Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges
Abstract
1. Introduction
2. Key Factors Influencing Immunotherapy and Vaccine Selection in Breast Cancer
2.1. Breast Cancer Classification
2.2. Tumor Mutation Burden (TMB)
2.3. Tumour-Infiltrating Lymphocytes (TILs)
2.4. PD-L1 Expression
2.5. HER2 Resistance
2.6. Tumor Microenvironment (TME)
2.6.1. Cancer Cells: Heterogeneity and Adaptability
2.6.2. Stromal Cells: The Supportive Niche
2.6.3. Vasculature: Tumor Angiogenesis and Hypoxia
2.6.4. Infiltrating Immune Cells: A Double-Edged Sword
2.6.5. Crosstalk Between TME Components
2.6.6. Therapeutic Implications: Targeting the Breast TME
2.6.7. Conclusions
3. Anticancer Immunotherapy Strategy Against Breast Cancer
3.1. HER2 Block: Targeting Oncogenic Signaling Pathways in Cancer Therapy
3.1.1. Monoclonal Antibodies
3.1.2. Small Molecule Tyrosine Kinase Inhibitors (TKIs)
3.1.3. Antibody-Drug Conjugates (ADCs)
3.1.4. Challenges and Future Directions
3.2. Immune Checkpoint Inhibition: Revolutionizing Cancer Immunotherapy
3.2.1. Mechanisms of Immune Checkpoint Inhibition
3.2.2. PD-1/PD-L1 Axis
3.2.3. CTLA-4 Pathway
3.2.4. Clinical Applications
3.3. Chimeric Antigen Receptor Macrophages (CAR-M) as a Novel Immunotherapeutic Approach in Breast Cancer
3.4. Advantages and Disadvantages of Immunotherapy for Breast Cancer
3.4.1. Advantages of Immunotherapy for Breast Cancer
- Enhanced tumor-specific response: unlike chemotherapy, which affects both cancerous and healthy cells, immunotherapy activates the immune system to specifically target cancer cells [193].
- Long-lasting immune memory: some immunotherapies, particularly immune checkpoint inhibitors (ICIs), can induce long-term immune memory, reducing the chances of recurrence.
- Reduced systemic toxicity: compared to chemotherapy, immunotherapies often have fewer severe side effects, as they do not directly damage healthy tissues [194].
- Effectiveness in triple-negative breast cancer (TNBC): TNBC lacks hormone receptors and HER2, making it resistant to targeted therapies. However, immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1) combined with chemotherapy have shown efficacy in TNBC [195].
- Personalized treatment approach: advances in biomarkers (e.g., PD-L1 expression, tumor mutational burden) enable patient selection for immunotherapy, increasing its effectiveness in suitable candidates [197].
3.4.2. Disadvantages of Immunotherapy for Breast Cancer
- Limited efficacy in some subtypes: immunotherapy is most effective in TNBC, but hormone receptor-positive (HR+) and HER2-positive breast cancers tend to have lower immunogenicity, making them less responsive [140].
- Immune-related adverse events (irAEs): the overactivation of the immune system can lead to autoimmune-like side effects, such as colitis, pneumonitis, hepatitis, and thyroid dysfunction [198].
- High cost of treatment: immunotherapies, particularly monoclonal antibodies and immune checkpoint inhibitors, are expensive and may not be accessible to all patients [199].
- Development of resistance: some tumors develop mechanisms to evade immune detection, leading to primary or acquired resistance [200].
- Delayed response or pseudo-progression: some patients experience an initial increase in tumor size (pseudo-progression) before seeing a clinical response, making treatment evaluation challenging [201].
- Need for biomarker identification: the effectiveness of immunotherapy depends on biomarkers like PD-L1 expression and tumor mutational burden, which may not always be present in breast cancer patients [202].
4. Anticancer Vaccines in Breast Cancer
4.1. Protein- or Whole-Cell-Based Vaccines
4.2. DNA and RNA Vaccines
4.2.1. DNA Vaccines
4.2.2. RNA Vaccines
4.3. Dendritic Cell (DC) Vaccines
4.4. Viral Vector-Based Vaccines
4.5. Epitope-Based Vaccine Against Breast Cancer
4.5.1. HER2
- Epitopes from HER2 extracellular domain
- Epitopes from HER2 transmembrane domain
- Epitopes from HER2 intracellular domain
4.5.2. MUC1
4.5.3. Multi-Peptide Vaccines
4.5.4. MAGEA
4.5.5. Triple Peptide Vaccination
4.6. Advantages and Disadvantages of Anticancer Vaccines in Breast Cancer Treatment
4.6.1. Advantages of Anticancer Vaccines for Breast Cancer
- Specific immune activation: anticancer vaccines stimulate the immune system to recognize and eliminate cancer cells, minimizing damage to normal tissues compared to chemotherapy or radiation [301].
- Potential for long-term protection: once trained, the immune system can maintain memory against cancer antigens, reducing the risk of recurrence and metastasis [302].
- Low toxicity compared to traditional therapies: unlike chemotherapy and radiation, vaccines generally have fewer systemic side effects, since they do not directly kill cells but rather enhance immune surveillance [303].
- Potential to target minimal residual disease: vaccines may help eliminate residual cancer cells after surgery, reducing the likelihood of relapse [304].
- Application in high-risk patients: individuals with high genetic risk (e.g., BRCA1/2 mutations) could benefit from prophylactic cancer vaccines in the future, similar to HPV vaccines for cervical cancer prevention [305].
- Combination potential with other therapies: cancer vaccines can be combined with immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1), chemotherapy, or radiotherapy to enhance therapeutic efficacy [306].
4.6.2. Disadvantages of Anticancer Vaccines for Breast Cancer
- Limited efficacy in established tumors: breast tumors often create an immunosuppressive microenvironment, making it difficult for vaccines to generate a strong immune response [307].
- Tumor antigen heterogeneity: breast cancer is highly heterogeneous, and different patients may express different tumor antigens, making it challenging to develop a universal vaccine [308].
- Slow onset of action: unlike chemotherapy or targeted therapy, which can show effects quickly, vaccines take time to stimulate an immune response, making them less effective for patients with rapidly progressing disease.
- Risk of autoimmune reactions: some tumor-associated antigens are also present in normal tissues, and immune activation against these antigens may cause autoimmune side effects [309].
- Need for effective biomarker identification: predicting which patients will respond to cancer vaccines is challenging, requiring the identification of biomarkers to select appropriate candidates [310].
- Limited success in clinical trials: while promising in preclinical studies, many anticancer vaccines for breast cancer have failed in clinical trials due to weak immunogenicity or lack of efficacy in advanced disease.
- High cost and regulatory challenges: developing personalized or peptide-based cancer vaccines is expensive, and regulatory approval can be slow due to the need for extensive clinical validation.
5. Future Directions
5.1. Enhancing Immunotherapy Efficacy Through Biomarker Integration
5.2. Addressing Tumor Heterogeneity, TME, and Resistance Mechanisms
5.3. Advancing Cancer Vaccine Development
5.4. Personalizing Treatment Through Molecular, Genomic, and Immunological Profiling
5.5. Exploring Combination Strategies to Overcome Resistance
5.6. Expanding Clinical Trials and Inclusive Research
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apantaku, L.M. Breast-conserving surgery for breast cancer. Am. Fam. Physician 2002, 66, 2271–2278. [Google Scholar] [PubMed]
- Lumachi, F. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231. [Google Scholar] [CrossRef]
- Verhoog, N.J.D.; Spies, L.-M.L. The anti-aromatase and anti-estrogenic activity of plant products in the treatment of estrogen receptor-positive breast cancer. J. Steroid Biochem. Mol. Biol. 2024, 243, 106581. [Google Scholar] [CrossRef]
- Sriramulu, S.; Thoidingjam, S.; Speers, C.; Nyati, S. Present and Future of Immunotherapy for Triple-Negative Breast Cancer. Cancers 2024, 16, 3250. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Shimizu, J.; Kubo, Y.; Tabata, N.; Aso, T. Quantitative classification of invasive and noninvasive breast cancer using dynamic magnetic resonance imaging of the mammary gland. J. Clin. Imaging Sci. 2022, 12, 45. [Google Scholar] [CrossRef]
- Ma, W.; Pham, B.; Li, T. Cancer neoantigens as potential targets for immunotherapy. Clin. Exp. Metastasis 2022, 39, 51–60. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. eClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J.; Hao, X.; Shi, H.; Li, X.; Wang, A.; Hu, Z.; Yang, Y.; Jiang, Z.; Wang, T. Efficacy relevance of PD-L1 expression on circulating tumor cells in metastatic breast cancer patients treated with anti-PD-1 immunotherapy. Breast Cancer Res. Treat. 2023, 200, 281–291. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Cheng, Y.; Yang, J.; Liu, S.; Ma, T.; Luo, L.; Hu, Y.; Cai, Y.; Yan, D. Case Report: Addition of PD-1 Antibody Camrelizumab Overcame Resistance to Trastuzumab Plus Chemotherapy in a HER2-Positive, Metastatic Gallbladder Cancer Patient. Front. Immunol. 2022, 12, 784861. [Google Scholar] [CrossRef]
- Akinsipe, T.; Mohamedelhassan, R.; Akinpelu, A.; Pondugula, S.R.; Mistriotis, P.; Avila, L.A.; Suryawanshi, A. Cellular interactions in tumor microenvironment during breast cancer progression: New frontiers and implications for novel therapeutics. Front. Immunol. 2024, 15, 1302587. [Google Scholar] [CrossRef]
- Sammons, S.; Elliott, A.; Barroso-Sousa, R.; Chumsri, S.; Tan, A.R.; Sledge, G.W.; Tolaney, S.M.; Torres, E.T.R. Concurrent predictors of an immune responsive tumor microenvironment within tumor mutational burden-high breast cancer. Front. Oncol. 2023, 13, 1235902. [Google Scholar] [CrossRef]
- Sammons, S.; Elliott, A.; Force, J.M.; DeVito, N.C.; Marcom, P.K.; Swain, S.M.; Tan, A.R.; Roussos Torres, E.T.; Zeng, J.; Khasraw, M.; et al. Genomic evaluation of tumor mutational burden-high (TMB-H) versus TMB-low (TMB-L) metastatic breast cancer to reveal unique mutational features. J. Clin. Oncol. 2021, 39 (Suppl. S15), 1091. [Google Scholar] [CrossRef]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef] [PubMed]
- Sobral-Leite, M.; Van de Vijver, K.; Michaut, M.; van der Linden, R.; Hooijer, G.K.J.; Horlings, H.M.; Severson, T.M.; Mulligan, A.M.; Weerasooriya, N.; Sanders, J.; et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1 -like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018, 7, e1509820. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Liu, Z.; Yu, Q.; Wang, X.; Bian, M.; Yu, Z.; Yu, J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 2019, 9, 14356. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Luo, M.; Huang, J.; Zhang, K.; Zheng, S.; Zhang, S.; Zhou, J. Progression from ductal carcinoma in situ to invasive breast cancer: Molecular features and clinical significance. Signal Transduct. Target. Ther. 2024, 9, 83. [Google Scholar] [CrossRef]
- Posner, M.C.; Wolmark, N. Non-invasive breast carcinoma. Breast Cancer Res. Treat. 1992, 21, 155–164. [Google Scholar] [CrossRef]
- Borst, M.J.; Ingold, J.A. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993, 114, 637–641, discussion 641–642. [Google Scholar]
- Yersal, O. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492. [Google Scholar] [CrossRef]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233–1239, discussion 1239–1240+1243. [Google Scholar] [PubMed]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Immunotherapy for triple-negative breast cancer: A molecular insight into the microenvironment, treatment, and resistance. J. Natl. Cancer Cent. 2021, 1, 75–87. [Google Scholar] [CrossRef]
- Mo, S.-F.; Cai, Z.-Z.; Kuai, W.-H.; Li, X.; Chen, Y.-T. Universal cutoff for tumor mutational burden in predicting the efficacy of anti-PD-(L)1 therapy for advanced cancers. Front. Cell Dev. Biol. 2023, 11, 1209243. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Luen, S.J.; Salgado, R.; Fox, S.; Savas, P.; Eng-Wong, J.; Clark, E.; Kiermaier, A.; Swain, S.M.; Baselga, J.; Michiels, S.; et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: A retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017, 18, 52–62. [Google Scholar] [CrossRef]
- Michaels, E.; Chen, N.; Nanda, R. The Role of Immunotherapy in Triple-Negative Breast Cancer (TNBC). Clin. Breast Cancer 2024, 24, 263–270. [Google Scholar] [CrossRef]
- Matsueda, S.; Chen, L.; Li, H.; Yao, H.; Yu, F. Recent clinical researches and technological development in TIL therapy. Cancer Immunol. Immunother. 2024, 73, 232. [Google Scholar] [CrossRef] [PubMed]
- June, C.H. Adoptive T cell therapy for cancer in the clinic. J. Clin. Investig. 2007, 117, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Chic, N.; Ciruelos, E.; Saura, C.; Gonzalez, E.-A.; Álvarez-Vallina, L.; Lasarte, J.J.; Gros, A.; Villanueva, L.; Canes, J.; Angelats, L.; et al. Abstract OT2-10-04: Treatment of advanced or metastatic triple-negative breast cancer with adoptive therapy of PD1+ tumor-infiltrating lymphocytes (TILS001 trial). Cancer Res. 2023, 83 (Suppl. S5), OT2-10-04. [Google Scholar] [CrossRef]
- L’Orphelin, J.-M.; Lancien, U.; Nguyen, J.-M.; Coronilla, F.J.S.; Saiagh, S.; Cassecuel, J.; Boussemart, L.; Dompmartin, A.; Dréno, B. NIVO-TIL: Combination anti-PD-1 therapy and adoptive T-cell transfer in untreated metastatic melanoma: An exploratory open-label phase I trial. Acta Oncol. 2024, 63, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front. Immunol. 2022, 13, 1018962. [Google Scholar] [CrossRef]
- Albarrán Fernández, V.; Ballestín Martínez, P.; Stoltenborg Granhøj, J.; Borch, T.H.; Donia, M.; Marie Svane, I. Biomarkers for response to TIL therapy: A comprehensive review. J. Immunother. Cancer 2024, 12, e008640. [Google Scholar] [CrossRef]
- Niu, Z.; Wu, J.; Zhao, Q.; Zhang, J.; Zhang, P.; Yang, Y. CAR-based immunotherapy for breast cancer: Peculiarities, ongoing investigations, and future strategies. Front. Immunol. 2024, 15, 1385571. [Google Scholar] [CrossRef]
- Qu, R.; Zhao, Y.; Zhang, Y. The mechanism of cytokine regulation of cancer occurrence and development in the tumor microenvironment and its application in cancer treatment: A narrative review. Transl. Cancer Res. 2024, 13, 5649–5663. [Google Scholar] [CrossRef]
- Grandal, B.; Mangiardi-Veltin, M.; Laas, E.; Laé, M.; Meseure, D.; Bataillon, G.; El-Alam, E.; Darrigues, L.; Dumas, E.; Daoud, E.; et al. PD-L1 Expression after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancers Is Associated with Aggressive Residual Disease, Suggesting a Potential for Immunotherapy. Cancers 2021, 13, 746. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/ neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Zheng, H.; Zhong, A.; Xie, S.; Wang, Y.; Sun, J.; Zhang, J.; Tong, Y.; Chen, M.; Zhang, G.; Ma, Q.; et al. Elevated serum HER-2 predicts poor prognosis in breast cancer and is correlated to ADAM10 expression. Cancer Med. 2019, 8, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Banin-Hirata, B.K.; de Oliveira, C.E.C.; Losi-Guembarovski, R.; Ozawa, P.M.M.; Vitiello, G.A.F.; de Almeida, F.C.; Derossi, D.R.; André, N.D.; Watanabe, M.A.E. The prognostic value of regulatory T cells infiltration in HER2-enriched breast cancer microenvironment. Int. Rev. Immunol. 2018, 37, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.S.; Dane, M.; Chin, K.; Tatarova, Z.; Liu, M.; Liby, T.; Thompson, W.; Smith, R.; Nederlof, M.; Bucher, E.; et al. Microenvironment-Mediated Mechanisms of Resistance to HER2 Inhibitors Differ between HER2+ Breast Cancer Subtypes. Cell Syst. 2018, 6, 329–342.e6. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.F.; De Angelis, C.; Schiff, R. Resistance to Anti-HER2 Therapies in Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e157–e164. [Google Scholar] [CrossRef]
- Pondé, N.; Brandão, M.; El-Hachem, G.; Werbrouck, E.; Piccart, M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat. Rev. 2018, 67, 10–20. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Ahn, S.; Woo, J.W.; Lee, K.; Park, S.Y. HER2 status in breast cancer: Changes in guidelines and complicating factors for interpretation. J. Pathol. Transl. Med. 2020, 54, 34–44. [Google Scholar] [CrossRef]
- Marín, A.; Mamun, A.A.; Patel, H.; Akamatsu, H.; Ye, D.; Sudhan, D.R.; Eli, L.; Marcelain, K.; Brown, B.P.; Meiler, J.; et al. Acquired Secondary HER2 Mutations Enhance HER2/MAPK Signaling and Promote Resistance to HER2 Kinase Inhibition in Breast Cancer. Cancer Res. 2023, 83, 3145–3158. [Google Scholar] [CrossRef]
- Madrid-Paredes, A.; Cañadas-Garre, M.; Sánchez-Pozo, A.; Calleja-Hernández, M.Á. Non-HER2 signaling pathways activated in resistance to anti-HER2 therapy in breast cancer. Breast Cancer Res. Treat. 2015, 153, 493–505. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered metabolism in cancer: Insights into energy pathways and therapeutic targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-M.; Tzeng, S.-F.; Ho, P.-C.; Tsai, C.-H. Immunosurveillance encounters cancer metabolism. EMBO Rep. 2024, 25, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Hachey, S.J.; Hatch, C.J.; Gaebler, D.; Mocherla, A.; Nee, K.; Kessenbrock, K.; Hughes, C.C.W. Targeting tumor–stromal interactions in triple-negative breast cancer using a human vascularized micro-tumor model. Breast Cancer Res. 2024, 26, 5. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.-H.; Tan, L.-D.; Wang, Q.-S.; Li, X.-Q.; Feng, Y.-M. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef]
- Wang, F.; Sun, W.; Zhang, J.; Fan, Y. Cancer-associated fibroblast regulation of tumor neo-angiogenesis as a therapeutic target in cancer (Review). Oncol. Lett. 2019, 17, 3055–3065. [Google Scholar] [CrossRef]
- Urban, L.; Novák, Š.; Čoma, M.; Dvořánková, B.; Lacina, L.; Šáchová, J.; Hradilová, M.; Svatoňová, P.; Kolář, M.; Strnad, H.; et al. Unravelling heterogeneous effects of cancer-associated fibroblasts on poor prognosis markers in breast cancer EM-G3 cell line: In vitro -targeted treatment (anti-IL-6, anti-VEGF-A, anti-MFGE8) based on transcriptomic profiling. Oncol. Rep. 2023, 51, 3. [Google Scholar] [CrossRef]
- Zaoui, M.; Morel, M.; Ferrand, N.; Fellahi, S.; Bastard, J.-P.; Lamazière, A.; Larsen, A.K.; Béréziat, V.; Atlan, M.; Sabbah, M. Breast-Associated Adipocytes Secretome Induce Fatty Acid Uptake and Invasiveness in Breast Cancer Cells via CD36 Independently of Body Mass Index, Menopausal Status and Mammary Density. Cancers 2019, 11, 2012. [Google Scholar] [CrossRef]
- Incio, J.; Ligibel, J.A.; McManus, D.T.; Suboj, P.; Jung, K.; Kawaguchi, K.; Pinter, M.; Babykutty, S.; Chin, S.M.; Vardam, T.D.; et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018, 10, eaag0945. [Google Scholar] [CrossRef]
- Delort, L.; Bougaret, L.; Cholet, J.; Vermerie, M.; Billard, H.; Decombat, C.; Bourgne, C.; Berger, M.; Dumontet, C.; Caldefie-Chezet, F. Hormonal Therapy Resistance and Breast Cancer: Involvement of Adipocytes and Leptin. Nutrients 2019, 11, 2839. [Google Scholar] [CrossRef] [PubMed]
- Vazquez Rodriguez, G.; Abrahamsson, A.; Jensen, L.D.E.; Dabrosin, C. Adipocytes Promote Early Steps of Breast Cancer Cell Dissemination via Interleukin-8. Front. Immunol. 2018, 9, 1767. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α) and Cancer: Mechanisms of Tumor Hypoxia and Therapeutic Targeting. Cureus 2024, 16, e70700. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Seo, E.-B.; Jeong, A.J.; Lee, S.-H.; Noh, K.H.; Lee, S.; Cho, C.-H.; Lee, C.-H.; Shin, H.M.; Kim, H.-R.; et al. The acidic tumor microenvironment enhances PD-L1 expression via activation of STAT3 in MDA-MB-231 breast cancer cells. BMC Cancer 2022, 22, 852. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 772615. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, X.; Zheng, M.; Yu, Y.; Jin, G.; Zhang, S. Regulatory T cells are associated with the tumor immune microenvironment and immunotherapy response in triple-negative breast cancer. Front. Immunol. 2023, 14, 1263537. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, X.; Zhang, L.; Lu, X.; Chaudhary, S.; Teng, R.; Frederickson, C.; Champion, M.M.; Zhao, R.; Cheng, L.; et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA 2018, 115, 10094–10099. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Datta, M.; Coussens, L.M.; Nishikawa, H.; Hodi, F.S.; Jain, R.K. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sugiyama, D.; Koseki, J.; Kojima, Y.; Hattori, S.; Sone, K.; Nishinakamura, H.; Ishikawa, T.; Ishikawa, Y.; Kato, T.; et al. Sustained inhibition of CSF1R signaling augments antitumor immunity through inhibiting tumor-associated macrophages. JCI Insight 2025, 10, e178146. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Lim, J.; Choo, J.; Ow, S.G.W.; Wong, A.; Lee, M.; Chan, C.W.; Hartman, M.; Lim, S.E.; Ngoi, N.; et al. Immunohistochemistry study of tumor vascular normalization and anti-angiogenic effects of sunitinib versus bevacizumab prior to dose-dense doxorubicin/cyclophosphamide chemotherapy in HER2-negative breast cancer. Breast Cancer Res. Treat. 2022, 192, 131–142. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, Y.; Yokota, Y.; Matsumoto, Y.; Zhai, B.; Maarouf, N.; Hayashi, H.; Carlson, R.; Zhang, S.; Sousa, A.; et al. Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade. J. Exp. Clin. Cancer Res. 2022, 41, 132. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Rai, A.; Deshpande, S.G.; Vaidya, A.; Shinde, R.K. Advancements in Immunotherapy for Breast Cancer: Mechanisms, Efficacy, and Future Directions. Cureus 2024, 16, e68351. [Google Scholar] [CrossRef]
- Schietinger, A.; Philip, M.; Schreiber, H. Specificity in cancer immunotherapy. Semin. Immunol. 2008, 20, 276–285. [Google Scholar] [CrossRef]
- Nami, B.; Maadi, H.; Wang, Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers 2018, 10, 342. [Google Scholar] [CrossRef]
- Gril, B.; Palmieri, D.; Bronder, J.L.; Herring, J.M.; Vega-Valle, E.; Feigenbaum, L.; Liewehr, D.J.; Steinberg, S.M.; Merino, M.J.; Rubin, S.D.; et al. Effect of Lapatinib on the Outgrowth of Metastatic Breast Cancer Cells to the Brain. JNCI J. Natl. Cancer Inst. 2008, 100, 1092–1103. [Google Scholar] [CrossRef]
- Sievers, E.L.; Senter, P.D. Antibody-Drug Conjugates in Cancer Therapy. Annu. Rev. Med. 2013, 64, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, Z.; Wang, Y.; Fan, X.; Cai, J.; Yao, X.; Liu, L.; Huang, J.; He, J.; Xie, C.; et al. Modulation of gut microbiota to overcome resistance to immune checkpoint blockade in cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, Y.; Li, Q.; Xu, Y.; Xue, Y.; Xu, S. CAR Macrophages: A promising novel immunotherapy for solid tumors and beyond. Biomark. Res. 2024, 12, 86. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Ruiz-Saenz, A.; Dreyer, C.; Campbell, M.R.; Steri, V.; Gulizia, N.; Moasser, M.M. HER2 Amplification in Tumors Activates PI3K/Akt Signaling Independent of HER3. Cancer Res. 2018, 78, 3645–3658. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Xu, Q.; Gao, Z.; Yang, M.; Wu, X.; Li, X. HER2/PI3K/AKT pathway in HER2-positive breast cancer: A review. Medicine 2024, 103, e38508. [Google Scholar] [CrossRef]
- Grillo, F.; Fassan, M.; Sarocchi, F.; Fiocca, R.; Mastracci, L. HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J. Gastroenterol. 2016, 22, 5879. [Google Scholar] [CrossRef]
- Verri, E.; Guglielmini, P.; Puntoni, M.; Perdelli, L.; Papadia, A.; Lorenzi, P.; Rubagotti, A.; Ragni, N.; Boccardo, F. HER2/neu Oncoprotein Overexpression in Epithelial Ovarian Cancer: Evaluation of its Prevalence and Prognostic Significance. Oncology 2005, 68, 154–161. [Google Scholar] [CrossRef]
- Koeppen, H.K.W.; Wright, B.D.; Burt, A.D.; Quirke, P.; McNicol, A.M.; Dybdal, N.O.; Sliwkowski, M.X.; Hillan, K.J. Overexpression of HER2/neu in solid tumours: An immunohistochemical survey. Histopathology 2001, 38, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control Release 2010, 146, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Braga, L.; Volpe, M.C.; Maiocchi, S.; Generali, D. The Predictive and Prognostic Role of RAS–RAF–MEK–ERK Pathway Alterations in Breast Cancer: Revision of the Literature and Comparison with the Analysis of Cancer Genomic Datasets. Cancers 2022, 14, 5306. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Codony-Servat, J.; Albanell, J.; Rojo, F.; Arribas, J.; Baselga, J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001, 61, 4744–4749. [Google Scholar]
- Collins, D.M.; O’Donovan, N.; McGowan, P.M.; O’Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann. Oncol. 2012, 23, 1788–1795. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Scher, N.S.; Cortazar, P.; Chattopadhyay, S.; Tang, S.; Song, P.; Liu, Q.; Ringgold, K.; Pilaro, A.M.; Tilley, A.; et al. First FDA Approval of Dual Anti-HER2 Regimen: Pertuzumab in Combination with Trastuzumab and Docetaxel for HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 4911–4916. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Eiermann, W. International Herceptin Study Group Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: Pivotal trial data. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12 (Suppl. S1), S57–S62. [Google Scholar]
- Sirhan, Z.; Thyagarajan, A.; Sahu, R.P. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil. Med. Res. 2022, 9, 39. [Google Scholar] [CrossRef]
- Konecny, G.E.; Pegram, M.D.; Venkatesan, N.; Finn, R.; Yang, G.; Rahmeh, M.; Untch, M.; Rusnak, D.W.; Spehar, G.; Mullin, R.J.; et al. Activity of the Dual Kinase Inhibitor Lapatinib (GW572016) against HER-2-Overexpressing and Trastuzumab-Treated Breast Cancer Cells. Cancer Res. 2006, 66, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.K.; Park, P.U.; Widdison, W.C.; Kovtun, Y.V.; Garrett, L.M.; Hoffman, K.; Lutz, R.J.; Goldmacher, V.S.; Blättler, W.A. Antibody-Maytansinoid Conjugates Are Activated in Targeted Cancer Cells by Lysosomal Degradation and Linker-Dependent Intracellular Processing. Cancer Res. 2006, 66, 4426–4433. [Google Scholar] [CrossRef] [PubMed]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab Ravtansine: A Novel Mesothelin-Targeting Antibody–Drug Conjugate Cures Tumors with Heterogeneous Target Expression Favored by Bystander Effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef]
- Okeley, N.M.; Miyamoto, J.B.; Zhang, X.; Sanderson, R.J.; Benjamin, D.R.; Sievers, E.L.; Senter, P.D.; Alley, S.C. Intracellular Activation of SGN-35, a Potent Anti-CD30 Antibody-Drug Conjugate. Clin. Cancer Res. 2010, 16, 888–897. [Google Scholar] [CrossRef]
- Tagawa, S.; Klute, K.; Nackos, E.; Tasaki, S.; Nguyen, D.; Bander, N. Microtubule inhibitor-based antibody–drug conjugates for cancer therapy. OncoTargets Ther. 2014, 7, 2227. [Google Scholar] [CrossRef]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLoS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef]
- Lopus, M.; Oroudjev, E.; Wilson, L.; Wilhelm, S.; Widdison, W.; Chari, R.; Jordan, M.A. Maytansine and Cellular Metabolites of Antibody-Maytansinoid Conjugates Strongly Suppress Microtubule Dynamics by Binding to Microtubules. Mol. Cancer Ther. 2010, 9, 2689–2699. [Google Scholar] [CrossRef]
- Zein, N.; Sinha, A.M.; McGahren, W.J.; Ellestad, G.A. Calicheamicin γ 1 I: An Antitumor Antibiotic That Cleaves Double-Stranded DNA Site Specifically. Science 1988, 240, 1198–1201. [Google Scholar] [CrossRef]
- Gerratana, B. Biosynthesis, synthesis, and biological activities of pyrrolobenzodiazepines. Med. Res. Rev. 2012, 32, 254–293. [Google Scholar] [CrossRef]
- Singh, A.P.; Sharma, S.; Shah, D.K. Quantitative characterization of in vitro bystander effect of antibody-drug conjugates. J. Pharmacokinet. Pharmacodyn. 2016, 43, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.M.; Arseniu, A.M.; Frum, A.; Rus, L.-L.; Cormos, G.; Georgescu, C.; Morgovan, C.; Butuca, A.; Gligor, F.G.; et al. Antibody–Drug Conjugates—Evolution and Perspectives. Int. J. Mol. Sci. 2024, 25, 6969. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Ansell, S.M. Brentuximab vedotin. Blood 2014, 124, 3197–3200. [Google Scholar] [CrossRef] [PubMed]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A.; et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef]
- Geng, W.; Thomas, H.; Chen, Z.; Yan, Z.; Zhang, P.; Zhang, M.; Huang, W.; Ren, X.; Wang, Z.; Ding, K.; et al. Mechanisms of acquired resistance to HER2-Positive breast cancer therapies induced by HER3: A comprehensive review. Eur. J. Pharmacol. 2024, 977, 176725. [Google Scholar] [CrossRef]
- Blangé, D.; Stroes, C.I.; Derks, S.; Bijlsma, M.F.; van Laarhoven, H.W.M. Resistance mechanisms to HER2-targeted therapy in gastroesophageal adenocarcinoma: A systematic review. Cancer Treat. Rev. 2022, 108, 102418. [Google Scholar] [CrossRef]
- Wu, X.; Huang, S.; He, W.; Song, M. Emerging insights into mechanisms of trastuzumab resistance in HER2-positive cancers. Int. Immunopharmacol. 2023, 122, 110602. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Tan, S.; Day, D.; Nicholls, S.J.; Segelov, E. Immune Checkpoint Inhibitor Therapy in Oncology. JACC Cardio Oncol. 2022, 4, 579–597. [Google Scholar] [CrossRef]
- Huang, A.C.; Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: Understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef]
- Sakamoto, H.; Tanaka, H.; Shiratori, T.; Baba, K.; Ishioka, Y.; Itoga, M.; Taima, K.; Hasegawa, Y.; Takanashi, S.; Tasaka, S. The efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer harboring driver mutations. Mol. Clin. Oncol. 2019, 10, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Lasorsa, F.; di Meo, N.A.; Rutigliano, M.; Milella, M.; Ferro, M.; Pandolfo, S.D.; Crocetto, F.; Tataru, O.S.; Autorino, R.; Battaglia, M.; et al. Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Molecular Basis and Rationale for Their Use in Clinical Practice. Biomedicines 2023, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Tao, Y.; Chen, H.; Li, X.; Wang, Y.; Xu, X.; Li, S.; Chen, H.; Cang, S.; Liu, Y. Real-world evaluation of the efficacy of immune checkpoint inhibitors in the treatment of metastatic breast cancer. Oncol. Lett. 2024, 29, 29. [Google Scholar] [CrossRef]
- Pauken, K.E.; Torchia, J.A.; Chaudhri, A.; Sharpe, A.H.; Freeman, G.J. Emerging concepts in PD-1 checkpoint biology. Semin. Immunol. 2021, 52, 101480. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.-F.; Li, S. PD-1-mediated inhibition of T cell activation: Mechanisms and strategies for cancer combination immunotherapy. Cell Insight 2024, 3, 100146. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Li, J.; Adhikari, R.; Fu, L. PD-1/PD-L1 Based Combinational Cancer Therapy: Icing on the Cake. Front. Pharmacol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhang, C.; Wang, Y.; Cheng, T.; Duan, L.; Tong, Z.; Tan, S.; Zhang, H.; Saw, P.E.; et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 6640–6650. [Google Scholar] [CrossRef]
- Deng, R.; Bumbaca, D.; Pastuskovas, C.V.; Boswell, C.A.; West, D.; Cowan, K.J.; Chiu, H.; McBride, J.; Johnson, C.; Xin, Y.; et al. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. MAbs 2016, 8, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, L.; Huang, Y.; Wu, Y.; Xie, N. Mechanisms and Strategies to Overcome PD-1/PD-L1 Blockade Resistance in Triple-Negative Breast Cancer. Cancers 2022, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef]
- Zou, Y.; Zou, X.; Zheng, S.; Tang, H.; Zhang, L.; Liu, P.; Xie, X. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940928. [Google Scholar] [CrossRef]
- Bondhopadhyay, B.; Hussain, S.; Kasherwal, V. The differential effect of the immune system in breast cancer. Explor. Med. 2023, 4, 1094–1108. [Google Scholar] [CrossRef]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers from Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Sternschuss, M.; Yerushalmi, R.; Saleh, R.R.; Amir, E.; Goldvaser, H. Efficacy and safety of neoadjuvant immune checkpoint inhibitors in early-stage triple-negative breast cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 3369–3379. [Google Scholar] [CrossRef]
- Cejuela, M.; Vethencourt, A.; Pernas, S. Immune Checkpoint Inhibitors and Novel Immunotherapy Approaches for Breast Cancer. Curr. Oncol. Rep. 2022, 24, 1801–1819. [Google Scholar] [CrossRef]
- Goldberg, J.; Pastorello, R.G.; Vallius, T.; Davis, J.; Cui, Y.X.; Agudo, J.; Waks, A.G.; Keenan, T.; McAllister, S.S.; Tolaney, S.M.; et al. The Immunology of Hormone Receptor Positive Breast Cancer. Front. Immunol. 2021, 12, 674192. [Google Scholar] [CrossRef]
- Rinnerthaler, G.; Egle, D.; Bartsch, R.; Schmitt, C.A.; Petzer, A.; Balic, M.; Petru, E.; Denison, U.; Singer, C.F.; Bjelic-Radisic, V.; et al. Neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin in women with early HER2-positive breast cancer: The randomized phase 2 ABCSG-52/ATHENE trial. Nat. Cancer 2025, 6, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.; Attig, J.; Dziadek, S.; Graefe, N.; Heller, A.; Rieder, N.; Gomes, B. Tumor beta2-microglobulin and HLA-A expression is increased by immunotherapy and can predict response to CIT in association with other biomarkers. Front. Immunol. 2024, 15, 1285049. [Google Scholar] [CrossRef]
- Gupta, R.G.; Li, F.; Roszik, J.; Lizée, G. Exploiting Tumor Neoantigens to Target Cancer Evolution: Current Challenges and Promising Therapeutic Approaches. Cancer Discov. 2021, 11, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, M.; Yu, W.; Li, H. Activation of Wnt/β-catenin signaling promotes immune evasion via the β-catenin/IKZF1/CCL5 axis in hepatocellular carcinoma. Int. Immunopharmacol. 2024, 138, 112534. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef]
- Coelho, M.A.; Cooper, S.; Strauss, M.E.; Karakoc, E.; Bhosle, S.; Gonçalves, E.; Picco, G.; Burgold, T.; Cattaneo, C.M.; Veninga, V.; et al. Base editing screens map mutations affecting interferon-γ signaling in cancer. Cancer Cell 2023, 41, 288–303.e6. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Chang, X.; Cheng, Y.; Cheng, H.; Ye, X.; Fu, T.; Chen, J.; Cui, H. Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin. J. Cancer Res. 2012, 24, 130–137. [Google Scholar] [CrossRef]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; Van Der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system (Review). Oncol. Lett. 2024, 27, 87. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Xiaoyu, P.; Chao, G.; Lihua, D.; Pengyu, C. Gut bacteria affect the tumoral immune milieu: Distorting the efficacy of immunotherapy or not? Gut Microbes 2020, 11, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Qin, S.; Chu, Q.; Wu, K. The role of gut microbiota in immune checkpoint inhibitor therapy. Hepatobiliary Surg. Nutr. 2018, 7, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Rebaudi, F.; De Franco, F.; Goda, R.; Obino, V.; Vita, G.; Baronti, C.; Iannone, E.; Pitto, F.; Massa, B.; Fenoglio, D.; et al. The landscape of combining immune checkpoint inhibitors with novel Therapies: Secret alliances against breast cancer. Cancer Treat. Rev. 2024, 130, 102831. [Google Scholar] [CrossRef]
- Morganti, S.; Curigliano, G. Combinations using checkpoint blockade to overcome resistance. Ecancermedicalscience 2020, 14, 1148. [Google Scholar] [CrossRef]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Rajan, A.; Kim, C.; Heery, C.R.; Guha, U.; Gulley, J.L. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum. Vaccin. Immunother. 2016, 12, 2219–2231. [Google Scholar] [CrossRef]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Nederlof, I.; Isaeva, O.I.; de Graaf, M.; Gielen, R.C.A.M.; Bakker, N.A.M.; Rolfes, A.L.; Garner, H.; Boeckx, B.; Traets, J.J.H.; Mandjes, I.A.M.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in early-stage triple-negative breast cancer: A phase 2 adaptive trial. Nat. Med. 2024, 30, 3223–3235. [Google Scholar] [CrossRef]
- Kyte, J.A.; Andresen, N.K.; Russnes, H.G.; Fretland, S.Ø.; Falk, R.S.; Lingjærde, O.C.; Naume, B. ICON: A randomized phase IIb study evaluating immunogenic chemotherapy combined with ipilimumab and nivolumab in patients with metastatic hormone receptor positive breast cancer. J. Transl. Med. 2020, 18, 269. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, C.; Huang, K. LCOR Reverses Immune-Checkpoint Inhibitors Therapy Resistance Out of IFN Constraint in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 911572. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Sun, Z.-J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef]
- Bhalla, N.; Brooker, R.; Brada, M. Combining immunotherapy and radiotherapy in lung cancer. J. Thorac. Dis. 2018, 10, S1447–S1460. [Google Scholar] [CrossRef]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef]
- Kim, C.-G.; Sang, Y.-B.; Lee, J.-H.; Chon, H.-J. Combining Cancer Vaccines with Immunotherapy: Establishing a New Immunological Approach. Int. J. Mol. Sci. 2021, 22, 8035. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.-J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Yuan, Y.; Kos, F.J.; He, T.-F.; Yin, H.H.; Li, M.; Hardwick, N.; Zurcher, K.; Schmolze, D.; Lee, P.; Pillai, R.K.; et al. Complete regression of cutaneous metastases with systemic immune response in a patient with triple negative breast cancer receiving p53MVA vaccine with pembrolizumab. Oncoimmunology 2017, 6, e1363138. [Google Scholar] [CrossRef]
- Pack, C.D.; Bommireddy, R.; Munoz, L.E.; Patel, J.M.; Bozeman, E.N.; Dey, P.; Radhakrishnan, V.; Vartabedian, V.F.; Venkat, K.; Ramachandiran, S.; et al. Tumor membrane-based vaccine immunotherapy in combination with anti-CTLA-4 antibody confers protection against immune checkpoint resistant murine triple-negative breast cancer. Hum. Vaccin. Immunother. 2020, 16, 3184–3193. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.; Rucki, A.A.; Wu, A.A.; Olino, K.; Xiao, Q.; Chai, Y.; Wamwea, A.; Bigelow, E.; Lutz, E.; Liu, L.; et al. PD-1/PD-L1 Blockade Together with Vaccine Therapy Facilitates Effector T-Cell Infiltration Into Pancreatic Tumors. J. Immunother. 2015, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Antonios, J.P.; Soto, H.; Everson, R.G.; Orpilla, J.; Moughon, D.; Shin, N.; Sedighim, S.; Yong, W.H.; Li, G.; Cloughesy, T.F.; et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight 2016, 1, e87059. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.E.; Latchman, Y.E.; Balint, J.P.; Lee, J.H.; Gabitzsch, E.S.; Jones, F.R. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther. 2015, 22, 454–462. [Google Scholar] [CrossRef]
- Wada, S.; Jackson, C.M.; Yoshimura, K.; Yen, H.-R.; Getnet, D.; Harris, T.J.; Goldberg, M.V.; Bruno, T.C.; Grosso, J.F.; Durham, N.; et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J. Transl. Med. 2013, 11, 89. [Google Scholar] [CrossRef]
- Lovatt, C.; Parker, A.L. Oncolytic Viruses and Immune Checkpoint Inhibitors: The “Hot” New Power Couple. Cancers 2023, 15, 4178. [Google Scholar] [CrossRef]
- Carter, M.E.; Hartkopf, A.D.; Wagner, A.; Volmer, L.L.; Brucker, S.Y.; Berchtold, S.; Lauer, U.M.; Koch, A. A Three-Dimensional Organoid Model of Primary Breast Cancer to Investigate the Effects of Oncolytic Virotherapy. Front. Mol. Biosci. 2022, 9, 826302. [Google Scholar] [CrossRef]
- Arab, A.; Behravan, N.; Razazn, A.; Barati, N.; Mosaffa, F.; Nicastro, J.; Slavcev, R.; Behravan, J. The viral approach to breast cancer immunotherapy. J. Cell. Physiol. 2019, 234, 1257–1267. [Google Scholar] [CrossRef]
- Chesney, J.A.; Puzanov, I.; Collichio, F.A.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 2023, 11, e006270. [Google Scholar] [CrossRef]
- Yin, J.; Gu, T.; Chaudhry, N.; Davidson, N.E.; Huang, Y. Epigenetic modulation of antitumor immunity and immunotherapy response in breast cancer: Biological mechanisms and clinical implications. Front. Immunol. 2024, 14, 1325615. [Google Scholar] [CrossRef]
- Orillion, A.; Hashimoto, A.; Damayanti, N.; Shen, L.; Adelaiye-Ogala, R.; Arisa, S.; Chintala, S.; Ordentlich, P.; Kao, C.; Elzey, B.; et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 5187–5201. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.S.; Zhou, M.; Krishnan, B.; Utsumi, T.; Manocha, U.; Stewart, K.G.; Beck, W.; Rose, T.L.; Milowsky, M.I.; He, X.; et al. Entinostat induces antitumor immune responses through immune editing of tumor neoantigens. J. Clin. Investig. 2021, 131, 138560. [Google Scholar] [CrossRef]
- Diab, A.; Tannir, N.M.; Bentebibel, S.-E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef]
- Huang, J.; Gong, C.; Zhou, A. Modulation of gut microbiota: A novel approach to enhancing the effects of immune checkpoint inhibitors. Ther. Adv. Med. Oncol. 2023, 15, 17588359231204854. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Z.; Wang, Z.; Chen, C.; Luo, Y. Chimeric antigen receptor macrophages activated through TLR4 or IFN-γ receptors suppress breast cancer growth by targeting VEGFR2. Cancer Immunol. Immunother. 2023, 72, 3243–3257. [Google Scholar] [CrossRef]
- Pierini, S.; Gabbasov, R.; Oliveira-Nunes, M.C.; Qureshi, R.; Worth, A.; Huang, S.; Nagar, K.; Griffin, C.; Lian, L.; Yashiro-Ohtani, Y.; et al. Chimeric antigen receptor macrophages (CAR-M) sensitize HER2+ solid tumors to PD1 blockade in pre-clinical models. Nat. Commun. 2025, 16, 706. [Google Scholar] [CrossRef]
- Hadiloo, K.; Taremi, S.; Heidari, M.; Esmaeilzadeh, A. The CAR macrophage cells, a novel generation of chimeric antigen-based approach against solid tumors. Biomark. Res. 2023, 11, 103. [Google Scholar] [CrossRef]
- Ebrahimabadi, S.; Kaufman, D.S. Next-generation macrophages: Repolarizing CAR-macrophages against cancer. Blood Sci. 2024, 6, e00201. [Google Scholar] [CrossRef]
- Samareh Salavatipour, M.; Poursalehi, Z.; Hosseini Rouzbahani, N.; Mohammadyar, S.; Vasei, M. CRISPR-Cas9 in basic and translational aspects of cancer therapy. BioImpacts 2024, 14, 30087. [Google Scholar] [CrossRef]
- Pierini, S.; Gabbasov, R.; Gabitova, L.; Ohtani, Y.; Shestova, O.; Gill, S.; Abramson, S.; Condamine, T.; Klichinsky, M. Abstract 63: Chimeric antigen receptor macrophages (CAR-M) induce anti-tumor immunity and synergize with T cell checkpoint inhibitors in pre-clinical solid tumor models. Cancer Res. 2021, 81 (Suppl. S13), 63. [Google Scholar] [CrossRef]
- Richardson, J.R.; Schöllhorn, A.; Gouttefangeas, C.; Schuhmacher, J. CD4+ T Cells: Multitasking Cells in the Duty of Cancer Immunotherapy. Cancers 2021, 13, 596. [Google Scholar] [CrossRef]
- Shao, J.; Wang, C.; Ren, P.; Jiang, Y.; Tian, P.; Li, W. Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer. Biosci. Rep. 2020, 40, BSR20192347. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice with Intracranial Gliomas. Int. J. Radiat. Oncol. 2013, 86, 343–349. [Google Scholar] [CrossRef]
- Maciejko, L.; Smalley, M.; Goldman, A. Cancer Immunotherapy and Personalized Medicine: Emerging Technologies and Biomarker Based Approaches. J. Mol. Biomark. Diagn. 2017, 8, 350. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Schaft, N.; Dörrie, J.; Schuler, G.; Schuler-Thurner, B.; Sallam, H.; Klein, S.; Eisenberg, G.; Frankenburg, S.; Lotem, M.; Khatib, A. The future of affordable cancer immunotherapy. Front. Immunol. 2023, 14, 1248867. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef]
- Waxman, E.S.; Gerber, C.D.L. Pseudoprogression and Immunotherapy Phenomena. J. Adv. Pract. Oncol. 2020, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.R.; Wang, J.; Silk, A.W.; Ganesan, S.; Kaufman, H.L.; Mehnert, J.M. Biomarkers for Immunotherapy: Current Developments and Challenges. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e493–e503. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Srivatsan, S.; Patel, J.M.; Bozeman, E.N.; Imasuen, I.E.; He, S.; Daniels, D.; Selvaraj, P. Allogeneic tumor cell vaccines. Hum. Vaccin. Immunother. 2014, 10, 52–63. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Hato, L.; Vizcay, A.; Eguren, I.; Pérez-Gracia, J.L.; Rodríguez, J.; Gállego Pérez-Larraya, J.; Sarobe, P.; Inogés, S.; Díaz de Cerio, A.L.; Santisteban, M. Dendritic Cells in Cancer Immunology and Immunotherapy. Cancers 2024, 16, 981. [Google Scholar] [CrossRef]
- D’Alise, A.M.; Brasu, N.; De Intinis, C.; Leoni, G.; Russo, V.; Langone, F.; Baev, D.; Micarelli, E.; Petiti, L.; Picelli, S.; et al. Adenoviral-based vaccine promotes neoantigen-specific CD8 + T cell stemness and tumor rejection. Sci. Transl. Med. 2022, 14, eabo7604. [Google Scholar] [CrossRef]
- Nicolás-Morales, M.L.; Luisa-Sanjuan, A.; Gutiérrez-Torres, M.; Vences-Velázquez, A.; Ortuño-Pineda, C.; Espinoza-Rojo, M.; Navarro-Tito, N.; Cortés-Sarabia, K. Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review. Vaccines 2022, 10, 1249. [Google Scholar] [CrossRef]
- Keenan, B.P.; Jaffee, E.M. Whole Cell Vaccines—Past Progress and Future Strategies. Semin. Oncol. 2012, 39, 276–286. [Google Scholar] [CrossRef]
- Chiang, C.L.-L.; Benencia, F.; Coukos, G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22, 132–143. [Google Scholar] [CrossRef]
- Gupta, I.; Hussein, O.; Sastry, K.S.; Bougarn, S.; Gopinath, N.; Chin-Smith, E.; Sinha, Y.; Korashy, H.M.; Maccalli, C. Deciphering the complexities of cancer cell immune evasion: Mechanisms and therapeutic implications. Adv. Cancer Biol.-Metastasis 2023, 8, 100107. [Google Scholar] [CrossRef]
- Disis, M.L.; Dang, Y.; Coveler, A.L.; Childs, J.S.; Higgins, D.M.; Liu, Y.; Zhou, J.; Mackay, S.; Salazar, L.G. A Phase I/II Trial of HER2 Vaccine–Primed Autologous T-Cell Infusions in Patients with Treatment Refractory HER2–Overexpressing Breast Cancer. Clin. Cancer Res. 2023, 29, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol. Ther. Exp. 2020, 68, 2. [Google Scholar] [CrossRef]
- Disis, M.L.; Wallace, D.R.; Gooley, T.A.; Dang, Y.; Slota, M.; Lu, H.; Coveler, A.L.; Childs, J.S.; Higgins, D.M.; Fintak, P.A.; et al. Concurrent Trastuzumab and HER2/ neu -Specific Vaccination in Patients with Metastatic Breast Cancer. J. Clin. Oncol. 2009, 27, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Maglakelidze, M.; Andrić, Z.; Ryspayeva, D.; Bulat, I.; Nikolić, I.; Petrović, Z.; Chawla, T.; Nagarkar, R.; Garner-Spitzer, E.; et al. Phase II Trial of HER-Vaxx, a B-cell Peptide-Based Vaccine, in HER2-Overexpressing Advanced Gastric Cancer Patients Under Platinum-Based Chemotherapy (HERIZON). Clin. Cancer Res. 2024, 30, 4044–4054. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Gu, J.; Gao, Y.; Wang, Z.; Zhang, K.; Mu, N.; Huang, T.; Li, W.; Hao, Q.; et al. Synergistic tumoricidal effect of combined hPD-L1 vaccine and HER2 gene vaccine. Biochem. Biophys. Res. Commun. 2018, 497, 394–400. [Google Scholar] [CrossRef]
- de Gruijl, T.D.; van den Eertwegh, A.J.M.; Pinedo, H.M.; Scheper, R.J. Whole-cell cancer vaccination: From autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol. Immunother. 2008, 57, 1569–1577. [Google Scholar] [CrossRef]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta-Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Diao, L.; Liu, M. Rethinking Antigen Source: Cancer Vaccines Based on Whole Tumor Cell/tissue Lysate or Whole Tumor Cell. Adv. Sci. 2023, 10, e2300121. [Google Scholar] [CrossRef]
- Chapman, P.B. Vaccinating Patients with Autologous Tumor. J. Clin. Oncol. 2002, 20, 4139–4140. [Google Scholar] [CrossRef]
- Pérez-Baños, A.; Gleisner, M.A.; Flores, I.; Pereda, C.; Navarrete, M.; Araya, J.P.; Navarro, G.; Quezada-Monrás, C.; Tittarelli, A.; Salazar-Onfray, F. Whole tumour cell-based vaccines: Tuning the instruments to orchestrate an optimal antitumour immune response. Br. J. Cancer 2023, 129, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Tadic, S.; Martínez, A. Nucleic acid cancer vaccines targeting tumor related angiogenesis. Could mRNA vaccines constitute a game changer? Front. Immunol. 2024, 15, 1433185. [Google Scholar] [CrossRef] [PubMed]
- Omabe, M.; Ahmed, S.; Sami, A.; Xie, Y.; Tao, M.; Xiang, J. HER2-Specific Vaccines for HER2-Positive Breast Cancer Immunotherapy. World J. Vaccines 2015, 5, 106–128. [Google Scholar] [CrossRef][Green Version]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.-F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Lee, S.-H.; Danishmalik, S.N.; Sin, J.-I. DNA vaccines, electroporation and their applications in cancer treatment. Hum. Vaccin. Immunother. 2015, 11, 1889–1900. [Google Scholar] [CrossRef]
- Mucker, E.M.; Karmali, P.P.; Vega, J.; Kwilas, S.A.; Wu, H.; Joselyn, M.; Ballantyne, J.; Sampey, D.; Mukthavaram, R.; Sullivan, E.; et al. Lipid Nanoparticle Formulation Increases Efficiency of DNA-Vectored Vaccines/Immunoprophylaxis in Animals Including Transchromosomic Bovines. Sci. Rep. 2020, 10, 8764. [Google Scholar] [CrossRef]
- Wu, C.; Qin, C.; Long, W.; Wang, X.; Xiao, K.; Liu, Q. Tumor antigens and immune subtypes of glioblastoma: The fundamentals of mRNA vaccine and individualized immunotherapy development. J. Big Data 2022, 9, 92. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Shan, K.; Dalal, S.; Vu, D.; Milillo-Naraine, A.; Guaqueta, D.; Ergle, A. Molecular Targeting of the Human Epidermal Growth Factor Receptor-2 (HER2) Genes across Various Cancers. Int. J. Mol. Sci. 2024, 25, 1064. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Q. mRNA vaccination in breast cancer: Current progress and future direction. J. Cancer Res. Clin. Oncol. 2023, 149, 9435–9450. [Google Scholar] [CrossRef]
- Pardi, N.; Weissman, D. Nucleoside Modified mRNA Vaccines for Infectious Diseases. In RNA Vaccinzes: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 109–121. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goedegebuure, S.P.; Chen, M.Y.; Mishra, R.; Zhang, F.; Yu, Y.Y.; Singhal, K.; Li, L.; Gao, F.; Myers, N.B.; et al. Neoantigen DNA vaccines are safe, feasible, and induce neoantigen-specific immune responses in triple-negative breast cancer patients. Genome Med. 2024, 16, 131. [Google Scholar] [CrossRef]
- Khattak, A.; Weber, J.S.; Meniawy, T.; Taylor, M.H.; Ansstas, G.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; Faries, M.B.; et al. Distant metastasis-free survival results from the randomized, phase 2 mRNA-4157-P201/KEYNOTE-942 trial. J. Clin. Oncol. 2023, 41 (Suppl. S17), LBA9503. [Google Scholar] [CrossRef]
- Sebastian, M.; Papachristofilou, A.; Weiss, C.; Früh, M.; Cathomas, R.; Hilbe, W.; Wehler, T.; Rippin, G.; Koch, S.D.; Scheel, B.; et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 2014, 14, 748. [Google Scholar] [CrossRef]
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the Treatment of Prostate Cancer: Novel Insights and Future Directions. Future Oncol. 2018, 14, 907–917. [Google Scholar] [CrossRef]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (provenge) injection: The first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Qian, D.; Li, J.; Huang, M.; Cui, Q.; Liu, X.; Sun, K. Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy. Biomed. Pharmacother. 2023, 162, 114685. [Google Scholar] [CrossRef]
- Sharma, A.; Koldovsky, U.; Xu, S.; Mick, R.; Roses, R.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 2012, 118, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Cen, Q.; Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Zanotta, S.; Galati, D.; De Filippi, R.; Pinto, A. Enhancing Dendritic Cell Cancer Vaccination: The Synergy of Immune Checkpoint Inhibitors in Combined Therapies. Int. J. Mol. Sci. 2024, 25, 7509. [Google Scholar] [CrossRef]
- Koeneman, B.J.; Schreibelt, G.; Gorris, M.A.J.; Hins-de Bree, S.; Westdorp, H.; Ottevanger, P.B.; de Vries, I.J.M. Dendritic cell vaccination combined with carboplatin/paclitaxel for metastatic endometrial cancer patients: Results of a phase I/II trial. Front. Immunol. 2024, 15, 1368103. [Google Scholar] [CrossRef]
- Kamigaki, T.; Takimoto, R.; Okada, S.; Ibe, H.; Oguma, E.; Goto, S. Personalized Dendritic-cell-based Vaccines Targeting Cancer Neoantigens. Anticancer Res. 2024, 44, 3713–3724. [Google Scholar] [CrossRef]
- Roy, S.; Sethi, T.K.; Taylor, D.; Kim, Y.J.; Johnson, D.B. Breakthrough concepts in immune-oncology: Cancer vaccines at the bedside. J. Leukoc. Biol. 2020, 108, 1455–1489. [Google Scholar] [CrossRef]
- Larocca, C.; Schlom, J. Viral Vector-Based Therapeutic Cancer Vaccines. Cancer J. 2011, 17, 359–371. [Google Scholar] [CrossRef]
- Madan, R.A.; Arlen, P.M.; Gulley, J.L. PANVACTM-VF: Poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert. Opin. Biol. Ther. 2007, 7, 543–554. [Google Scholar] [CrossRef]
- Esteban, J.M.; Felder, B.; Ahn, C.; Simpson, J.F.; Battifora, H.; Shively, J.E. Prognostic relevance of carcinoembryonic antigen and estrogen receptor status in breast cancer patients. Cancer 1994, 74, 1575–1583. [Google Scholar] [CrossRef]
- Kankanala, V.L.; Zubair, M.; Mukkamalla, S.K.R. Carcinoembryonic Antigen; 2025. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16708907 (accessed on 20 March 2025).
- Allum, W.H.; Stokes, H.J.; Macdonald, F.; Fielding, J.W. Demonstration of carcinoembryonic antigen (CEA) expression in normal, chronically inflamed, and malignant pancreatic tissue by immunohistochemistry. J. Clin. Pathol. 1986, 39, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Boyce, R.W.G.; Abd El-Rehim, D.; Kurien, T.; Green, A.R.; Paish, E.C.; Robertson, J.F.R.; Ellis, I.O. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 2005, 18, 1295–1304. [Google Scholar] [CrossRef]
- Heery, C.R.; Ibrahim, N.K.; Arlen, P.M.; Mohebtash, M.; Murray, J.L.; Koenig, K.; Madan, R.A.; McMahon, S.; Marté, J.L.; Steinberg, S.M.; et al. Docetaxel Alone or in Combination with a Therapeutic Cancer Vaccine (PANVAC) in Patients with Metastatic Breast Cancer. JAMA Oncol. 2015, 1, 1087. [Google Scholar] [CrossRef]
- Seclì, L.; Leoni, G.; Ruzza, V.; Siani, L.; Cotugno, G.; Scarselli, E.; D’Alise, A.M. Personalized Cancer Vaccines Go Viral: Viral Vectors in the Era of Personalized Immunotherapy of Cancer. Int. J. Mol. Sci. 2023, 24, 16591. [Google Scholar] [CrossRef]
- de Paula Peres, L.; da Luz, F.A.C.; dos Anjos Pultz, B.; Brígido, P.C.; de Araújo, R.A.; Goulart, L.R.; Silva, M.J.B. Peptide vaccines in breast cancer: The immunological basis for clinical response. Biotechnol. Adv. 2015, 33, 1868–1877. [Google Scholar] [CrossRef]
- Jalali, S.A.; Sankian, M.; Tavakkol-Afshari, J.; Jaafari, M.R. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 692–701. [Google Scholar] [CrossRef]
- Miyako, H.; Kametani, Y.; Katano, I.; Ito, R.; Tsuda, B.; Furukawa, A.; Saito, Y.; Ishikawa, D.; Ogino, K.; Sasaki, S.; et al. Antitumor effect of new HER2 peptide vaccination based on B cell epitope. Anticancer. Res. 2011, 31, 3361–3368. [Google Scholar]
- Vincent, B.G.; File, D.M.; McKinnon, K.P.; Moore, D.T.; Frelinger, J.A.; Collins, E.J.; Ibrahim, J.G.; Bixby, L.; Reisdorf, S.; Laurie, S.J.; et al. Efficacy of a Dual-Epitope Dendritic Cell Vaccine as Part of Combined Immunotherapy for HER2-Expressing Breast Tumors. J. Immunol. 2023, 211, 219–228. [Google Scholar] [CrossRef]
- Tobias, J.; Jasinska, J.; Baier, K.; Kundi, M.; Ede, N.; Zielinski, C.; Wiedermann, U. Enhanced and long term immunogenicity of a Her-2/neu multi-epitope vaccine conjugated to the carrier CRM197 in conjunction with the adjuvant Montanide. BMC Cancer 2017, 17, 118. [Google Scholar] [CrossRef]
- Tobias, J.; Drinić, M.; Högler, S.; Ambroz, K.; Baier, K.; Kodajova, P.; Tomasich, E.; Berghoff, A.S.; Schmid, A.; Garner-Spitzer, E.; et al. Active immunization with a Her-2/neu-targeting Multi-peptide B cell vaccine prevents lung metastases formation from Her-2/neu breast cancer in a mouse model. Transl. Oncol. 2022, 19, 101378. [Google Scholar] [CrossRef]
- Gil, E.-Y.; Jo, U.-H.; Lee, H.J.; Kang, J.; Seo, J.H.; Lee, E.S.; Kim, Y.H.; Kim, I.; Phan-Lai, V.; Disis, M.L.; et al. Vaccination with ErbB-2 peptides prevents cancer stem cell expansion and suppresses the development of spontaneous tumors in MMTV-PyMT transgenic mice. Breast Cancer Res. Treat. 2014, 147, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Peoples, G.E.; Goedegebuure, P.S.; Smith, R.; Linehan, D.C.; Yoshino, I.; Eberlein, T.J. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc. Natl. Acad. Sci. USA 1995, 92, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Besmer, D.M.; Erick, T.K.; Steuerwald, N.; Das Roy, L.; Grover, P.; Rao, S.; Nath, S.; Ferrier, J.W.; Reid, R.W.; et al. Indomethacin enhances anti-tumor efficacy of a MUC1 peptide vaccine against breast cancer in MUC1 transgenic mice. PLoS ONE 2019, 14, e0224309. [Google Scholar] [CrossRef] [PubMed]
- Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I.G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int. J. Oncol. 2016, 48, 1369–1378. [Google Scholar] [CrossRef]
- Shi, W.; Tong, Z.; Chen, S.; Qiu, Q.; Zhou, J.; Qian, H. Development of novel self-assembled vaccines based on tumour-specific antigenic peptide and TLR2 agonist for effective breast cancer immunotherapy via activating CD8 + T cells and enhancing their function. Immunology 2023, 169, 454–466. [Google Scholar] [CrossRef]
- Cen, L.; Zhang, Z.; Sun, Y.; Wu, N.; Shao, J.; Qian, Z.; Tian, M.; Ke, Y.; Liu, B. Efficacy of MAGE-A4 long peptide as a universal immunoprevention cancer vaccine. Cancer Cell Int. 2024, 24, 232. [Google Scholar] [CrossRef]
- Takahashi, R.; Toh, U.; Iwakuma, N.; Takenaka, M.; Otsuka, H.; Furukawa, M.; Fujii, T.; Seki, N.; Kawahara, A.; Kage, M.; et al. Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients. Breast Cancer Res. 2014, 16, R70. [Google Scholar] [CrossRef]
- Toh, U.; Sakurai, S.; Saku, S.; Takao, Y.; Okabe, M.; Iwakuma, N.; Shichijo, S.; Yamada, A.; Itoh, K.; Akagi, Y. Early phase II study of mixed 19-peptide vaccine monotherapy for refractory triple-negative breast cancer. Cancer Sci. 2020, 111, 2760–2769. [Google Scholar] [CrossRef]
- Ménard, S.; Pupa, S.M.; Campiglio, M.; Tagliabue, E. Biologic and therapeutic role of HER2 in cancer. Oncogene 2003, 22, 6570–6578. [Google Scholar] [CrossRef]
- Sanomachi, T.; Okuma, H.S.; Kitadai, R.; Kawachi, A.; Yazaki, S.; Tokura, M.; Arakaki, M.; Saito, A.; Kita, S.; Yamamoto, K.; et al. Low HER2 expression is a predictor of poor prognosis in stage I triple-negative breast cancer. Front. Oncol. 2023, 13, 1157789. [Google Scholar] [CrossRef]
- Black, L.E.; Longo, J.F.; Carroll, S.L. Mechanisms of Receptor Tyrosine-Protein Kinase ErbB-3 (ERBB3) Action in Human Neoplasia. Am. J. Pathol. 2019, 189, 1898–1912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, L.; Yazdani, M.; Momtazi-Borojeni, A.A.; Razazan, A.; Shariat, S.; Mansourian, M.; Arab, A.; Barati, N.; Arabsalmani, M.; Abbasi, A.; et al. Preparation of nanoliposomes containing HER2/neu (P5+435) peptide and evaluation of their immune responses and anti-tumoral effects as a prophylactic vaccine against breast cancer. PLoS ONE 2020, 15, e0243550. [Google Scholar] [CrossRef]
- Hu, H.; Steinmetz, N.F. Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine. Cancers 2021, 13, 2909. [Google Scholar] [CrossRef]
- Disis, M.L.; Gad, E.; Herendeen, D.R.; Lai, V.P.-; Park, K.H.; Cecil, D.L.; O’Meara, M.M.; Treuting, P.M.; Lubet, R.A. A Multiantigen Vaccine Targeting Neu, IGFBP-2, and IGF-IR Prevents Tumor Progression in Mice with Preinvasive Breast Disease. Cancer Prev. Res. 2013, 6, 1273–1282. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinić, M.; Wiedermann, U. Vaccination against Her-2/neu, with focus on peptide-based vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef]
- Tobias, J.; Kundi, M.; Garner-Spitzer, E.; Zielinski, C.; Maglakelidze, M.; Andric, Z.; Petrovic, Z.; Nagarkar, R.; Chawla, T.; Chong, L.; et al. PD-8 HERIZON: A phase 2 study of HER-Vaxx (IMU-131), a HER2-targeting peptide vaccine plus SOC chemotherapy in patients with HER2+ advanced stomach cancer—Correlation of the antibody responses and clinical outcome. Ann. Oncol. 2023, 34, S4. [Google Scholar] [CrossRef]
- Maglakelidze, M.; Ryspayeva, D.E.; Andric, Z.; Petrovic, Z.; Bulat, I.; Nikolic, I.; Nagarkar, R.; Wiedermann, U.; Blumenstein, B.A.; Chong, L.M.O.; et al. HERIZON: A phase 2 study of HER-Vaxx (IMU-131), a HER2-targeting peptide vaccine, plus standard of care chemotherapy in patients with HER2-overexpressing metastatic or advanced gastric/GEJ adenocarcinoma—Overall survival analysis. J. Clin. Oncol. 2023, 41 (Suppl. S4), 289. [Google Scholar] [CrossRef]
- O’Shea, A.E.; Clifton, G.T.; Peoples, G.E. Results from a randomized trial combining trastuzumab with a peptide vaccine suggest a role for HER2-targeted therapy in triple-negative breast cancer. Oncotarget 2021, 12, 2318–2319. [Google Scholar] [CrossRef]
- Chick, R.C.; Clifton, G.T.; Hale, D.F.; Vreeland, T.J.; Hickerson, A.T.; Kemp Bohan, P.M.; McCarthy, P.M.; Litton, J.K.; Alatrash, G.; Murthy, R.K.; et al. Subgroup analysis of nelipepimut-S plus GM-CSF combined with trastuzumab versus trastuzumab alone to prevent recurrences in patients with high-risk, HER2 low-expressing breast cancer. Clin. Immunol. 2021, 225, 108679. [Google Scholar] [CrossRef]
- Fisk, B.; Blevins, T.L.; Wharton, J.T.; Ioannides, C.G. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J. Exp. Med. 1995, 181, 2109–2117. [Google Scholar] [CrossRef]
- Razazan, A.; Behravan, J.; Arab, A.; Barati, N.; Arabi, L.; Gholizadeh, Z.; Hatamipour, M.; Reza Nikpoor, A.; Momtazi-Borojeni, A.A.; Mosaffa, F.; et al. Conjugated nanoliposome with the HER2/neu-derived peptide GP2 as an effective vaccine against breast cancer in mice xenograft model. PLoS ONE 2017, 12, e0185099. [Google Scholar] [CrossRef]
- Razazan, A.; Nicastro, J.; Slavcev, R.; Barati, N.; Arab, A.; Mosaffa, F.; Jaafari, M.R.; Behravan, J. Lambda bacteriophage nanoparticles displaying GP2, a HER2/neu derived peptide, induce prophylactic and therapeutic activities against TUBO tumor model in mice. Sci. Rep. 2019, 9, 2221. [Google Scholar] [CrossRef]
- Carmichael, M.G.; Benavides, L.C.; Holmes, J.P.; Gates, J.D.; Mittendorf, E.A.; Ponniah, S.; Peoples, G.E. Results of the first phase 1 clinical trial of the HER-2/ neu peptide (GP2) vaccine in disease-free breast cancer patients. Cancer 2010, 116, 292–301. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Ardavanis, A.; Litton, J.K.; Shumway, N.M.; Hale, D.F.; Murray, J.L.; Perez, S.A.; Ponniah, S.; Baxevanis, C.N.; Papamichail, M.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 2016, 7, 66192–66201. [Google Scholar] [CrossRef]
- Clive, K.S.; Tyler, J.A.; Clifton, G.T.; Holmes, J.P.; Ponniah, S.; Peoples, G.E.; Mittendorf, E.A. The GP2 peptide: A HER2/ neu -based breast cancer vaccine. J. Surg. Oncol. 2012, 105, 452–458. [Google Scholar] [CrossRef]
- Brown, T.A.; Mittendorf, E.A.; Hale, D.F.; Myers, J.W.; Peace, K.M.; Jackson, D.O.; Greene, J.M.; Vreeland, T.J.; Clifton, G.T.; Ardavanis, A.; et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res. Treat. 2020, 181, 391–401. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Storrer, C.E.; Shriver, C.D.; Ponniah, S.; Peoples, G.E. Investigating the Combination of Trastuzumab and HER2/neu Peptide Vaccines for the Treatment of Breast Cancer. Ann. Surg. Oncol. 2006, 13, 1085–1098. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Storrer, C.E.; Foley, R.J.; Harris, K.; Jama, Y.; Shriver, C.D.; Ponniah, S.; Peoples, G.E. Evaluation of the HER2/ neu -derived peptide GP2 for use in a peptide-based breast cancer vaccine trial. Cancer 2006, 106, 2309–2317. [Google Scholar] [CrossRef]
- Manjili, M.H.; Wang, X.-Y.; Chen, X.; Martin, T.; Repasky, E.A.; Henderson, R.; Subjeck, J.R. HSP110-HER2/ neu Chaperone Complex Vaccine Induces Protective Immunity Against Spontaneous Mammary Tumors in HER-2/ neu Transgenic Mice. J. Immunol. 2003, 171, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.N.; Guthrie, K.A.; Liu, Y.; Coveler, A.L.; Higgins, D.M.; Childs, J.S.; Dang, Y.; Salazar, L.G. Safety and Outcomes of a Plasmid DNA Vaccine Encoding the ERBB2 Intracellular Domain in Patients with Advanced-Stage ERBB2-Positive Breast Cancer. JAMA Oncol. 2023, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Pizzatti, L.; Corrêa, S.; Binato, R.; Lemos, G.F.; da Silva do Amaral Herrera, A.C.; Seixas, T.F.; Cecchini, R.; Abdelhay, E. The positive is inside the negative: HER2-negative tumors can express the HER2 intracellular domain and present a HER2-positive phenotype. Cancer Lett. 2015, 357, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Gillogly, M.E.; Kallinteris, N.L.; Xu, M.; Gulfo, J.V.; Humphreys, R.E.; Murray, J.L. Ii-Key/HER-2/ neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol. Immunother. 2004, 53, 490–496. [Google Scholar] [CrossRef]
- Perez, S.A.; Kallinteris, N.L.; Bisias, S.; Tzonis, P.K.; Georgakopoulou, K.; Varla-Leftherioti, M.; Papamichail, M.; Thanos, A.; von Hofe, E.; Baxevanis, C.N. Results from a Phase I Clinical Study of the Novel Ii-Key/HER-2/neu(776–790) Hybrid Peptide Vaccine in Patients with Prostate Cancer. Clin. Cancer Res. 2010, 16, 3495–3506. [Google Scholar] [CrossRef]
- Yang, C.; Murray, J.L.; Ibrahim, N.K. MUC1 and Cancer Immunotherapy. In Immunology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–240. [Google Scholar] [CrossRef]
- Shim, J.Y.; An, H.J.; Lee, Y.H.; Kim, S.K.; Lee, K.P.; Lee, K.S. Overexpression of Cyclooxygenase-2 Is Associated with Breast Carcinoma and Its Poor Prognostic Factors. Mod. Pathol. 2003, 16, 1199–1204. [Google Scholar] [CrossRef]
- Pockaj, B.A.; Basu, G.D.; Pathangey, L.B.; Gray, R.J.; Hernandez, J.L.; Gendler, S.J.; Mukherjee, P. Reduced T-Cell and Dendritic Cell Function Is Related to Cyclooxygenase-2 Overexpression and Prostaglandin E2 Secretion in Patients with Breast Cancer. Ann. Surg. Oncol. 2004, 11, 328–339. [Google Scholar] [CrossRef]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Antigen-specific vaccines for cancer treatment. Hum. Vaccin. Immunother. 2014, 10, 3332–3346. [Google Scholar] [CrossRef]
- Suekane, S.; Yutani, S.; Toh, U.; Yoshiyama, K.; Itoh, K. Immune responses of patients without cancer recurrence after a cancer vaccine over a long term. Mol. Clin. Oncol. 2022, 16, 112. [Google Scholar] [CrossRef]
- Emens, L.A. Cancer vaccines: On the threshold of success. Expert. Opin. Emerg. Drugs 2008, 13, 295–308. [Google Scholar] [CrossRef]
- Tucker, Z.C.G.; Laguna, B.A.; Moon, E.; Singhal, S. Adjuvant immunotherapy for non-small cell lung cancer. Cancer Treat. Rev. 2012, 38, 650–661. [Google Scholar] [CrossRef]
- Enokida, T.; Moreira, A.; Bhardwaj, N. Vaccines for immunoprevention of cancer. J. Clin. Investig. 2021, 131, e146956. [Google Scholar] [CrossRef]
- Kerr, M.D.; McBride, D.A.; Chumber, A.K.; Shah, N.J. Combining therapeutic vaccines with chemo- and immunotherapies in the treatment of cancer. Expert. Opin. Drug Discov. 2021, 16, 89–99. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Pounraj, S.; Chen, S.; Ma, L.; Mazzieri, R.; Dolcetti, R.; Rehm, B.H.A. Targeting Tumor Heterogeneity with Neoantigen-Based Cancer Vaccines. Cancer Res. 2024, 84, 353–363. [Google Scholar] [CrossRef]
- Gianneschi, G.; Scolpino, A.; Oleske, J. A systematic review of the risk of autoimmunity, cancer seeding, and adverse events in human trials of whole-tissue autologous therapeutic vaccines. Cancer Pathog. Ther. 2024, 3, 129–134. [Google Scholar] [CrossRef]
- Copier, J.; Whelan, M.; Dalgleish, A. Biomarkers for the Development of Cancer Vaccines. Mol. Diagn. Ther. 2006, 10, 337–343. [Google Scholar] [CrossRef]
- Hawsawi, Y.M.; Khoja, B.; Aljaylani, A.O.; Jaha, R.; AlDerbi, R.M.; Alnuman, H.; Khan, M.I. Recent progress and applications of single-cell sequencing technology in breast cancer. Front. Genet. 2024, 15, 1417415. [Google Scholar] [CrossRef]
- An, J.; Lu, Y.; Chen, Y.; Chen, Y.; Zhou, Z.; Chen, J.; Peng, C.; Huang, R.; Peng, F. Spatial transcriptomics in breast cancer: Providing insight into tumor heterogeneity and promoting individualized therapy. Front. Immunol. 2024, 15, 1499301. [Google Scholar] [CrossRef]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, H.; Cao, H.; Mao, J.; Chen, X.; Wang, L.; Zhang, N.; Luo, P.; Xue, J.; et al. Liquid biopsy for human cancer: Cancer screening, monitoring, and treatment. MedComm 2024, 5, e564. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.; Khushaish, S.; Luqmani, Y. The effect of lactate dehydrogenase inhibitors on proliferation, motility and invasion of breast cancer cells in vitro highlights a new role for lactate. Mol. Med. Rep. 2023, 29, 12. [Google Scholar] [CrossRef]
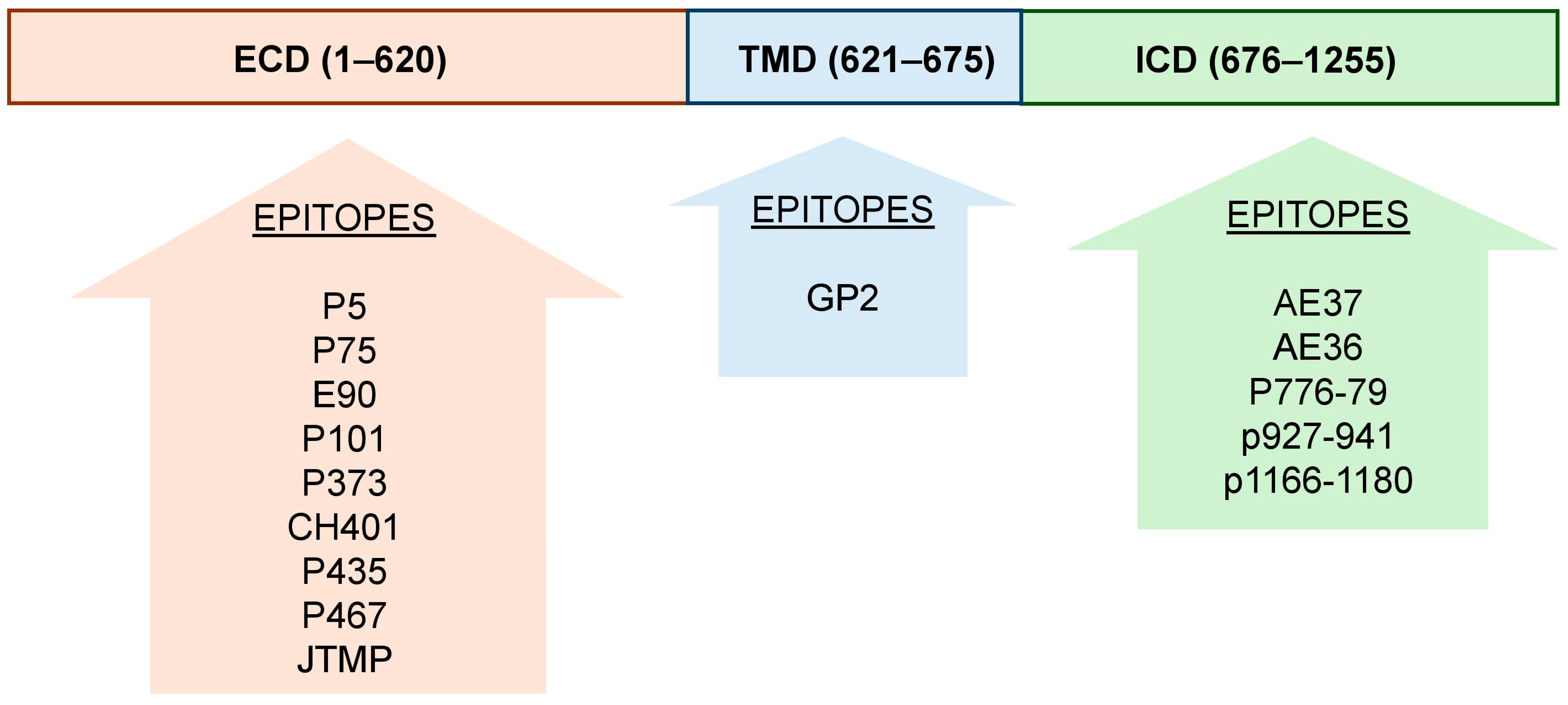
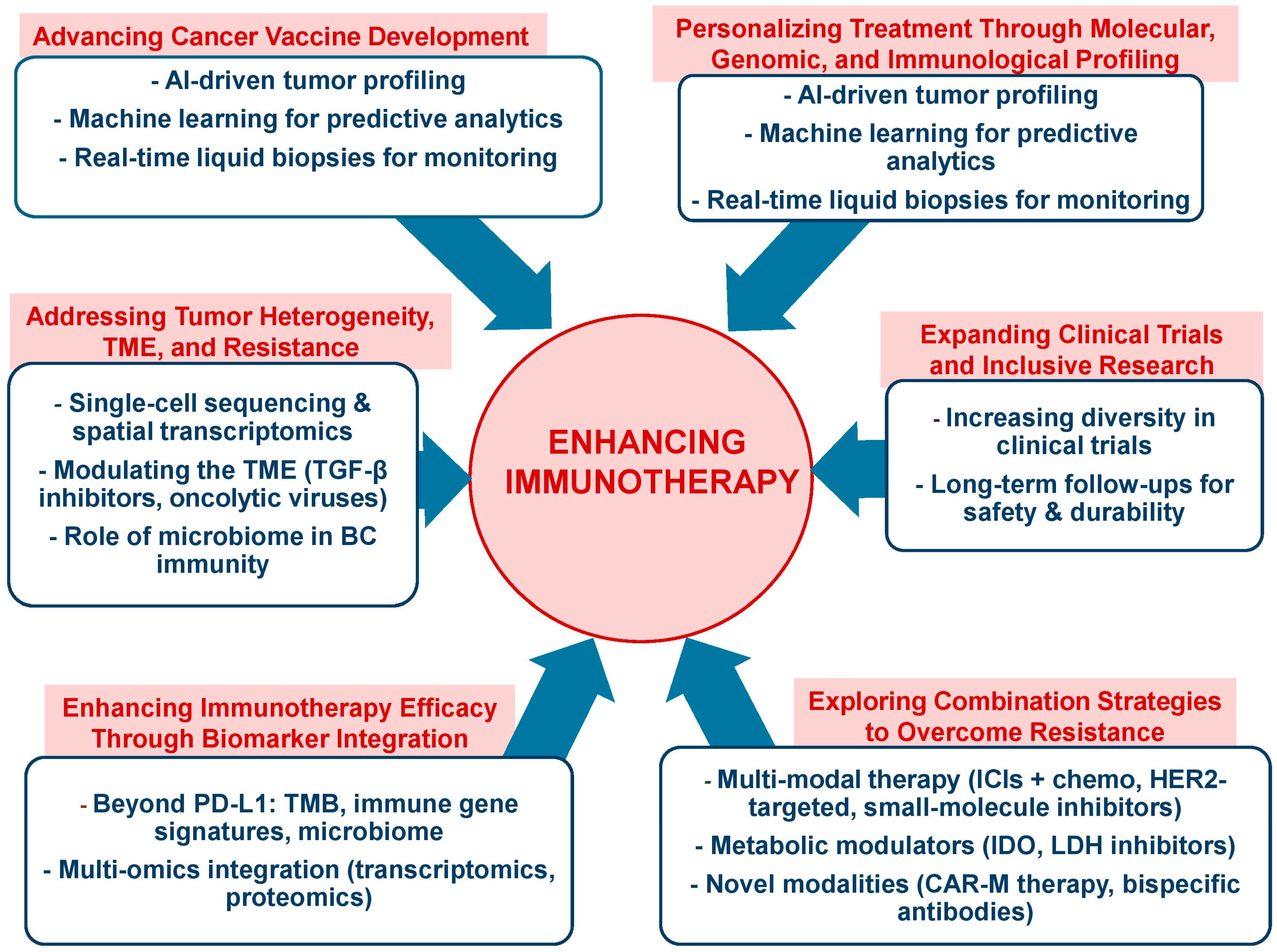
| Factors | Description | Reference |
|---|---|---|
| BC type | By determining immune landscape and treatment response. TNBC responds best to immunotherapy, while HER2+ and HR+ subtypes may require combination approaches for enhanced efficacy. | [5] |
| TMB | By influencing neoantigen production and immune activation. High TMB enhances response to immune checkpoint inhibitors, while low TMB may indicate resistance to immunotherapy. | [6] |
| TILs | By reflecting antitumor immune activity. High TIL levels correlate with better response to immune checkpoint inhibitors, while low TILs may indicate immune evasion and reduced efficacy. | [7] |
| PD-L1 expression | By predicting response to immune checkpoint inhibitors. High PD-L1 levels enhance immunotherapy efficacy, while low expression may indicate limited benefit from checkpoint blockade therapies. | [8] |
| HER2 resistance | By limiting response to HER2-targeted therapies and altering tumor immune evasion. Overcoming resistance may require combination strategies, including immune checkpoint inhibitors and novel immunotherapies. | [9] |
| TME | By comprising cancer cells, stromal components, blood vessels, and immune cells, all of which dynamically interact to influence tumor progression and therapy response. | [10] |
| Factor | Type BC | Value | Luminal A (HR+, HER2−) | Luminal B (HR+, HER2+) | HER2-Enriched (HR−, HER2+) | TNBC (HR−, HER2−) | Ductal | Lobular | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TMB threshold ≥ 10 mut/MB | Metastatic BC | %High (total number) | 7.8% (3087) | 8.3% (266) | 7.8% (179) | 8.6% (1476) | 5.1% (2273) | 16% (349) | [11,12] |
| TIL | Early breast cancer | %Low/%High (total number) | 83%/17% (46) | 45%/55% (42) | 27%/73% (92) | [13] | |||
| Invasive breast cancers | %TIL density (total number) | 11.1% (162) | 31.7% (101) | 38.1% (147) | [14] | ||||
| PD-1 Expression | Invasive breast tumor | Percent (total number) | 40% (10) | 47.8% (23) | 60% (10) | 50% (4) | [15] | ||
| Metastatic lymph node | 20% (10) | 14% | 5% | 75% | [15] | ||||
| Early breast cancer | 13% (46) | 19% (42) | 28% (92) | [13] | |||||
| PD-L1 Expression | Invasive breast cancers | Percent (total number) | 53.1% (162) | 73.3% (101) | 84.4% (147) | [14] | |||
| Invasive breast tumor | 20% (10) | 34.7% (23) | 20% (10) | 50% (4) | [15] | ||||
| Metastatic lymph node | 0% (10) | 17.4% (23) | 10% (10) | 50% (4) | [15] | ||||
| Early breast cancer | 20% (46) | 31% (42) | 43% (92) | [13] | |||||
| TME | Metastatic BC | Median T-cell -imflamed score | 3.96 | 4.02 | 4.2 | 4.17 | 4.14 | 3.90 | [11] |
| Therapy/Drug | Description | Reference |
|---|---|---|
| HER2 Block | ||
| Monoclonal Antibodies | Trastuzumab binds to subdomain IV, blocking HER2 dimerization and activating antibody-dependent cellular cytotoxicity (ADCC). It is a key treatment for HER2-positive breast and gastric cancers, significantly improving survival. Pertuzumab targets subdomain II, preventing HER2/HER3 dimerization and enhancing trastuzumab’s effects when combined with chemotherapy. This combination is a standard treatment for HER2-positive metastatic breast cancer, offering superior outcomes. | [79] |
| Small Molecule TKIs | Lapatinib and tucatinib inhibit HER2’s intracellular kinase domain, blocking tumor growth. Tucatinib is FDA-approved for combination therapy, while lapatinib helps overcome trastuzumab resistance in HER2-positive breast cancer. | [80] |
| ADCs | ADCs combine monoclonal antibodies with cytotoxic agents for targeted cancer therapy. They selectively bind tumor antigens, internalize, and release potent drugs, minimizing toxicity. ADCs offer precision treatment, bystander effects, and immune activation, enhancing efficacy in resistant cancers. | [81] |
| Immune Checkpoint Inhibition | ||
| PD-1/PD-L1 | PD-1, an inhibitory receptor on T-cells, suppresses antitumor immunity via interactions with PD-L1/PD-L2. Checkpoint inhibitors, like pembrolizumab and atezolizumab, block this pathway, restoring T-cell function and enhancing cancer immunotherapy efficacy. | [82] |
| CTLA-4 | CTLA-4 inhibits T-cell activation by outcompeting CD28 for B7 ligands on APCs. Ipilimumab blocks CTLA-4, restoring CD28 signaling and enhancing T-cell activation, leading to stronger antitumor immune responses in cancer immunotherapy. | [83] |
| CAR-M | CAR-M harness macrophages’ phagocytic and antigen-presenting abilities to target tumors and reshape the immunosuppressive tumor microenvironment. CAR-M therapy enhances immune activation, overcomes resistance to immunotherapy, and shows promise in breast cancer treatment. | [84] |
| Therapy/Drug | Description | Reference |
|---|---|---|
| Protein- or Whole-Cell-Based Vaccines | Protein-based vaccines, like HER2 vaccines, enhance antibody and T-cell activation, while whole-cell vaccines provide broad antigen coverage, reducing immune escape. These vaccines hold promise for cancer immunotherapy, especially in combination therapies. | [204] |
| DNA and RNA Vaccines | DNA and RNA vaccines use genetic material to stimulate immune responses against tumor-associated antigens. DNA vaccines rely on plasmid DNA to encode tumor antigens, while mRNA vaccines provide direct instructions for antigen production. mRNA vaccines have shown promise, particularly for HER2-positive cancers, due to their rapid development and strong immune activation. | [205] |
| Dendritic Cell (DC) Vaccines | Dendritic cell (DC) vaccines utilize the antigen-presenting abilities of dendritic cells to stimulate targeted anticancer immune responses. These vaccines are created by isolating a patient’s dendritic cells, exposing them to tumor antigens, and reintroducing them to activate T-cells against cancer. | [206] |
| Viral Vector-Based Vaccines | Viral vector-based vaccines use modified viruses, such as adenoviruses or poxviruses, to deliver tumor-associated antigen (TAA) genes into host cells, stimulating both humoral and cellular immune responses against cancer. | [207] |
| Epitope-Based Vaccines | Peptide-based vaccines target tumor-associated (TAAs) and tumor-specific antigens (TSAs) to stimulate immune responses, while minimizing damage to healthy tissues. | [208] |
| Name | Origin Protein | Position | Amino Acid Sequence | Reference |
|---|---|---|---|---|
| P5 | HER2/neu | ECD | ELAAWCRWGFLLALLPPGIAG | [258] |
| P435 | HER2 | ECD | IRGRILHDGAYSLTLQGLGIH | [258] |
| P5+435 | HER2 | ECD | ELAAWCRWGFLLALLPPGIAGRRIRGRILHDGAYSLTLQGLGIHGGGC | [258] |
| CH401 | HER2 | 163–182 | YQDMVLWKDVFRKNNQLAPV | [259] |
| E75 or NPS or ErbB2368–377 | HER2 | 366–379 | KIFGSLAFL | [260] |
| E90 | HER2 | ECD | CLTSTVQLV | [260] |
| P467 | HER2 | ECD | PESFDGDPASNTAPLQPRVLQGLPREYVNARHSLPYMPIWKFPDEEGAC | [261] |
| JTMP | HER2/neu | 260–301 | HSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACPY | [262] |
| P101 | HER2 | ECD | RLRIVRGQLFEDKYAL | [263] |
| P373 | HER2 | ECD | KIFGSLAFLPESFDGDPS | [263] |
| GP2 | HER2 | 654–662 | IISAVVGIL | [264] |
| AE36, AE37, p776–790 | HER2 | 776–790 | GVGSPYVSRLLGICL | [212] |
| p927–941 | HER2 | 927–941 | PAREIPDLLEKGERL | |
| p1166–1180 | HER2 | 166–1180 | TLERPKTLSPGKNGV | |
| MUC1159–167 | MUC1 | 159–167 | SAPDNRPAL | [265] |
| CEA605–613 | CEA | 605–613 | YLSGADLNL | [266] |
| MAGE-A1 | MAGEA | SQYGEPRKL | [267] | |
| MLP1 | MAGE-A4 | 102−130 | AESLFREALSNKVDELAHFLLRKYRAKEL | [268] |
| MLP2 | MAGE-A4 | 134−160 | AEMLERVIKNYKRCFPVIFGKASESLK | [268] |
| MLP3 | MAGE-A4 | 274−306 | RALAETSYVKVLEHVVRVNARVRIAYPSLR | [268] |
| SART3 | ||||
| SART3-302 | SART3 | 302–310 | LLQAEAPRL | [269] |
| SART3-309 | SART3 | 309–317 | RLAEYQAYI | [269] |
| SART3-109 | SART3 | 109–118 | VYDYNCHVDL | [269] |
| SART3-734 | SART3 | 734–742 | QIRPIFSNR | [269] |
| CypB-129 | Cyclophilin B | 129–138 | KLKHYGPGWV | [269] |
| WHSC2 | ||||
| WHSC2-103 | WHSC2 | 103–111 | ASLDSDPWV | [269] |
| WHSC2-141 | WHSC2 | 141–149 | ILGELREKV | |
| UBE2V-43 | UBE2V | 43–51 | RLQEWCSVI | [269] |
| HNRPL-140 | HNRPL | 140–148 | ALVEFEDVL | [269] |
| Lck | ||||
| Lck-246 | p56lck | 246–254 | KLVERLGAA | [269] |
| Lck-208 | p56lck | 208–216 | HYTNASDGL | [269] |
| Lck-486 | p56lck | 486–494 | TFDYLRSVL | [269] |
| Lck-488 | p56lck | 488–497 | DYLRSVLEDF | [269] |
| Lck-90 | p56lck | 90–99 | ILEQSGEWWK | [269] |
| Lck-449 | p56lck | 449–458 | VIQNLERGYR | [269] |
| MRP3-1293 | MRP3 | 1293–1302 | NYSVRYRPGL | |
| PSA-248 | PSA | 248–257 | HYRKWIKDTI | [270] |
| PAP-213 | PAP | 213–221 | LYCESVHNF | [270] |
| EGFR-800 | EGFR | 800–809 | DYVREHKDNI | [270] |
| PTHrP-102 | PTHrP | 102–111 | RYLTQETNKV | [270] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, M. Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges. Vaccines 2025, 13, 344. https://doi.org/10.3390/vaccines13040344
Palma M. Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges. Vaccines. 2025; 13(4):344. https://doi.org/10.3390/vaccines13040344
Chicago/Turabian StylePalma, Marco. 2025. "Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges" Vaccines 13, no. 4: 344. https://doi.org/10.3390/vaccines13040344
APA StylePalma, M. (2025). Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges. Vaccines, 13(4), 344. https://doi.org/10.3390/vaccines13040344






