Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Recombinant Constructs and Protein Preparation
2.3. Protein Stability
2.4. Mouse Immunization and Sample Collection
2.5. ELISA
2.6. Pseudovirus Generation and Neutralization Assay
2.7. Virus Challenge and Evaluation Studies
2.8. Quantification and Statistical Analysis
3. Results
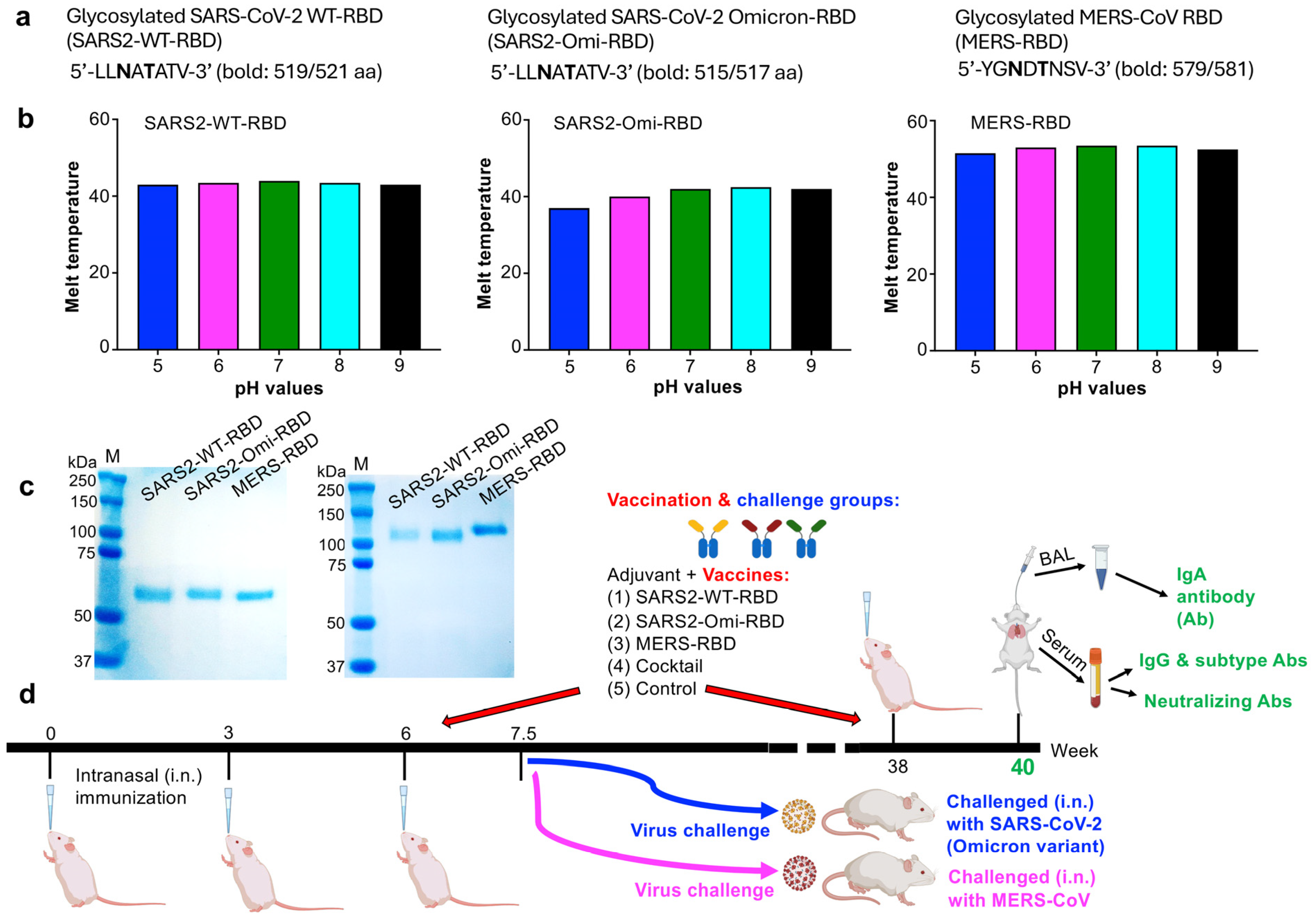
3.1. Characterization of the Glycosylated RBD Subunit Vaccines and Mucosal Immunization
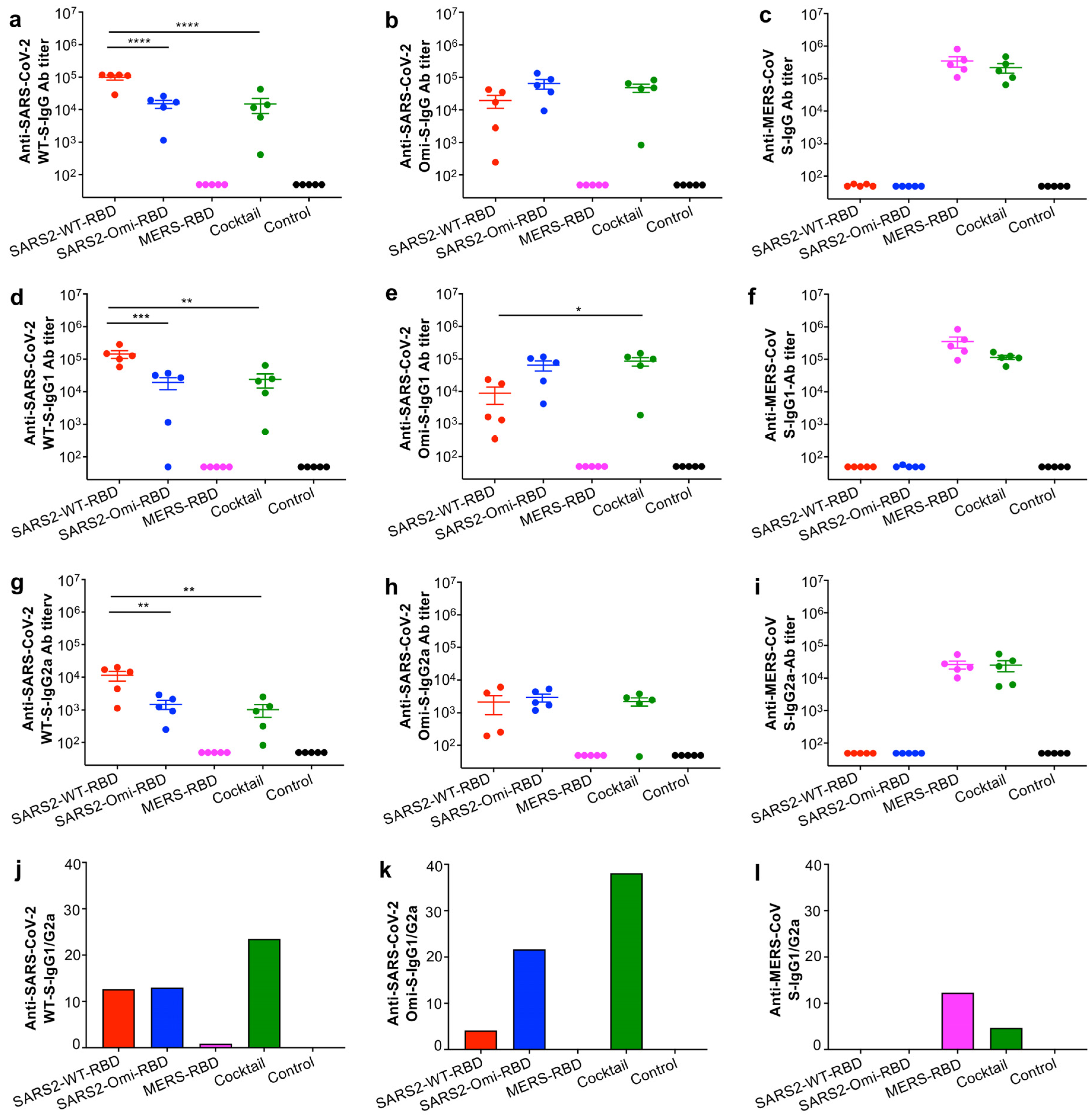
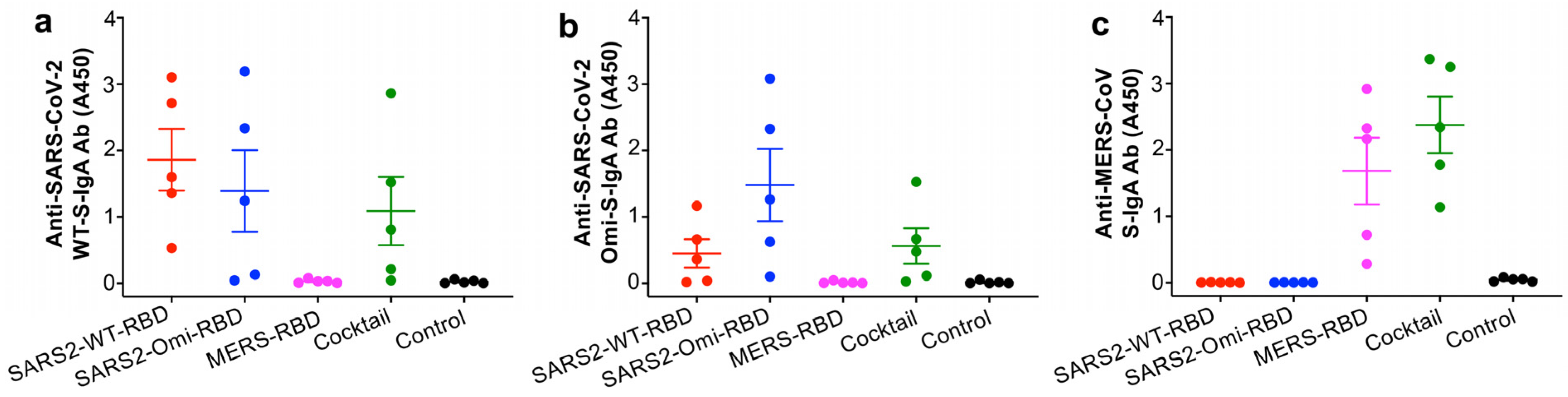
3.2. The Glycosylated RBD Mucosal Subunit Vaccines Elicited Durable Systemic and Mucosal Antibody Responses
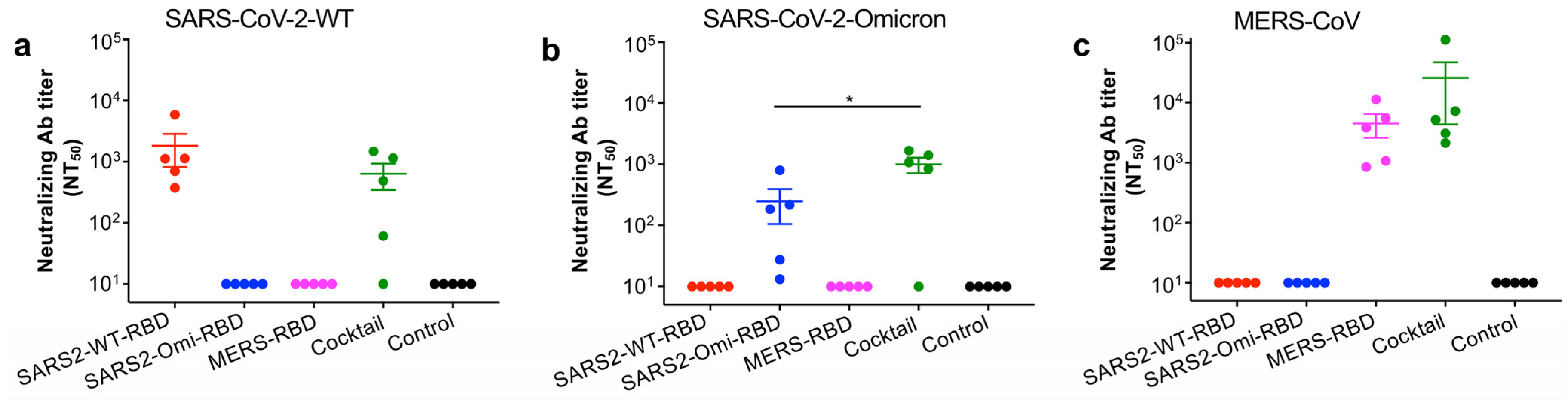
3.3. The Glycosylated RBD Cocktail Subunit Mucosal Vaccine Elicits Durable and Broadly Neutralizing Antibody Responses Against SARS-CoV-2 and MERS-CoV
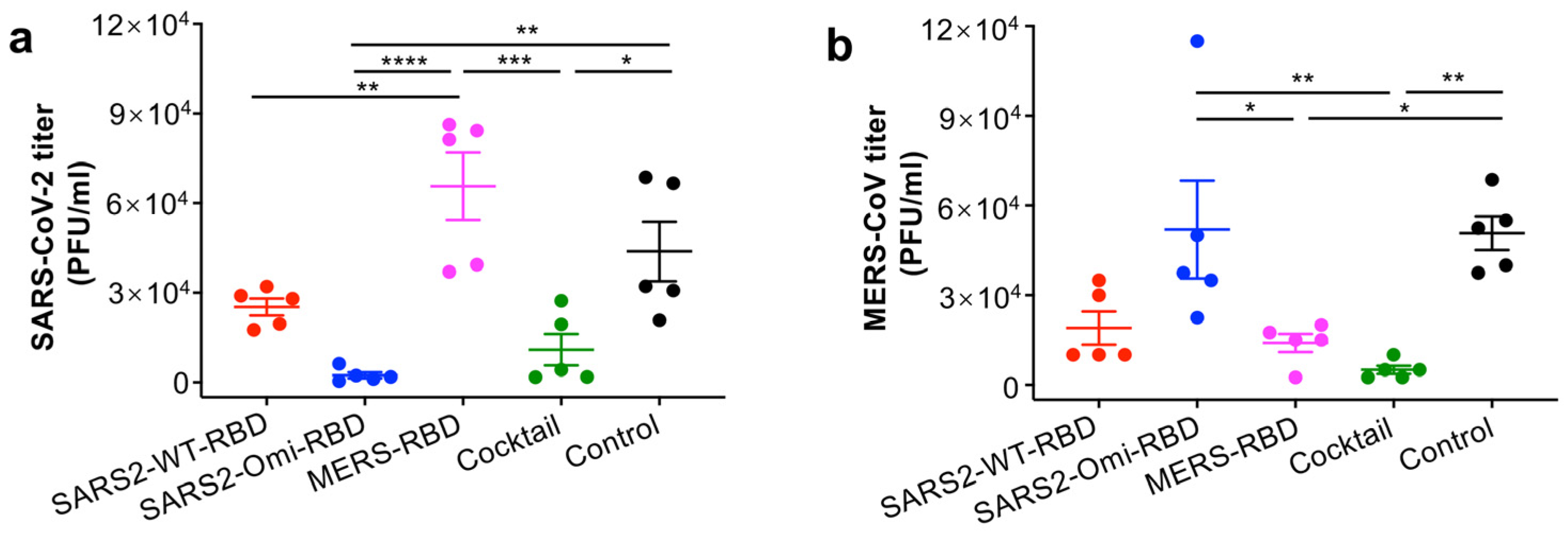
3.4. The Glycosylated RBD Cocktail Subunit Mucosal Vaccine Protects Mice from Challenge with SARS-CoV-2 Omicron and MERS-CoV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A global challenge with old history, epidemiology and progress so far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus (COVID-19) Dashboard. 2025. Available online: https://covid19.who.int/ (accessed on 2 February 2025).
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Manathunga, S.S.; Abeyagunawardena, I.A.; Dharmaratne, S.D. A comparison of transmissibility of SARS-CoV-2 variants of concern. Virol. J. 2023, 20, 59. [Google Scholar] [CrossRef]
- Sarkar, M.; Madabhavi, I. SARS-CoV-2 variants of concern: A review. Monaldi Arch. Chest Dis. 2022, 93. [Google Scholar] [CrossRef]
- Bouhaddou, M.; Reuschl, A.K.; Polacco, B.J.; Thorne, L.G.; Ummadi, M.R.; Ye, C.; Rosales, R.; Pelin, A.; Batra, J.; Jang, G.M.; et al. SARS-CoV-2 variants evolve convergent strategies to remodel the host response. Cell 2023, 186, 4597–4614.e4526. [Google Scholar] [CrossRef]
- Sah, R.; Rais, M.A.; Mohanty, A.; Chopra, H.; Chandran, D.; Bin Emran, T.; Dhama, K. Omicron (B.1.1.529) variant and its subvariants and lineages may lead to another COVID-19 wave in the world? -An overview of current evidence and counteracting strategies. Int. J. Surg. Open 2023, 55, 100625. [Google Scholar] [CrossRef]
- World Helath Organization. Tracking SARS-CoV-2 Variants. 2025. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 3 February 2025).
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Li, F.; Du, L. MERS coronavirus: An emerging zoonotic virus. Viruses 2019, 11, 663. [Google Scholar] [CrossRef]
- Haagmans, B.L.; Al Dhahiry, S.H.; Reusken, C.B.; Raj, V.S.; Galiano, M.; Myers, R.; Godeke, G.J.; Jonges, M.; Farag, E.; Diab, A.; et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect. Dis. 2014, 14, 140–145. [Google Scholar] [CrossRef]
- Azhar, E.I.; El-Kafrawy, S.A.; Farraj, S.A.; Hassan, A.M.; Al-Saeed, M.S.; Hashem, A.M.; Madani, T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014, 370, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Dudas, G.; Carvalho, L.M.; Rambaut, A.; Bedford, T. MERS-CoV spillover at the camel-human interface. Elife 2018, 7, e31257. [Google Scholar] [CrossRef] [PubMed]
- Ogoti, B.M.; Riitho, V.; Wildemann, J.; Mutono, N.; Tesch, J.; Rodon, J.; Harichandran, K.; Emanuel, J.; Möncke-Buchner, E.; Kiambi, S.; et al. Biphasic MERS-CoV incidence in nomadic dromedaries with putative transmission to humans, Kenya, 2022–2023. Emerg. Infect. Dis. 2024, 30, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, A.A.; Grant, R.; Assiri, A.; Elhakim, M.; Malik, M.R.; Van Kerkhove, M.D. MERS-CoV infection among healthcare workers and risk factors for death: Retrospective analysis of all laboratory-confirmed cases reported to WHO from 2012 to 2 June 2018. J. Infect. Public Health 2020, 13, 418–422. [Google Scholar] [CrossRef]
- Drosten, C.; Meyer, B.; Müller, M.A.; Corman, V.M.; Al-Masri, M.; Hossain, R.; Madani, H.; Sieberg, A.; Bosch, B.J.; Lattwein, E.; et al. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014, 371, 828–835. [Google Scholar] [CrossRef]
- Oboho, I.K.; Tomczyk, S.M.; Al-Asmari, A.M.; Banjar, A.A.; Al-Mugti, H.; Aloraini, M.S.; Alkhaldi, K.Z.; Almohammadi, E.L.; Alraddadi, B.M.; Gerber, S.I.; et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N. Engl. J. Med. 2015, 372, 846–854. [Google Scholar] [CrossRef]
- Petersen, E.; Pollack, M.M.; Madoff, L.C. Health-care associate transmission of Middle East respiratory syndrome coronavirus, MERS-CoV, in the Kingdom of Saudi Arabia. Int. J. Infect. Dis. 2014, 29, 299–300. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control. MERS-CoV Worldwide Overview. 2025. Available online: https://www.ecdc.europa.eu/en/middle-east-respiratory-syndrome-coronavirus-mers-cov-situation-update (accessed on 5 February 2025).
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wong, G.; Lu, G.; Yan, J.; Gao, G.F. MERS-CoV spike protein: Targets for vaccines and therapeutics. Antiviral Res. 2016, 133, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Chen, Y.; Rajashankar, K.R.; Yang, Y.; Agnihothram, S.S.; Liu, C.; Lin, Y.L.; Baric, R.S.; Li, F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013, 87, 10777–10783. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef]
- Martina, C.E.; Crowe, J.E.; Meiler, J. Glycan masking in vaccine design: Targets, immunogens and applications. Front. Immunol. 2023, 14, 1126034. [Google Scholar] [CrossRef]
- Guan, X.; Yang, Y.; Du, L. Advances in SARS-CoV-2 receptor-binding domain-based COVID-19 vaccines. Expert. Rev. Vaccines 2023, 22, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, J.; Tai, W.; Verma, A.K.; Zhang, X.; Geng, Q.; Wang, G.; Guan, X.; Malisheni, M.M.; Odle, A.E.; et al. A glycosylated RBD protein induces enhanced neutralizing antibodies against Omicron and other variants with improved protection against SARS-CoV-2 infecnion. J. Virol. 2022, 96, e0011822. [Google Scholar] [CrossRef]
- Du, L.; Tai, W.; Yang, Y.; Zhao, G.; Zhu, Q.; Sun, S.; Liu, C.; Tao, X.; Tseng, C.K.; Perlman, S.; et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat. Commun. 2016, 7, 13473. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.M. Mucosal immunity in COVID-19: A comprehensive review. Front. Immunol. 2024, 15, 1433452. [Google Scholar] [CrossRef]
- Kaiser, J.A.; Nelson, C.E.; Liu, X.; Park, H.S.; Matsuoka, Y.; Luongo, C.; Santos, C.; Ahlers, L.R.H.; Herbert, R.; Moore, I.N.; et al. Mucosal prime-boost immunization with live murine pneumonia virus-vectored SARS-CoV-2 vaccine is protective in macaques. Nat. Commun. 2024, 15, 3553. [Google Scholar] [CrossRef]
- Park, S.I.; Park, S.; Lee, K.; Kwak, H.W.; Kim, Y.K.; Park, H.J.; Bang, Y.J.; Kim, J.Y.; Kim, D.; Seo, K.W.; et al. Intranasal immunization with the recombinant measles virus encoding the spike protein of SARS-CoV-2 confers protective immunity against COVID-19 in hamsters. Vaccine 2024, 42, 69–74. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, E.; Guo, J.; Zhang, X.; Wang, L.; Chen, D.; Fang, X.; Zhu, J.; Li, F.; Sun, T.; et al. Immunogenicity of mucosal COVID-19 vaccine candidates based on the highly attenuated vesicular stomatitis virus vector (VSV). Acta Pharm. Sin. B 2023, 13, 4856–4874. [Google Scholar] [CrossRef]
- Gagne, M.; Flynn, B.J.; Andrew, S.F.; Marquez, J.; Flebbe, D.R.; Mychalowych, A.; Lamb, E.; Davis-Gardner, M.E.; Burnett, M.R.; Serebryannyy, L.A.; et al. Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates. Nat. Immunol. 2024, 25, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.; Lee, D.Y.; Lee, S.H.; Jeong, J.H.; Kim, J.Y.; Kim, J.; Choi, Y.K.; Lee, J.B.; Park, S.Y.; et al. Intranasal administration of recombinant Newcastle Disease virus expressing SARS-CoV-2 spike protein protects hACE2 TG mice against lethal SARS-CoV-2 infection. Vaccines 2024, 12, 921. [Google Scholar] [CrossRef]
- Liu, X.; Ng, W.H.; Zusinaite, E.; Freitas, J.; Taylor, A.; Yerragunta, V.; Aavula, S.M.; Gorriparthi, S.; Ponsekaran, S.; Bonda, R.L.; et al. A single-dose intranasal live-attenuated codon deoptimized vaccine provides broad protection against SARS-CoV-2 and its variants. Nat. Commun. 2024, 15, 7225. [Google Scholar] [CrossRef]
- Honda, T.; Toyama, S.; Matsumoto, Y.; Sanada, T.; Yasui, F.; Koseki, A.; Kono, R.; Yamamoto, N.; Kamishita, T.; Kodake, N.; et al. Intranasally inoculated SARS-CoV-2 spike protein combined with mucoadhesive polymer induces broad and long-lasting immunity. Vaccines 2024, 12, 794. [Google Scholar] [CrossRef]
- Leekha, A.; Saeedi, A.; Sefat, K.M.S.R.; Kumar, M.; Martinez-Paniagua, M.; Damian, A.; Kulkarni, R.; Reichel, K.; Rezvan, A.; Masoumi, S.; et al. Multi-antigen intranasal vaccine protects against challenge with sarbecoviruses and prevents transmission in hamsters. Nat. Commun. 2024, 15, 6193. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Dahlke, C.; Krähling, V.; Kupke, A.; Okba, N.M.A.; Raadsen, M.P.; Heidepriem, J.; Müller, M.A.; Paris, G.; Lassen, S.; et al. Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome. Nat. Commun. 2022, 13, 4182. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Morsy, M.A.; Abd El-Lateef, H.M.; Marzok, M.; El-Beltagi, H.S.; Al Khodair, K.M.; Albokhadaim, I.; Venugopala, K.N. Safety and immunogenicity of the ChAdOx1, MVA-MERS-S, and GLS-5300 DNA MERS-CoV vaccines. Int. Immunopharmacol. 2023, 118, 109998. [Google Scholar] [CrossRef] [PubMed]
- Bosaeed, M.; Balkhy, H.H.; Almaziad, S.; Aljami, H.A.; Alhatmi, H.; Alanazi, H.; Alahmadi, M.; Jawhary, A.; Alenazi, M.W.; Almasoud, A.; et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): An open-label, non-randomised, dose-escalation, phase 1b trial. Lancet Microbe 2022, 3, e11–e20. [Google Scholar] [CrossRef]
- Patel, A.; Reuschel, E.L.; Xu, Z.; Zaidi, F.I.; Kim, K.Y.; Scott, D.P.; Mendoza, J.; Ramos, S.; Stoltz, R.; Feldmann, F.; et al. Intradermal delivery of a synthetic DNA vaccine protects macaques from Middle East respiratory syndrome coronavirus. JCI Insight 2021, 6, e146082. [Google Scholar] [CrossRef]
- Chang, C.C.; Algaissi, A.; Lai, C.C.; Chang, C.K.; Lin, J.S.; Wang, Y.S.; Chang, B.H.; Chang, Y.C.; Chen, W.T.; Fan, Y.Q.; et al. Subunit vaccines with a saponin-based adjuvant boost humoral and cellular immunity to MERS coronavirus. Vaccine 2023, 41, 3337–3346. [Google Scholar] [CrossRef]
- Almansour, I.; Jermy, B.R. Nucleic acid vaccine candidates encapsulated with mesoporous silica nanoparticles against MERS-CoV. Hum. Vaccin. Immunother. 2024, 20, 2346390. [Google Scholar] [CrossRef]
- Gutiérrez-Álvarez, J.; Honrubia, J.M.; Sanz-Bravo, A.; González-Miranda, E.; Fernández-Delgado, R.; Rejas, M.T.; Zúñiga, S.; Sola, I.; Enjuanes, L. Middle East respiratory syndrome coronavirus vaccine based on a propagation-defective RNA replicon elicited sterilizing immunity in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2111075118. [Google Scholar] [CrossRef]
- Tai, W.; Zheng, J.; Zhang, X.; Shi, J.; Wang, G.; Guan, X.; Zhu, J.; Perlman, S.; Du, L. MERS-CoV RBD-mRNA vaccine induces potent and broadly neutralizing antibodies with protection against MERS-CoV infection. Virus Res. 2023, 334, 199156. [Google Scholar] [CrossRef]
- Wang, G.; Verma, A.K.; Guan, X.; Bu, F.; Odle, A.E.; Li, F.; Liu, B.; Perlman, S.; Du, L. Pan-beta-coronavirus subunit vaccine prevents SARS-CoV-2 Omicron, SARS-CoV, and MERS-CoV challenge. J. Virol. 2024, 98, e0037624. [Google Scholar] [CrossRef]
- Tai, W.; Wang, Y.; Fett, C.A.; Zhao, G.; Li, F.; Perlman, S.; Jiang, S.; Zhou, Y.; Du, L. Recombinant receptor-binding domains of multiple Middle East respiratory syndrome coronaviruses (MERS-CoVs) induce cross-neutralizing antibodies against divergent human and camel MERS-CoVs and antibody escape mutants. J. Virol. 2016, 91, e01651-16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shang, J.; Li, C.; Zhou, K.; Du, L. An overview of Middle East respiratory syndrome coronavirus vaccines in preclinical studies. Expert. Rev. Vaccines 2020, 19, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, J.H.; Park, J.Y.; Jun, S.H.; Shin, H.J. A chimeric MERS-CoV virus-like particle vaccine protects mice against MERS-CoV challenge. Virol. J. 2022, 19, 112. [Google Scholar] [CrossRef]
- Lee, P.; Kim, J.; Oh, H.; Kim, C.U.; Jeong, A.Y.; Lee, M.S.; Jang, M.S.; Hong, J.J.; Park, J.E.; Kim, D.J. Coronavirus nucleocapsid-based vaccine provides partial protection against hetero-species coronavirus in murine models. Antiviral Res. 2024, 231, 105991. [Google Scholar] [CrossRef]
- Veit, S.; Jany, S.; Fux, R.; Sutter, G.; Volz, A. CD8+ T cells responding to the Middle East respiratory syndrome coronavirus nucleocapsid protein delivered by vaccinia virus MVA in mice. Viruses 2018, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Generotti, A.; Contreras, R.; Zounes, B.; Schade, E.; Kemme, A.; Rane, Y.; Liu, X.; Elwood, D.; Schultheis, K.; Marston, J.; et al. Intradermal DNA vaccine delivery using vacuum-controlled, needle-free electroporation. Mol. Ther. Nucleic Acids 2023, 34, 102070. [Google Scholar] [CrossRef]
- Guan, X.; Verma, A.K.; Wang, G.; Shi, J.; Perlman, S.; Du, L. Glycosylated Delta-receptor-binding domain mucosal vaccine elicits broadly neutralizing antibodies with protection against SARS-CoV-2 challenge. iScience 2023, 26, 108033. [Google Scholar] [CrossRef]
- Pilewski, K.A.; Kramer, K.J.; Georgiev, I.S. Simultaneous immunization with multiple diverse immunogens alters development of antigen-specific antibody-mediated immunity. Vaccines 2021, 9, 964. [Google Scholar] [CrossRef]
- Ramirez, S.I.; Lopez, P.G.; Faraji, F.; Parikh, U.M.; Heaps, A.; Ritz, J.; Moser, C.; Eron, J.J.; Wohl, D.; Currier, J.; et al. Early antiviral CD4+ and CD8+ T cells are associated with upper airway clearance of SARS-CoV-2. JCI Insight 2024, 9, e186078. [Google Scholar] [CrossRef]
- Shrwani, K.J.; Mahallawi, W.H.; Mohana, A.I.; Algaissi, A.; Dhayhi, N.; Sharwani, N.J.; Gadour, E.; Aldossari, S.M.; Asiri, H.; Kameli, N.; et al. Mucosal immunity in upper and lower respiratory tract to MERS-CoV. Front. Immunol. 2024, 15, 1358885. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Liu, W.C.; Lin, C.Y.; Liu, C.C.; Jan, J.T.; Spearman, M.; Butler, M.; Wu, S.C. Glycan-masking hemagglutinin antigens from stable CHO cell clones for H5N1 avian influenza vaccine development. Biotechnol. Bioeng. 2019, 116, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Carnell, G.W.; Billmeier, M.; Vishwanath, S.; Suau, S.M.; Wein, H.; George, C.L.; Neckermann, P.; Del Rosario, J.M.M.; Sampson, A.T.; Einhauser, S.; et al. Glycan masking of a non-neutralising epitope enhances neutralising antibodies targeting the RBD of SARS-CoV-2 and its variants. Front. Immunol. 2023, 14, 1118523. [Google Scholar] [CrossRef]
- Anish, C.; Beurret, M.; Poolman, J. Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines. NPJ Vaccines 2021, 6, 150. [Google Scholar] [CrossRef]
- Liang, Z.; Li, C.; Gong, X.; Ye, G.; Jiang, Y.; Shi, H.; Hussain, A.; Zhao, M.; Li, M.; Tian, Y.; et al. Development of Glycan-masked SARS-CoV-2 RBD vaccines against SARS-related coronaviruses. PLoS Pathog. 2024, 20, e1012599. [Google Scholar] [CrossRef]
- Wei, Z.; Angrisano, F.; Eriksson, E.M.; Mazhari, R.; Van, H.; Zheng, S.; Center, R.J.; Boo, I.; McMahon, J.; Lau, J.; et al. Serological assays to measure dimeric IgA antibodies in SARS-CoV-2 infections. Immunol. Cell Biol. 2023, 101, 857–866. [Google Scholar] [CrossRef]
- Stowell, N.C.; Seideman, J.; Raymond, H.A.; Smalley, K.A.; Lamb, R.J.; Egenolf, D.D.; Bugelski, P.J.; Murray, L.A.; Marsters, P.A.; Bunting, R.A.; et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir. Res. 2009, 10, 43. [Google Scholar] [CrossRef]
- Tian, X.; Xu, F.; Lung, W.Y.; Meyerson, C.; Ghaffari, A.A.; Cheng, G.; Deng, J.C. Poly I:C enhances susceptibility to secondary pulmonary infections by gram-positive bacteria. PLoS ONE 2012, 7, e41879. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos, J.S.; Firmino-Cruz, L.; da Fonseca-Martins, A.M.; Oliveira-Maciel, D.; Perez, G.G.; Roncaglia-Pereira, V.A.; Dumard, C.H.; Guedes-da-Silva, F.H.; Santos, A.C.V.; Leandro, M.D.S.; et al. Immunogenicity of SARS-CoV-2 trimeric spike protein associated to Poly(I:C) plus Alum. Front. Immunol. 2022, 13, 884760. [Google Scholar] [CrossRef]
- Becker, W.; Rebbani, K.; Duan, Z.; Valkov, E.; Bryant, S.; Ho, M.; Berzofsky, J.A.; Olkhanud, P.B. Adjuvants to the S1-subunit of the SARS-CoV-2 spike protein vaccine improve antibody and T cell responses and surrogate neutralization in mice. Sci. Rep. 2024, 14, 29609. [Google Scholar] [CrossRef]
- Migliorini, D.; Dutoit, V.; Allard, M.; Grandjean Hallez, N.; Marinari, E.; Widmer, V.; Philippin, G.; Corlazzoli, F.; Gustave, R.; Kreutzfeldt, M.; et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro. Oncol. 2019, 21, 923–933. [Google Scholar] [CrossRef]
- Nakajima, M.; Hazama, S.; Tamada, K.; Udaka, K.; Kouki, Y.; Uematsu, T.; Arima, H.; Saito, A.; Doi, S.; Matsui, H.; et al. A phase I study of multi-HLA-binding peptides derived from heat shock protein 70/glypican-3 and a novel combination adjuvant of hLAG-3Ig and Poly-ICLC for patients with metastatic gastrointestinal cancers: YNP01 trial. Cancer Immunol. Immunother. 2020, 69, 1651–1662. [Google Scholar] [CrossRef]
- De Waele, J.; Verhezen, T.; van der Heijden, S.; Berneman, Z.N.; Peeters, M.; Lardon, F.; Wouters, A.; Smits, E.L.J.M. A systematic review on poly(I:C) and poly-ICLC in glioblastoma: Adjuvants coordinating the unlocking of immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 213. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Longet, S.; Lundahl, M.L.E.; Lavelle, E.C. Targeted strategies for mucosal vaccination. Bioconjug. Chem. 2018, 29, 613–623. [Google Scholar] [CrossRef]
- Xi, J.; Lei, L.R.; Zouzas, W.; April Si, X. Nasally inhaled therapeutics and vaccination for COVID-19: Developments and challenges. MedComm 2021, 2, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Bellier, B.; Saura, A.; Luján, L.A.; Molina, C.R.; Luján, H.D.; Klatzmann, D. A thermostable oral SARS-CoV-2 vaccine induces mucosal and protective immunity. Front. Immunol. 2022, 13, 837443. [Google Scholar] [CrossRef]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Verma, A.K.; Liu, Q.; Palacios, M.; Odle, A.E.; Perlman, S.; Du, L. Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV. Vaccines 2025, 13, 293. https://doi.org/10.3390/vaccines13030293
Guan X, Verma AK, Liu Q, Palacios M, Odle AE, Perlman S, Du L. Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV. Vaccines. 2025; 13(3):293. https://doi.org/10.3390/vaccines13030293
Chicago/Turabian StyleGuan, Xiaoqing, Abhishek K. Verma, Qian Liu, Melissa Palacios, Abby E. Odle, Stanley Perlman, and Lanying Du. 2025. "Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV" Vaccines 13, no. 3: 293. https://doi.org/10.3390/vaccines13030293
APA StyleGuan, X., Verma, A. K., Liu, Q., Palacios, M., Odle, A. E., Perlman, S., & Du, L. (2025). Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV. Vaccines, 13(3), 293. https://doi.org/10.3390/vaccines13030293





