The Past, Present, and Future of Cervical Cancer Vaccines
Abstract
1. Introduction
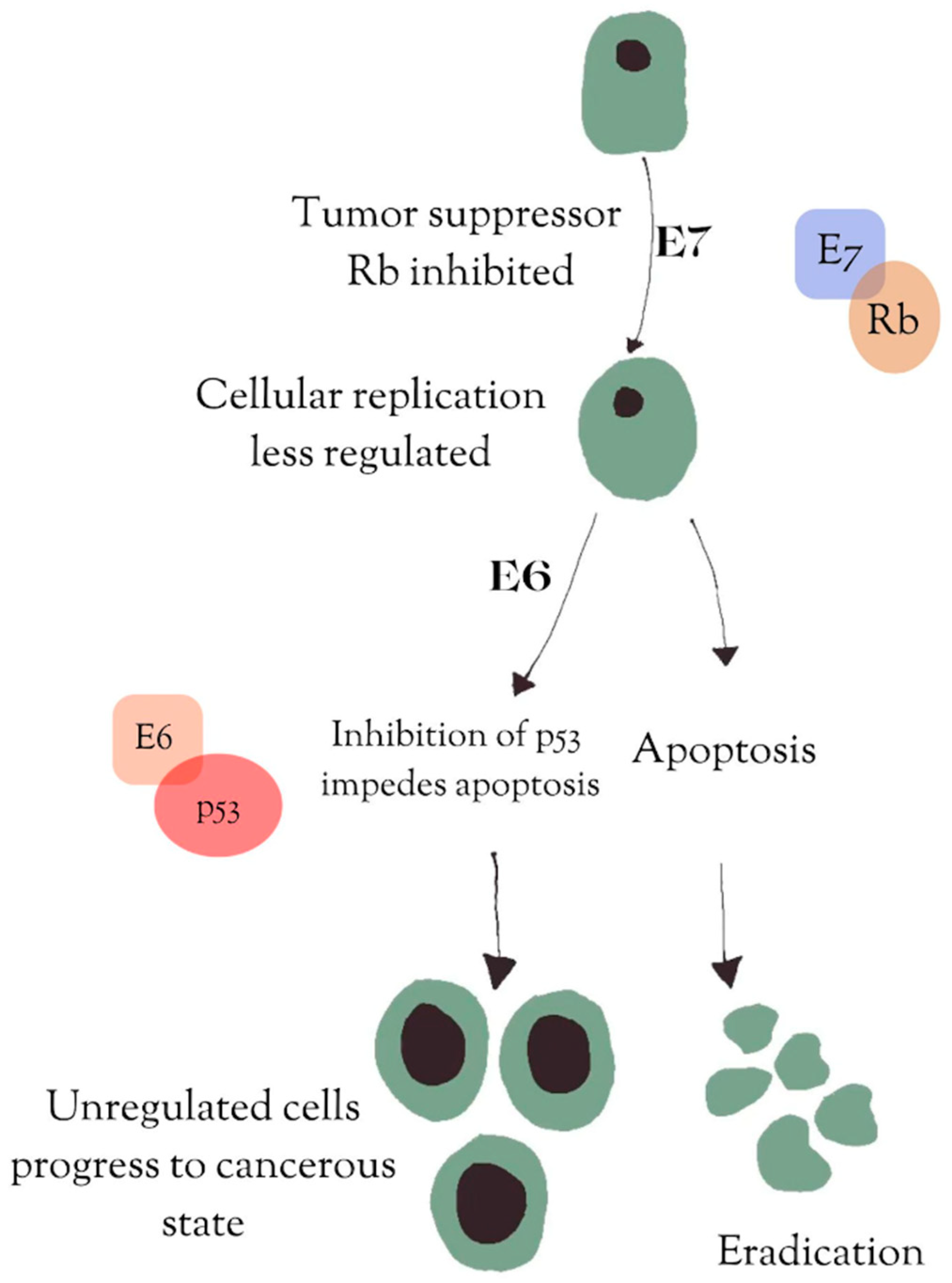
2. HPV
3. HPV Vaccines
4. Current Cervical Cancer Treatments
5. Rationale for Vaccines as a Therapeutic Treatment
6. Accum™ and Vaccines
7. DNA Vaccines and Adenovirus Vectors
8. Cervical Precancerous Vaccines Used in Combination Therapy
9. Lacticaseibacillus Oral Vaccine
10. mRNA Vaccines
11. Areas in Need of Continued Advancement
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Disease Control and Prevention. About HPV, Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/hpv/about/?CDC_AAref_Val=https%3A%2F%2Fwww.cdc.gov%2Fhpv%2Fparents%2Fabout-hpv.html (accessed on 16 July 2024).
- zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. HPV Vaccination, Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/hpv/vaccines/?CDC_AAref_Val=https%3A%2F%2Fwww.cdc.gov%2Fhpv%2Fparents%2Fvaccine-for-hpv.html (accessed on 16 July 2024).
- González-Rodríguez, J.C.; Cruz-Valdez, A.; Madrid-Marina, V. Cervical cancer prevention by vaccination: Review. Front Oncol. 2024, 14, 1386167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO. Cervical Cancer, World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 16 July 2024).
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, S.; Gao, K.; Gu, S.; You, L.; Qian, S.; Tang, M.; Wang, J.; Chen, K.; Jin, M. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer 2021, 127, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Münger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallin, K.L.; Wiklund, F.; Angström, T.; Bergman, F.; Stendahl, U.; Wadell, G.; Hallmans, G.; Dillner, J. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N. Engl. J. Med. 1999, 341, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Taub, J.; Greenstone, H.; Roden, R.; Dürst, M.; Gissmann, L.; Lowy, D.R.; Schiller, J. T Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 1993, 67, 6929–6936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Münger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Shamseddine, A.A.; Burman, B.; Lee, N.Y.; Zamarin, D.; Riaz, N. Tumor Immunity and Immunotherapy for HPV-Related Cancers. Cancer Discov. 2021, 11, 1896–1912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otter, S.J.; Chatterjee, J.; Stewart, A.J.; Michael, A. The Role of Biomarkers for the Prediction of Response to Checkpoint Immunotherapy and the Rationale for the Use of Checkpoint Immunotherapy in Cervical Cancer. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; van der Velden, J.; Kenter, G.G.; de Gruijl, T.D.; Jordanova, E.S. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. 2016, 29, 753–763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feltkamp, M.C.; Vreugdenhil, G.R.; Vierboom, M.P.; Ras, E.; van der Burg, S.H.; ter Schegget, J.; Melief, C.J.; Kast, W.M. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur. J. Immunol. 1995, 25, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Ault, K.A.; Giuliano, A.R.; Wheeler, C.M.; Koutsky, L.A.; Malm, C.; Lehtinen, M.; et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005, 6, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Franco, E.L.; Wheeler, C.M.; Moscicki, A.B.; Romanowski, B.; Roteli-Martins, C.M.; Jenkins, D.; Schuind, A.; Costa Clemens, S.A.; Dubin, G. HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet 2006, 367, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S.; et al. GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, M.K.; Sheikha, H.; Nyhan, K.; Oliveira, C.R.; Niccolai, L.M. Human papillomavirus vaccine effectiveness by age at vaccination: A systematic review. Hum. Vaccines Immunother. 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Gullo, G.; Basile, G.; Gulia, C.; Porrello, A.; Cucinella, G.; Marinelli, E.; Zaami, S. Circulating miRNAs as a Tool for Early Diagnosis of Endometrial Cancer—Implications for the Fertility-Sparing Process: Clinical, Biological, and Legal Aspects. Int. J. Mol. Sci. 2023, 24, 11356. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society Medical and Editorial Content Team. Cervical Cancer Treatment Options: Treatment Choices by Stage, Cervical Cancer Treatment Options|Treatment Choices by Stage|American Cancer Society. 2024. Available online: https://www.cancer.org/cancer/types/cervical-cancer/treating/by-stage.html# (accessed on 16 July 2024).
- Poddar, P.; Maheshwari, A. Surgery for cervical cancer: Consensus & controversies. Indian J. Med. Res. 2021, 154, 284–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M.; Williams, C.J. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001, 358, 781–786. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society Medical and Editorial Content Team. Cervical Cancer Chemotherapy: Chemo for Cervical, Cervical Cancer Chemotherapy|Chemo for Cervical|American Cancer Society. 2024. Available online: https://www.cancer.org/cancer/types/cervical-cancer/treating/chemotherapy.html (accessed on 16 July 2024).
- Cancer Research UK. Chemotherapy for Cervical Cancer, Chemotherapy for Cervical Cancer|Cancer Research, U.K. 2023. Available online: https://www.cancerresearchuk.org/about-cancer/cervical-cancer/treatment/chemotherapy-treatment#:~:text=Cisplatin%20is%20the%20most%20common,cisplatin%20and%20paclitaxel%20(Taxol) (accessed on 18 July 2024).
- The American Cancer Society Medical and Editorial Content Team. Cervical Cancer Survival Rates: Cancer 5 Year Survival Rates, Cervical Cancer Survival Rates|Cancer 5 Year Survival Rates|American Cancer Society. 2024. Available online: https://www.cancer.org/cancer/types/cervical-cancer/detection-diagnosis-staging/survival.html#references (accessed on 18 July 2024).
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.; Beaudoin, S.; Plouffe, S.; Rafei, M. Accum™ Technology: A Novel Conjugable Primer for Onco-Immunotherapy. Molecules 2022, 27, 3807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bikorimana, J.P.; Abusarah, J.; Gonçalves, M.; Farah, R.; Saad, W.; Talbot, S.; Stanga, D.; Beaudoin, S.; Plouffe, S.; Rafei, M. An engineered Accum-E7 protein-based vaccine with dual anti-cervical cancer activity. Cancer Sci. 2024, 115, 1102–1113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, Z. DNA vaccine. Adv. Genet. 2005, 54, 257–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Gao, Z.; Cheng, Y.; Wu, S.; Chen, J.; Zhang, W. A Therapeutic DNA Vaccine Targeting HPV16 E7 in Combination with Anti-PD-1/PD-L1 Enhanced Tumor Regression and Cytotoxic Immune Responses. Int. J. Mol. Sci. 2023, 24, 15469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ewer, K.J.; Lambe, T.; Rollier, C.S.; Spencer, A.J.; Hill, A.V.; Dorrell, L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr. Opin. Immunol. 2016, 41, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rahmanzade, R. Redefinition of tumor capsule: Rho-dependent clustering of cancer-associated fibroblasts in favor of tensional homeostasis. Med. Hypotheses 2020, 135, 109425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seo, S.H.; Jin, H.T.; Park, S.H.; Youn, J.I.; Sung, Y.C. Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine 2009, 27, 5906–5912. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hur, S.Y.; Kim, T.J.; Hong, S.R.; Lee, J.K.; Cho, C.H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Jin, H.T.; Hur, S.Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.W.; Kim, S.; Woo, J.W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Youn, J.W.; Hur, S.Y.; Woo, J.W.; Kim, Y.M.; Lim, M.C.; Park, S.Y.; Seo, S.S.; No, J.H.; Kim, B.G.; Lee, J.K.; et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: Interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Kobayashi, O.; Ikeda, Y.; Yahata, H.; Iwata, T.; Satoh, T.; Akiyama, A.; Maeda, D.; Hori-Hirose, Y.; Uemura, Y.; et al. Phase I and II randomized clinical trial of an oral therapeutic vaccine targeting human papillomavirus for treatment of cervical intraepithelial neoplasia 2 and 3. JNCI Cancer Spectr. 2023, 7, pkad101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, R.; Xie, C.; Xia, X. Recent progress in mRNA cancer vaccines. Hum. Vaccin Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, W.; Jiang, L.; Liao, S.; Wu, F.; Yang, G.; Hou, L.; Liu, L.; Pan, X.; Jia, W.; Zhang, Y. Vaccines’ New Era-RNA Vaccine. Viruses 2023, 15, 1760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA vaccines: Clinical advances and future opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.; Lu, X.; Lu, B.; Gullo, G.; Chen, L. Measuring the composition of the tumor microenvironment with transcriptome analysis: Past, present and future. Future Oncol. 2024, 20, 1207–1220. [Google Scholar] [CrossRef]

| Vaccine Name (FDA Approved) | Target HPV Strains | Efficacy | FIDA Approval (Current vs. Previous) |
|---|---|---|---|
| Gardasil | 6, 11, 16, 18 | 93-100% | Previous |
| Gardasil 9 | 6, 11, 16, 18, 31, 33, 45, 52, 58 | 97-100% | Current |
| Cervarix | 16, 18 | 93% | Previous |
| Vaccine Strategies | Unique Advantages |
|---|---|
| AccumTM Vaccines | Increased vaccine delivery to APCs |
| Adenovirus Vector Vaccines | Historical success |
| Oral Vaccines | Ease of administration |
| mRNA Vaccines | High efficacy and rapid development |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, A.C.; Johnson, G.S.; Guan, T.; Burns, C.P.; Parker, J.M.; Dong, L.; Wakefield, M.R.; Fang, Y. The Past, Present, and Future of Cervical Cancer Vaccines. Vaccines 2025, 13, 201. https://doi.org/10.3390/vaccines13020201
Lien AC, Johnson GS, Guan T, Burns CP, Parker JM, Dong L, Wakefield MR, Fang Y. The Past, Present, and Future of Cervical Cancer Vaccines. Vaccines. 2025; 13(2):201. https://doi.org/10.3390/vaccines13020201
Chicago/Turabian StyleLien, Alexander C., Grace S. Johnson, Tianyun Guan, Caitlin P. Burns, Jacob M. Parker, Lijun Dong, Mark R. Wakefield, and Yujiang Fang. 2025. "The Past, Present, and Future of Cervical Cancer Vaccines" Vaccines 13, no. 2: 201. https://doi.org/10.3390/vaccines13020201
APA StyleLien, A. C., Johnson, G. S., Guan, T., Burns, C. P., Parker, J. M., Dong, L., Wakefield, M. R., & Fang, Y. (2025). The Past, Present, and Future of Cervical Cancer Vaccines. Vaccines, 13(2), 201. https://doi.org/10.3390/vaccines13020201







