Profile of Humoral Immunity and B Cell Pool in Infection with the SARS-CoV-2 Prototype Strain and AZD1222 (ChAdOx nCoV-19) Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Blood Sample Collection
2.2. PBMC and Plasma Isolation
2.3. IgG ELISPOT Assay
2.4. B Cell Profile Assessment with Extracellular Staining Using Flow Cytometry
2.5. BAFF, APRIL, and sCD40L Quantification
2.6. SARS-CoV-2 Serology
2.7. Statistical Analysis
3. Results
3.1. Recovered Individuals Exhibit a Robust Spike-1-Specific ASC Response
3.2. Plasmablast Cells Are Commonly Increased in the Acute, Recovered, and Vaccinated Groups, Although the Frequency of Total B Cells or B Cell Subsets Did Not Vary When Comparing the Four Groups
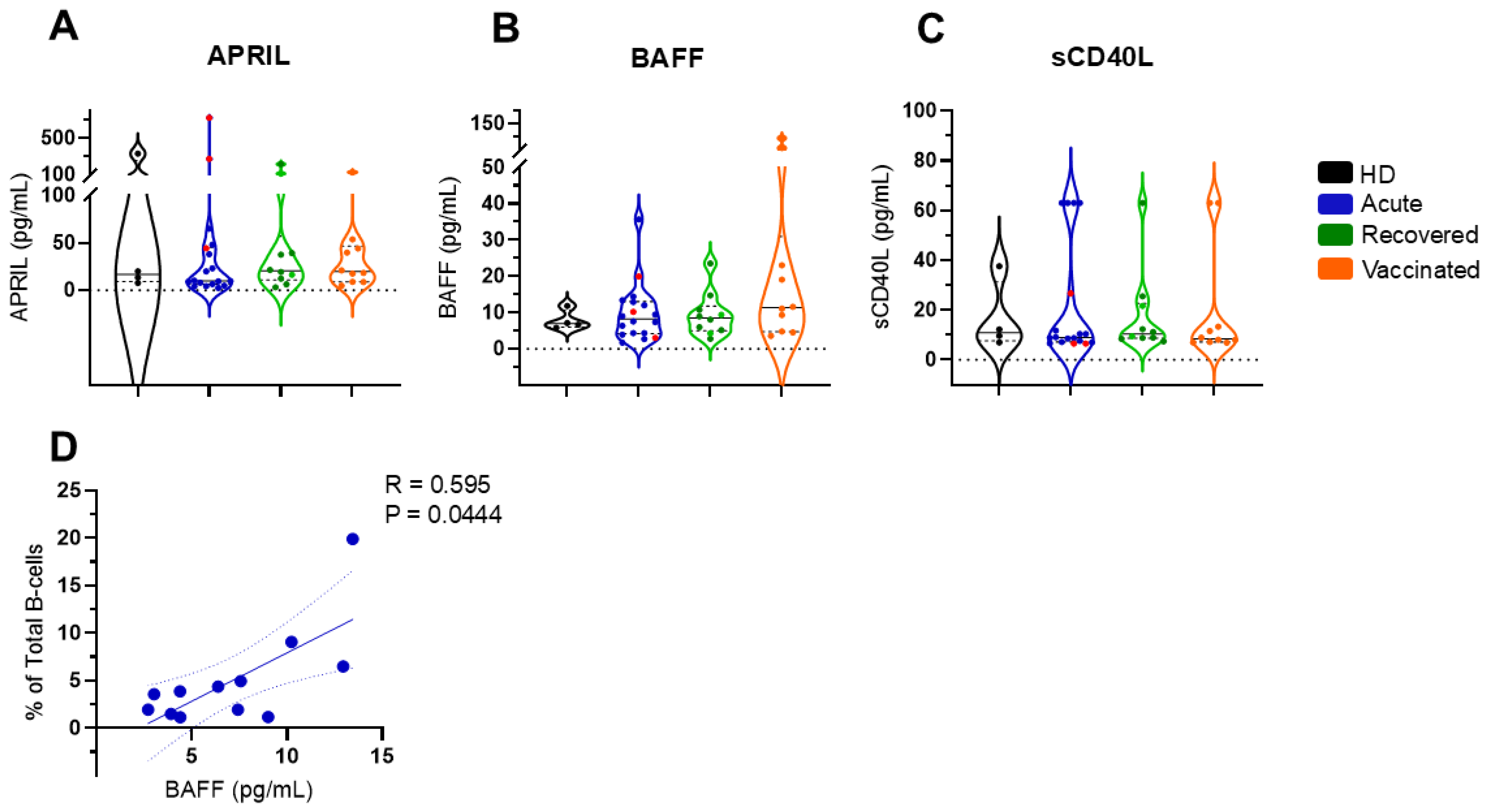
3.3. Soluble BAFF Appears to Regulate Total B Cells in Patients with Acute COVID-19
3.4. Differential Profile of IgA and IgG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef]
- Sepand, M.R.; Bigdelou, B.; Ho, J.Q.; Sharaf, M.; Lannigan, A.J.; Sullivan, I.M.; da Silva, A.P.; Barrett, L.O.; McGoldrick, S.; Lnu, Y.; et al. Long-Term Immunity and Antibody Response: Challenges for Developing Efficient COVID-19 Vaccines. Antibodies 2022, 11, 35. [Google Scholar] [CrossRef]
- Khan, W.H.; Hashmi, Z.; Goel, A.; Ahmad, R.; Gupta, K.; Khan, N.; Alam, I.; Ahmed, F.; Ansari, M.A. COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions. Front. Cell Infect. Microbiol. 2021, 11, 690621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, C.Q.; Vishwanath, S.; Carnell, G.W.; Chan, A.C.Y.; Heeney, J.L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 2023, 8, 1971–1985. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Q.; Gu, X.; Ren, L.; Huang, T.; Li, Y.; Zhang, H.; Liu, Y.; Zhong, J.; Wang, X.; et al. Durability and cross-reactive immune memory to SARS-CoV-2 in individuals 2 years after recovery from COVID-19: A longitudinal cohort study. Lancet Microbe 2024, 5, e24–e33. [Google Scholar] [CrossRef]
- Bortnick, A.; Chernova, I.; Quinn, W.J., 3rd; Mugnier, M.; Cancro, M.P.; Allman, D. Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J. Immunol. 2012, 188, 5389–5396. [Google Scholar] [CrossRef]
- Landsverk, O.J.; Snir, O.; Casado, R.B.; Richter, L.; Mold, J.E.; Réu, P.; Horneland, R.; Paulsen, V.; Yaqub, S.; Aandahl, E.M.; et al. Antibody-secreting plasma cells persist for decades in human intestine. J. Exp. Med. 2017, 214, 309–317. [Google Scholar] [CrossRef]
- Slifka, M.K.; Antia, R.; Whitmire, J.K.; Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 1998, 8, 363–372. [Google Scholar] [CrossRef]
- Wilmore, J.R.; Allman, D. Here, There, and Anywhere? Arguments for and against the Physical Plasma Cell Survival Niche. J. Immunol. 2017, 199, 839–845. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Yilmaz, B.; Limenitakis, J.P.; Ganal-Vonarburg, S.C. IgA Function in Relation to the Intestinal Microbiota. Annu. Rev. Immunol. 2018, 36, 359–381. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Bossen, C.; Schneider, P. BAFF, APRIL and their receptors: Structure, function and signaling. Semin. Immunol. 2006, 18, 263–275. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Brink, R. Regulation of B cell self-tolerance by BAFF. Semin. Immunol. 2006, 18, 276–283. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef]
- De-Oliveira-Pinto, L.M.; Fiestas Solórzano, V.E.; de Lourdes Martins, M.; Fernandes-Santos, C.; Damasco, P.H.; de Siqueira, M.A.M.T.; Dias, H.G.; Pauvolid-Corrêa, A.; Damasco, P.V.; de Azeredo, E.L. Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients. Viruses 2022, 14, 455. [Google Scholar] [CrossRef]
- Ministério da Saúde. Guia de Vigilância Epidemiológica Emergência de Saúde Pública de Importância Nacional Pela Doença Pelo Coronavirus; Ministério da Saúde: Brasília, Brasil, 2022.
- Familiar-Macedo, D.; Vieira Damasco, P.; Fiestas Solórzano, V.E.; Carnevale Rodrigues, J.; Sampaio de Lemos, E.R.; Barreto Dos Santos, F.; Agudo Mendonça Teixeira de Siqueira, M.; de Azeredo, E.L.; de-Oliveira-Pinto, L.M. Inflammatory and cytotoxic mediators in COVID-19 patients and in ChAdOx1 nCoV-19 (AZD1222) vaccine recipients. Cytokine 2023, 171, 156350. [Google Scholar] [CrossRef]
- Badolato-Corrêa, J.; Carvalho, F.R.; Paiva, I.A.; Familiar-Macedo, D.; Dias, H.G.; Pauvolid-Corrêa, A.; Fernandes-Santos, C.; Lima, M.D.R.Q.; Gandini, M.; Silva, A.A.; et al. Differential Longevity of Memory CD4 and CD8 T Cells in a Cohort of the Mothers with a History of ZIKV Infection and Their Children. Front. Immunol. 2021, 12, 610456. [Google Scholar] [CrossRef]
- Familiar-Macedo, D.; Amancio Paiva, I.; Badolato-Corrêa da Silva, J.; de Carvalho, F.R.; Dias, H.G.; Pauvolid-Corrêa, A.; Dos Santos, C.F.; Gandini, M.; Silva, A.A.; Baeta Cavalcanti, S.M.; et al. Evaluation of the Expression of CCR5 and CX3CR1 Receptors and Correlation with the Functionality of T Cells in Women infected with ZIKV during Pregnancy. Viruses 2021, 13, 191. [Google Scholar] [CrossRef]
- Paiva, I.A.; Familiar-Macedo, D.; Badolato-Corrêa, J.; Carvalho, F.R.; Dias, H.G.; Pauvolid-Corrêa, A.; Santos, C.F.D.; Silva, A.A.; de Azeredo, E.L.; Vianna, R.A.O.; et al. Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS). Viruses 2022, 14, 250. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Luu, L.D.W.; Li, J.; Cui, X.; Yao, H.; Chen, S.; Fu, J.; Wang, L.; Wang, C.; et al. Single-cell transcriptomic atlas reveals distinct immunological responses between COVID-19 vaccine and natural SARS-CoV-2 infection. J. Med. Virol. 2022, 94, 5304–5324. [Google Scholar] [CrossRef]
- Lo Tartaro, D.; Paolini, A.; Mattioli, M.; Swatler, J.; Neroni, A.; Borella, R.; Santacroce, E.; Di Nella, A.; Gozzi, L.; Busani, S.; et al. Detailed characterization of SARS-CoV-2-specific T and B cells after infection or heterologous vaccination. Front. Immunol. 2023, 14, 1123724. [Google Scholar] [CrossRef]
- Inoue, T.; Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 2024, 24, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, P.; Wang, T.T. Immunity after SARS-CoV-2 infections. Nat. Immunol. 2021, 22, 539–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byazrova, M.; Yusubalieva, G.; Spiridonova, A.; Efimov, G.; Mazurov, D.; Baranov, K.; Baklaushev, V.; Filatov, A. Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19. Clin. Transl. Immunology. 2021, 10, e1245. [Google Scholar] [CrossRef]
- Lee, T.; Kim, Y.; Kim, H.J.; Ha, N.Y.; Lee, S.; Chin, B.; Cho, N.H. Acute Surge of Atypical Memory and Plasma B-Cell Subsets Driven by an Extrafollicular Response in Severe COVID-19. Front. Cell Infect. Microbiol. 2022, 12, 909218, Erratum in: Front. Cell Infect. Microbiol. 2023, 13, 1178630. [Google Scholar] [CrossRef]
- Pušnik, J.; Richter, E.; Schulte, B.; Dolscheid-Pommerich, R.; Bode, C.; Putensen, C.; Hartmann, G.; Alter, G.; Streeck, H. Memory B cells targeting SARS-CoV-2 spike protein and their dependence on CD4+ T cell help. Cell Rep. 2021, 35, 109320. [Google Scholar] [CrossRef]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; van Zelm, M.C. Humoral immunity and B-cell memory in response to SARS-CoV-2 infection and vaccination. Biochem. Soc. Trans. 2022, 50, 1643–1658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Brieva, J.A.; Roldán, E.; Rodríguez, C.; Navas, G. Human tonsil, blood and bone marrow in vivo-induced B cells capable of spontaneous and high-rate immunoglobulin secretion in vitro: Differences in the requirements for factors and for adherent and bone marrow stromal cells, as well as distinctive adhesion molecule expression. Eur. J. Immunol. 1994, 24, 362–366. [Google Scholar] [CrossRef]
- Schultheiß, C.; Paschold, L.; Simnica, D.; Mohme, M.; Willscher, E.; von Wenserski, L.; Scholz, R.; Wieters, I.; Dahlke, C.; Tolosa, E.; et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity 2020, 53, 442–455.e4. [Google Scholar] [CrossRef]
- Wang, H.; Yan, D.; Li, Y.; Gong, Y.; Mai, Y.; Li, B.; Zhu, X.; Wan, X.; Xie, L.; Jiang, H.; et al. Clinical and antibody characteristics reveal diverse signatures of severe and non-severe SARS-CoV-2 patients. Infect. Dis. Poverty 2022, 11, 15. [Google Scholar] [CrossRef]
- Alturaiki, W.; Alkadi, H.; Alamri, S.; Awadalla, M.E.; Alfaez, A.; Mubarak, A.; Alanazi, M.A.; Alenzi, F.Q.; Flanagan, B.F.; Alosaimi, B. Association between the expression of toll-like receptors, cytokines, and homeostatic chemokines in SARS-CoV-2 infection and COVID-19 severity. Heliyon 2023, 9, e12653. [Google Scholar] [CrossRef]
- Gupta, S.; Clark, E.S.; Termini, J.M.; Boucher, J.; Kanagavelu, S.; LeBranche, C.C.; Abraham, S.; Montefiori, D.C.; Khan, W.N.; Stone, G.W. DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J. Virol. 2015, 89, 4158–4169. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478, Erratum in: Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Bidgood, S.R.; Tam, J.C.; McEwan, W.A.; Mallery, D.L.; James, L.C. Translocalized IgA mediates neutralization and stimulates innate immunity inside infected cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13463–13468. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsaeng, R.; Thongmee, T.; Vichaiwattana, P.; Auphimai, C.; Wongsrisang, L.; Klinfueng, S.; Sudhinaraset, N.; et al. Immunogenicity of heterologous inactivated and adenoviral-vectored COVID-19 vaccine: Real-world data. Vaccine 2022, 40, 3203–3209. [Google Scholar] [CrossRef]
- Shaw, R.H.; Stuart, A.; Greenland, M.; Liu, X.; Nguyen Van-Tam, J.S.; Snape, M.D.; Com-COV Study Group. Heterologous prime-boost COVID-19 vaccination: Initial reactogenicity data. Lancet 2021, 397, 2043–2046. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]



| HD | COVID-19 | ADZ1222 | p | ||
|---|---|---|---|---|---|
| Acute | Recovered | Vaccinated | |||
| Total | 11 | 28 | 15 | 11 | |
| Female (%) | 64.7 | 50 | 87.5 | 63.6 | |
| Age (years) a | 32 (25–61) | 55 (24–97) | 35 (23–51) | 33 (25–61) | HD vs acute * acute vs. vaccinated * |
| Days after symptom onset b | NA | 7 (2–19) | 23 (17–38) | NA | |
| Hospitalization (%) | NA | 32 | 0 | NA | |
| Death (%) | NA | 28.6 | 0 | NA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Familiar-Macedo, D.; de Azeredo, E.L.; de Lemos, E.R.S.; Damasco, P.V.; de-Oliveira-Pinto, L.M. Profile of Humoral Immunity and B Cell Pool in Infection with the SARS-CoV-2 Prototype Strain and AZD1222 (ChAdOx nCoV-19) Vaccination. Vaccines 2025, 13, 101. https://doi.org/10.3390/vaccines13020101
Familiar-Macedo D, de Azeredo EL, de Lemos ERS, Damasco PV, de-Oliveira-Pinto LM. Profile of Humoral Immunity and B Cell Pool in Infection with the SARS-CoV-2 Prototype Strain and AZD1222 (ChAdOx nCoV-19) Vaccination. Vaccines. 2025; 13(2):101. https://doi.org/10.3390/vaccines13020101
Chicago/Turabian StyleFamiliar-Macedo, Débora, Elzinandes Leal de Azeredo, Elba Regina Sampaio de Lemos, Paulo Vieira Damasco, and Luzia Maria de-Oliveira-Pinto. 2025. "Profile of Humoral Immunity and B Cell Pool in Infection with the SARS-CoV-2 Prototype Strain and AZD1222 (ChAdOx nCoV-19) Vaccination" Vaccines 13, no. 2: 101. https://doi.org/10.3390/vaccines13020101
APA StyleFamiliar-Macedo, D., de Azeredo, E. L., de Lemos, E. R. S., Damasco, P. V., & de-Oliveira-Pinto, L. M. (2025). Profile of Humoral Immunity and B Cell Pool in Infection with the SARS-CoV-2 Prototype Strain and AZD1222 (ChAdOx nCoV-19) Vaccination. Vaccines, 13(2), 101. https://doi.org/10.3390/vaccines13020101








