N.; project administration, S.G.N.; funding acquisition, S.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NIH-NIAID R01AI153522 (to SGN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The figures in this review article were prepared using BioRender software (https://www.biorender.com/, accessed on 24 June 2024).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Data and Statistics on Fungal Diseases. Available online: https://www.cdc.gov/fungal/data-research/facts-stats/index.html (accessed on 8 July 2024).
- Robbins, N.; Wright, G.D.; Cowen, L.E. Antifungal Drugs: The Current Armamentarium and Development of New Agents. Microbiol. Spectr. 2016, 4, FUNK-0002-2016. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Overcoming antifungal resistance. Drug Discov. Today Technol. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- Thomas, C.M.; Shae, W.; Koestler, D.; DeFor, T.; Bahr, N.C.; Alpern, J.D. Antifungal drug price increases in the United States, 2000–2019. Mycoses 2022, 65, 859–865. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Steinman, R.M. Dendritic cells and the control of immunity: Enhancing the efficiency of antigen presentation. Mt. Sinai J. Med. 2001, 68, 160–166. [Google Scholar]
- Guilliams, M.; Lambrecht, B.N.; Hammad, H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013, 6, 464–473. [Google Scholar] [CrossRef]
- Bosteels, C.; Neyt, K.; Vanheerswynghels, M.; van Helden, M.J.; Sichien, D.; Debeuf, N.; De Prijck, S.; Bosteels, V.; Vandamme, N.; Martens, L.; et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity 2020, 52, 1039–1056.E9. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 860915. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, B.; Rossi, S.A.; Santos, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Front. Cell Infect. Microbiol. 2020, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front. Cell Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.M.; Madl, A.K.; Pinkerton, K.E. Respiratory Health Effects of Exposure to Ambient Particulate Matter and Bioaerosols. Compr. Physiol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- von Garnier, C.; Filgueira, L.; Wikstrom, M.; Smith, M.; Thomas, J.A.; Strickland, D.H.; Holt, P.G.; Stumbles, P.A. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 2005, 175, 1609–1618. [Google Scholar] [CrossRef]
- Condon, T.V.; Sawyer, R.T.; Fenton, M.J.; Riches, D.W. Lung dendritic cells at the innate-adaptive immune interface. J. Leukoc. Biol. 2011, 90, 883–895. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Randall, T.D.; Silva-Sanchez, A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front. Immunol. 2016, 7, 258. [Google Scholar] [CrossRef]
- Lysen, A.; Gudjonsson, A.; Tesfaye, D.Y.; Bobic, S.; Bern, M.; Bogen, B.; Fossum, E. Intranasal delivery of a cDC1 targeted influenza vaccine with poly(I:C) enhances T cell responses and protects against influenza infection. Scand. J. Immunol. 2022, 95, e13128. [Google Scholar] [CrossRef]
- Guilliams, M.; Henri, S.; Tamoutounour, S.; Ardouin, L.; Schwartz-Cornil, I.; Dalod, M.; Malissen, B. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur. J. Immunol. 2010, 40, 2089–2094. [Google Scholar] [CrossRef]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Desch, A.N.; Randolph, G.J.; Murphy, K.; Gautier, E.L.; Kedl, R.M.; Lahoud, M.H.; Caminschi, I.; Shortman, K.; Henson, P.M.; Jakubzick, C.V. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 2011, 208, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Dammeijer, F.; Aerts, J.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018, 9, 2804. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.M.; Chan, A.; Rock, K.L. Pathways of MHC I cross-presentation of exogenous antigens. Semin. Immunol. 2023, 66, 101729. [Google Scholar] [CrossRef]
- Raymond, M.; Rubio, M.; Fortin, G.; Shalaby, K.H.; Hammad, H.; Lambrecht, B.N.; Sarfati, M. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of TH2-mediated allergic inflammation. J. Allergy Clin. Immunol. 2009, 124, 1333–1342.E1. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Roy, R.M.; Klein, B.S. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 2012, 11, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. moDCs, Less Problems. Immunity 2018, 48, 6–8. [Google Scholar] [CrossRef]
- Banki, Z.; Werner, R.; Riepler, L.; Rossler, A.; Mullauer, B.; Hegen, V.; Bayer, W.; Verbeek, J.S.; Dittmer, U.; Stoiber, H. Fcgamma Receptor Type I (CD64)-Mediated Impairment of the Capacity of Dendritic Cells to Activate Specific CD8 T Cells by IgG-opsonized Friend Virus. Viruses 2019, 11, 145. [Google Scholar] [CrossRef]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012, 42, 3150–3166. [Google Scholar] [CrossRef]
- Langlet, C.; Tamoutounour, S.; Henri, S.; Luche, H.; Ardouin, L.; Gregoire, C.; Malissen, B.; Guilliams, M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J. Immunol. 2012, 188, 1751–1760. [Google Scholar] [CrossRef]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef]
- Wu, X.; Briseno, C.G.; Durai, V.; Albring, J.C.; Haldar, M.; Bagadia, P.; Kim, K.W.; Randolph, G.J.; Murphy, T.L.; Murphy, K.M. Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J. Exp. Med. 2016, 213, 2553–2565. [Google Scholar] [CrossRef]
- Backer, R.A.; Probst, H.C.; Clausen, B.E. Classical DC2 subsets and monocyte-derived DC: Delineating the developmental and functional relationship. Eur. J. Immunol. 2023, 53, e2149548. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Victora, G.D.; Schwickert, T.A.; Guermonprez, P.; Meredith, M.M.; Yao, K.; Chu, F.F.; Randolph, G.J.; Rudensky, A.Y.; Nussenzweig, M. In vivo analysis of dendritic cell development and homeostasis. Science 2009, 324, 392–397. [Google Scholar] [CrossRef]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.I.; Booth, J.L.; Duggan, E.S.; Cate, S.; White, V.L.; Hutchings, D.; Kovats, S.; Burian, D.M.; Dozmorov, M.; Metcalf, J.P. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J. Immunol. 2017, 198, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A., III; Dutertre, C.A.; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 2021, 21, 101–115. [Google Scholar] [CrossRef]
- Diao, J.; Winter, E.; Cantin, C.; Chen, W.; Xu, L.; Kelvin, D.; Phillips, J.; Cattral, M.S. In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J. Immunol. 2006, 176, 7196–7206. [Google Scholar] [CrossRef]
- Maraskovsky, E.; Brasel, K.; Teepe, M.; Roux, E.R.; Lyman, S.D.; Shortman, K.; McKenna, H.J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: Multiple dendritic cell subpopulations identified. J. Exp. Med. 1996, 184, 1953–1962. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Schraml, B.U.; van Blijswijk, J.; Zelenay, S.; Whitney, P.G.; Filby, A.; Acton, S.E.; Rogers, N.C.; Moncaut, N.; Carvajal, J.J.; Reis e Sousa, C. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 2013, 154, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Boonstra, A.; Paturel, C.; Antonenko, S.; Xu, X.L.; Trinchieri, G.; O’Garra, A.; Liu, Y.J. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002, 195, 953–958. [Google Scholar] [CrossRef]
- Adams, N.M.; Das, A.; Yun, T.J.; Reizis, B. Ontogeny and Function of Plasmacytoid Dendritic Cells. Annu. Rev. Immunol. 2024, 42, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Tirard, A.; Villani, A.C. Plasmacytoid dendritic cells: Welcome back to the DC fold. Immunity 2022, 55, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ohteki, T.; Ginhoux, F.; Shortman, K.; Spits, H. Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature. Nat. Rev. Immunol. 2023, 23, 338–339. [Google Scholar] [CrossRef]
- Nagasawa, M.; Schmidlin, H.; Hazekamp, M.G.; Schotte, R.; Blom, B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur. J. Immunol. 2008, 38, 2389–2400. [Google Scholar] [CrossRef]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Holt, P.G.; Haining, S.; Nelson, D.J.; Sedgwick, J.D. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 1994, 153, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Liu, K.; Helft, J.; Bogunovic, M.; Greter, M.; Hashimoto, D.; Price, J.; Yin, N.; Bromberg, J.; Lira, S.A.; et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009, 206, 3115–3130. [Google Scholar] [CrossRef]
- Segura, E.; Villadangos, J.A. Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 2009, 21, 105–110. [Google Scholar] [CrossRef]
- McGinnis, M.R.; Tyring, S.K. Introduction to Mycology. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Bozza, S.; Gaziano, R.; Spreca, A.; Bacci, A.; Montagnoli, C.; di Francesco, P.; Romani, L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 2002, 168, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N. Spreading the load: Antigen transfer between migratory and lymph node-resident dendritic cells promotes T-cell priming. Eur. J. Immunol. 2017, 47, 1798–1801. [Google Scholar] [CrossRef]
- Xu, J.; Hissong, R.; Bareis, R.; Creech, A.; Goughenour, K.D.; Freeman, C.M.; Olszewski, M.A. Batf3-dependent orchestration of the robust Th1 responses and fungal control during cryptococcal infection, the role of cDC1. mBio 2024, 15, e0285323. [Google Scholar] [CrossRef]
- Guasconi, L.; Beccacece, I.; Volpini, X.; Burstein, V.L.; Mena, C.J.; Silvane, L.; Almeida, M.A.; Musri, M.M.; Cervi, L.; Chiapello, L.S. Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice. J. Fungi 2022, 8, 792. [Google Scholar] [CrossRef]
- Van Prooyen, N.; Henderson, C.A.; Hocking Murray, D.; Sil, A. CD103+ Conventional Dendritic Cells Are Critical for TLR7/9-Dependent Host Defense against Histoplasma capsulatum, an Endemic Fungal Pathogen of Humans. PLoS Pathog. 2016, 12, e1005749. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015, 11, e1004701. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Curtis, J.L.; Polak, T.; Ames, T.; Chen, G.H.; McDonald, R.; Huffnagle, G.B.; Toews, G.B. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J. Immunol. 2008, 181, 610–620. [Google Scholar] [CrossRef]
- Ersland, K.; Wuthrich, M.; Klein, B.S. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe 2010, 7, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kasahara, S.; Jhingran, A.; Tosini, N.L.; Zhai, B.; Aufiero, M.A.; Mills, K.A.M.; Gjonbalaj, M.; Espinosa, V.; Rivera, A.; et al. During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk. Cell Host Microbe 2020, 28, 104–116.E4. [Google Scholar] [CrossRef]
- Trautwein-Weidner, K.; Gladiator, A.; Kirchner, F.R.; Becattini, S.; Rulicke, T.; Sallusto, F.; LeibundGut-Landmann, S. Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis. PLoS Pathog. 2015, 11, e1005164. [Google Scholar] [CrossRef]
- Teitz-Tennenbaum, S.; Viglianti, S.P.; Roussey, J.A.; Levitz, S.M.; Olszewski, M.A.; Osterholzer, J.J. Autocrine IL-10 Signaling Promotes Dendritic Cell Type-2 Activation and Persistence of Murine Cryptococcal Lung Infection. J. Immunol. 2018, 201, 2004–2015. [Google Scholar] [CrossRef]
- Fei, M.; Bhatia, S.; Oriss, T.B.; Yarlagadda, M.; Khare, A.; Akira, S.; Saijo, S.; Iwakura, Y.; Fallert Junecko, B.A.; Reinhart, T.A.; et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5360–5365. [Google Scholar] [CrossRef]
- Huang, S.; Deepe, G.S., Jr. Notch regulates Histoplasma capsulatum clearance in mouse lungs during innate and adaptive immune response phases in primary infection. J. Leukoc. Biol. 2022, 112, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Bacci, A.; Montagnoli, C.; Perruccio, K.; Bozza, S.; Gaziano, R.; Pitzurra, L.; Velardi, A.; d’Ostiani, C.F.; Cutler, J.E.; Romani, L. Dendritic cells pulsed with fungal RNA induce protective immunity to Candida albicans in hematopoietic transplantation. J. Immunol. 2002, 168, 2904–2913. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, C.; Bacci, A.; Bozza, S.; Gaziano, R.; Fiorucci, S.; Spreca, A.; Romani, L. The plasticity of dendritic cells at the host/fungal interface. Immunobiology 2001, 204, 582–589. [Google Scholar] [CrossRef]
- d’Ostiani, C.F.; Del Sero, G.; Bacci, A.; Montagnoli, C.; Spreca, A.; Mencacci, A.; Ricciardi-Castagnoli, P.; Romani, L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000, 191, 1661–1673. [Google Scholar] [CrossRef]
- Mezger, M.; Kneitz, S.; Wozniok, I.; Kurzai, O.; Einsele, H.; Loeffler, J. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 2008, 197, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Hohl, T.M.; Collins, N.; Leiner, I.; Gallegos, A.; Saijo, S.; Coward, J.W.; Iwakura, Y.; Pamer, E.G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 2011, 208, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Karnam, A.; Fontaine, T.; Beauvais, A.; Das, M.; Hegde, P.; Prakhar, P.; Holla, S.; Balaji, K.N.; Kaveri, S.V.; et al. Aspergillus fumigatus Cell Wall alpha-(1,3)-Glucan Stimulates Regulatory T-Cell Polarization by Inducing PD-L1 Expression on Human Dendritic Cells. J. Infect. Dis. 2017, 216, 1281–1294. [Google Scholar] [CrossRef]
- Rivera, A.; Van Epps, H.L.; Hohl, T.M.; Rizzuto, G.; Pamer, E.G. Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect. Immun. 2005, 73, 7170–7179. [Google Scholar] [CrossRef] [PubMed]
- Hohl, T.M.; Van Epps, H.L.; Rivera, A.; Morgan, L.A.; Chen, P.L.; Feldmesser, M.; Pamer, E.G. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005, 1, e30. [Google Scholar] [CrossRef]
- Heung, L.J.; Wiesner, D.L.; Wang, K.; Rivera, A.; Hohl, T.M. Immunity to fungi in the lung. Semin. Immunol. 2023, 66, 101728. [Google Scholar] [CrossRef]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018, 10, 373–397. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, Z.G.; Lee, C.K.; Wang, J.P.; Boon, L.; Specht, C.A.; Levitz, S.M. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 2011, 9, 415–424. [Google Scholar] [CrossRef]
- Loures, F.V.; Rohm, M.; Lee, C.K.; Santos, E.; Wang, J.P.; Specht, C.A.; Calich, V.L.; Urban, C.F.; Levitz, S.M. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog. 2015, 11, e1004643. [Google Scholar] [CrossRef]
- Araujo, E.F.; Medeiros, D.H.; Galdino, N.A.; Condino-Neto, A.; Calich, V.L.; Loures, F.V. Tolerogenic Plasmacytoid Dendritic Cells Control Paracoccidioides brasiliensis Infection by Inducting Regulatory T Cells in an IDO-Dependent Manner. PLoS Pathog. 2016, 12, e1006115. [Google Scholar] [CrossRef]
- Joo, H.; Upchurch, K.; Zhang, W.; Ni, L.; Li, D.; Xue, Y.; Li, X.H.; Hori, T.; Zurawski, S.; Liu, Y.J.; et al. Opposing Roles of Dectin-1 Expressed on Human Plasmacytoid Dendritic Cells and Myeloid Dendritic Cells in Th2 Polarization. J. Immunol. 2015, 195, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Albiston, A.L.; Wicks, I.P.; Chai, S.Y.; Villadangos, J.A. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20377–20381. [Google Scholar] [CrossRef] [PubMed]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976, 143, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Yang, C.W.; Wang, D.W.; Wu-Hsieh, B.A. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J. Immunol. 2005, 174, 6282–6291. [Google Scholar] [CrossRef]
- Carvalho, A.; De Luca, A.; Bozza, S.; Cunha, C.; D’Angelo, C.; Moretti, S.; Perruccio, K.; Iannitti, R.G.; Fallarino, F.; Pierini, A.; et al. TLR3 essentially promotes protective class I-restricted memory CD8+ T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 2012, 119, 967–977. [Google Scholar] [CrossRef]
- Backer, R.; van Leeuwen, F.; Kraal, G.; den Haan, J.M. CD8- dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur. J. Immunol. 2008, 38, 370–380. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, A.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Yamagishi, Y.; Ota, S. Phase I/II Pilot Study of Wilms’ Tumor 1 Peptide-Pulsed Dendritic Cell Vaccination Combined with Conventional Chemotherapy in Patients with Head and Neck Cancer. Ther. Apher. Dial. 2019, 23, 279–288. [Google Scholar] [CrossRef]
- Tanyi, J.L.; George, E. Personalized vaccination against ovarian cancer: What are the possibilities? Expert. Rev. Vaccines 2018, 17, 955–958. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Avivi, I.; Vasir, B.; Uhl, L.; Munshi, N.C.; Katz, T.; Dey, B.R.; Somaiya, P.; Mills, H.; Campigotto, F.; et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin. Cancer Res. 2013, 19, 3640–3648. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef]
- Tel, J.; Aarntzen, E.H.; Baba, T.; Schreibelt, G.; Schulte, B.M.; Benitez-Ribas, D.; Boerman, O.C.; Croockewit, S.; Oyen, W.J.; van Rossum, M.; et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013, 73, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.M.; Moreira, A.L.E.; Cruz-Leite, V.R.M.; Mattos, K.; Silva, L.O.S.; Venturini, J.; Ruiz, O.H.; Ribeiro-Dias, F.; Weber, S.S.; Soares, C.M.A.; et al. Fungal Vaccine Development: State of the Art and Perspectives Using Immunoinformatics. J. Fungi 2023, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; Zeelenberg, I.S.; Cruz, L.J.; van Hout-Kuijer, M.A.; van de Glind, G.; Fokkink, R.G.; Lambeck, A.J.; Figdor, C.G. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011, 118, 6836–6844. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Huang, H.; Levitz, S.M. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS ONE 2007, 2, e1009. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef]
- Carter, R.W.; Thompson, C.; Reid, D.M.; Wong, S.Y.; Tough, D.F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 2006, 177, 2276–2284. [Google Scholar] [CrossRef]
- Dutta, O.; Masso-Silva, J.A.; Wang, K.; Rivera, A. Host response to pulmonary fungal infections: A highlight on cell-driven immunity to Cryptococcus species and Aspergillus fumigatus. Curr. Pharmacol. Rep. 2017, 3, 335–345. [Google Scholar] [CrossRef]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R.; Clavaud, C.; Paris, S.; Brakhage, A.A.; Kaveri, S.V.; et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Lodge, J.; Xue, C. Harnessing the Immune Response to Fungal Pathogens for Vaccine Development. Annu. Rev. Microbiol. 2022, 76, 703–726. [Google Scholar] [CrossRef]
- Oliveira, L.V.N.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for human fungal diseases: Close but still a long way to go. NPJ Vaccines 2021, 6, 33. [Google Scholar] [CrossRef]
- Bozza, S.; Gaziano, R.; Lipford, G.B.; Montagnoli, C.; Bacci, A.; Di Francesco, P.; Kurup, V.P.; Wagner, H.; Romani, L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002, 4, 1281–1290. [Google Scholar] [CrossRef]
- Bozza, S.; Perruccio, K.; Montagnoli, C.; Gaziano, R.; Bellocchio, S.; Burchielli, E.; Nkwanyuo, G.; Pitzurra, L.; Velardi, A.; Romani, L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 2003, 102, 3807–3814. [Google Scholar] [CrossRef]
- Shao, C.; Qu, J.; He, L.; Zhang, Y.; Wang, J.; Zhou, H.; Wang, Y.; Liu, X. Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes. Immun. 2005, 6, 103–114. [Google Scholar] [CrossRef]
- Perruccio, K.; Bozza, S.; Montagnoli, C.; Bellocchio, S.; Aversa, F.; Martelli, M.; Bistoni, F.; Velardi, A.; Romani, L. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol. Dis. 2004, 33, 248–255. [Google Scholar] [CrossRef]
- Awasthi, S. Dendritic cell-based vaccine against coccidioides infection. Ann. N. Y. Acad. Sci. 2007, 1111, 269–274. [Google Scholar] [CrossRef]
- Chechi, J.L.; da Costa, F.A.C.; Figueiredo, J.M.; de Souza, C.M.; Valdez, A.F.; Zamith-Miranda, D.; Camara, A.C.; Taborda, C.P.; Nosanchuk, J.D. Vaccine development for pathogenic fungi: Current status and future directions. Expert. Rev. Vaccines 2023, 22, 1136–1153. [Google Scholar] [CrossRef]
- Zou, G.; Wei, Y. World Health Organization’s first-ever release of a fungal priority pathogens list: A reply action proposal for the prevention and treatment of fungal pathogens. Eco-Environ. Health 2023, 2, 43–44. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.Y. Enlightenment of World Health Organization fungal priority pathogens list. Zhonghua Liu Xing Bing Xue Za Zhi 2023, 44, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Sondermeyer, G.L.; Lee, L.A.; Gilliss, D.; Vugia, D.J. Coccidioidomycosis-Associated Deaths in California, 2000–2013. Public Health Rep. 2016, 131, 531–535. [Google Scholar] [CrossRef]
- Loh, J.T.; Lam, K.P. Fungal infections: Immune defense, immunotherapies and vaccines. Adv. Drug Deliv. Rev. 2023, 196, 114775. [Google Scholar] [CrossRef] [PubMed]
- Peyclit, L.; Yousfi, H.; Rolain, J.M.; Bittar, F. Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals 2021, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [CrossRef]
- Awasthi, S.; Awasthi, V.; Magee, D.M.; Coalson, J.J. Efficacy of antigen 2/proline-rich antigen cDNA-transfected dendritic cells in immunization of mice against Coccidioides posadasii. J. Immunol. 2005, 175, 3900–3906. [Google Scholar] [CrossRef]
- Awasthi, S.; Vilekar, P.; Conkleton, A.; Rahman, N. Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response. Vaccine 2019, 37, 1685–1691. [Google Scholar] [CrossRef]
- Awasthi, S. Intranasal Antifungal Vaccination Using DNA-Transfected Dendritic Cells. Methods Mol. Biol. 2017, 1625, 75–83. [Google Scholar] [CrossRef]
- Vilekar, P.; Awasthi, V.; Lagisetty, P.; King, C.; Shankar, N.; Awasthi, S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunol. 2010, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.B.R.; Taborda, C.P.; Nosanchuk, J.D. Advances in Fungal Peptide Vaccines. J. Fungi 2020, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: A review from 1996 to 2006. Memórias Do Inst. Oswaldo Cruz 2009, 104, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Bastos, K.R.; Russo, M.; Almeida, S.R. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and toll-like receptor-2 expression: Possible mechanisms of susceptibility. J. Infect. Dis. 2007, 196, 1108–1115. [Google Scholar] [CrossRef][Green Version]
- Silva, L.B.R.; Taira, C.L.; Cleare, L.G.; Martins, M.; Junqueira, M.; Nosanchuk, J.D.; Taborda, C.P. Identification of Potentially Therapeutic Immunogenic Peptides from Paracoccidioides lutzii Species. Front. Immunol. 2021, 12, 670992. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. Linear Epitopes of Paracoccidioides brasiliensis and Other Fungal Agents of Human Systemic Mycoses As Vaccine Candidates. Front. Immunol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Jannuzzi, G.P.; de Almeida, J.R.F.; Dos Santos, S.S.; de Almeida, S.R.; Ferreira, K.S. Notch Signaling is Required for Dendritic Cell Maturation and T Cell Expansion in Paracoccidioidomycosis. Mycopathologia 2018, 183, 739–749. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. New advances in the development of a vaccine against paracoccidioidomycosis. Front. Microbiol. 2012, 3, 212. [Google Scholar] [CrossRef]
- Magalhaes, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Silva, L.B.R.; Taira, C.L.; Dias, L.S.; Souza, A.C.O.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Experimental Therapy of Paracoccidioidomycosis Using P10-Primed Monocyte-Derived Dendritic Cells Isolated From Infected Mice. Front. Microbiol. 2019, 10, 1727. [Google Scholar] [CrossRef]
- Silva, L.B.R.; Dias, L.S.; Rittner, G.M.G.; Munoz, J.E.; Souza, A.C.O.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Dendritic Cells Primed with Paracoccidioides brasiliensis Peptide P10 Are Therapeutic in Immunosuppressed Mice with Paracoccidioidomycosis. Front. Microbiol. 2017, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, J.; Escandon, P.; Agudelo, C.I.; Firacative, C.; Meyer, W.; Castaneda, E. Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997–2011. PLoS Negl. Trop. Dis. 2014, 8, e3272. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Emergence of Cryptococcus gattii—Pacific Northwest, 2004–2010. Morb. Mortal. Wkly. Rep. (MMWR) 2010, 59, 865–868. [Google Scholar]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Smith, R.M.; Mba-Jonas, A.; Tourdjman, M.; Schimek, T.; DeBess, E.; Marsden-Haug, N.; Harris, J.R. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS ONE 2014, 9, e88875. [Google Scholar] [CrossRef]
- Ueno, K.; Kinjo, Y.; Okubo, Y.; Aki, K.; Urai, M.; Kaneko, Y.; Shimizu, K.; Wang, D.N.; Okawara, A.; Nara, T.; et al. Dendritic cell-based immunization ameliorates pulmonary infection with highly virulent Cryptococcus gattii. Infect. Immun. 2015, 83, 1577–1586. [Google Scholar] [CrossRef]
- Goughenour, K.D.; Nair, A.S.; Xu, J.; Olszewski, M.A.; Wozniak, K.L. Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections. J. Fungi 2023, 9, 1050. [Google Scholar] [CrossRef]
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 2019, 10, 2955. [Google Scholar] [CrossRef]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed. Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef]
- Caballero Van Dyke, M.C.; Wormley, F.L., Jr. A Call to Arms: Quest for a Cryptococcal Vaccine. Trends Microbiol. 2018, 26, 436–446. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Ravi, S.; Macias, S.; Young, M.L.; Olszewski, M.A.; Steele, C.; Wormley, F.L. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS ONE 2009, 4, e6854. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Urai, M.; Sadamoto, S.; Shinozaki, M.; Takatsuka, S.; Abe, M.; Otani, Y.; Yanagihara, N.; Shimizu, K.; Iwakura, Y.; et al. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal Immunol. 2019, 12, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Chaze, T.; Miranda, K.; Roberson, R.W.; Gorgette, O.; Nimrichter, L.; Matondo, M.; Latge, J.P.; Beauvais, A.; Rodrigues, M.L. Characterization of Extracellular Vesicles Produced by Aspergillus fumigatus Protoplasts. mSphere 2020, 5, e00476-20. [Google Scholar] [CrossRef]
- Marina, C.L.; Burgel, P.H.; Agostinho, D.P.; Zamith-Miranda, D.; Las-Casas, L.O.; Tavares, A.H.; Nosanchuk, J.D.; Bocca, A.L. Nutritional Conditions Modulate C. neoformans Extracellular Vesicles’ Capacity to Elicit Host Immune Response. Microorganisms 2020, 8, 1815. [Google Scholar] [CrossRef] [PubMed]
- Joffe, L.S.; Nimrichter, L.; Rodrigues, M.L.; Del Poeta, M. Potential Roles of Fungal Extracellular Vesicles during Infection. mSphere 2016, 1, e00099-16. [Google Scholar] [CrossRef]
- Sandbrink, J.B.; Shattock, R.J. RNA Vaccines: A Suitable Platform for Tackling Emerging Pandemics? Front. Immunol. 2020, 11, 608460. [Google Scholar] [CrossRef]
- Oda, Y.; Kumagai, Y.; Kanai, M.; Iwama, Y.; Okura, I.; Minamida, T.; Yagi, Y.; Kurosawa, T.; Greener, B.; Zhang, Y.; et al. Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: A double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Infect. Dis. 2024, 24, 351–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).