Immune Cell Profiles of Patients with Sickle Cell Disease during Parvovirus B19–Induced Transient Red Cell Aplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Selection and Data Collection
2.2. Total Serum Immunoglobulin Measurements
2.3. Cytokine/Chemokine Measurements
2.4. Flow Cytometry
2.5. Statistical Analyses
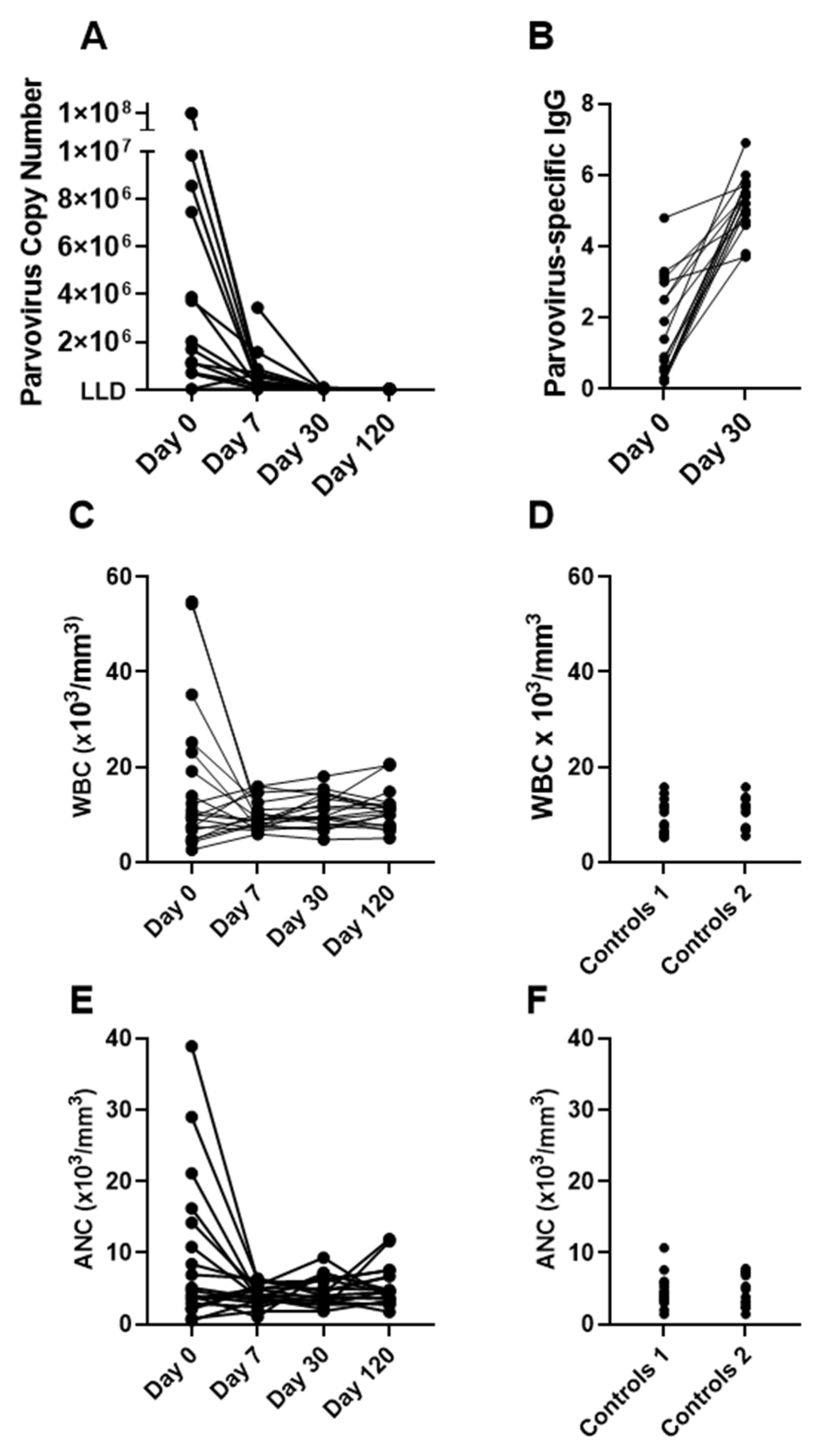
3. Results
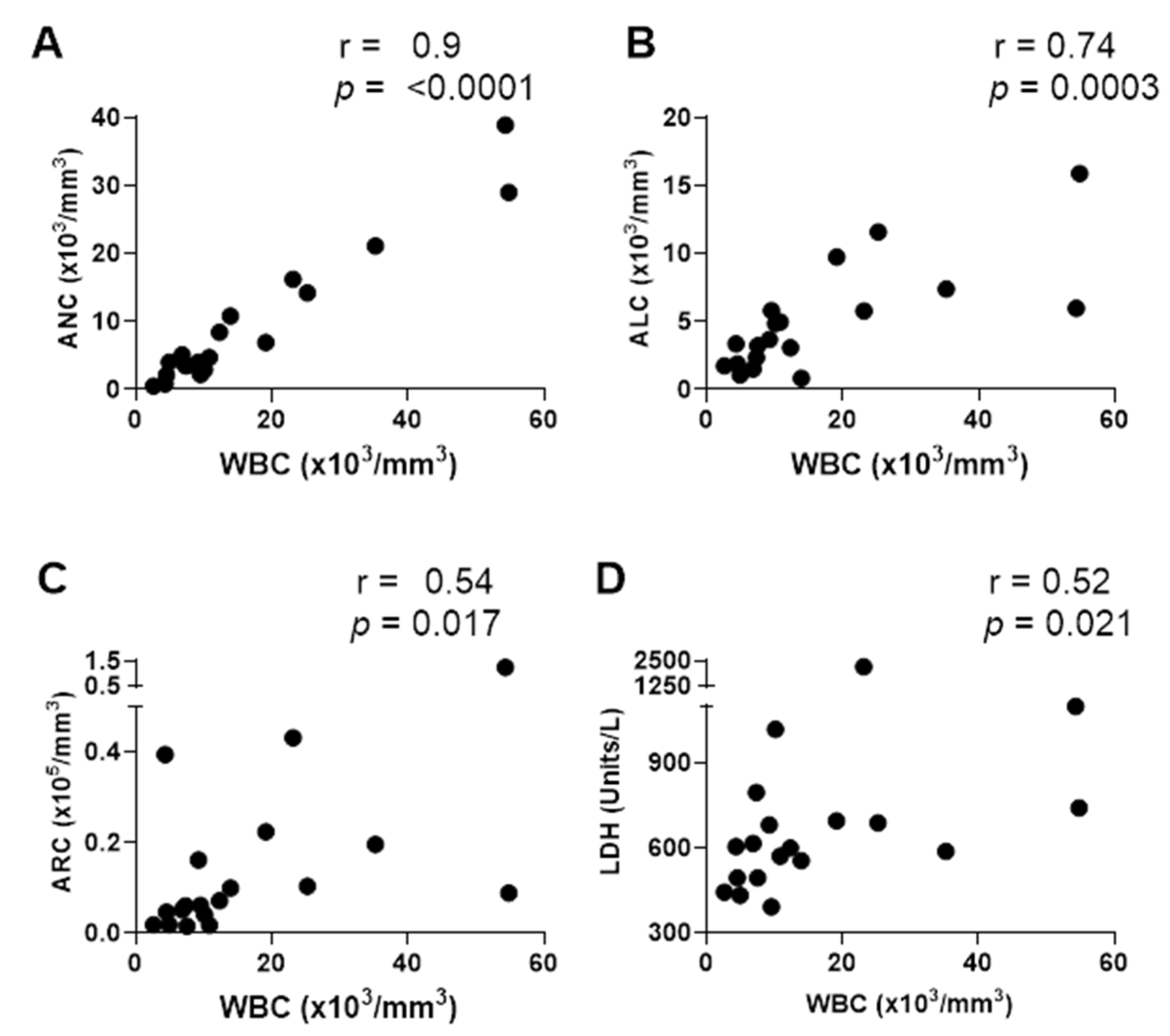
3.1. Cases of Elevated WBC and ANC upon Hospitalization of Patients with SCD and Parvovirus B19 Infections
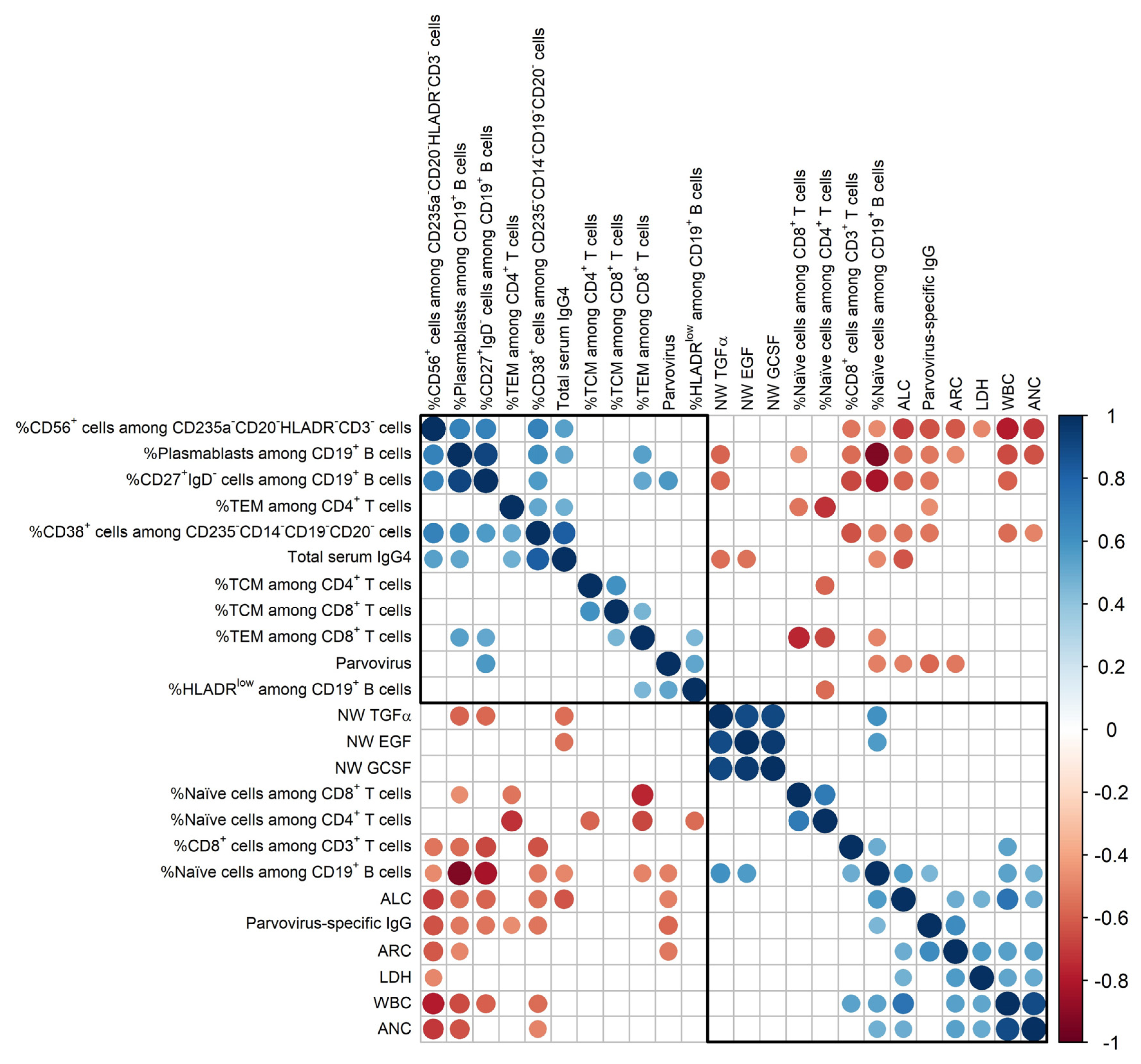
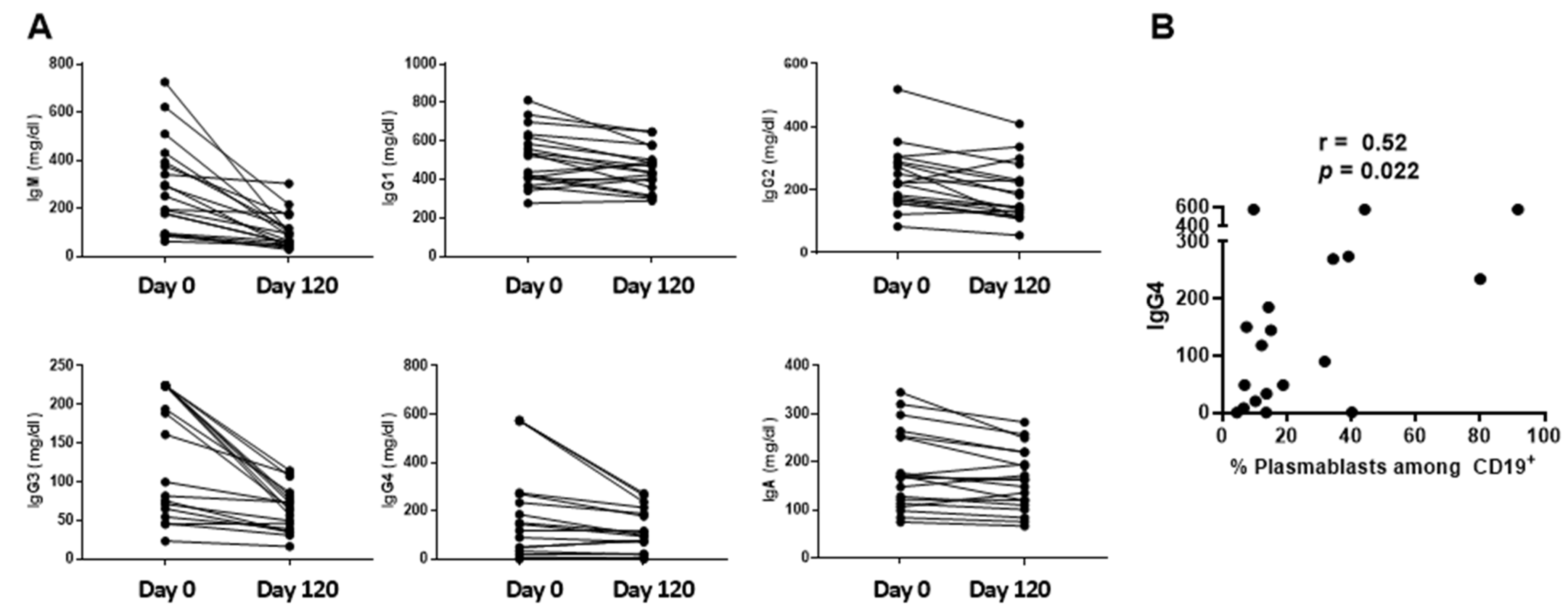
3.2. Two Mutually Exclusive Phenotype Clusters on Day 0
4. Discussion
4.1. Immunophenotypes on Day 0
4.2. Might Acute Immune Abnormalities Following Parvovirus B19 Infections Exacerbate Chronic Inflammatory Disease in Patients with SCD?
4.3. Study Limitations
4.4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Young, N.S.; Brown, K.E. Parvovirus B19. N. Engl. J. Med. 2004, 350, 586–597. [Google Scholar] [CrossRef]
- Kelleher, J.F., Jr.; Luban, N.L.; Cohen, B.J.; Mortimer, P.P. Human serum parvovirus as the cause of aplastic crisis in sickle cell disease. Am. J. Dis. Child. 1984, 138, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Miller, S.T.; Cohen, B.J. Transient aplastic crisis in patients with sickle cell disease. B19 parvovirus studies during a 7-year period. Am. J. Dis. Child. 1992, 146, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Hankins, J.S.; Penkert, R.R.; Lavoie, P.; Tang, L.; Sun, Y.; Hurwitz, J.L. Original Research: Parvovirus B19 infection in children with sickle cell disease in the hydroxyurea era. Exp. Biol. Med. 2016, 241, 749–754. [Google Scholar] [CrossRef]
- Krishnamurti, L.; Lanford, L.; Munoz, R. Life threatening parvovirus B19 and herpes simplex virus associated acute myocardial dysfunction in a child with homozygous sickle cell disease. Pediatr. Blood Cancer 2007, 49, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Rayburg, M.; Kalinyak, K.A.; Towbin, A.J.; Baker, P.B.; Joiner, C.H. Fatal bone marrow embolism in a child with hemoglobin SE disease. Am. J. Hematol. 2010, 85, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Balkaran, B.; Char, G.; Morris, J.S.; Thomas, P.W.; Serjeant, B.E.; Serjeant, G.R. Stroke in a cohort of patients with homozygous sickle cell disease. J. Pediatr. 1992, 120, 360–366. [Google Scholar] [CrossRef]
- Kurtzman, G.J.; Gascon, P.; Caras, M.; Cohen, B.; Young, N.S. B19 parvovirus replicates in circulating cells of acutely infected patients. Blood 1988, 71, 1448–1454. [Google Scholar] [CrossRef]
- Li, W.; Pucka, A.Q.; Debats, C.; Reyes, B.A.; Syed, F.; O’Brien, A.R.W.; Mehta, R.; Manchanda, N.; Jacob, S.A.; Hardesty, B.M.; et al. Inflammation and autoimmunity are interrelated in patients with sickle cell disease at a steady-state condition: Implications for vaso-occlusive crisis, pain, and sensory sensitivity. Front. Immunol. 2024, 15, 1288187. [Google Scholar] [CrossRef]
- Penkert, R.R.; Azul, M.; Sealy, R.E.; Jones, B.G.; Dowdy, J.; Hayden, R.T.; Tang, L.; Ross, A.C.; Hankins, J.S.; Hurwitz, J.L. Hypothesis: Low Vitamin A and D Levels Worsen Clinical Outcomes When Children with Sickle Cell Disease Encounter Parvovirus B19. Nutrients 2022, 14, 3415. [Google Scholar] [CrossRef]
- Penkert, R.R.; Chandramouli, S.; Dormitzer, P.R.; Settembre, E.C.; Sealy, R.E.; Wong, S.; Young, N.S.; Sun, Y.; Tang, L.; Cotton, A.; et al. Novel Surrogate Neutralizing Assay Supports Parvovirus B19 Vaccine Development for Children with Sickle Cell Disease. Vaccines 2021, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Ellebedy, A.H.; Jackson, K.J.; Kissick, H.T.; Nakaya, H.I.; Davis, C.W.; Roskin, K.M.; McElroy, A.K.; Oshansky, C.M.; Elbein, R.; Thomas, S.; et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 2016, 17, 1226–1234. [Google Scholar] [CrossRef]
- Nobutoki, T.; Hori, H.; Higashigawa, M.; Azuma, E.; Sakurai, M.; Yoshizumi, T.; Nunoue, T. A case of prolonged human parvovirus B19 DNA-emia associated with polyclonal B cell activation. Acta Paediatr. Jpn. 1996, 38, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Onlamoon, N.; Akondy, R.S.; Perng, G.C.; Polsrila, K.; Chandele, A.; Kwissa, M.; Pulendran, B.; Wilson, P.C.; Wittawatmongkol, O.; et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 2012, 86, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, A.M.; Mei, H.; Hoyer, B.F.; Mumtaz, I.M.; Thiele, K.; Radbruch, A.; Burmester, G.R.; Hiepe, F.; Dorner, T. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2010, 69, 305–308. [Google Scholar] [CrossRef]
- Wrammert, J.; Smith, K.; Miller, J.; Langley, W.A.; Kokko, K.; Larsen, C.; Zheng, N.Y.; Mays, I.; Garman, L.; Helms, C.; et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453, 667–671. [Google Scholar] [CrossRef]
- von Borstel, A.; Land, J.; Abdulahad, W.H.; Rutgers, A.; Stegeman, C.A.; Diepstra, A.; Heeringa, P.; Sanders, J.S. CD27(+)CD38(hi) B Cell Frequency During Remission Predicts Relapsing Disease in Granulomatosis With Polyangiitis Patients. Front. Immunol. 2019, 10, 2221. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef]
- Krasselt, M.; Baerwald, C.; Wagner, U.; Rossol, M. CD56+ monocytes have a dysregulated cytokine response to lipopolysaccharide and accumulate in rheumatoid arthritis and immunosenescence. Arthritis Res. Ther. 2013, 15, R139. [Google Scholar] [CrossRef]
- Grip, O.; Bredberg, A.; Lindgren, S.; Henriksson, G. Increased subpopulations of CD16(+) and CD56(+) blood monocytes in patients with active Crohn’s disease. Inflamm. Bowel Dis. 2007, 13, 566–572. [Google Scholar] [CrossRef]
- Ferrara, F.; Morabito, F.; Martino, B.; Specchia, G.; Liso, V.; Nobile, F.; Boccuni, P.; Di Noto, R.; Pane, F.; Annunziata, M.; et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J. Clin. Oncol. 2000, 18, 1295–1300. [Google Scholar] [CrossRef]
- Harrington, A.M.; Hari, P.; Kroft, S.H. Utility of CD56 immunohistochemical studies in follow-up of plasma cell myeloma. Am. J. Clin. Pathol. 2009, 132, 60–66. [Google Scholar] [CrossRef][Green Version]
- Miyazaki, K.; Suzuki, K. CD56 for Multiple Myeloma: Lack of CD56 May Be Associated with Worse Prognosis. Acta Haematol. 2018, 140, 40–41. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef]
- Van Camp, B.; Durie, B.G.; Spier, C.; De Waele, M.; Van Riet, I.; Vela, E.; Frutiger, Y.; Richter, L.; Grogan, T.M. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19). Blood 1990, 76, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Dobi, A.; Dubernet, A.; Rakoto, M.L.; Seteyen, A.S.; Vagner, D.; Lebeau, G.; Raffray, L.; Gasque, P. Low levels of the key B cell activation marker, HLA-DR, in COVID-19 hospitalized cases are associated with disease severity, dexamethasone treatment, and circulating IL-6 levels. Immunol. Res. 2022, 70, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Currin, A.; Griffin, B.D.; Shannon-Lowe, C.; Thomas, W.A.; Ressing, M.E.; Wiertz, E.J.; Rowe, M. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 2009, 5, e1000255. [Google Scholar] [CrossRef]
- Musa, B.O.; Onyemelukwe, G.C.; Hambolu, J.O.; Mamman, A.I.; Isa, A.H. Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clin. Vaccine Immunol. CVI 2010, 17, 602–608. [Google Scholar] [CrossRef]
- Nagant, C.; Barbezange, C.; Dedeken, L.; Besse-Hammer, T.; Thomas, I.; Mahadeb, B.; Efira, A.; Ferster, A.; Corazza, F. Alteration of humoral, cellular and cytokine immune response to inactivated influenza vaccine in patients with Sickle Cell Disease. PLoS ONE 2019, 14, e0223991. [Google Scholar] [CrossRef]
- Penkert, R.R.; Hurwitz, J.L.; Thomas, P.; Rosch, J.; Dowdy, J.; Sun, Y.; Tang, L.; Hankins, J.S. Inflammatory molecule reduction with hydroxyurea therapy in children with sickle cell anemia. Haematologica 2018, 103, e50–e54. [Google Scholar] [CrossRef] [PubMed]
- Conran, N.; Embury, S.H. Sickle cell vaso-occlusion: The dialectic between red cells and white cells. Exp. Biol. Med. 2021, 246, 1458–1472. [Google Scholar] [CrossRef]
- Yousif, T.Y.E. Impact of Abnormal Leukocyte Count in the Pathophysiology of Sickle Cell Anemia. J. Blood Med. 2022, 13, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.R.; Glader, B.E. Leukocyte counts in children with sickle cell disease. Comparative values in the steady state, vaso-occlusive crisis, and bacterial infection. Am. J. Dis. Child. 1978, 132, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Turhan, A.; Weiss, L.A.; Mohandas, N.; Coller, B.S.; Frenette, P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proc. Natl. Acad. Sci. USA 2002, 99, 3047–3051. [Google Scholar] [CrossRef]
- Curtis, S.A.; Danda, N.; Etzion, Z.; Cohen, H.W.; Billett, H.H. Elevated Steady State WBC and Platelet Counts Are Associated with Frequent Emergency Room Use in Adults with Sickle Cell Anemia. PLoS ONE 2015, 10, e0133116. [Google Scholar] [CrossRef] [PubMed]
- Olenscki Gilli, S.C.; Pericole, F.V.; Benites, B.D.; Sippert, E.A.; Castilho, L.M.; Addas-Carvalho, M.; Olalla Saad, S.T. Cytokine polymorphisms in sickle cell disease and the relationship with cytokine expression. Exp. Hematol. 2016, 44, 583–589. [Google Scholar] [CrossRef]
- Lehmann, H.W.; von Landenberg, P.; Modrow, S. Parvovirus B19 infection and autoimmune disease. Autoimmun. Rev. 2003, 2, 218–223. [Google Scholar] [CrossRef]
- Tsay, G.J.; Zouali, M. Unscrambling the role of human parvovirus B19 signaling in systemic autoimmunity. Biochem. Pharmacol. 2006, 72, 1453–1459. [Google Scholar] [CrossRef]
- Fink, K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front. Immunol. 2012, 3, 78. [Google Scholar] [CrossRef]
- Hoffman, S.J.; Polack, F.P.; Hauer, D.A.; Griffin, D.E. Measles virus infection of rhesus macaques affects neutrophil expression of IL-12 and IL-10. Viral Immunol. 2003, 16, 369–379. [Google Scholar] [CrossRef]
- Adam, Z.; Zeman, D.; Cermak, A.; Dastych, M.; Doubkova, M.; Horvath, T.; Skorkovska, S.; Adamova, Z.; Rehak, Z.; Koukalova, R.; et al. IgG4-related disease. Clinical manifestation differential diagnosis and recent International Diagnostic Criteria for IgG4-related disease. Vnitr. Lek. 2022, 68, 4–19. [Google Scholar] [CrossRef]
- Peyronel, F.; Fenaroli, P.; Maritati, F.; Schleinitz, N.; Vaglio, A. IgG4-related disease: Advances in pathophysiology and treatment. Expert. Rev. Clin. Immunol. 2023, 19, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, P.; Chen, H.; Chen, Y.; Yang, H.; Zheng, W.; Zhang, X.; Zhang, F.; Zhang, W.; Lipsky, P.E. Circulating plasmablasts/plasma cells: A potential biomarker for IgG4-related disease. Arthritis Res. Ther. 2017, 19, 25. [Google Scholar] [CrossRef]
- Lin, W.; Jin, L.; Chen, H.; Wu, Q.; Fei, Y.; Zheng, W.; Wang, Q.; Li, P.; Li, Y.; Zhang, W.; et al. B cell subsets and dysfunction of regulatory B cells in IgG4-related diseases and primary Sjogren’s syndrome: The similarities and differences. Arthritis Res. Ther. 2014, 16, R118. [Google Scholar] [CrossRef]
- Iaccarino, L.; Talarico, R.; Bozzalla-Cassione, E.; Burmester, G.R.; Culver, E.L.; Doria, A.; Ebbo, M.; van Hagen, P.M.; Hachulla, E.; van Laar, J.A.M.; et al. Blood biomarkers recommended for diagnosing and monitoring IgG4-related disease. Considerations from the ERN ReCONNET and collaborating partners. Clin. Exp. Rheumatol. 2022, 40, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Elenitsas, R.; Abell, E.; Lee, Y.Y.; Huang, J.; Deng, J.S. Comparison of IgG subclass autoantibodies in patients with systemic lupus erythematosus and subacute cutaneous lupus erythematosus. J. Dermatol. Sci. 1990, 1, 207–215. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J. Clin. Pathol. 2016, 69, 279–291. [Google Scholar] [CrossRef]
- Li-Thiao-Te, V.; Uettwiller, F.; Quartier, P.; Lacaille, F.; Bader-Meunier, B.; Brousse, V.; de Montalembert, M. Coexistent sickle-cell anemia and autoimmune disease in eight children: Pitfalls and challenges. Pediatr. Rheumatol. Online J. 2018, 16, 5. [Google Scholar] [CrossRef]
- Chaplin, H., Jr.; Zarkowsky, H.S. Combined sickle cell disease and autoimmune hemolytic anemia. Arch. Intern. Med. 1981, 141, 1091–1093. [Google Scholar] [CrossRef]
- Piccin, A.; O’Connor-Byrne, N.; Daves, M.; Lynch, K.; Farshbaf, A.D.; Martin-Loeches, I. Autoimmune disease and sickle cell anaemia: ‘Intersecting pathways and differential diagnosis’. Br. J. Haematol. 2022, 197, 518–528. [Google Scholar] [CrossRef]
- Waisbourd-Zinman, O.; Frenklak, R.; Hakakian, O.; Hilmara, D.; Lin, H. Autoimmune Liver Disease in Patients With Sickle Cell Disease. J. Pediatr. Hematol. Oncol. 2021, 43, 254–257. [Google Scholar] [CrossRef]
- Kerr, J.R. Pathogenesis of human parvovirus B19 in rheumatic disease. Ann. Rheum. Dis. 2000, 59, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Mattey, D.L. The role of parvovirus B19 and the immune response in the pathogenesis of acute leukemia. Rev. Med. Virol. 2015, 25, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Barah, F.; Cunniffe, V.S.; Smith, J.; Vallely, P.J.; Will, A.M.; Wynn, R.F.; Stevens, R.F.; Taylor, G.M.; Cleator, G.M.; et al. Association of acute parvovirus B19 infection with new onset of acute lymphoblastic and myeloblastic leukaemia. J. Clin. Pathol. 2003, 56, 873–875. [Google Scholar] [CrossRef]
- Mustafa, M.M.; McClain, K.L. Diverse hematologic effects of parvovirus B19 infection. Pediatr. Clin. N. Am. 1996, 43, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.C.; Tsay, G.J. Human parvovirus B19 infection in patients with systemic lupus erythematosus. Rheumatology 2001, 40, 152–157. [Google Scholar] [CrossRef]
- von Poblotzki, A.; Gerdes, C.; Reischl, U.; Wolf, H.; Modrow, S. Lymphoproliferative responses after infection with human parvovirus B19. J. Virol. 1996, 70, 7327–7330. [Google Scholar] [CrossRef]
- Scheurlen, W.; Ramasubbu, K.; Wachowski, O.; Hemauer, A.; Modrow, S. Chronic autoimmune thrombopenia/neutropenia in a boy with persistent parvovirus B19 infection. J. Clin. Virol. 2001, 20, 173–178. [Google Scholar] [CrossRef]
- Hemauer, A.; Beckenlehner, K.; Wolf, H.; Lang, B.; Modrow, S. Acute parvovirus B19 infection in connection with a flare of systemic lupus erythematodes in a female patient. J. Clin. Virol. 1999, 14, 73–77. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, E.K.; Penkert, R.R.; Hankins, J.S.; Surman, S.L.; Van de Velde, L.-A.; Cotton, A.; Hayden, R.T.; Tang, L.; Yuan, X.; Zheng, Y.; et al. Immune Cell Profiles of Patients with Sickle Cell Disease during Parvovirus B19–Induced Transient Red Cell Aplasia. Vaccines 2024, 12, 984. https://doi.org/10.3390/vaccines12090984
Allen EK, Penkert RR, Hankins JS, Surman SL, Van de Velde L-A, Cotton A, Hayden RT, Tang L, Yuan X, Zheng Y, et al. Immune Cell Profiles of Patients with Sickle Cell Disease during Parvovirus B19–Induced Transient Red Cell Aplasia. Vaccines. 2024; 12(9):984. https://doi.org/10.3390/vaccines12090984
Chicago/Turabian StyleAllen, E. Kaitlynn, Rhiannon R. Penkert, Jane S. Hankins, Sherri L. Surman, Lee-Ann Van de Velde, Alyssa Cotton, Randall T. Hayden, Li Tang, Xiaomeng Yuan, Ying Zheng, and et al. 2024. "Immune Cell Profiles of Patients with Sickle Cell Disease during Parvovirus B19–Induced Transient Red Cell Aplasia" Vaccines 12, no. 9: 984. https://doi.org/10.3390/vaccines12090984
APA StyleAllen, E. K., Penkert, R. R., Hankins, J. S., Surman, S. L., Van de Velde, L.-A., Cotton, A., Hayden, R. T., Tang, L., Yuan, X., Zheng, Y., Thomas, P. G., & Hurwitz, J. L. (2024). Immune Cell Profiles of Patients with Sickle Cell Disease during Parvovirus B19–Induced Transient Red Cell Aplasia. Vaccines, 12(9), 984. https://doi.org/10.3390/vaccines12090984





