A Phase II Study of Denileukin Diftitox in Patients with Advanced Treatment Refractory Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Study Design
2.3. Evaluation of Regulatory T Cells
2.4. Evaluation of Anti-Diphtheria Toxin Antibodies
2.5. Statistical Methods
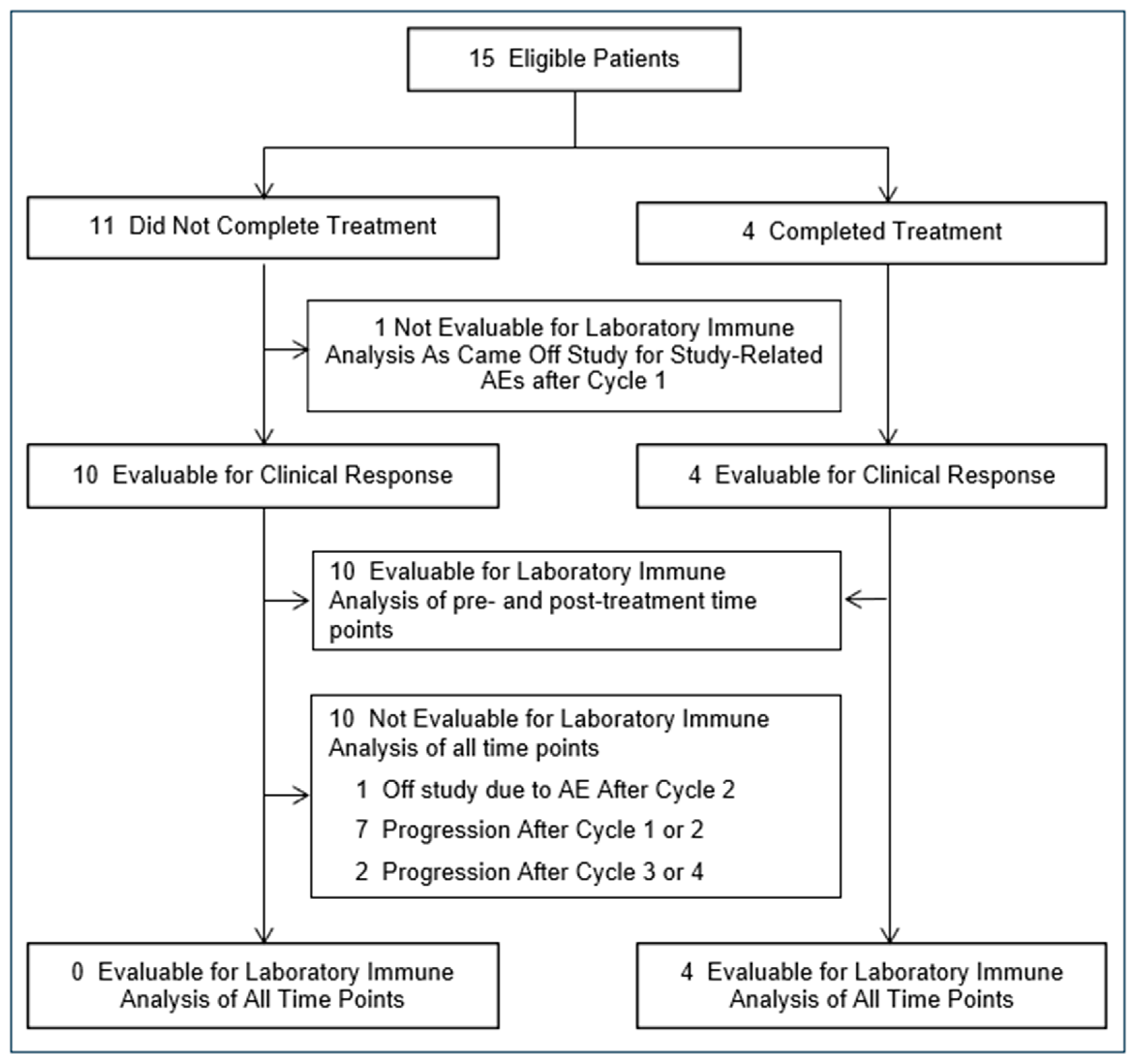
3. Results
3.1. Patient Characteristics
3.2. Feasibility and Toxicity of Administering Denileukin Diftitox for Treatment Refractory Breast Cancer
3.3. Clinical Response to Denileukin Diftitox Therapy in Advanced Breast Cancer
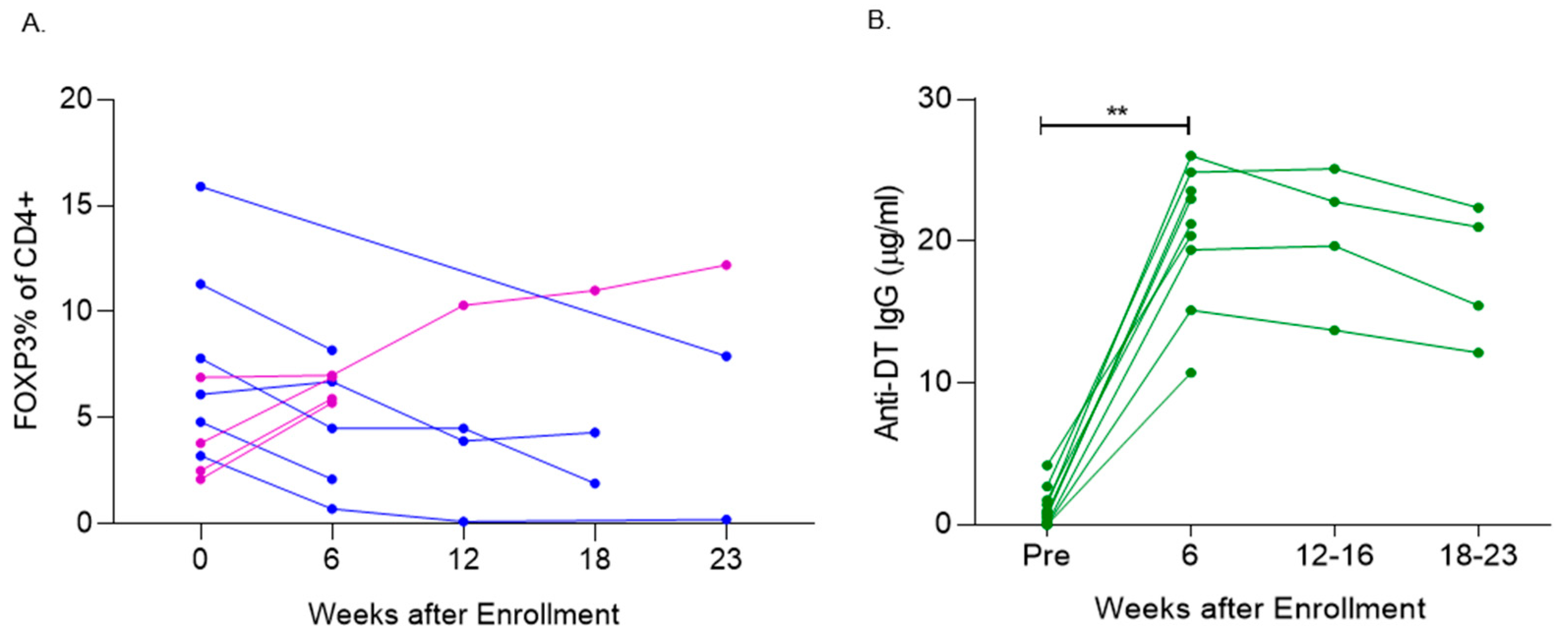
3.4. Denileukin Diftitox Therapy Modulated Peripheral Blood FOXP3+ T Cells
3.5. Denileukin Diftitox Therapy Induced Anti-Diphtheria Toxin Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimino-Mathews, A.; Foote, J.B.; Emens, L.A. Immune targeting in breast cancer. Oncology 2015, 29, 375–385. [Google Scholar] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. J. Am. Med. Assoc. Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Re, G.G.; Waters, C.; Poisson, L.; Willingham, M.C.; Sugamura, K.; Frankel, A.E. Interleukin 2 (IL-2) receptor expression and sensitivity to diphteria fusion toxin DAB389IL-2 in cultured hematopoietic cells. Cancer Res. 1996, 56, 2590–2595. [Google Scholar] [PubMed]
- Foss, F.M.; Kim, Y.H.; Prince, H.M.; Kuzel, T.M.; Yannakou, C.K.; Ooi, C.E.; Xing, D.; Sauter, N.; Singh, P.; Czuczman, M.; et al. Efficacy and Safety of E7777 (improved purity Denileukin diftitox [ONTAK]) in Patients with Relapsed or Refractory Cutaneous T-Cell Lymphoma: Results from Pivotal Study 302. Blood 2022, 140 (Suppl. S1), 1491–1492. [Google Scholar] [CrossRef]
- Barnett, B.; Kryczek, I.; Cheng, P.; Zou, W.; Curiel, T.J. Regulatory T cells in ovarian cancer: Biology and therapeutic potential. Am. J. Reprod. Immunol. 2005, 54, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, K.; Schonfeld, K.; Fondel, S.; Ring, S.; Karakhanova, S.; Wiedemeyer, K.; Bedke, T.; Johnson, T.S.; Storn, V.; Schallenberg, S.; et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: Kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int. J. Cancer 2007, 120, 2723–2733. [Google Scholar] [CrossRef]
- Dannull, J.; Su, Z.; Rizzieri, D.; Yang, B.K.; Coleman, D.; Yancey, D.; Zhang, A.; Dahm, P.; Chao, N.; Gilboa, E.; et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Investig. 2005, 115, 3623–3633. [Google Scholar] [CrossRef]
- Knutson, K.L.; Dang, Y.; Lu, H.; Lukas, J.; Almand, B.; Gad, E.; Azeke, E.; Disis, M.L. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J. Immunol. 2006, 177, 84–91. [Google Scholar] [CrossRef]
- Disis, M.L.; Dang, Y.; Coveler, A.L.; Marzbani, E.; Kou, Z.C.; Childs, J.S.; Fintak, P.; Higgins, D.M.; Reichow, J.; Waisman, J.; et al. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol. Immunother. CII 2014, 63, 101–109. [Google Scholar] [CrossRef]
- Murphy, J.R.; Bacha, P.; Teng, M. Determination of Corynebacterium diphtheriae toxigenicity by a colorimetric tissue culture assay. J. Clin. Microbiol. 1978, 7, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zucker, D.R.; Murphy, J.R. Monoclonal antibody analysis of diphtheria toxin--I. Localization of epitopes and neutralization of cytotoxicity. Mol. Immunol. 1984, 21, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Goodell, V.; Disis, M.L. Human tumor cell lysates as a protein source for the detection of cancer antigen-specific humoral immunity. J. Immunol. Methods 2005, 299, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Schiffman, K.; Guthrie, K.; Salazar, L.G.; Knutson, K.L.; Goodell, V.; dela Rosa, C.; Cheever, M.A. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein--based vaccine. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 1916–1925. [Google Scholar] [CrossRef]
- LeMaistre, C.F.; Meneghetti, C.; Rosenblum, M.; Reuben, J.; Parker, K.; Shaw, J.; Deisseroth, A.; Woodworth, T.; Parkinson, D.R. Phase I trial of an interleukin-2 (IL-2) fusion toxin (DAB486IL-2) in hematologic malignancies expressing the IL-2 receptor. Blood 1992, 79, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Shevach, E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998, 188, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.F.; Duggan, P.J.; Ponchel, F.; Matarese, G.; Lombardi, G.; Edwards, A.D.; Isaacs, J.D.; Lechler, R.I. Human CD4(+)CD25(+) cells: A naturally occurring population of regulatory T cells. Blood 2001, 98, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Yamaguchi, Y.; Matsuura, K.; Murakami, S.; Arihiro, K.; Okada, M. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol. Immunother. CII 2009, 58, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bense, R.D.; Sotiriou, C.; Piccart-Gebhart, M.J.; Haanen, J.B.; van Vugt, M.A.; de Vries, E.G.; Schroder, C.P.; Fehrmann, R.S. Relevance of Tumor-Infiltrating Immune Cell Composition and Functionality for Disease Outcome in Breast Cancer. J. Natl. Cancer Inst. 2017, 109, djw192. [Google Scholar] [CrossRef]
- Petricevic, B.; Laengle, J.; Singer, J.; Sachet, M.; Fazekas, J.; Steger, G.; Bartsch, R.; Jensen-Jarolim, E.; Bergmann, M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Leatherman, J.M.; Sunay, M.E.; Emens, L.A. Abstract 4749: Cyclophosphamide induces dose dependent apoptosis of CD4+FoxP3+ regulatory T cells relative to CD4+FoxP3- effector T cells in breast cancer patients. Cancer Res. 2013, 73, 4749. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Serra, D.; Niedzwiecki, D.; Lyerly, H.K.; Clay, T.M. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 2008, 112, 610–618. [Google Scholar] [CrossRef] [PubMed]
- de Vries, I.J.; Castelli, C.; Huygens, C.; Jacobs, J.F.; Stockis, J.; Schuler-Thurner, B.; Adema, G.J.; Punt, C.J.; Rivoltini, L.; Schuler, G.; et al. Frequency of circulating Tregs with demethylated FOXP3 intron 1 in melanoma patients receiving tumor vaccines and po-tentially Treg-depleting agents. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Attia, P.; Maker, A.V.; Haworth, L.R.; Rogers-Freezer, L.; Rosenberg, S.A. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J. Immunother. 2005, 28, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Foss, F.M.; Bacha, P.; Osann, K.E.; Demierre, M.F.; Bell, T.; Kuzel, T. Biological correlates of acute hypersensitivity events with DAB(389)IL-2 (denileukin diftitox, ONTAK) in cutaneous T-cell lymphoma: Decreased frequency and severity with steroid premedication. Clin. Lymphoma 2001, 1, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Fleming, D.R.; Hall, P.D.; Powell, B.L.; Black, J.H.; Leftwich, C.; Gartenhaus, R. A phase II study of DT fusion protein denileukin diftitox in patients with fludarabine-refractory chronic lymphocytic leukemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 3555–3561. [Google Scholar]
- LeMaistre, C.F.; Saleh, M.N.; Kuzel, T.M.; Foss, F.; Platanias, L.C.; Schwartz, G.; Ratain, M.; Rook, A.; Freytes, C.O.; Craig, F.; et al. Phase I trial of a ligand fusion-protein (DAB389IL-2) in lymphomas expressing the receptor for interleukin-2. Blood 1998, 91, 399–405. [Google Scholar] [PubMed]
| Characteristic | No. of Patients | % | |
|---|---|---|---|
| Age (Median, Range) | 58 (32–69) | 15 | 100 |
| Number of Disease Site(s) | |||
| Bone, LN and soft tissue only (including skin) | 6 | 40 | |
| Visceral | 4 | 26 | |
| Mixed | 4 | 26 | |
| Breast Cancer Subtype | |||
| ER and/or PR+ * | 6 | 40 | |
| HER2+ (overexpressing/amplified) | 5 | 33 | |
| TNBC (ER/PR/HER2-) | 4 | 26 | |
| Number of Prior Anti-Estrogen | |||
| 1–2 regimens | 5 | 33 | |
| ≥ 3 regimens | 5 | 33 | |
| Number of Prior Cytotoxic Chemotherpay | |||
| 2–3 regimens | 7 | 47% | |
| ≥4 | 8 | ||
| Number of Prior HER2 therapies | |||
| 1–2 | 4 | 26 | |
| Months from Last Therapy to Enrollment | |||
| <1 month | 8 | ||
| 2–3 months | 4 | 26 | |
| ≥4 months | 3 | ||
| Protocol 127 Adverse Events (n = 589) | ||||
|---|---|---|---|---|
| Possibly, Probably, or Definitely Related | All AEs | |||
| Most Common | No. | % of Related AEs | No. | % of All AEs |
| Fatigue | 36 | 10 | 36 | 6 |
| Hypoalbuminemia | 27 | 8 | 27 | 5 |
| Hypokalemia | 26 | 7 | 31 | 5 |
| ALT, SGPT elevated | 22 | 6 | 33 | 6 |
| Hypocalcemia | 21 | 6 | 28 | 5 |
| AST, SGOT elevated | 19 | 5 | 34 | 6 |
| Acute vascular leak syndrome | 19 | 5 | 19 | 3 |
| Nausea | 15 | 4 | 22 | 4 |
| Constipation | 12 | 3 | 26 | 4 |
| Hyperglycemia | 4 | 1 | 21 | 4 |
| AE Gradings | ||||
| 1 | 242 | 67 | 416 | 71 |
| 2 | 87 | 24 | 142 | 24 |
| 3 | 30 | 8 | 30 | 5 |
| 4 | 1 | 0 | 1 | 0 |
| 5 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwin, W.R., III; Salazar, L.G.; Dai, J.Y.; Higgins, D.; Coveler, A.L.; Childs, J.S.; Blancas, R.; Dang, Y.; Reichow, J.; Slota, M.; et al. A Phase II Study of Denileukin Diftitox in Patients with Advanced Treatment Refractory Breast Cancer. Vaccines 2025, 13, 117. https://doi.org/10.3390/vaccines13020117
Gwin WR III, Salazar LG, Dai JY, Higgins D, Coveler AL, Childs JS, Blancas R, Dang Y, Reichow J, Slota M, et al. A Phase II Study of Denileukin Diftitox in Patients with Advanced Treatment Refractory Breast Cancer. Vaccines. 2025; 13(2):117. https://doi.org/10.3390/vaccines13020117
Chicago/Turabian StyleGwin, William R., III, Lupe G. Salazar, James Y. Dai, Doreen Higgins, Andrew L. Coveler, Jennifer S. Childs, Rosie Blancas, Yushe Dang, Jessica Reichow, Meredith Slota, and et al. 2025. "A Phase II Study of Denileukin Diftitox in Patients with Advanced Treatment Refractory Breast Cancer" Vaccines 13, no. 2: 117. https://doi.org/10.3390/vaccines13020117
APA StyleGwin, W. R., III, Salazar, L. G., Dai, J. Y., Higgins, D., Coveler, A. L., Childs, J. S., Blancas, R., Dang, Y., Reichow, J., Slota, M., Lu, H., & Disis, M. L. (2025). A Phase II Study of Denileukin Diftitox in Patients with Advanced Treatment Refractory Breast Cancer. Vaccines, 13(2), 117. https://doi.org/10.3390/vaccines13020117






