Immunogenicity and Neutralization of Recombinant Vaccine Candidates Expressing F and G Glycoproteins against Nipah Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Recombinant Vaccine Design and Phylogenetic Analysis
2.3. Plasmid Preparation and Expression of Recombinant Proteins
2.4. SDS-PAGE and Western Blot
2.5. Immunization of Mice
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Generation of Pseudovirus Expressing Nipah Virus F or G Glycoproteins
2.8. Pseudovirus-Based Neutralization Assay
2.9. Statistical Analysis
3. Results
3.1. Nipah Virus Strain Selection
3.2. Design of NiV-F/G Vaccine Candidates
3.3. Expression of Recombinant NiV Proteins
3.4. Immunogenity of Recombinant NiV Proteins
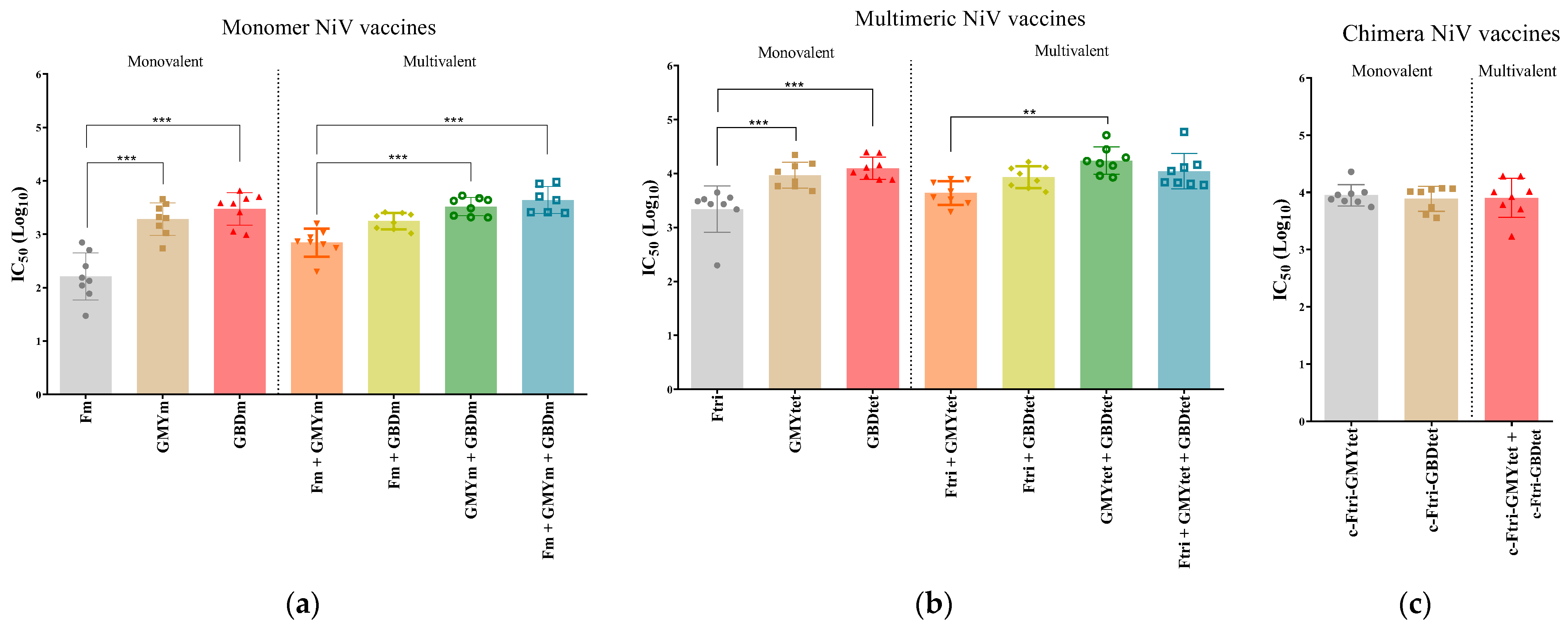
3.5. Neutralization Efficacy of Recombinant NiV Proteins
3.6. Immunogenicity of Bivalent and Trivalent NiV Vaccines Using Different Adjuvants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.S.P.; Lim, T.C.C.; Wang, L. Nipah Virus Infection. J. Clin. Microbiol. 2018, 56, e01875-17. [Google Scholar] [CrossRef]
- Sherrini, B.A.; Chong, T.T. Nipah encephalitis—An update. Med. J. Malays. 2014, 69 (Suppl. A), 103–111. [Google Scholar]
- Srivastava, S.; Deb, N.; Roy, P.; Jaiswal, V.; Sah, S.; Pandey, Y.; Reddy Edara, R.S.; Mohanty, A.; Henao-Martinez, A.F.; Sah, R. Recent Nipah virus outbreak in India: Lessons and imperatives. Ther. Adv. Infect. Dis. 2023, 10, 20499361231208535. [Google Scholar] [CrossRef] [PubMed]
- Nazmunnahar; Ahmed, I.; Roknuzzaman, A.S.M.; Islam, M.R. The recent Nipah virus outbreak in Bangladesh could be a threat for global public health: A brief report. Health Sci. Rep. 2023, 6, e1423. [Google Scholar] [CrossRef] [PubMed]
- Ching, P.K.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E.; Columna-Vingno, A.B.; Malbas, F.F., Jr.; Bolo, G.C., Jr.; Sejvar, J.J.; Eagles, D.; Playford, G.; et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg. Infect. Dis. 2015, 21, 328–331. [Google Scholar] [CrossRef]
- Mazzola, L.T.; Kelly-Cirino, C. Diagnostics for Nipah virus: A zoonotic pathogen endemic to Southeast Asia. BMJ Glob. Health 2019, 4, e001118. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Zacharski, M.; Bernaciak, Z.; Gospodarek-Komkowska, E. Nipah Virus-Another Threat From the World of Zoonotic Viruses. Front. Microbiol. 2021, 12, 811157. [Google Scholar] [CrossRef]
- Field, H.; Young, P.; Yob, J.M.; Mills, J.; Hall, L.; Mackenzie, J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001, 3, 307–314. [Google Scholar] [CrossRef]
- Joshi, J.; Shah, Y.; Pandey, K.; Ojha, R.P.; Joshi, C.R.; Bhatt, L.R.; Dumre, S.P.; Acharya, P.R.; Joshi, H.R.; Rimal, S.; et al. Possible high risk of transmission of the Nipah virus in South and South East Asia: A review. Trop. Med. Health 2023, 51, 44. [Google Scholar] [CrossRef]
- Hayman, D.T.; Wang, L.F.; Barr, J.; Baker, K.S.; Suu-Ire, R.; Broder, C.C.; Cunningham, A.A.; Wood, J.L. Antibodies to henipavirus or henipa-like viruses in domestic pigs in Ghana, West Africa. PLoS ONE 2011, 6, e25256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Hickey, A.C.; Zhang, Y.; Li, Y.; Wu, Y.; Zhang, H.; Yuan, J.; Han, Z.; McEachern, J.; et al. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerg. Infect. Dis. 2008, 14, 1974–1976. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H.; Anthony, S.J.; Islam, A.; Kilpatrick, A.M.; Ali Khan, S.; Balkey, M.D.; Ross, N.; Smith, I.; Zambrana-Torrelio, C.; Tao, Y.; et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl. Acad. Sci. USA 2020, 117, 29190–29201. [Google Scholar] [CrossRef] [PubMed]
- Field, H.; de Jong, C.E.; Halpin, K.; Smith, C.S. Henipaviruses and fruit bats, Papua New Guinea. Emerg. Infect. Dis. 2013, 19, 670–671. [Google Scholar] [CrossRef]
- Breed, A.C.; Meers, J.; Sendow, I.; Bossart, K.N.; Barr, J.A.; Smith, I.; Wacharapluesadee, S.; Wang, L.; Field, H.E. The distribution of henipaviruses in Southeast Asia and Australasia: Is Wallace’s line a barrier to Nipah virus? PLoS ONE 2013, 8, e61316. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Ghai, S.; Duengkae, P.; Manee-Orn, P.; Thanapongtharm, W.; Saraya, A.W.; Yingsakmongkon, S.; Joyjinda, Y.; Suradhat, S.; Ampoot, W.; et al. Two decades of one health surveillance of Nipah virus in Thailand. One Health Outlook 2021, 3, 12. [Google Scholar] [CrossRef]
- Byrne, P.O.; Fisher, B.E.; Ambrozak, D.R.; Blade, E.G.; Tsybovsky, Y.; Graham, B.S.; McLellan, J.S.; Loomis, R.J. Structural basis for antibody recognition of vulnerable epitopes on Nipah virus F protein. Nat. Commun. 2023, 14, 1494. [Google Scholar] [CrossRef]
- Chua, K.B.; Lam, S.K.; Goh, K.J.; Hooi, P.S.; Ksiazek, T.G.; Kamarulzaman, A.; Olson, J.; Tan, C.T. The presence of Nipah virus in respiratory secretions and urine of patients during an outbreak of Nipah virus encephalitis in Malaysia. J. Infect. 2001, 42, 40–43. [Google Scholar] [CrossRef]
- Islam, M.S.; Sazzad, H.M.S.; Satter, S.M.; Sultana, S.; Hossain, M.J.; Hasan, M.; Rahman, M.; Campbell, S.; Cannon, D.L.; Ströher, U.; et al. Nipah Virus Transmission from Bats to Humans Associated with Drinking Traditional Liquor Made from Date Palm Sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 2016, 22, 664–670. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Bandyopadhyay, B.T.; Ramdasi, A.Y.; Jadi, R.; Patil, D.R.; Rahman, M.; Majumdar, M.; Banerjee, P.S.; Hati, A.K.; Goswami, R.P.; et al. Genomic characterization of Nipah virus, West Bengal, India. Emerg. Infect. Dis. 2011, 17, 907–909. [Google Scholar] [CrossRef]
- Bruno, L.; Nappo, M.A.; Ferrari, L.; Di Lecce, R.; Guarnieri, C.; Cantoni, A.M.; Corradi, A. Nipah Virus Disease: Epidemiological, Clinical, Diagnostic and Legislative Aspects of This Unpredictable Emerging Zoonosis. Animals 2022, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, B.H.; Lowe, L.; Tamin, A.; Liu, X.; Bankamp, B.; Bowden, N.; Rollin, P.E.; Comer, J.A.; Ksiazek, T.G.; Hossain, M.J.; et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1594–1597. [Google Scholar] [CrossRef]
- Sun, B.; Jia, L.; Liang, B.; Chen, Q.; Liu, D. Phylogeography, Transmission, and Viral Proteins of Nipah Virus. Virol. Sin. 2018, 33, 385–393. [Google Scholar] [CrossRef]
- Bishop, K.A.; Stantchev, T.S.; Hickey, A.C.; Khetawat, D.; Bossart, K.N.; Krasnoperov, V.; Gill, P.; Feng, Y.R.; Wang, L.; Eaton, B.T.; et al. Identification of Hendra Virus G Glycoprotein Residues That Are Critical for Receptor Binding. J. Virol. 2007, 81, 5893–5901. [Google Scholar] [CrossRef]
- Walpita, P.; Cong, Y.; Jahrling, P.B.; Rojas, O.; Postnikova, E.; Yu, S.; Johns, L.; Holbrook, M.R. A VLP-based vaccine provides complete protection against Nipah virus challenge following multiple-dose or single-dose vaccination schedules in a hamster model. npj Vaccines 2017, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.L.; Woolsey, C.; Borisevich, V.; Agans, K.N.; Prasad, A.N.; Deer, D.J.; Geisbert, J.B.; Dobias, N.S.; Fenton, K.A.; Cross, R.W.; et al. A recombinant VSV-vectored vaccine rapidly protects nonhuman primates against lethal Nipah virus disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2200065119. [Google Scholar] [CrossRef]
- Welch, S.R.; Spengler, J.R.; Genzer, S.C.; Coleman-McCray, J.D.; Harmon, J.R.; Sorvillo, T.E.; Scholte, F.E.M.; Rodriguez, S.E.; O’Neal, T.J.; Ritter, J.M.; et al. Single-dose mucosal replicon-particle vaccine protects against lethal Nipah virus infection up to 3 days after vaccination. Sci. Adv. 2023, 9, eadh4057. [Google Scholar] [CrossRef]
- Srivastava, S.; Verma, S.; Kamthania, M.; Saxena, A.K.; Pandey, K.C.; Pande, V.; Kolbe, M. Exploring the structural basis to develop efficient multi-epitope vaccines displaying interaction with HLA and TAP and TLR3 molecules to prevent NIPAH infection, a global threat to human health. PLoS ONE 2023, 18, e0282580. [Google Scholar] [CrossRef] [PubMed]
- Pedrera, M.; McLean, R.K.; Medfai, L.; Thakur, N.; Todd, S.; Marsh, G.; Bailey, D.; Donofrio, G.; Muramatsu, H.; Pardi, N.; et al. Evaluation of the immunogenicity of an mRNA vectored Nipah virus vaccine candidate in pigs. Front. Immunol. 2024, 15, 1384417. [Google Scholar] [CrossRef]
- Loomis, R.J.; Stewart-Jones, G.B.E.; Tsybovsky, Y.; Caringal, R.T.; Morabito, K.M.; McLellan, J.S.; Chamberlain, A.L.; Nugent, S.T.; Hutchinson, G.B.; Kueltzo, L.A.; et al. Structure-Based Design of Nipah Virus Vaccines: A Generalizable Approach to Paramyxovirus Immunogen Development. Front. Immunol. 2020, 11, 842. [Google Scholar] [CrossRef]
- Jardetzky, T.S.; Lamb, R.A. Activation of paramyxovirus membrane fusion and virus entry. Curr. Opin. Virol. 2014, 5, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Jones, G.B.E.; Chuang, G.Y.; Xu, K.; Zhou, T.; Acharya, P.; Tsybovsky, Y.; Ou, L.; Zhang, B.; Fernandez-Rodriguez, B.; Gilardi, V.; et al. Structure-based design of a quadrivalent fusion glycoprotein vaccine for human parainfluenza virus types 1-4. Proc. Natl. Acad. Sci. USA 2018, 115, 12265–12270. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schafer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.; Kim, J.; No, J.S.; Park, K.; Budhathoki, S.; Lee, S.H.; Lee, J.; Cho, S.H.; Cho, S.; et al. Discovery and Genetic Characterization of Novel Paramyxoviruses Related to the Genus Henipavirus in Crocidura Species in the Republic of Korea. Viruses 2021, 13, 2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lu, M.; Yao, Y.; Zhang, X.; Liu, H.; Gao, G.; Peng, Y.; Chen, M.; Zhao, J.; Zhang, X.; Yin, C.; et al. Both chimpanzee adenovirus-vectored and DNA vaccines induced long-term immunity against Nipah virus infection. npj Vaccines 2023, 8, 170. [Google Scholar] [CrossRef]
- Watanabe, S.; Yoshikawa, T.; Kaku, Y.; Kurosu, T.; Fukushi, S.; Sugimoto, S.; Nishisaka, Y.; Fuji, H.; Marsh, G.; Maeda, K.; et al. Construction of a recombinant vaccine expressing Nipah virus glycoprotein using the replicative and highly attenuated vaccinia virus strain LC16m8. PLoS Negl. Trop. Dis. 2023, 17, e0011851. [Google Scholar] [CrossRef]
- Anasir, M.I.; Poh, C.L. Structural Vaccinology for Viral Vaccine Design. Front. Microbiol. 2019, 10, 738. [Google Scholar] [CrossRef]
- Sesterhenn, F.; Bonet, J.; Correia, B.E. Structure-based immunogen design-leading the way to the new age of precision vaccines. Curr. Opin. Struct. Biol. 2018, 51, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Islam, M.M.; Hossain, M.E.; Rahman, M.M.; Islam, A.; Siddika, A.; Hossain, M.S.S.; Sultana, S.; Islam, A.; Rahman, M.; et al. Genetic diversity of Nipah virus in Bangladesh. Int. J. Infect. Dis. 2021, 102, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mire, C.E.; Satterfield, B.A.; Geisbert, J.B.; Agans, K.N.; Borisevich, V.; Yan, L.; Chan, Y.P.; Cross, R.W.; Fenton, K.A.; Broder, C.C.; et al. Pathogenic Differences between Nipah Virus Bangladesh and Malaysia Strains in Primates: Implications for Antibody Therapy. Sci. Rep. 2016, 6, 30916. [Google Scholar] [CrossRef] [PubMed]
- Clayton, B.A.; Middleton, D.; Arkinstall, R.; Frazer, L.; Wang, L.F.; Marsh, G.A. The Nature of Exposure Drives Transmission of Nipah Viruses from Malaysia and Bangladesh in Ferrets. PLoS Negl. Trop. Dis. 2016, 10, e0004775. [Google Scholar] [CrossRef] [PubMed]
- Loomis, R.J.; DiPiazza, A.T.; Falcone, S.; Ruckwardt, T.J.; Morabito, K.M.; Abiona, O.M.; Chang, L.A.; Caringal, R.T.; Presnyak, V.; Narayanan, E.; et al. Chimeric Fusion (F) and Attachment (G) Glycoprotein Antigen Delivery by mRNA as a Candidate Nipah Vaccine. Front. Immunol. 2021, 12, 772864. [Google Scholar] [CrossRef]
- Gao, Z.; Li, T.; Han, J.; Feng, S.; Li, L.; Jiang, Y.; Xu, Z.; Hao, P.; Chen, J.; Hao, J.; et al. Assessment of the immunogenicity and protection of a Nipah virus soluble G vaccine candidate in mice and pigs. Front. Microbiol. 2022, 13, 1031523. [Google Scholar] [CrossRef]
- Tamin, A.; Harcourt, B.H.; Ksiazek, T.G.; Rollin, P.E.; Bellini, W.J.; Rota, P.A. Functional properties of the fusion and attachment glycoproteins of Nipah virus. Virology 2002, 296, 190–200. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Berhane, Y.; Caswell, J.L.; Loosmore, S.; Audonnet, J.C.; Roth, J.A.; Czub, M. Recombinant nipah virus vaccines protect pigs against challenge. J. Virol. 2006, 80, 7929–7938. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garcon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Neeli, P.; Chai, D.F.; Wang, X.; Sobhani, N.; Udeani, G.; Li, Y. Comparison of DNA vaccines with AS03 as an adjuvant and an mRNA vaccine against SARS-CoV-2. Iscience 2023, 26, 107120. [Google Scholar] [CrossRef] [PubMed]
- Charland, N.; Gobeil, P.; Pillet, S.; Boulay, I.; Séguin, A.; Makarkov, A.; Heizer, G.; Bhutada, K.; Mahmood, A.; Trépanier, S.; et al. Safety and immunogenicity of an AS03-adjuvanted plant-based SARS-CoV-2 vaccine in Adults with and without Comorbidities. npj Vaccines 2022, 7, 142. [Google Scholar] [CrossRef] [PubMed]
| Name | Target Sequence | Structure | Mutation | Tag | Multimeric Domain | Accession Number |
|---|---|---|---|---|---|---|
| NiV_Fmo | F | Monomer | I114C, L104C, S191P, L172F | 6× his | - | In this study (OR947674) |
| NiV_Ftri | F | Trimer | I114C, L104C, S191P, L172F | 6× his | GCN4 | |
| NiV_GMYm | G | Monomer | - | 6× his | - | In this study (OR947675) |
| NiV_GBDm | G | Monomer | - | 6× his | - | In this study (OR947676) |
| NiV_GMYtet | G | Tetramer | - | 6× his | - | In this study (OR947675) |
| NiV_GBDtet | G | Tetramer | - | 6× his | - | In this study (OR947676) |
| C_Ftri_GMYtet | F + G | Chimeric † | - | 6× his | GCN4 | |
| C_Ftri_GBDtet | F + G | Chimeric † | - | 6× his | GCN4 |
| Group | Vaccine | Dose | Description | Target | Route | Sample Size (n) |
|---|---|---|---|---|---|---|
| 1 | PBS | 20 μg | Control | IM | 8 | |
| 2 | Fm | 20 μg | F monomer | F | IM | 8 |
| 3 | GMYm | 20 μg | GMY monomer | GMY | IM | 8 |
| 4 | GBDm | 20 μg | GBD monomer | GBD | IM | 8 |
| 5 | Fm + GMYm | 20 μg + 20 μg | F monomer + GMY monomer | F, GMY | IM | 8 |
| 6 | Fm + GBDm | 20 μg + 20 μg | F monomer + GMY monomer | F, GBD | IM | 8 |
| 7 | Ftri | 20 μg | F trimer | F | IM | 8 |
| 8 | GMYtet | 20 μg | GMY tetramer | GMY | IM | 8 |
| 9 | GBDtet | 20 μg | GBD tetramer | GBD | IM | 8 |
| 10 | Ftri + GMYtet | 20 μg + 20 μg | F trimer + GMY tetramer | F, GMY | IM | 8 |
| 11 | Ftri + GBDtet | 20 μg + 20 μg | F trimer + GBD tetramer | F, GBD | IM | 8 |
| 12 | c-Ftri-GMYtet | 20 μg | Chimeric F trimer-GMY tetramer | F, GMY | IM | 8 |
| 13 | c-Ftri-GBDtet | 20 μg | Chimeric F trimer- GBD tetramer | F, GBD | IM | 8 |
| 14 | GMYm + GBDm | 20 μg + 20 μg | GMY monomer + GBD monomer | GMY, GBD | IM | 8 |
| 15 | Fm + GMYm + GBDm | 20 μg + 20 μg + 20 μg | F monomer + GMY monomer + GBD monomer | F, GMY, GBD | IM | 8 |
| 16 | GMYtet + GBDtet | 20 μg + 20 μg | GMY tetramer + GBD tetramer | GMY, GBD | IM | 8 |
| 17 | Ftri + GMYtet + GBDtet | 20 μg + 20 μg + 20 μg | F trimer + GMY tetramer + GBD tetramer | F, GMY, GBD | IM | 8 |
| 18 | c-Ftri-GMYtet + c-Ftri-GBDtet | 20 μg + 20 μg | Chimeric F trimer-GMY tetramer + Chimeric F trimer- GBD tetramer | F, GMY, GBD | IM | 8 |
| Group | Vaccine | NiV-Vaccine | Dose | Adjuvant | Route | Sample Size (n) |
|---|---|---|---|---|---|---|
| 1 | PBS | IM | 6 | |||
| 2 | G-Phos | GMYtet + GBDtet | 20 µg + 20 µg | Phos | IM | 6 |
| 3 | G-Alum | GMYtet + GBDtet | 20 µg + 20 µg | Alum | IM | 6 |
| 4 | G-MPLA | GMYtet + GBDtet | 20 µg + 20 µg | MPLA | IM | 6 |
| 5 | G-AS03 | GMYtet + GBDtet | 20 µg + 20 µg | AS03 | IM | 6 |
| 6 | G-Vax | GMYtet + GBDtet | 20 µg + 20 µg | Vax | IM | 6 |
| 7 | FG-Phos | Ftri + GMYtet + GBDtet | 20 µg + 20 µg + 20 µg | Phos | IM | 6 |
| 8 | FG-Alum | Ftri + GMYtet + GBDtet | 20 µg + 20 µg + 20 µg | Alum | IM | 6 |
| 9 | FG-MPLA | Ftri + GMYtet + GBDtet | 20 µg + 20 µg + 20 µg | MPLA | IM | 6 |
| 10 | FG-AS03 | Ftri + GMYtet + GBDtet | 20 µg + 20 µg + 20 µg | AS03 | IM | 6 |
| 11 | FG-Vax | Ftri + GMYtet + GBDtet | 20 µg + 20 µg + 20 µg | Vax | IM | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.Y.; Flores, R.A.; Yim, M.S.; Lim, H.; Kim, S.; Lee, S.Y.; Lee, Y.-k.; Kim, J.-O.; Park, H.; Bae, S.E.; et al. Immunogenicity and Neutralization of Recombinant Vaccine Candidates Expressing F and G Glycoproteins against Nipah Virus. Vaccines 2024, 12, 999. https://doi.org/10.3390/vaccines12090999
Moon SY, Flores RA, Yim MS, Lim H, Kim S, Lee SY, Lee Y-k, Kim J-O, Park H, Bae SE, et al. Immunogenicity and Neutralization of Recombinant Vaccine Candidates Expressing F and G Glycoproteins against Nipah Virus. Vaccines. 2024; 12(9):999. https://doi.org/10.3390/vaccines12090999
Chicago/Turabian StyleMoon, Seo Young, Rochelle A. Flores, Min Su Yim, Heeji Lim, Seungyeon Kim, Seung Yun Lee, Yoo-kyoung Lee, Jae-Ouk Kim, Hyejin Park, Seong Eun Bae, and et al. 2024. "Immunogenicity and Neutralization of Recombinant Vaccine Candidates Expressing F and G Glycoproteins against Nipah Virus" Vaccines 12, no. 9: 999. https://doi.org/10.3390/vaccines12090999






