Impact of Oil-in-Water Adjuvanted β-Glucan on Innate Immune Memory in Piglets
Abstract
1. Introduction
2. Material and Methods
2.1. In Vitro Assessment of the Immunomodulatory Effects of Various β-Glucans
2.2. Ethics and Animal Experimental Conditions
2.3. Treatment Groups
2.4. Blood Sample Preparation
2.5. Cytokine Measurements
2.6. Antibody Measurements
2.7. Bulk-RNA Sequencing and Analysis
2.8. Statistical Analysis
3. Results
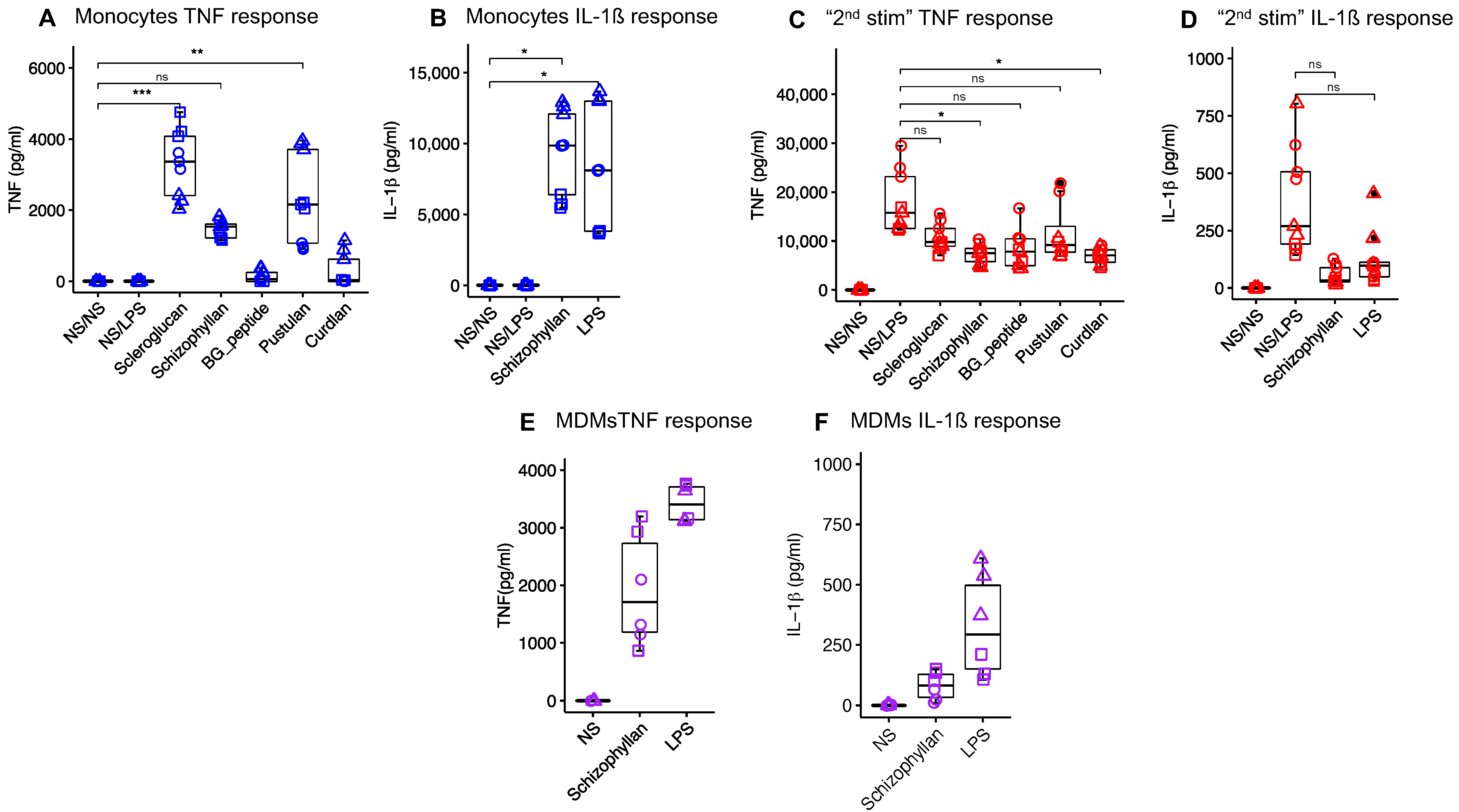
3.1. In Vitro Selection of Immunostimulatory β-Glucans
3.2. Safety Parameters
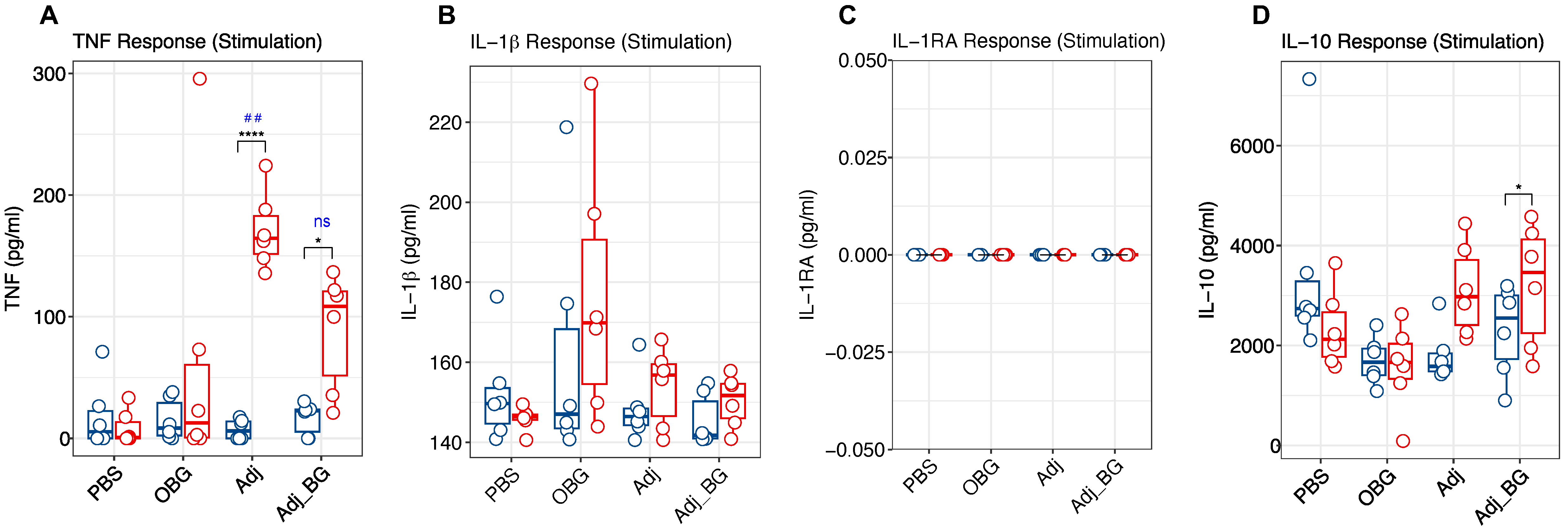
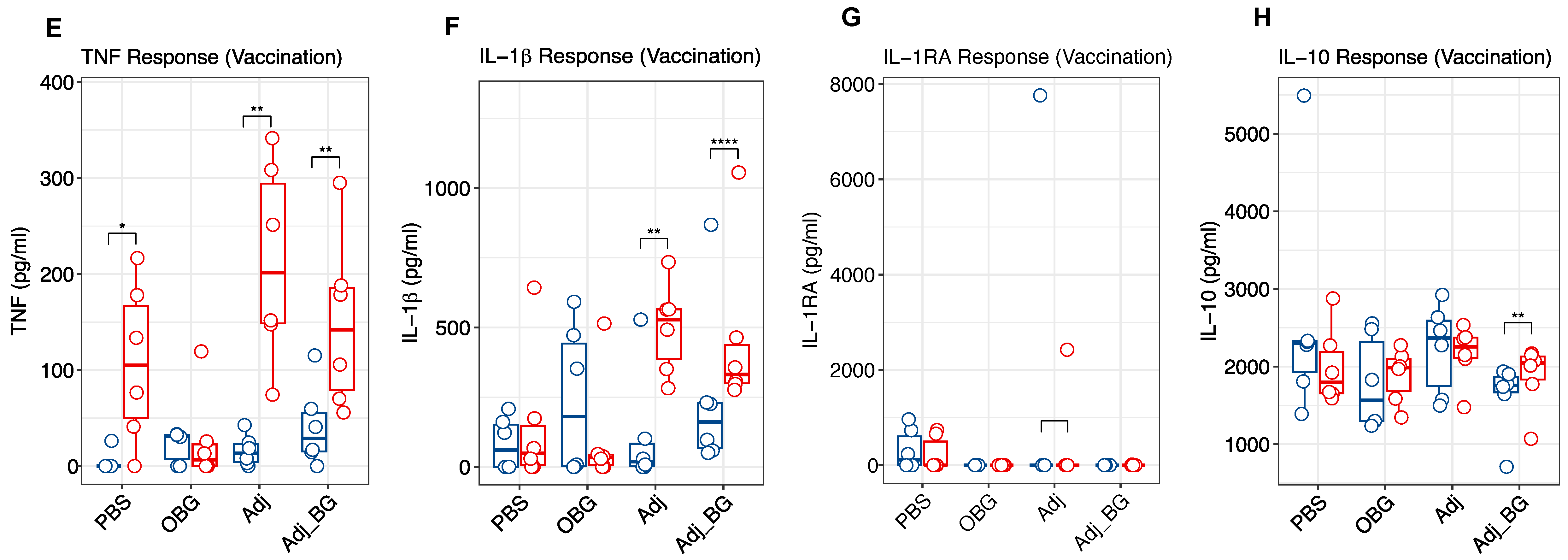
3.3. Cytokine and Antibody Profiles
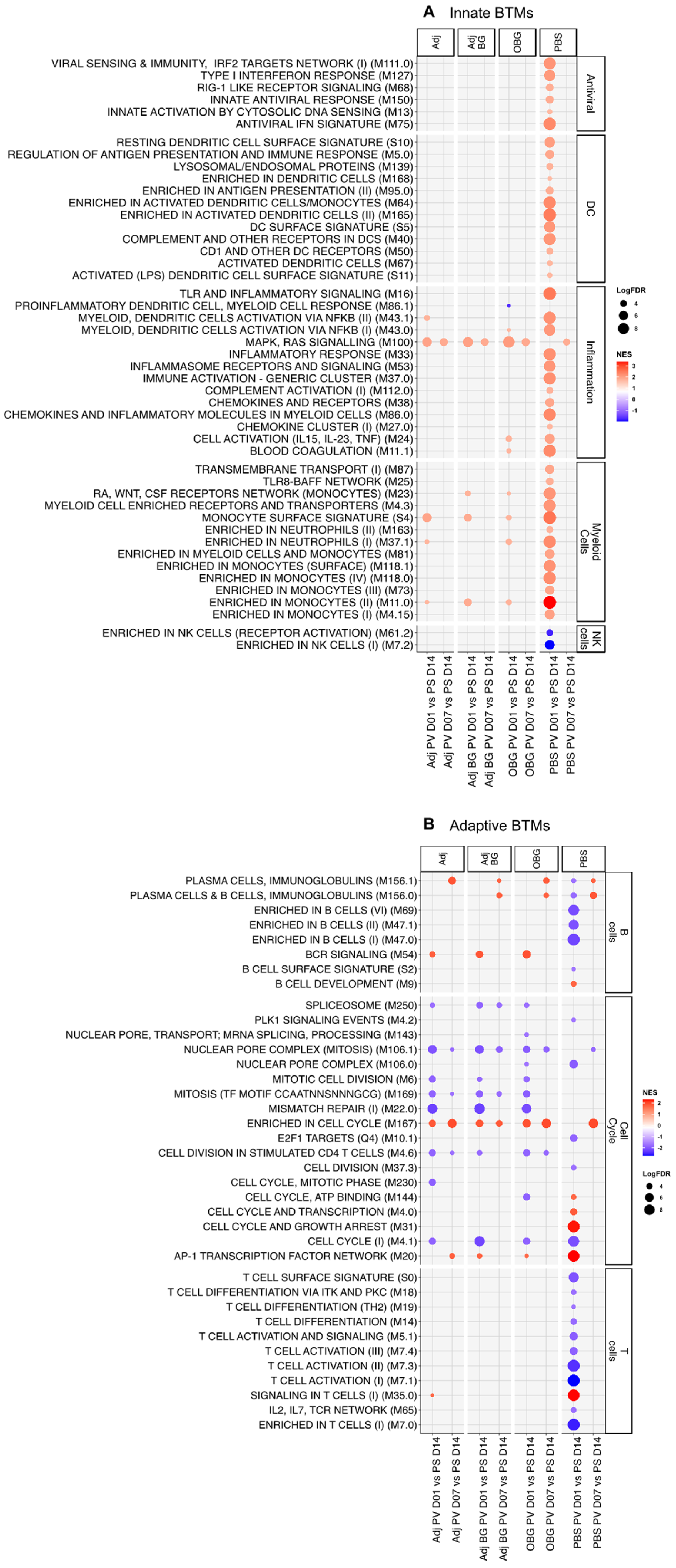
3.4. Transcriptomic Profile of Leukocytes Post-Stimulation
3.5. Transcriptomic Profile of Leukocytes Post-Vaccination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PrabhuDas, M.; Adkins, B.; Gans, H.; King, C.; Levy, O.; Ramilo, O.; Siegrist, C.-A. Challenges in infant immunity: Implications for responses to infection and vaccines. Nat. Immunol. 2011, 12, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Troy, N.M.; Strickland, D.; Serralha, M.; de Jong, E.; Jones, A.C.; Read, J.; Galbraith, S.; Islam, Z.; Kaur, P.; Mincham, K.T. Protection against severe infant lower respiratory tract infections by immune training: Mechanistic studies. J. Allergy Clin. Immunol. 2022, 150, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Malinczak, C.-A.; Lukacs, N.W.; Fonseca, W. Early-life respiratory syncytial virus infection, trained immunity and subsequent pulmonary diseases. Viruses 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Wynn, J.L. A prime time for trained immunity: Innate immune memory in newborns and infants. Neonatology 2014, 105, 136–141. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Burelbach, K.R.; McBride, M.A.; Stothers, C.L.; Owen, A.M.; Hernandez, A.; Patil, N.K.; Williams, D.L.; Bohannon, J.K. Innate immune memory and the host response to infection. J. Immunol. 2022, 208, 785–792. [Google Scholar] [CrossRef]
- Prentice, S.; Nassanga, B.; Webb, E.L.; Akello, F.; Kiwudhu, F.; Akurut, H.; Elliott, A.M.; Arts, R.J.; Netea, M.G.; Dockrell, H.M.; et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: An investigator-blind randomised controlled trial. Lancet Infect. Dis. 2021, 21, 993–1003. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.-C. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Moorlag, S.J.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [PubMed]
- Bannister, S.; Kim, B.; Domínguez-Andrés, J.; Kilic, G.; Ansell, B.R.; Neeland, M.R.; Moorlag, S.J.; Matzaraki, V.; Vlahos, A.; Shepherd, R. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci. Adv. 2022, 8, eabn4002. [Google Scholar] [CrossRef]
- Kazmin, D.; Clutterbuck, E.A.; Napolitani, G.; Wilkins, A.L.; Tarlton, A.; Thompson, A.J.; Montomoli, E.; Lapini, G.; Bihari, S.; White, R. Memory-like innate response to booster vaccination with MF-59 adjuvanted influenza vaccine in children. npj Vaccines 2023, 8, 100. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J. Induction of trained immunity by influenza vaccination-impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Katzmarski, N.; Domínguez-Andrés, J.; Cirovic, B.; Renieris, G.; Ciarlo, E.; Le Roy, D.; Lepikhov, K.; Kattler, K.; Gasparoni, G.; Händler, K. Transmission of trained immunity and heterologous resistance to infections across generations. Nat. Immunol. 2021, 22, 1382–1390. [Google Scholar] [CrossRef]
- Wynn, J.L.; Scumpia, P.O.; Winfield, R.D.; Delano, M.J.; Kelly-Scumpia, K.; Barker, T.; Ungaro, R.; Levy, O.; Moldawer, L.L. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood J. Am. Soc. Hematol. 2008, 112, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Moorlag, S.J.; Khan, N.; Novakovic, B.; Kaufmann, E.; Jansen, T.; van Crevel, R.; Divangahi, M.; Netea, M.G. β-Glucan induces protective trained immunity against Mycobacterium tuberculosis infection: A key role for IL-1. Cell Rep. 2020, 31, 107634. [Google Scholar] [CrossRef]
- Paris, S.; Chapat, L.; Martin-Cagnon, N.; Poulet, H.; Freyburger, L.; De Luca, K. β-glucan as trained immunity-based adjuvants for rabies vaccines in dogs. Front. Immunol. 2020, 11, 564497. [Google Scholar] [CrossRef]
- Darroch, H.; Astin, J.W.; Hall, C.J. Towards a new model of trained immunity: Exposure to bacteria and β-glucan protects larval zebrafish against subsequent infections. Dev. Comp. Immunol. 2022, 132, 104400. [Google Scholar] [CrossRef] [PubMed]
- Ciarlo, E.; Heinonen, T.; Théroude, C.; Asgari, F.; Le Roy, D.; Netea, M.G.; Roger, T. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. 2020, 222, 1869–1881. [Google Scholar] [CrossRef]
- Ochando, J.; Mulder, W.J.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity—Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef]
- Chethan, G.E.; Garkhal, J.; Sircar, S.; Malik, Y.P.S.; Mukherjee, R.; Sahoo, N.R.; Agarwal, R.K.; De, U.K. Immunomodulatory potential of β-glucan as supportive treatment in porcine rotavirus enteritis. Vet. Immunol. Immunopathol. 2017, 191, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Amorim, A.B.; Grecco, H.A.; Berto, D.A.; Padovani, C.R.; Orsi, R.d.O.; Marcos, L. Effects of β-(1→ 3, 1→ 6)-d-glucan and density of diets on the blood profiles of immunologically challenged weaned piglets. Int. J. Biol. Macromol. 2015, 80, 659–667. [Google Scholar] [CrossRef]
- Stuyven, E.; Cox, E.; Vancaeneghem, S.; Arnouts, S.; Deprez, P.; Goddeeris, B. Effect of β-glucans on an ETEC infection in piglets. Vet. Immunol. Immunopathol. 2009, 128, 60–66. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.; Wang, R.; Qin, L.; Peng, X.; Hu, L.; Liu, Y.; Fang, Z.; Lin, Y.; Xu, S. Effects of dietary β-glucan supplementation on growth performance and immunological and metabolic parameters of weaned pigs administered with Escherichia coli lipopolysaccharide. Food Funct. 2018, 9, 3338–3343. [Google Scholar] [CrossRef]
- Sautter, C.A.; Auray, G.; Python, S.; Liniger, M.; Summerfield, A. Phenotypic and functional modulations of porcine macrophages by interferons and interleukin-4. Dev. Comp. Immunol. 2018, 84, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Scalisi, N.; Kuhnert, P.; Amado, M.E.V.; Overesch, G.; Stärk, K.D.; Ruggli, N.; Jores, J. Seroprevalence of Mycoplasma hyopneumoniae in sows fifteen years after implementation of a control programme for enzootic pneumonia in Switzerland. Vet. Microbiol. 2022, 270, 109455. [Google Scholar] [CrossRef]
- Love, M.I.; Anders, S.; Kim, V.; Huber, W. RNA-Seq workflow: Gene-level exploratory analysis and differential expression. F1000Research 2015, 4, 1070. [Google Scholar] [CrossRef]
- Matthijs, A.M.; Auray, G.; Jakob, V.; García-Nicolás, O.; Braun, R.O.; Bruggman, R.; Devriendt, B.; Boyen, F.; Guzman, C.A.; Haesebrouck, F. Systems immunology characterization of novel vaccine formulations for Mycoplasma hyopneumoniae bacterins. Front. Immunol. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Braun, R.O.; Brunner, L.; Wyler, K.; Auray, G.; García-Nicolás, O.; Python, S.; Zumkehr, B.; Gaschen, V.; Stoffel, M.H.; Collin, N. System immunology-based identification of blood transcriptional modules correlating to antibody responses in sheep. npj Vaccines 2018, 3, 41. [Google Scholar] [CrossRef]
- Bocard, L.V.; Kick, A.R.; Hug, C.; Lischer, H.E.L.; Käser, T.; Summerfield, A. Systems immunology analyses following porcine respiratory and reproductive syndrome virus infection and vaccination. Front. Immunol. 2021, 12, 779747. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Rouphael, N.; Duraisingham, S.; Romero-Steiner, S.; Presnell, S.; Davis, C.; Schmidt, D.S.; Johnson, S.E.; Milton, A.; Rajam, G. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 2014, 15, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, E.; Mehinagic, K.; Wüthrich, T.; Hilty, M.; Posthaus, H.; Summerfield, A.; Ruggli, N.; Benarafa, C. The baseline immunological and hygienic status of pigs impact disease severity of African swine fever. PLoS Pathog. 2022, 18, e1010522. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.; Majhen, D.; Van Der Ley, P.; Thakur, A.; Summerfield, A.; Berisio, R.; Nativi, C.; Fernández-Tejada, A.; Alvarez-Dominguez, C.; Gizurarson, S. Rational vaccine design in times of emerging diseases: The critical choices of immunological correlates of protection, vaccine antigen and immunomodulation. Pharmaceutics 2021, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.-S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Scott, M.K.; Wimmers, F.; Arunachalam, P.S.; Luo, W.; Fox, C.B.; Tomai, M.; Khatri, P.; Pulendran, B. A molecular atlas of innate immunity to adjuvanted and live attenuated vaccines, in mice. Nat. Commun. 2022, 13, 549. [Google Scholar] [CrossRef]
- Fourati, S.; Tomalin, L.E.; Mulè, M.P.; Chawla, D.G.; Gerritsen, B.; Rychkov, D.; Henrich, E.; Miller, H.E.; Hagan, T.; Diray-Arce, J. Pan-vaccine analysis reveals innate immune endotypes predictive of antibody responses to vaccination. Nat. Immunol. 2022, 23, 1777–1787. [Google Scholar] [CrossRef]
- Borriello, F.; Poli, V.; Shrock, E.; Spreafico, R.; Liu, X.; Pishesha, N.; Carpenet, C.; Chou, J.; Di Gioia, M.; McGrath, M.E. An adjuvant strategy enabled by modulation of the physical properties of microbial ligands expands antigen immunogenicity. Cell 2022, 185, 614–629.e21. [Google Scholar] [CrossRef]
- Olafsdottir, T.A.; Lindqvist, M.; Nookaew, I.; Andersen, P.; Maertzdorf, J.; Persson, J.; Christensen, D.; Zhang, Y.; Anderson, J.; Khoomrung, S. Comparative systems analyses reveal molecular signatures of clinically tested vaccine adjuvants. Sci. Rep. 2016, 6, 39097. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.L.; Yang, C.-F.; Liu, Z.; Sweetwood, R.; Zhao, J.; Cheng, L.; Jin, H.; Woo, J. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS ONE 2012, 7, e51618. [Google Scholar] [CrossRef]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; Donato, M.; Kuo, A.; Ashuach, T.; Gupta, S.; Li, C.; Dvorak, M.; Foecke, M.H.; Chang, S.E.; Hagan, T. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 2021, 184, 3915–3935.e21. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Pettini, E.; Kazmin, D.; Ciabattini, A.; Fiorino, F.; Gilfillan, G.D.; Evenroed, I.M.; Andersen, P.; Pozzi, G.; Medaglini, D. Transcriptomics of the vaccine immune response: Priming with adjuvant modulates recall innate responses after boosting. Front. Immunol. 2018, 9, 344280. [Google Scholar] [CrossRef] [PubMed]
- Feraoun, Y.; Tchitchek, N.; Dereuddre-Bosquet, N.; Contreras, V.; Martinon, F.; Le Grand, R.; Beignon, A.-S. The route of vaccine administration determines whether blood neutrophils undergo long-term phenotypic modifications. Front. Immunol. 2022, 12, 784813. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, D.C.; Quintin, J.; Joosten, L.A.; Jacobs, C.; Jansen, T.; Jacobs, L.; Gow, N.A.; Williams, D.L.; Van Der Meer, J.W.; Netea, M.G. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vaccine Immunol. 2014, 21, 534–545. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin effects, a β-(1, 3)-glucan, on skin cell inflammation and oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Byrne, K.A.; Tuggle, C.K.; Loving, C.L. Differential induction of innate memory in porcine monocytes by β-glucan or bacillus Calmette-Guerin. Innate Immun. 2021, 27, 448–460. [Google Scholar] [CrossRef]
- Benn, C.S.; Fisker, A.B.; Rieckmann, A.; Sørup, S.; Aaby, P. Vaccinology: Time to change the paradigm? Lancet Infect. Dis. 2020, 20, e274–e283. [Google Scholar] [CrossRef]




| Score | Description | |
|---|---|---|
| 0 | Normal | no more than a visible injection site less than about 0.5 diameter zone of redness surrounding injection site |
| 1 | Mild | 0.5–2 cm diameter discoloration no distinct palpable swelling may be irritation (occasional rubbing at injection site) |
| 2 | Moderate | 2–5 cm diameter discoloration (or) palpable swelling may be irritation (persistent rubbing at injection site) |
| 3 | Sever | >5 cm diameter discoloration (and) visible and palpable swelling irritation and pain (persistent rubbing at injection site, withdrawal and vocalization upon palpation may be abscess, exudate |
| Treatment | PBS | Adj | Adj_BG | OBG |
|---|---|---|---|---|
| Post-stimulation | ||||
| D1_PS | 978 | 1045 | 1376 | 2617 |
| D3_PS | 703 | 6034 | 3771 | 3263 |
| D7_PS | 5939 | 5057 | 4149 | 4153 |
| D14_PS | 5008 | 5412 | 3891 | 4001 |
| Post-vaccination | ||||
| D1_PV | 1471 | 5119 | 4377 | 5238 |
| D7_PV | 3934 | 3972 | 3650 | 4222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardali, R.; Garcia-Nicolas, O.; Ollagnier, C.; Sánchez Carvajal, J.M.; Levy, M.; Yvernault, P.; de Aboim Borges Fialho de Brito, F.; Summerfield, A. Impact of Oil-in-Water Adjuvanted β-Glucan on Innate Immune Memory in Piglets. Vaccines 2024, 12, 982. https://doi.org/10.3390/vaccines12090982
Ardali R, Garcia-Nicolas O, Ollagnier C, Sánchez Carvajal JM, Levy M, Yvernault P, de Aboim Borges Fialho de Brito F, Summerfield A. Impact of Oil-in-Water Adjuvanted β-Glucan on Innate Immune Memory in Piglets. Vaccines. 2024; 12(9):982. https://doi.org/10.3390/vaccines12090982
Chicago/Turabian StyleArdali, Razieh, Obdulio Garcia-Nicolas, Catherine Ollagnier, José María Sánchez Carvajal, Maria Levy, Pauline Yvernault, Francisco de Aboim Borges Fialho de Brito, and Artur Summerfield. 2024. "Impact of Oil-in-Water Adjuvanted β-Glucan on Innate Immune Memory in Piglets" Vaccines 12, no. 9: 982. https://doi.org/10.3390/vaccines12090982
APA StyleArdali, R., Garcia-Nicolas, O., Ollagnier, C., Sánchez Carvajal, J. M., Levy, M., Yvernault, P., de Aboim Borges Fialho de Brito, F., & Summerfield, A. (2024). Impact of Oil-in-Water Adjuvanted β-Glucan on Innate Immune Memory in Piglets. Vaccines, 12(9), 982. https://doi.org/10.3390/vaccines12090982






